Abstract
Candida dubliniensis is often found in mixed culture with C. albicans, but its recognition is hampered as the color of its colonies in primary culture on CHROMagar Candida varies. Furthermore, definite identification of C. dubliniensis is difficult to achieve, time-consuming, and expensive. Therefore, a method to discriminate between these two closely related yeast species by fatty acid methyl ester (FAME) analysis using gas-liquid chromatography (Sherlock Microbial Identification System [MIS]; MIDI, Inc., Newark, Del.) was developed. Although the chromatograms of these two species revealed no obvious differences when applying FAME analysis, a new library (CADLIB) was successfully created using Sherlock Library Generation Software (MIDI). The amount and frequency of FAME was analyzed using library training files (n = 10 for each species), preferentially those comprising reference strains. For testing the performance of the CADLIB, clinical isolates genetically assigned to the respective species (C. albicans, n = 32; C. dubliniensis, n = 28) were chromatographically analyzed. For each isolate tested, MIS computed a similarity index (SI) indicating a hierarchy of possible strain fits. When using the newly created library CADLIB, the SIs for C. albicans and C. dubliniensis ranged from 0.11 to 0.96 and 0.53 to 0.93 (for all but one), respectively. Only three isolates of C. albicans (9.4%) were misidentified as C. dubliniensis, whereas all isolates of C. dubliniensis were correctly identified. Resulting differentiation accuracy was 90.6% for C. albicans and 100% for C. dubliniensis. Cluster analysis and principal component analysis of the resulting FAME profiles showed two clearly distinguishable clusters matching up with two assigned species for the strains tested. Thus, the created library proved to be well suited to discriminate between these two species.
Various reports of the recently described yeast species Candida dubliniensis indicate a worldwide occurrence of this fungus which is phylogenetically closely related to C. albicans (7, 16, 20, 21). C. dubliniensis has so far been mainly recovered from the oral cavity of human immunodeficiency virus (HIV)-infected patients (12) and is rarely present in cases of candidemia in HIV-negative patients. Evidence for the inducibility of a stable fluconazole resistance in vitro in C. dubliniensis strains may indicate an emerging pathogen for immunocompromised patients receiving long-term fluconazole prophylaxis (11). However, the pathogenic potential of C. dubliniensis remains unknown (20).
Since C. dubliniensis is normally found in mixed culture with C. albicans, the color of their colonies during primary culture on CHROMagar Candida, a medium particularly recommended for uncovering mixed yeast cultures, has been investigated (19). Dark green colonies were found to be indicative of C. dubliniensis, in contrast to light green colonies, which indicate the presence C. albicans (19). However, this phenomenon has been found to be nonreproducible after subculture and storage of isolates (19, 20, 24). Recently it was also reported that only 30 of 53 proven C. dubliniensis isolates showed a typical dark green color upon primary culture, whereas C. albicans colonies could display every shade of green on CHROMagar Candida (24). These findings indicate that the color of the colonies on CHROMagar Candida is unreliable for selection of C. dubliniensis.
Various characteristic features to identify C. dubliniensis and to discriminate it from C. albicans have been reported; these include abundant typical chlamydospore formation (21), lack of intracellular β-d-glucosidase activity (19), no or highly restricted growth at 42°C (20), and a distinctive carbohydrate assimilation pattern obtained by application of the API ID 32C system (14, 17). As some C. albicans strains can also exhibit these phenotypical traits, none of these phenotypical characteristics have proved to be fully reliable for discrimination between these two species (7, 22, 24). Recently, Fourier transform-infrared (FT-IR) spectroscopy (23) combined with hierarchical clustering proved to be equally reliable to discriminate between the two species' genotypes (24). Unfortunately however, these methods are labor-intensive and, in the case of FT-IR, mostly unavailable in the average microbiological laboratory. Detection and identification of microorganisms strongly depends on the availability of easy-to-perform screening methods. Fatty acid methyl ester (FAME) analysis using gas-liquid chromatography (Sherlock Microbial Identification System [MIS]; MIDI, Inc., Newark, Del.), is a method widely available in microbiological laboratories and which has successfully been applied to the identification of clinically important yeast species (2). In this study, we test FAME analysis using gas-liquid chromatography for its ability to discriminate between these closely related Candida species, with the objective of providing a reliable method to discriminate between colonies showing different shades of green on CHROMagar Candida.
MATERIALS AND METHODS
Strains and identification.
Stock cultures of yeast isolates were kept on ceramic beads (Microbank; PRO-LAB Diagnostics, Richmond Hill, Ontario, Canada) at −70°C. When cultured on CHROMagar Candida (CHROMagar Company, Paris, France; distributed by Mast Diagnostica, Rheinfeld, Germany) all colonies of the strains used showed various shades of green.
In the case of C. albicans, chromatographic analysis data of nine reference strains (ATCC 90028, DSM 5817, DSM 1665, DSM 11943, DSM 11944, DSM 11945, DSM 11225, DSM 11948, and DSM 1386) and one clinical isolate (GHP 1707, isolated in our routine laboratory from urine of a patient) were used for generation of training files. Details of strains used for the subsequent chromatographic analysis to evaluate the newly created library are given in Table 1 (strains 1 to 32). All clinical isolates of C. albicans had been primarily identified using standard methods, including application of the API ID 32C system (bioMérieux, Nürtingen, Germany) (24) and testing of germ tube formation (13).
TABLE 1.
Comparison of identification and SI revealed by searching the respective databases using FAME profiles of C. albicans and C. dubliniensisa
| Strain no.b | Origin | CADLIB
|
YST28
|
YSTCLN
|
||||
|---|---|---|---|---|---|---|---|---|
| Identification | SI | Distance to next SI | Identification | SI | Identification | SI | ||
| 1 | CBS 1905 (former “C. stellatoidea” type strain) | C. albicansc | 0.180 | —d | C. parapsilosis | 0.502 | C. parapsilosis | 0.454 |
| 2 | Tietz et al. (22) | C. albicans | 0.114 | — | C. albicans | 0.601 | C. albicans | 0.665 |
| 3 | Tietz et al. (22) | C. albicansc | 0.156 | — | C. tropicalis | 0.507 | C. tropicalis | 0.507 |
| 4 | HIV patient (oral rinse) | C. albicans | 0.792 | 0.285 | C. albicans | 0.858 | C. albicans | 0.858 |
| 5 | HIV patient (oral rinse) | C. albicans | 0.838 | — | C. albicans | 0.877 | C. albicans | 0.877 |
| 6 | HIV patient (oral rinse) | C. albicans | 0.556 | 0.175 | C. albicans | 0.661 | C. albicans | 0.661 |
| 7 | Patient (vaginal swab) | C. albicans | 0.800 | 0.341 | C. albicans | 0.576 | C. albicans | 0.576 |
| 8 | Patient (vaginal swab) | C. albicans | 0.246 | — | C. albicans | 0.318 | No match | |
| 9 | Patient (vaginal swab) | C. albicans | 0.920 | 0.447 | C. albicans | 0.751 | C. albicans | 0.751 |
| 10 | Patient (vaginal swab) | C. albicans | 0.839 | — | C. albicans | 0.639 | C. albicans | 0.639 |
| 11 | Patient (vaginal swab) | C. albicans | 0.768 | — | C. albicans | 0.724 | C. albicans | 0.724 |
| 12 | Patient (urine) | C. albicans | 0.901 | 0.39 | C. albicans | 0.808 | C. albicans | 0.808 |
| 13 | Patient (urine) | C. albicans | 0.969 | — | C. albicans | 0.857 | C. albicans | 0.857 |
| 14 | Patient (vaginal swab) | C. albicans | 0.783 | — | C. albicans | 0.544 | C. albicans | 0.544 |
| 15 | Patient (blood) | C. dubliniensise | 0.911 | — | C. albicans | 0.516 | C. albicans | 0.516 |
| 16 | Patient (urine) | C. albicans | 0.860 | 0.423 | C. albicans | 0.808 | C. albicans | 0.808 |
| 17 | Patient (urine) | C. albicans | 0.710 | 0.146 | C. albicans | 0.558 | C. albicans | 0.558 |
| 18 | Patient (urine) | C. albicans | 0.786 | 0.322 | C. albicans | 0.889 | C. albicans | 0.889 |
| 19 | Patient (vaginal swab) | C. albicans | 0.691 | 0.221 | C. albicans | 0.578 | C. albicans | 0.578 |
| 20 | Patient (vaginal swab) | C. albicans | 0.577 | — | C. albicans | 0.626 | C. albicans | 0.626 |
| 21 | Patient (urine) | C. albicans | 0.790 | 0.210 | C. albicans | 0.697 | C. albicans | 0.697 |
| 22 | Patient (blood) | C. dubliniensise | 0.933 | — | C. albicans | 0.567 | C. albicans | 0.567 |
| 23 | Patient (urine) | C. albicans | 0.081 | — | C. albicans | 0.524 | C. albicans | 0.524 |
| 24 | Patient (urine) | C. albicans | 0.853 | 0.305 | C. albicans | 0.778 | C. albicans | 0.778 |
| 25 | Patient (vaginal swab) | C. albicans | 0.642 | 0.291 | C. albicans | 0.702 | C. albicans | 0.702 |
| 26 | Patient (vaginal swab) | C. albicans | 0.728 | 0.330 | C. albicans | 0.782 | C. albicans | 0.782 |
| 27 | Patient (vaginal swab) | C. albicans | 0.854 | 0.264 | C. albicans | 0.783 | C. albicans | 0.783 |
| 28 | DSM 70014 | C. albicans | 0.480 | — | C. sake | 0.627 | C. albicans | 0.626 |
| 29 | ATCC 10261 | C. albicans | 0.869 | — | C. guilliermondii | 0.664 | C. guilliermondii | 0.703 |
| 30 | HIV patient (oral rinse) | C. albicans | 0.968 | — | C. albicans | 0.791 | C. albicans | 0.791 |
| 31 | HIV patient (oral rinse) | C. albicans | 0.182 | — | C. albicans | 0.337 | No match | |
| 32 | Tietz et al. (22) | C. dubliniensisc,e | 0.809 | — | C. albicans | 0.299 | No match | |
| 33 | Patient (throat swab) | C. dubliniensis | 0.008 | — | Zygosaccharomyces fermentati | 0.649 | No match | |
| 34 | HIV patient (oral rinse) | C. dubliniensis | 0.824 | — | C. albicans | 0.616 | C. albicans | 0.616 |
| 35 | HIV patient (oral rinse) | C. dubliniensis | 0.879 | — | C. albicans | 0.567 | C. albicans | 0.567 |
| 36 | HIV patient (oral rinse) | C. dubliniensis | 0.900 | — | C. albicans | 0.468 | C. albicans | 0.468 |
| 37 | HIV patient (oral rinse) | C. dubliniensis | 0.790 | — | C. albicans | 0.430 | C. albicans | 0.430 |
| 38 | HIV patient (oral rinse) | C. dubliniensis | 0.931 | — | C. albicans | 0.658 | C. albicans | 0.658 |
| 39 | HIV patient (oral rinse) | C. dubliniensis | 0.828 | — | C. albicans | 0.693 | C. albicans | 0.693 |
| 40 | HIV patient (oral rinse) | C. dubliniensis | 0.859 | — | C. albicans | 0.625 | C. albicans | 0.625 |
| 41 | HIV patient (oral rinse) | C. dubliniensis | 0.847 | — | C. albicans | 0.425 | C. albicans | 0.425 |
| 42 | HIV patient (oral rinse) | C. dubliniensis | 0.890 | — | C. albicans | 0.333 | No match | |
| 43 | HIV patient (oral rinse) | C. dubliniensis | 0.826 | — | C. albicans | 0.527 | C. albicans | 0.527 |
| 44 | HIV patient (oral rinse) | C. dubliniensis | 0.755 | — | C. albicans | 0.590 | C. albicans | 0.590 |
| 45 | HIV patient (oral rinse) | C. dubliniensis | 0.889 | — | C. albicans | 0.567 | C. albicans | 0.567 |
| 46 | HIV patient (oral rinse) | C. dubliniensis | 0.796 | — | C. albicans | 0.443 | C. albicans | 0.443 |
| 47 | HIV patient (oral rinse) | C. dubliniensis | 0.584 | — | C. albicans | 0.348 | No match | |
| 48 | HIV patient (oral rinse) | C. dubliniensis | 0.587 | — | C. albicans | 0.432 | C. albicans | 0.432 |
| 49 | HIV patient (oral rinse) | C. dubliniensis | 0.922 | — | C. albicans | 0.497 | C. albicans | 0.497 |
| 50 | HIV patient (oral rinse) | C. dubliniensis | 0.814 | — | C. albicans | 0.351 | No match | |
| 51 | HIV patient (oral rinse) | C. dubliniensis | 0.583 | — | C. albicans | 0.445 | C. albicans | 0.445 |
| 52 | HIV patient (oral rinse) | C. dubliniensis | 0.906 | — | C. albicans | 0.61 | C. albicans | 0.610 |
| 53 | HIV patient (oral rinse) | C. dubliniensis | 0.863 | — | C. albicans | 0.502 | C. albicans | 0.502 |
| 54 | HIV patient (oral rinse) | C. dubliniensis | 0.547 | — | C. albicans | 0.258 | No match | |
| 55 | NCPF 3108 | C. dubliniensis | 0.869 | — | C. albicans | 0.550 | C. albicans | 0.550 |
| 56 | HIV patient (oral rinse) | C. dubliniensis | 0.905 | — | C. albicans | 0.465 | C. albicans | 0.465 |
| 57 | HIV patient (oral rinse) | C. dubliniensis | 0.877 | — | C. albicans | 0.454 | C. albicans | 0.454 |
| 58 | HIV patient (oral rinse) | C. dubliniensis | 0.825 | — | C. albicans | 0.447 | C. albicans | 0.447 |
| 59 | HIV patient (oral rinse) | C. dubliniensis | 0.531 | — | C. albicans | 0.605 | C. albicans | 0.605 |
| 60 | Patient (sputum) | C. dubliniensis | 0.749 | — | C. albicans | 0.689 | C. albicans | 0.689 |
C. albicans and C. dubliniensis identified as indicated by the respective FAME library.
Numbers 1 to 32, C. albicans identified genetically as stated in the text; numbers 33 to 60, C. dubliniensis identified genetically. Numbering in the dendrogram (Fig. 2) and the dot plot (Fig. 3) is done accordingly.
C. stellatoidea revealed by sequencing.
No next SI reported, thereby signifying good identification.
Isolate of C. albicans falsely identified as C. dubliniensis when using the CADLIB.
For C. dubliniensis chromatographic analysis, data on two C. dubliniensis reference strains (CBS 7987T and CBS 7988), and eight clinical isolates (GHP 1244, GHP 1321, GHP 1342, GHP 1343, GHP 1345, GHP 1465, GHP 1456, and GHP 1317) cultured from oral rinses of HIV-infected patients attending an outpatient clinic for infectious diseases at the Humboldt University in Berlin, Germany (24), were used as training files for library development. These clinical isolates have been extensively characterized phenotypically for chlamydospore formation, no or highly restricted growth at 42°C, carbohydrate assimilation patterns (API ID 32C), and by FT-IR spectroscopy (24). Details of these strains used for evaluation are shown in Table 1 (strains 33 to 60).
All strains used in the present study, with the exception of reference strains of C. albicans (n = 12) (see above and Table 1) were genotypically assigned to the respective species (C. albicans, n = 30; C. dubliniensis, n = 38) by sequencing 500 bp of the 5′ end of the nuclear large ribosomal subunit (LSU) rRNA gene (rDNA) using recently designed primers (10). Details of the methods used have been previously published (6, 24). Concatenation of the resulting sequences and alignment, using the respective sequence of C. albicans (EMBL X70659) as a reference sequence, were done with the help of the DSCE software package (4). As distinctive signature nucleotides, the following were used: for C. albicans (position in EMBL X70659), A 278, G 288, U 492, C 494, G 496; the homolog positions of C. albicans (position in EMBL Z48345; former type strain of “C. stellatoidea”) A 126, G 136, U 340, U 342, G 344; and in the case of C. dubliniensis (position in EMBL U57685) G 176, A 186, A 390, U 392, A 394. Accordingly, three of the C. albicans strains could be identified as “C. stellatoidea,” which has now being identified as C. albicans.
Library development and chromatographic analysis.
For library development respective strains were unfrozen and, to induce fatty acid production, subcultured twice on Sabouraud dextrose agar (SDA) (Becton Dickinson, Heidelberg, Germany) and then incubated aerobically at 28°C for 24 h. The SDA had been purchased in dehydrated form comprising different lots and was freshly prepared in-house prior to use.
Ten strains of each species (n = 20) were used for library generation, and as recommended (MIDI technical note 103) they comprised preferentially reference strains (C. albicans, n = 9; C. dubliniensis, n = 3). These reference strains and some clinical isolates (n = 8), were cultured on SDA and incubated accordingly. The biomass (40 ± 2 mg) recovered from the third quadrant was saponified (sodium hydroxide in methanol). Cellular fatty acids were methylated (hydrochloric acid in methanol), extracted (hexane in methyl tert-butyl ether), and cleaned (sodium hydroxide) as specified by Sasser (18).
Chromatographic analysis was performed using the MIS along with the YEAST28 method. The MIS includes a gas chromatograph (6890 series; Hewlett-Packard, Avondale, Pa.) with a flame ionization detector along with an autosampler and an integrator, coupled to a computer system. The Sherlock computer software (version 2.95; MIDI, Inc.) automatically sets the operating parameters of the gas chromatograph each time a sample is processed. Coupled to Sherlock is the ChemStation software (version 4.02; Hewlett-Packard) used for operating sampling, analysis, and integration of the chromatographic samples.
FAME profiles of the primarily analyzed strains (n = 10 for each species) were used as training files to create a new library entry (CADLIB, short for C. albicans-C. dubliniensis library) (Table 2) using the Library Generation Software (LGS) (MIDI, Inc.) following the Sherlock guidelines (18). The FAME analysis of the library training files was performed in duplicate, revealing reproducible FAME profiles (data not shown).
TABLE 2.
Data profile used for the newly created librarya entry (CADLIB) enabling the discrimination of C. albicans and C. dubliniensis
| Fatty acid |
C. albicans
|
C. dubliniensis
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nb | Mean (%) | SD | Minimum value (strain no.c) | Maximum value (strain no.c) | nb | Mean (%) | SD | Minimum value (strain no.c) | Maximum value (strain no.c) | |
| 14:0 | 9 | 0.91 | 0.37 | 0.00 (3) | 1.30 (7) | 6 | 0.50 | 0.44 | 0.00 (3) | 1.30 (7) |
| 16:1 cis 9 (ω7) | 10 | 8.41 | 1.17 | 6.35 (10) | 11.33 (7) | 10 | 8.91 | 2.17 | 6.14 (6) | 13.17 (5) |
| 16:0 | 10 | 14.96 | 0.81 | 13.71 (10) | 16.48 (5) | 10 | 15.01 | 1.57 | 10.69 (7) | 16.05 (9) |
| 17:1 cis 9 (ω8) | 9 | 1.66 | 0.87 | 0.00 (2) | 3.28 (8) | 10 | 2.22 | 0.51 | 1.47 (4) | 3.26 (5) |
| 18:2 cis 9, 12/18:0a | 10 | 27.85 | 1.83 | 25.47 (3) | 30.54 (10) | 10 | 18.65 | 1.96 | 14.52 (5) | 22.24 (7) |
| 18:0 | 10 | 4.20 | 0.80 | 3.00 (7) | 5.42 (4) | 10 | 5.70 | 0.86 | 4.11 (2) | 6.84 (6) |
| 18:1 cis 8 (ω9) | 10 | 41.43 | 2.08 | 38.34 (8) | 45.06 (3) | 10 | 47.31 | 2.25 | 45.41 (1) | 52.69 (7) |
Library training parameters set for the CADLIB entry according to the MIS instructions as follows: quality threshold, 0.25 (mathematical tool for standardizing the impact of a fatty acid in a library entry); maximum misses, 3; maximum standard deviation, 4.0.
Number of strains used.
Numbering in order of strains listed in Materials and Methods.
Performance of the CADLIB was tested by loading the newly created CADLIB along with the two commercially available yeast libraries (YST28 and YSTCLN [both version 3.8]) using 32 C. albicans and 28 C. dubliniensis strains (Table 1). The calibration mixture (MIDI, Inc.) was chromatographically analyzed at the beginning and at least every 10 runs, as a measure of quality control.
Cluster analysis by unweighted pair matching and principal component analysis of the chromatographic data was performed using the implemented software of the LGS.
RESULTS
Library development.
Figure 1 shows typical chromatograms and MIS reports for one C. albicans and C. dubliniensis strain respectively, which were used as training files for the new library entry CADLIB. When judged by the naked eye no obvious differences could be seen. Nevertheless, after entering all the training files, two subgroups corresponding to both of the respective species could be identified by data entries displayed as histogram distance (data not shown). Each subgroup was renamed accordingly. As recommended the relationship of the samples of the respective subgroup was edited to reveal all samples within 3 standard deviations from the mean (MIDI technical note 103). This was achieved by creating a histogram for each fatty acid detected showing the distance of each sample from the mean sample value. As recommended, samples farther than 3 standard deviations away from the mean were removed (18). The final fatty acid profiles used for each subgroup showing the relationship of the data files used in the new library entry CADLIB are shown in Table 2.
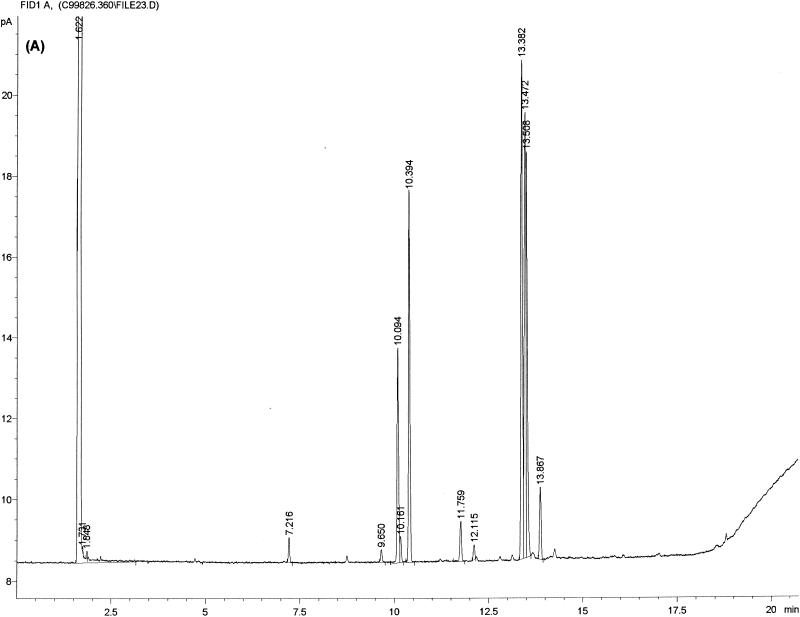
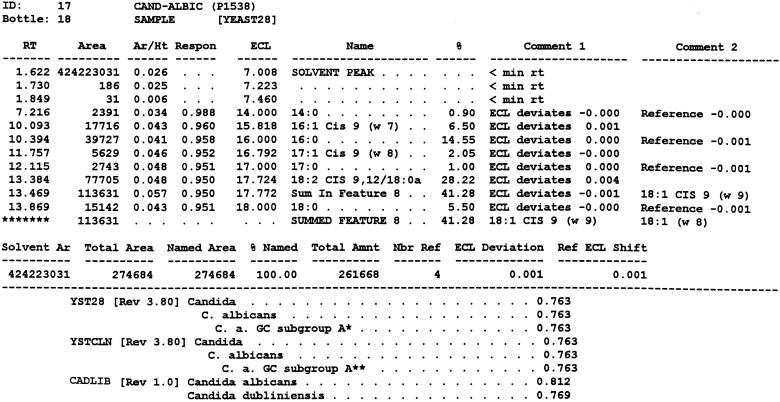
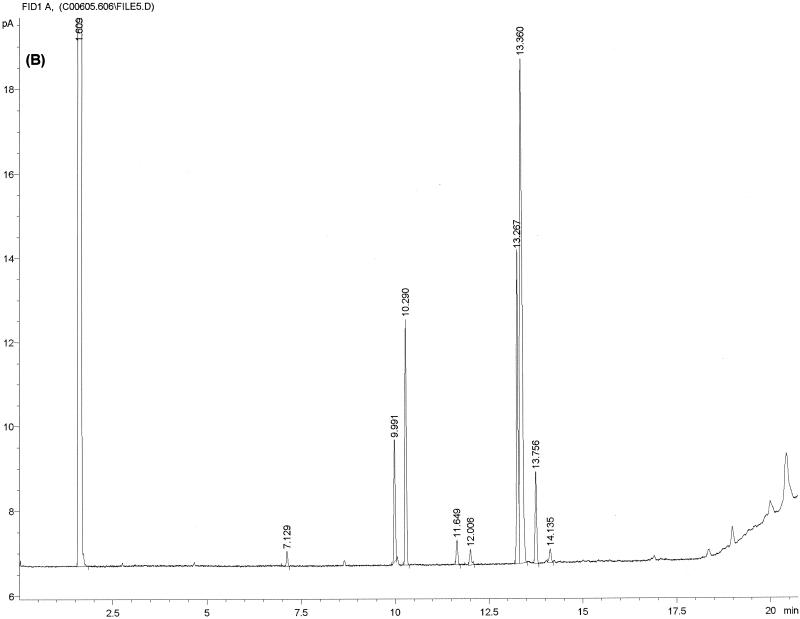
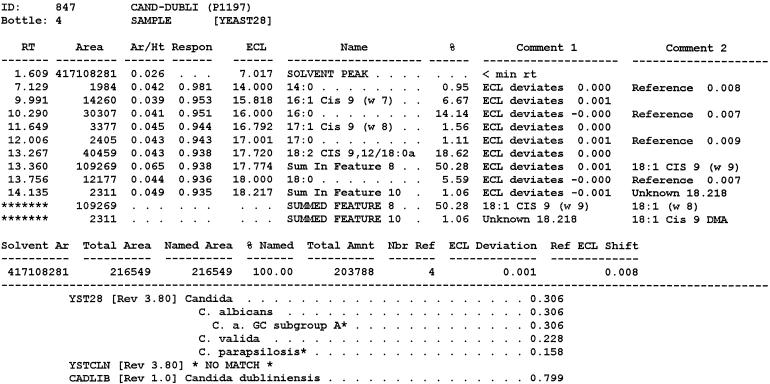
FIG. 1.
Typical chromatogram and MIS report using FAME analysis and YEAST28 method. (A) C. albicans DSM 11943; (B) C. dubliniensis CBS 7987T. The MIS report comprises the chromatogram, the composition report (text above the continuous dotted line) and library search report (text below the continuous dotted line). In the composition report each peak of the chromatogram is listed by retention time (RT), area, and area/height ratio (Ar/Ht). A linear interpolation of each peak's retention between two saturated straight-chain FAME reference peaks is referred to here as equivalent chain length (ECL). The MIS software compares the ECL of each peak in the analysis with the expected ECL of the fatty acids (results are found in the column Name). After the peak areas are modified by the quantitative response factor (Respon) and normalized to 100%, the resulting weight is listed as a percentage (%). The library search report lists the most likely matches by searching the loaded libraries and provides an SI (for details, see Discussion) for each of them. A high SI (>0.5) indicates a good match. The commercially available yeast libraries Yeast28 (YST28) (preferentially for identification of environmental yeast isolates) and Yeast Clinical (YSTCLN, preferentially for identification of clinical yeast isolates) and the newly created entry CADLIB (intended for differentiation between C. albicans and C. dubliniensis) were used.
Performance of the newly created library.
Similarity indices (SIs) indicating the range of choice (see also Discussion) of the subsequent chromatographic analysis of 60 isolates comprising 32 C. albicans and 28 C. dubliniensis strains using the new CADLIB entry in comparison with the results of the two commercially available databases (YST 28 and YSTCLN) (see also Discussion) are shown in Table 1. When using the CADLIB, 3 of 32 C. albicans isolates tested (strains 15, 22, and 32) were misidentified as C. dubliniensis. In the remaining cases of correctly identified C. albicans isolates (n = 29) the SI ranged from 0.114 to 0.96 and was ≥0.5 in the case of 22 isolates. Because for 15 C. albicans strains (Table 1) no further species was listed, identification was concluded as unequivocal. In the other cases (n = 14) the SI distance to C. dubliniensis was always ≥0.14 (Table 1).
In comparison, the commercially available libraries, YST28 and YSTCLN, misidentified four and three of the C. albicans isolates, respectively (Table 1). Additionally, three C. albicans strains were not identified by YSTCLN, which reported no match. The resulting predictive values for identification of C. albicans by the respective libraries were 90.7% for the newly created library, 87.5% for YST28, and 82.3% for YSTCLN.
When using the newly created CADLIB entry, all C. dubliniensis strains tested (n = 28) were definitely identified (predictive value, 100%) to the species level (SI ranging from 0.531 to 0.93, with one extreme value of 0.008 in the case of strain 33) without listing C. albicans as a secondary choice. The commercially available libraries, YST28 and YSTCLN, misidentified C. dubliniensis as C. albicans in 96.4 and 82.1% of these strains, respectively. Additionally, YSTCLN failed to identify (no match) any of the five C. dubliniensis isolates.
Using API ID 32C, none of the C. dubliniensis isolates tested (n = 28) was correctly identified due to at least one weak positive reaction in the negative key reactions described as indicative for the identification of C. dubliniensis (14): lactate (83%), methyl-α-d-glucoside (MDG) (58%), d-trehalose (92%), and d-xylose (75%).
Analysis of the chromatographic data of the 60 yeast strains tested using cluster analysis (unweighted pair matching) and principal component analysis are shown in Fig. 2 and 3, respectively. Both revealed two clearly distinguishable clusters precisely representing the C. albicans and C. dubliniensis strains.
FIG. 2.
Dendrogram revealed by cluster analysis of FAME profile data using C. albicans (n = 32) and C. dubliniensis (n = 28). Numbers in italics (1 to 32), C. albicans genotypically assigned to the respective species; numbers in boldface type (33 to 60), C. dubliniensis genotypically assigned to the respective species; circled numbers (n = 3), isolates falsely identified by FAME analysis using CADLIB; ∗, C. stellatoidea revealed by analysis of a partial LSU rDNA sequence (500 bp).
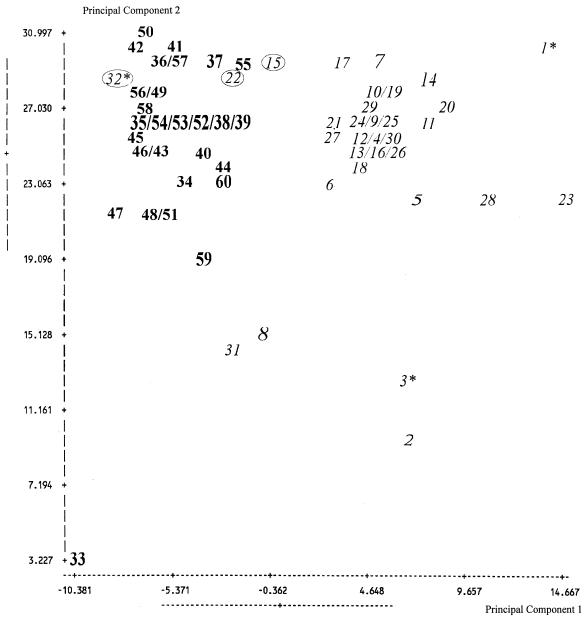
FIG. 3.
A 2-D plot was revealed by principal component analysis of FAME profile data using C. albicans (n = 32) and C. dubliniensis (n = 28). Total of samples used n = 60; mean x, −0.098 (standard deviation, 6.177); mean y, 24.863 (standard deviation, 5.105); numbers in italics (1 to 32), C. albicans genotypically assigned to the respective species; numbers in boldface type (33 to 60), C. dubliniensis genotypically assigned to the respective species; circled numbers (n = 3), isolates falsely identified by FAME analysis using CADLIB; ∗, C. stellatoidea revealed by analysis of a partial LSU rDNA sequence (500 bp).
DISCUSSION
In microbiology, gas-liquid chromatography is mainly used for taxonomic research and for detecting metabolic products in cultures, analyzing cellular constituents or investigating metabolic products (15). Analysis of whole-cell fatty acids is also used for taxonomic purposes, and recently the MIS software package for the MIDI system has been released and includes a computer database (YSTCLN, version 3.8) especially designed for the identification of yeasts from clinical specimens (18). In contrast to labor-intensive or expensive methods, e.g., molecular genetic methods and FT-IR spectroscopy, FAME analysis using MIS software provides a reasonable, accurate, rapid, and cost-effective alternative for identification. The system analyzes long-chain fatty acids containing 9 to 20 C atoms, identifying and quantifying the FAMEs of microorganisms. The database library searches for fatty acid compositions and compares the FAME profile of the isolate with those of well-characterized strains. The software can then define the most likely species of the isolate as well as the extent of correlation of the isolate's profile with a species entry in the database (Fig. 1) (18).
It is known that fatty acids with 16 to 18 carbon atoms generally predominate in yeasts: 16:0 (palmitic) constitutes usually between 15 and 20%; 18:0 (stearic) rarely exceeds 10%; 18:1 (oleic) is usually the most abundant fatty acid; 18:2 (linoleic) can be the second most abundant fatty acid; and 18:3 (linolenic) usually comprises only a minor component (15). The fatty acids occur as esters in triacylglycerol, phopholipids, glycolipids, or sterols in membranes and other cytoplasmatic organelles such as the mitochondria, plasmalemma, endoplasmatic reticulum, nuclei, vacuoles, spores and lipid particles. The 14:0 fatty acids are only seen as trace fatty acyl residues (15).
In previous studies, gas-liquid chromatography of whole-cell FAME profiles has proved to be suitable for reliable and rapid identification of clinically and industrially important yeast species (2). Efforts have been made to standardize culture conditions giving reproducible FAME profiles (2). Especially in the food and beverage industry, this technique has been found to be an inexpensive and reliable means of distinguishing between strains of Saccharomyces cerevisiae and other specific yeast species from the environment (2). Furthermore, it is used in a quality control process to determine fungal contaminants (2). The library YST28 of the MIS has been especially created for identification of environmental species and is therefore used in biotechnological processes.
The MIDI system has also been proposed for rapid identification of clinical yeast isolates by using the YSTCLIN library (3). In a recent report, it was concluded that the YSTCLIN library needs to be improved (8); for example, C. dubliniensis is not included in the latest version. This is why we created the additional library, CADLIB, in an attempt to be able to discriminate C. dubliniensis from C. albicans.
An easy-to-perform method for identification of C. dubliniensis, which mostly occurs in mixed cultures with C. albicans, still does not exist (21). C. albicans and C. dubliniensis are phenotypically as well as genetically closely related species (1, 5). Therefore, throughout this study the strains used were genetically characterized by sequencing enabling unequivocal species assignment (10). Especially in the case of C. dubliniensis, the main problem in selection and identification has been the lack of a reliable discriminative phenotypic marker (24). For clarification of the epidemiological role of C. dubliniensis, an easy-to-perform screening method capable of selective identification of this yeast is mandatory; e.g., in mixed cultures with C. albicans, both species show various shades of green on CHROMagar, disabling species identification (24). In the present study, we developed a new library entry of FAME profiles (CADLIB) that enables identification of these green colonies. Thus, in contrast to genetically based methods, an easy-to-perform and inexpensive tool enabling reliable discrimination between these two species is also available for the routine microbiology laboratory that is skilled in FAME analysis. By applying FAME analysis (as done here), epidemiological studies can be carried out enabling accurate estimation of C. dubliniensis frequency.
The FAME profiles of C. albicans and C. dubliniensis library training files are in accordance with the general description of FAME profiles of yeasts (15) (Table 2). Since the profiles are very similar (Fig. 1), only differences in the frequency and the amount of FAME as evaluated by the MIS software enable discrimination of and therefore between these two species (Table 2). However, the variability of lipid content in yeasts depends heavily on growth modalities. Recently it has been shown that the culture medium used can impair the accuracy of the MIS for identification when using prepoured media from different sources (9). To provide comparable results, the recommended commercial source of SDA used here was in strict adherence to the MIS instructions, as were the recommended culture conditions (18).
In the library search report (Fig. 1, text below the continuous dotted line) the Sherlock MIS software computed an SI by principal-component analysis of detected cellular fatty acid content ratio for each isolate tested. The SI is a numerical value which expresses how closely the fatty acid composition of the measured sample compares with the mean fatty acid composition of the strains used to create the library entry listed as its match. An exact match of the fatty acid composition with the mean of the library entry would result in an SI of 1.0. The range of choice is reported in descending similarity, and “no match” signifies that the isolate was not identified (Table 1). According to MIS guidelines, strains with an SI of 0.5 or higher or a separation of ≥0.1 between the first and second choice are considered good library comparisons (18).
When using the newly created CADLIB, all SIs (except for one, namely, strain 33) revealed that for C. dubliniensis isolates good library comparisons were obtained (SI ≥ 0.5). For correctly identified C. albicans isolates, in 22 cases the SI was ≥0.5 and the SI distance of the remaining (n = 7) correctly identified isolates to the second choice listed was always ≥0.1. Thus, besides the three falsely identified C. albicans isolates, strains were still well separated and can therefore be considered well matched with the library according to the MIS instructions. The newly created CADLIB enabled us to successfully discriminate between clinical isolates of C. albicans (n = 32; predictive value, 93.5%) and C. dubliniensis (n = 28; predictive value, 100%) by comparison of FAME profiles.
Relatedness among the isolates tested was determined by applying cluster analysis (Fig. 2) and principal component analysis (Fig. 3), in conjunction with LGS software. Linkage of isolates is shown by Euclidean distance (ED), i.e., the resulting distance in a two-dimensional (2-D) space between two strains when comparing their two main fatty acid compositions. Samples linked in the dendrogram with an ED of ≤10 (2-D plot multiplied respective lengths of both the x and y axes ≤110) are generally considered to belong to the same species. Although these EDs were higher for several strains (Fig. 3, i.e., strains 33 and 46), the respective strains could be unequivocally assigned to the two species by analysis of the LSU rDNA. The cluster analysis of all isolates tested in the present study as well as in case of the principal component analysis both revealed two distinct clusters corresponding to the two species. The main clusters in the dendrogram are linked with an ED of >10, thus confirming that C. albicans and C. dubliniensis are not only subgroups but distinct species. There are several isolates, e.g., strains 2 and 3 (Table 1), that cluster distantly to the main clusters (Fig. 2). Strains 2 and 3 are atypical isolates of C. albicans which do not form chlamydospores or utilize the amino sugars glucosamine and N-acetylglucosamine. These atypical isolates have been found in vaginal specimens of Angolan women (22). By analysis of the LSU rDNA, one of these strains (strain 3) could be assigned to the former species “C. stellatoidea,” which is now considered as a synonym of C. albicans. Recently, a study assessing the genetic structure of typical and atypical populations of these C. albicans strains from Africa revealed that they were closely related to C. albicans but formed a monophyletic group, perhaps indicating an early stage of speciation (5). The third strain of these atypical C. albicans isolates (strain 32), genetically determined by us to be C. albicans, was falsely identified by CADLIB as C. dubliniensis and was not identified by API ID 32C (profile, 1000 0000 01). In the case of the remaining two isolates falsely identified by CADLIB, only strain 15 was clearly identified by API ID 32C (profile, 7147 3400 11), whereas strain 22 revealed doubtful results in two key reactions, namely d-trehalose and MDG [profile, 7142 (?) 3400 15]. Probably the other strains that clustered distantly to the main clusters (e.g., strains 8, 31, and 33 [Table 1]) or were falsely identified are in other traits somehow phenotypically atypical. Therefore, the high discriminative power of FAME analysis, which is comparable to that of genetically based methods, offers a powerful tool for the identification of different yeast species.
In the present study, none of the C. dubliniensis isolates could be identified by API ID 32C and the code numbers described (14). This was mainly due to weak positive reactions to MDG, d-trehalose, d-xylose, and lactate, which has also been previously observed (24). As with all methods, cost, the skills required by the technical assistant, and the time needed play an important role. For API ID 32C (13), it has been estimated that about $3.15 is spent on consumables. The average time needed by a technician for inoculation and evaluation of this test (manual reading and searching of the code book) has been calculated to be 5 min. In comparison, single sample analysis by FAME has been calculated to be about $1.30 for consumables (MIDI technical note 101) and to take an average of 6 min per sample preparation after the initial extraction, which requires a block of time. Therefore, FAME analysis may prove to be even less expensive than identification using biochemical based test kits regarding cost in regard to consumables.
Other phenotypical methods, e.g., reduced or lack of growth at 42°C, abundant chlamydospore formation, and absence of intracellular β-d-glucosidase activity, have also been only considered as presumptive for the identification of C. dubliniensis, due to the varying response as observed in C. albicans strains (14). Besides being relatively inexpensive, FAME analysis seems to be a useful phenotypic tool enabling discrimination between these two species. Thus, for microbiological laboratories skilled in FAME analysis, the introduction of this method allows large epidemiological studies to be performed.
In conclusion, comparison of FAME profiles using gas-liquid chromatography could be successfully applied to the differentiation of C. albicans and C. dubliniensis, both of which produce green colonies on CHROMagar Candida. Therefore, by using FAME analysis, larger epidemiological studies can be performed which will contribute to a greater understanding of the epidemiology of the recently described yeast species, C. dubliniensis.
ACKNOWLEDGMENTS
We thank Ralph Paisley for his critical reading of the manuscript, Kirstin Becker for her excellent technical assistance, and Hans-Jürgen Tietz for kindly providing the atypical C. albicans isolates.
REFERENCES
- 1.Abdelghani B A, Wuyts J, De Wachter R, Meyer A, Van de Peer Y. Construction of a variability map for eukaryotic large subunit ribosomal RNA. Nucleic Acids Res. 1999;27:2825–2831. doi: 10.1093/nar/27.14.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botha A, Kock J L. Application of fatty acid profiles in the identification of yeasts. Int J Food Microbiol. 1993;19:39–51. doi: 10.1016/0168-1605(93)90122-w. [DOI] [PubMed] [Google Scholar]
- 3.Crist A E, Jr, Johnson L M, Burke P J. Evaluation of the Microbial Identification System for identification of clinically isolated yeasts. J Clin Microbiol. 1996;34:2408–2410. doi: 10.1128/jcm.34.10.2408-2410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Rijk P, De Wachter R. DCSE, an interactive tool for sequence alignment and secondary structure research. Comput Applic Biosci. 1993;9:735–740. doi: 10.1093/bioinformatics/9.6.735. [DOI] [PubMed] [Google Scholar]
- 5.Forche A, Schönian G, Gräser Y, Vilgalys R, Mitchell T G. Genetic structure of typical and atypical populations of Candida albicans from Africa. Fung Gen Biol. 1999;28:107–125. doi: 10.1006/fgbi.1999.1164. [DOI] [PubMed] [Google Scholar]
- 6.Haase G, Sonntag L, van de Peer Y, Uijthof J M, Podbielski A, Melzer-Krick B. Phylogenetic analysis of ten black yeast species using nuclear small subunit rRNA gene sequences. Antonie Leeuwenhoek. 1995;68:19–33. doi: 10.1007/BF00873289. [DOI] [PubMed] [Google Scholar]
- 7.Jabra-Rizk M A, Baqui A CA, Kelley J I, Falkler W A, Jr, Merz W G, Meiller T F. Identification of Candida dubliniensis in a prospective study of patients in the United States. J Clin Microbiol. 1999;37:321–326. doi: 10.1128/jcm.37.2.321-326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellogg J A, Bankert D A, Chaturvedi V. Limitations of the current microbial identification system for identification of clinical yeast isolates. J Clin Microbiol. 1998;36:1197–1200. doi: 10.1128/jcm.36.5.1197-1200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellogg J A, Bankert D A, Chaturvedi V. Variation in Microbial Identification System accuracy for yeast identification depending on commercial source of Sabouraud dextrose agar. J Clin Microbiol. 1999;37:2080–2083. doi: 10.1128/jcm.37.6.2080-2083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtzman C P, Robnett C J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek. 1998;73:331–371. doi: 10.1023/a:1001761008817. [DOI] [PubMed] [Google Scholar]
- 11.Moran G P, Sanglard D, Donnelly S M, Shanley D B, Sullivan D J, Coleman D C. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob Agents Chemother. 1998;42:1819–1830. doi: 10.1128/aac.42.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odds F C, Van Nuffel L, Dams G. Prevalence of Candida dubliniensis isolates in a yeast stock collection. J Clin Microbiol. 1998;36:2869–2873. doi: 10.1128/jcm.36.10.2869-2873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peltroche-Llacsahuanga H, Jenster A, Lütticken R, Haase G. Novel microtiter plate format for testing germ tube formation and proposal of a cost-effective scheme for yeast identification in a clinical laboratory. Diagn Microbiol Infect Dis. 1999;35:197–204. doi: 10.1016/s0732-8893(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 14.Pincus D H, Coleman D C, Pruitt W R, Padhye A CA, Salkin I F, Geimer M, Bassel A, Sullivan D J, Clarke M, Hearn V. Rapid identification of Candida dubliniensis with commercial yeast identification systems. J Clin Microbiol. 1999;37:3533–3539. doi: 10.1128/jcm.37.11.3533-3539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratledge C. Yeasts, moulds, algae and bacteria as sources of lipids. In: Kamel B A, Kakuda Y, editors. Technological advances in improved and alternative sources of lipids. London, United Kingdom: Blackie Academic and Professional; 1994. pp. 235–291. [Google Scholar]
- 16.Ruhnke M, Grosch-Worner I, Steinmüller A, Neubauer A. Molecular epidemiology of Candida infections in HIV-infected mothers and their offspring. Wien Med Wochenschr. 1997;147:446–449. [PubMed] [Google Scholar]
- 17.Salkin I F, Pruitt W R, Padhye A A, Sullivan D, Coleman D, Pincus D H. Distinctive carbohydrate assimilation profiles used to identify the first clinical isolates of Candida dubliniensis recovered in the United States. J Clin Microbiol. 1998;36:1467. doi: 10.1128/jcm.36.5.1467-1467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasser M. MIS whole cell fatty acid analysis by gas chromatography. Newark, Del: MIDI, Inc.; 1991. [Google Scholar]
- 19.Schoofs A, Odds F C, Colebunders R, Ieven M, Goossens H. Use of specialised isolation media for recognition and identification of Candida dubliniensis isolates from HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1997;16:296–300. doi: 10.1007/BF01695634. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman C. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 22.Tietz H J, Kussner A, Thanos M, De Andrade M P, Presber W, Schönian G. Phenotypic and genotypic characterization of unusual vaginal isolates of Candida albicans from Africa. J Clin Microbiol. 1995;33:2462–2465. doi: 10.1128/jcm.33.9.2462-2465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timmins E M, Howell S A, Alsberg B K, Noble W C, Goodacre R. Rapid differentiation of closely related Candida species and strains by pyrolysis-mass spectrometry and Fourier transform-infrared spectroscopy. J Clin Microbiol. 1998;36:367–374. doi: 10.1128/jcm.36.2.367-374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tintelnot K, Haase G, Seibold M, Bergmann F, Staemmler M, Franz T, Naumann D. Evaluation of phenotypic markers for selection and identification of C. dubliniensis. J Clin Microbiol. 2000;38:1599–1608. doi: 10.1128/jcm.38.4.1599-1608.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]