Objective:
To assess the association between the timing of surgery relative to the development of Covid-19 and the risks of postoperative complications.
Summary Background Data:
It is unknown whether patients who recovered from Covid-19 and then underwent a major elective operation have an increased risk of developing postoperative complications.
Methods:
The risk of postoperative complications for patients with Covid-19 undergoing 18 major types of elective operations in the Covid-19 Research Database was evaluated using multivariable logistic regression. Patients were grouped by time of surgery relative to SARS-CoV-2 infection; that is, surgery performed: (1) before January 1, 2020 (“pre-Covid-19”), (2) 0 to 4 weeks after SARS-CoV-2 infection (“peri-Covid-19”), (3) 4 to 8 weeks after infection (“early post-Covid-19”), and (4) ≥8 weeks after infection (“late post-Covid-19”).
Results:
Of the 5479 patients who met study criteria, patients with peri-Covid-19 had an elevated risk of developing postoperative pneumonia [adjusted odds ratio (aOR), 6.46; 95% confidence interval (CI): 4.06–10.27], respiratory failure (aOR, 3.36; 95% CI: 2.22–5.10), pulmonary embolism (aOR, 2.73; 95% CI: 1.35–5.53), and sepsis (aOR, 3.67; 95% CI: 2.18–6.16) when compared to pre-Covid-19 patients. Early post-Covid-19 patients had an increased risk of developing postoperative pneumonia when compared to pre-Covid-19 patients (aOR, 2.44; 95% CI: 1.20–4.96). Late post-Covid-19 patients did not have an increased risk of postoperative complications when compared to pre-Covid-19 patients.
Conclusions:
Major, elective surgery 0 to 4 weeks after SARS-CoV-2 infection is associated with an increased risk of postoperative complications. Surgery performed 4 to 8 weeks after SARS-CoV-2 infection is still associated with an increased risk of postoperative pneumonia, whereas surgery 8 weeks after Covid-19 diagnosis is not associated with increased complications.
Keywords: COVID-19, operation, postoperative pulmonary complications, SARS-CoV-2, surgery
As of July 2021, over 20 million people in the United States (U.S.) have recovered from coronavirus disease 2019 (Covid-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2 During active SARS-CoV-2 infection, many of these patients had pneumonia,3,4 respiratory failure,3,5 arrhythmias,6–9 and thrombotic complications such as pulmonary embolism (PE)10,11 and deep vein thrombosis (DVT).10–12 Over 60% of patients may continue to have persistent symptoms after these complications.13–15
It is unclear whether patients who recovered from Covid-19 can undergo an elective major operation safely. Studies of elective3,5,9,16 or emergent3,16 operations on Covid-19 patients during the perioperative period report pulmonary complications ranging from 24.2% to 51.2%,3,16,17 thromboembolic events from 6.8% to 13.4%,16,18,19 shock from 11% to 13.9%,17,19 and 30-day mortality from 9.1% to 32.6%.3,17–20 However, there are few data on the risks of postoperative complications after surgery following recovery from Covid-19. The objective of this study is to assess the association between the timing of surgery relative to the development of Covid-19 and the risks of postoperative pulmonary complications and other major complications.
METHODS
Data Source
The data for this study were derived from Symphony Health through the Covid-19 Research Database. The Covid-19 Research Database is a pro-bono, public-private consortium composed of institutions that contribute de-identified data of patients with Covid-19. Symphony Health, a partnering institution, contains longitudinal patient data sources via its “Integrated Dataverse” that captures adjudicated prescription, medical, and hospital claims across the U.S. for all payment types, including commercial plans, Medicare Part D, cash, assistance programs, and Medicaid. Symphony Health is an open dataset from participating hospitals and health plans that utilize clearinghouses associated with Symphony Health. Data are continuously updated with new claims, with coverage beginning May 2019 and inclusion of over 1500 hospitals, 800 outpatient facilities, and 280 million patients at the time of this study.
Study Population
All patients with a confirmed Covid-19 diagnosis between March 1, 2020 and May 30, 2021 using the International Classification of Diseases for Oncology (ICD-10-CM) code U07.1 were included in the study. The Covid-19 diagnosis date (ie, the date that the patient is confirmed to have Covid-19) in this study cohort was defined as the date of the first Covid-19 Reverse Transcription Polymerase Chain Reaction (RT-PCR) test taken by the patient. Because all patients in the study cohort had a confirmed Covid-19 diagnosis (with ICD-10-CM code U07.1), this date is assumed to represent the start of Covid-19 for the patient, although the true infection date is unknown. Covid-19 severity was categorized as mild/moderate, severe (eg, requiring supplemental oxygen), and critical (eg, shock and acute respiratory distress syndrome [ARDS]). We did not include patients who had received the Covid-19 vaccine before surgery or during the 90-day period after surgery.
Patients who underwent one operation based on Current Procedural Terminology codes were included. These operations are all considered major elective operations in the U.S., which include lung resection (lobectomy, pneumonectomy, and segmentectomy), esophagectomy, mastectomy, colorectal resection for cancer (colectomy and proctectomy), prostatectomy, pancreatic resections (Whipple, total pancreatectomy, and distal pancreatectomies), hepatectomy, gastrectomy, hip replacement, coronary artery bypass grafting, knee replacement, laminectomy, hysterectomy, spinal fusion, elective open repair of abdominal aortic aneurysm (AAA), elective endovascular repair of abdominal aortic aneurysm (EVAR), brain tumor resection, and carotid endarterectomy (Supplemental Digital Content Table S3). Exclusion criteria included patients who underwent multiple operations and patients who did not have any of the above-specified operations. We specifically excluded operations that could have been performed for emergent reasons, including Caesarian section, cholecystectomy, appendectomy, colectomy for perforated diverticulitis, hernia repair in the setting of incarceration, AAA repair for ruptured aneurysm, and any type of trauma surgery. To assess the risk of postoperative complications among patients who underwent operations for more urgent reasons, a subgroup analysis was conducted. The operations included in this subgroup analysis were EVAR, open repair of AAA, carotid endarterectomy, gastrectomy, hepatectomy, lung resection, neurosurgical resection of tumor, pancreatic surgery, colorectal surgery, and esophagectomy.
Timing of Surgery Relative to Covid-19 Diagnosis
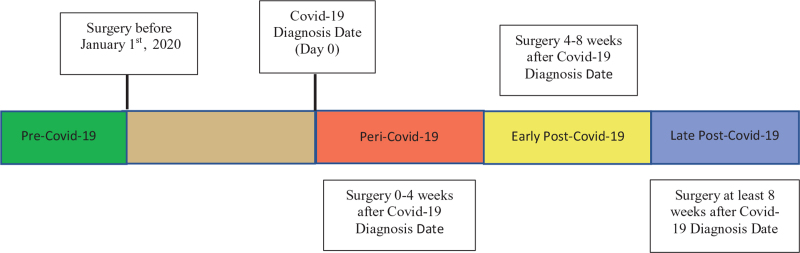
Patients were categorized into four groups based on the time of surgery relative to the Covid-19 diagnosis date (Fig. 2). The “peri-Covid-19” group was defined as surgery performed 0 to 4 weeks after the Covid-19 diagnosis date. These are patients with perioperative SARS-CoV-2 infection. The “early post-Covid-19” group was composed of patients who underwent surgery between 4 and 8 weeks after the Covid-19 diagnosis date. “Late post-Covid-19” was defined as surgery performed 8 weeks or greater after the Covid-19 diagnosis date.
FIGURE 2.
Surgery timing categories relative to Covid-19 diagnosis date.
For our control group, we selected patients who underwent surgery at least 30 days before their Covid-19 diagnosis date and had surgery between the dates of May 1, 2019 to January 1, 2020. This “pre-Covid-19” group was selected as our reference group because they had not been infected with SARS-CoV-2 before surgery and any 30-day postoperative complication they developed could not be attributed to sequelae of SARS-CoV-2 infection. For this “pre-Covid-19” control group, we did not select patients who never developed Covid-19 because patients who never developed Covid-19 during the pandemic may have a different racial, ethnic, socioeconomic, and/or geographic background from patients in the peri-Covid-19, early post-Covid-19 and late post-Covid-19.21
Complications
The primary outcomes of interest for the present study were postoperative pneumonia and respiratory failure. Secondary outcomes included DVT, PE, arrhythmia, and sepsis (Supplemental Digital Content Table S4).
Covariates
Comorbidities were identified using ICD-10-CM codes for the following underlying conditions: diabetes, hypertension, chronic obstructive pulmonary disease, gastroesophageal reflux disease, obesity, coronary artery disease, congestive heart failure, chronic kidney disease, ulcerative colitis, Crohn disease, mild liver disease, severe liver disease, history of stroke, and depression (Supplemental Digital Content Table S5).
Statistical Analysis
Patient characteristics and unadjusted outcomes were assessed using Pearson chi-square test for categorical variables and t test or Wilcoxon Rank Sum test where appropriate for continuous variables. A multivariable logistic regression model was used to evaluate the risk of developing any postoperative complication, adjusting for covariates determined a priori to be clinically relevant. These covariates included time of surgery relative to Covid-19 diagnosis date, age, sex, race, first digit zip code region, diabetes, hypertension, chronic obstructive pulmonary disease, gastroesophageal reflux disease, obesity, coronary artery disease, congestive heart failure, chronic kidney disease, ulcerative colitis, Crohn's disease, mild liver disease, severe liver disease, history of stroke, and depression. We also used separate multivariable logistic regression models to evaluate the risk of pneumonia, respiratory failure, PE, sepsis, DVT, arrhythmia, renal failure, and urinary tract infection, adjusting for covariates listed in Supplemental Digital Content Tables S4 and S5.
The dataset used for analysis was prepared using Snowflake (Snowflake Inc, San Mateo, CA). Statistical analysis was performed using STATA/MP software, version 16.1 for Windows (Statacorp, College Station, TX). Statistical significance was defined at P values <0.05. This retrospective study was approved by the Institutional Review Board of the Massachusetts General Hospital (IRB# 2020P004110). Additionally, the Covid-19 Research Database was established with IRB approval.
RESULTS
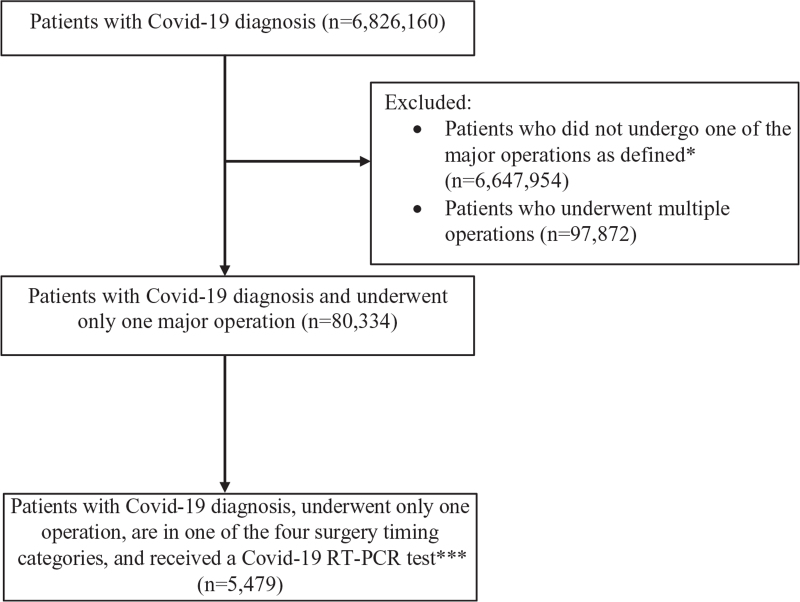
There were 5479 patients who met study criteria (Fig. 1). Of these patients, 2621 (47.8%) patients underwent surgery before January 1, 2020, at least 30 days before being diagnosed with Covid-19 (“pre-Covid-19”), 780 (14.2%) patients underwent surgery 0 to 4 weeks after their Covid-19 diagnosis date (“peri-Covid-19”), 445 (8.1%) patients underwent surgery 4 to 8 weeks after their Covid-19 diagnosis date (“early post-Covid-19”), and 1633 (29.8%) patients underwent surgery 8 weeks or more after their Covid-19 diagnosis date (“late post-Covid-19”).
FIGURE 1.
Flow diagram detailing schema of study subject selection. ∗Operations included: abdominal aortic aneurysm repair (endovascular approach), abdominal aortic aneurysm repair (open approach), brain tumor resection, carotid endarterectomy, colon surgery, coronary artery bypass grafting, esophagectomy, gastrectomy, mastectomy, hepatectomy, hip replacement, hysterectomy, knee replacement, laminectomy, lung resection (lobectomy, pneumonectomy, and segmentectomy), pancreatic surgery (Whipple, total pancreatectomy, and distal pancreatectomy), prostatectomy, and spinal fusion. ∗∗Surgery timing categories: “pre-Covid-19”: patients underwent surgery before January 1, 2020 before their Covid-19 diagnosis; “peri-Covid-19”: patients underwent surgery 0 days to 4 weeks after their Covid-19 diagnosis; “early post-Covid-19”: patients underwent surgery 4 to 8 weeks after their Covid-19 diagnosis; “late post-Covid-19”: patients underwent surgery ≥ 8 weeks after their Covid-19 diagnosis.
Supplemental Digital Content Table S1 details patient characteristics. The majority of patients had mild Covid-19 with only 0.4% and 0.9% having severe or critical Covid-19, respectively. Across all groups, the most common comorbidities were obesity and hypertension. The most common operation for the pre-Covid-19 group was hysterectomy, the most common operation for the peri-Covid-19 group was knee arthroplasty, and the most common operation for both the early and late post-Covid-19 groups was hysterectomy.
The postoperative outcomes for each group are detailed in Table 1. Peri-Covid-19 patients (ie, patients with perioperative SARS-CoV-2 infection) had the highest rates of pneumonia, respiratory failure, PE, renal failure and sepsis when compared to the other groups. Peri-Covid-19 patients had a 480% and 208% higher risk of developing postoperative pneumonia and respiratory failure, respectively, when compared to pre-Covid-19 patients. Early post-Covid-19 patients had a 96% higher risk of developing postoperative pneumonia when compared to pre-Covid-19 patients. Late post-Covid-19 patients had similar risks of developing postoperative pneumonia, respiratory failure and other major complications when compared to pre-Covid-19 patients. Similar trends were also observed among a subgroup of patients who underwent operations considered to be more urgent (Supplemental Digital Content Table S8).
TABLE 1.
Postoperative Outcomes
| Outcomes | Pre-Covid-19 Surgery Before January 1, 2020 (n = 2621) | Peri-Covid-19 Surgery 0 to 4 Weeks After Covid-19 (n = 780) | Early Post-Covid-19 Surgery 4 to 8 Weeks After Covid-19 (n = 445) | Late Post-Covid-19 Surgery at Least 8 Weeks After Covid-19 (n = 1633) | P Value |
| Postoperative pneumonia | 33 (1.3%) | 57 (7.3%) | 11 (2.5%) | 23 (1.4%) | <0.001 |
| Postoperative respiratory failure | 62 (2.4%) | 57 (7.3%) | 11 (2.5%) | 45 (2.8%) | <0.001 |
| Postoperative pulmonary embolism | 18 (0.7%) | 14 (1.8%) | 3 (0.7%) | 16 (1.0%) | 0.039 |
| Postoperative sepsis | 30 (1.1%) | 33 (4.2%) | 9 (2.0%) | 20 (1.2%) | <0.001 |
| Postoperative arrhythmia | 50 (1.9%) | 13 (1.7%) | 13 (2.9%) | 37 (2.3%) | 0.41 |
| Postoperative renal failure | 68 (2.6%) | 36 (4.6%) | 10 (2.3%) | 58 (3.6%) | 0.017 |
| Postoperative urinary tract infection | 69 (2.6%) | 22 (2.8%) | 11 (2.5%) | 46 (2.8%) | 0.097 |
| Postoperative deep vein thrombosis | 35 (1.3%) | 15 (1.9%) | 6 (1.4%) | 28 (1.7%) | 0.59 |
| Any postoperative complication | 270 (10.3%) | 131 (16.8%) | 52 (11.7%) | 183 (11.2%) | <0.001 |
Multivariable logistic regression modeling demonstrating the risk of different complications for each time period of surgery is shown in Supplemental Digital Content Table S2. After adjustment for patient characteristics and type of surgery, peri-Covid-19 patients had a significantly higher risk of developing postoperative pneumonia [adjusted odds ratio (aOR), 6.46; 95% confidence interval (CI), 4.06–10.27], respiratory failure (aOR, 3.36; 95% CI, 2.22–5.10), PE (aOR, 2.73; 95% CI, 1.35–5.53) and sepsis (aOR, 3.67; 95% CI, 2.18–6.16) when compared to pre-Covid-19 patients. For most complications, early post-Covid-19 patients did not have a higher risk when compared to pre-Covid-19 patients; however, early post-Covid-19 patients did have a higher risk of developing postoperative pneumonia (aOR, 2.44; 95% CI: 1.20–4.96). Notably, late post-Covid-19 patients did not have a higher risk of developing postoperative complications when compared to patients in the pre-Covid-19 group (Supplemental Digital Content Table S2). Among a subgroup of patients who underwent operations considered to be more urgent, peri-Covid-19 patients had a higher risk of postoperative pneumonia, respiratory failure, and sepsis, whereas early post-Covid-19 patients had an higher risk of postoperative pneumonia; late post-covid-19 patients did not have a higher risk of postoperative complications (Supplemental Digital Content Table S9).
DISCUSSION
We assessed the impact of timing of surgery relative to SARS-CoV-2 infection on the risk of developing postoperative complications among 5479 patients undergoing 18 types of common, major, elective operations. In this large population-based study, patients who underwent surgery close to the time of a SARS-CoV-2 infection had increased risks of developing postoperative pneumonia, respiratory failure, PE and sepsis. Surgery performed 4 to 8 weeks after SARS-CoV-2 infection was still associated with an increased risk of postoperative pneumonia, whereas surgery performed approximately 8 weeks after infection was not associated with increased risks of postoperative complications. Of note, the vast majority of the patients in the study cohort had mild to moderate Covid-19.
In the coming months, the number of patients who have recovered from SARS-CoV-2 infection and require major surgery will continue to increase. Previous studies have shown that patients with perioperative SARS-CoV-2 infection who undergo surgery have an increased risk of postoperative complications and 30-day mortality.3,18,22 However, there are only a few studies assessing the impact of the timing of elective major surgery relative to SARS-CoV-2 infection on the risk of postoperative complications. Thus far, there have only been two studies, one performed in Brazil23 and one multicenter study of mostly European patients evaluating postoperative risk after recovery from Covid-19.20 Baiocchi and colleagues23 assessed the postoperative outcomes of 49 Brazilian patients who underwent delayed elective surgery after a confirmed preoperative diagnosis of asymptomatic Covid-19. The authors found that asymptomatic Covid-19 patients who underwent surgery delayed by a median of 25 days did not have an increased risk of postoperative complications when compared to patients with a negative preoperative Covid-19 test. The Covid-19 Surg Collaborative evaluated 3127 predominately European patients from 1674 hospitals and 116 countries who had a Covid-19 diagnosis and then underwent surgery after different timepoints (eg, within 2 weeks of diagnosis, 3 to 4 weeks, 5 to 6 weeks, and ≥ 7 weeks after diagnosis). The authors found that the pulmonary complication rate and the mortality rate were the lowest among patients who underwent surgery ≥ 7 weeks after diagnosis. Our findings, which pertain to elective major operations in the U.S., are consistent with the results first reported in these studies.
Of note, Baiocchi et al23 and the Covid-19 Surg Collaborative included major and minor operations in their analysis, and the COVID-19 Surg Collaborative also included emergent operations and trauma surgeries. In the present study, we evaluated only elective major operations and did not include minor procedures or emergent operations in the analysis.
The findings from this study have important implications for surgical care as we begin to recover from the Covid-19 pandemic, which has caused widespread and numerous delays in surgical care. At the height of the Covid-19 pandemic, approximately 100,000 elective operations were canceled or postponed weekly in the U.S.24 To avoid similar delays and cancellations during the recovery from the pandemic, evidence-based guidelines to safely restore surgical activity are greatly needed. In preparation for an increasing number of patients who have recovered from Covid-19 who subsequently require surgery, guidelines and preoperative protocols for the evaluation of these patients have been published.25,26 However, due to the limited data available to inform the timeframe of recovery, recommendations for delaying surgery are based largely on expert opinion or previous data from other post-viral syndromes.25,26 The findings from our study could inform future guidelines on the timing of surgery in patients with recent SARS-CoV-2 infection. Importantly, our findings suggest that for most patients with confirmed Covid-19, delaying surgery for approximately 8 weeks may reduce the risk of developing major postoperative complications. Based on these findings, we recommend delaying surgery, if possible, for at least 8 weeks after confirmed SARS-CoV-2 infection. However, it is important to note that for certain cancer patients, delayed surgical treatment during non-pandemic settings has been shown to be associated with worse overall survival when compared to timely surgical treatment.27 As such, the balance between the risk of postoperative complications and the risk of worse overall survival associated with delayed surgical treatment should be carefully discussed in a multi-disciplinary setting before deciding on whether surgery should be delayed.
There are several limitations to our study. First, because of its retrospective study design, results are subject to residual confounding. Second, the Covid-19 research database did not have data on whether a patient with confirmed SARS-CoV2 infection was asymptomatic or symptomatic. Depending on the region in the U.S., anywhere from 18% to 45% of individuals with Covid-19 have been reported to be asymptomatic.28–30 Given that our study includes a nationally representative sample of Covid-19 cases, we assume the percentage of asymptomatic Covid-19 cases in our study is similar in range. The Covid-19 research database did have data on Covid-19 severity [mild/moderate vs severe (eg, requiring supplemental oxygen) vs critical (eg, shock and ARDS)] and we were able to adjust by Covid-19 severity in our models. Third, we were not able to precisely determine when the patient developed Covid-19. In the present study, we used the date of the PCR test to estimate the Covid-19 diagnosis date; however, the patient could have been infected by SARS-CoV-2 or developed Covid-19 several days before this date. Fourth, the Covid-19 research database does not include mortality information and we were unable to assess the risk of 30- or 90-day mortality associated with surgery at different timepoints with respect to SARS-CoV-2 infection. Fifth, quality of life measures and long-term survival data were not captured in the database. Sixth, we were unable to assess preoperative measurements of frailty that may have been important in determining when a patient would be sufficiently recovered from Covid-19 to undergo surgery. The association between measurements of frailty and recovery from Covid-19 should be further investigated. In addition, future studies should evaluate how measurements of frailty could be used to inform preoperative assessments of surgical risk among patients recovered from Covid-19.
In conclusion, surgery performed during or near the time of SARS-CoV-2 infection is associated with an increased risk of developing postoperative complications, including postoperative pneumonia, respiratory failure, PE and sepsis. Surgery performed 4 to 8 weeks after confirmed SARS-CoV-2 infection continues to carry an elevated risk of developing postoperative pneumonia. However, surgery performed 8 weeks or later after confirmed SARS-CoV-2 infection is not associated with an increased risk of developing postoperative complications. Based on these findings, we recommend that the safe period for patients with recent SARS-CoV-2 infection in whom elective, non-emergent surgery is indicated should be at least 8 weeks after the first date of confirmed SARS-CoV-2 infection.
Supplementary Material
Acknowledgment
The data, technology, and services used in the generation of these research findings were generously supplied pro bono by the COVID-19 Research Database partners, who are acknowledged at https://covid19researchdatabase.org/.
Footnotes
The data, technology, and services used in the generation of these research findings were generously supplied pro bono by the Covid-19 Research Database partners, who are acknowledged at https://covid19researchdatabase.org/.
David C. Chang and Chi-Fu Jeffrey Yang are co-senior authors.
The authors report no conflicts of interest.
Supplemental digital content is available for this article.
REFERENCES
- 1. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSEE) at Johns Hopkins University (JHU). Johns Hopkins University. Available at: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed January 18, 2021. [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nepogodiev D, Bhangu A, Glasbey JC, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020; 396:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aminian A, Safari S, Razeghian-Jahromi A, et al. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg 2020; 272:e27–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020; 382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolis AS, Manolis AA, Manolis TA, et al. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med 2020; 30:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine 2020; 21:100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020; 324:799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rali P, O’Corragain O, Oresanya L, et al. Incidence of venous thromboembolism in coronavirus disease 2019: an experience from a single large academic center. J Vasc Surg Venous Lymphat Disord 2021; 9:585–591. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Zhang D, Zheng T, et al. DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis 2021; 51:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser E. Long term respiratory complications of covid-19. BMJ 2020; 370:m3001. [DOI] [PubMed] [Google Scholar]
- 14.Leung TYM, Chan AYL, Chan EW, et al. Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg Microbes Infect 2020; 9:2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonker PKC, van der Plas WY, Steinkamp PJ, et al. Perioperative SARS-CoV-2 infections increase mortality, pulmonary complications, and thromboembolic events: a Dutch, multicenter, matched-cohort clinical study. Surgery 2021; 169:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knisely A, Zhou ZN, Wu J, et al. Perioperative morbidity and mortality of patients with COVID-19 who undergo urgent and emergent surgical procedures. Ann Surg 2021; 273:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg 2020; 155:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayani B, Onochie E, Patil V, et al. The effects of COVID-19 on perioperative morbidity and mortality in patients with hip fractures. Bone Joint J 2020; 102-B:1136–1145. [DOI] [PubMed] [Google Scholar]
- 20.Collaborative CO, GlobalSurg C. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia 2021; 76:748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmakar M, Lantz PM, Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open 2021; 4:e2036462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SH, Chen D, Paone D, et al. Thoracic surgery outcomes for patients with coronavirus disease 2019. J Thorac Cardiovasc Surg 2021; 162:1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baiocchi G, Aguiar S, Jr, Duprat JP, et al. Early postoperative outcomes among patients with delayed surgeries after preoperative positive test for SARS-CoV-2: a case-control study from a single institution. J Surg Oncol 2021; 123:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collaborative C. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg 2020; 107:1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anesthesia Patient Safety Foundation. COVID-19 and Anesthesia FAQ. [Updated 4 Aug 2020; Cited 25 Nov 2020.] Available at: https://www.apsf.org/COVID-19-and-anesthesia-faq/. Accessed July 19, 2021. [Google Scholar]
- 26.Bui N, Coetzer M, Schenning KJ, et al. Preparing previously COVID-19-positive patients for elective surgery: a framework for preoperative evaluation. Perioper Med (Lond) 2021; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayne NR, Elser HC, Darling AJ, et al. Estimating the impact of extended delay to surgery for stage I non-small-cell lung cancer on survival. Ann Surg 2021; 273:850–857. [DOI] [PubMed] [Google Scholar]
- 28.Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020; 25:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med 2020; 17:e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med 2020; 173:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.