Abstract
The Echinococcus Western Blot IgG (LDBIO Diagnostics, Lyon, France), using a whole larval antigen from Echinococcus multilocularis, was evaluated for serodiagnosis and differentiation between two human parasitic infections of worldwide importance: cystic echinococcosis, due to Echinococcus granulosus, and alveolar echinococcosis, due to E. multilocularis. Fifty and 61 serum samples from patients with cystic and alveolar echinococcosis, respectively, were used for assessing diagnostic sensitivity. The sensitivity of the assay was compared with those of screening tests used for these applications. Sera used for assessing cross-reactivities were from 154 patients with other diseases, either parasitic or not. The assay allowed the detection of serum immunoglobulin G antibodies in 97% of Echinococcus-infected patients. It had a higher sensitivity than screening assays for the detection for each echinococcosis. The assay allowed us to correctly distinguish between E. granulosus- and E. multilocularis-infected patients in 76% of cases. It did not allow us to distinguish active from inactive forms of both echinococcoses. The occurrence of cross-reactivities with neurocysticercosis indicates the necessity for retesting sera with species-specific antigens, for rare patients with neurologic disorders. This study shows the usefulness of the commercially available Echinococcus Western Blot IgG for the serological confirmation of human echinococcosis.
Echinococcosis is mainly caused by larvae of two species of tapeworms, Echinococcus granulosus (cystic echinococcosis) and Echinococcus multilocularis (alveolar echinococcosis). E. granulosus occurs worldwide, and E. multilocularis is found in the Northern Hemisphere. Humans can develop the diseases when they ingest eggs excreted with the feces of the final hosts (dogs and foxes). E. granulosus larvae then grow as large cysts with internal budding of brood capsules. The E. multilocularis larva develops by external budding to form an infiltrative growing tumor. In most cases, the liver is the primary organ affected, whatever the parasite species. Surgical removal of the parasitic tissue is the most efficient therapy for the diseases, and the efficiency of antiparasitic drugs depends upon size of the larvae (13). Thus, early diagnosis and subsequent treatment may reduce mortality. Because the symptoms vary according to the rate of parasite growth, the clinical diagnosis of echinococcosis is not easy. There is rarely any parasitological evidence of infection. Ultrasound-guided fine-needle aspiration of the lesions sometimes fails to detect parasites in patients with alveolar echinococcosis (10). Furthermore, the distinction between cystic and alveolar echinococcoses, which is desirable with regard to treatment and prognosis, is not always easy in those countries where both species occur sympatrically. Interpretation of data is sometimes difficult. The diagnosis is mainly suggested by imaging techniques, but they must therefore be combined with serological assays. Serological screening is usually based upon the use of crude antigens of E. granulosus or E. multilocularis, which results in problems with nonspecific reactions. The use of highly purified antigens improves the specificity of serological assays but leads to a loss of sensitivity (1, 6). An immunoblot using a whole E. multilocularis larval antigen has been developed and commercialized as a confirmation technique for the diagnosis of both parasitic infections, since E. multilocularis antigens show extensive cross-reactivity with E. granulosus-infected serum samples (2, 6). The aims of the present study were threefold: to assess the diagnostic sensitivity of this immunoblot in routine use, to evaluate its usefulness for differentiation between cystic and alveolar echinococcoses, and to assess cross-reactivities in a hospital setting.
MATERIALS AND METHODS
Patients and sera.
For assessing diagnostic sensitivity, 111 serum samples from patients with echinococcosis, confirmed by means of histology and/or positive imaging, were used. Fifty serum samples were from patients who had contracted an E. granulosus infection in Mediterranean countries (48 samples) or in Central Europe (2 samples). Twenty-eight patients suffered from cysts in the liver, 11 patients had pulmonary cysts, 8 patients had cysts in the peritoneum, 3 patients suffered from cysts in the liver and lung, and 1 patient had a cyst in the kidney. Sixty-one serum samples were from French patients with alveolar echinococcosis, either active or assumed abortive on the basis of radiologic findings as described by Bresson-Hadni et al. (1). All of these patients had liver lesions. Seven were clinically asymptomatic. In 10 cases, liver lesions were associated with other parasite localizations: in the lung (eight cases), skin (one case), and adrenal gland (one case). One serum sample had been obtained from a patient with an Echinococcus vogeli infection. Serum samples used for assessing cross-reactivities were from 113 patients with other proven parasitic diseases: Taenia solium neurocysticercosis (20 patients), Taenia saginata taeniosis (1), schistosomosis (21 patients with Schistosoma mansoni and 5 with Schistosoma haematobium), fasciolosis (10 patients), filariosis (8 patients), trichinellosis (8 patients), stongyloidosis (2 patients), toxocarosis (10 patients), liver amoebosis (10 patients), leishmaniosis (4 patients), toxoplasmosis (4 patients), malaria (6 patients), and candidosis (4 patients). Twenty serum samples used for assessing nonspecific reactions related to other hepatic diseases were from patients with an atypic biliary cyst (one sample), steatosis (two samples), alcoholic cirrhosis (three), primary biliary cirrhosis (two), viral hepatitis (three), autoimmune hepatitis (two), and hepatocellular carcinoma (seven). Twenty-one serum samples used for assessing nonspecific reactions related to systemic disorders included samples from patients with collagenosis (five) or rheumatoid arthritis (eight), one sample with anticardiolipid antibodies, and eight samples with antinuclear antibodies. All were stored at −80°C until use.
Screening tests.
An indirect hemagglutination assay (IHA) using antigens from E. granulosus (Fumouze, Levallois-Perret, France) was carried out on each serum sample from Echinococcus-infected patients. Serum samples with IHA titers equal to or greater than 320 were considered positive, according to the manufacturer's instructions. Serum samples with IHA titers of 80 or 160 were classified as doubtful. Samples were also examined for immunoglobulin G (IgG) levels using enzyme-linked immunosorbent assays (ELISAs) with crude antigens of E. granulosus and E. multilocularis, both obtained from experimentally infected Meriones unguiculatus. These tests, performed with an alkaline phosphatase-labeled rabbit anti-human IgG, are described elsewhere (5). Values were expressed as percentages relative to a positive reference serum (taken as 100%). Sera were considered positive at indices 20% greater than the value of the negative control. Sera from patients with cystic echinococcosis were examined for total Ig antibodies using an immunofluorescence assay (IFA) with protoscolices obtained from E. granulosus-infected sheep as the antigen. Frozen protoscolex sections, 5 μm thick, were overlaid with patient sera for 30 min, then washed and incubated with a fluorescein isothiocyanate-labeled antibody to human total Ig (Sanofi, Marnes la Coquette, France) for 30 min. After washing, a Zeiss fluorescence microscope was used for reading. Sera with titers equal to or greater than 100 were considered positive.
Immunoblotting.
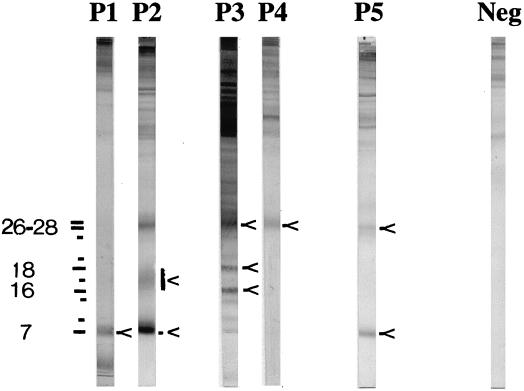
The Echinococcus Western Blot IgG (LDBIO Diagnostics, Lyon, France), an immunoblot assay using a whole larval extract from E. multilocularis as the antigen, was carried out according to the manufacturer's instructions. Echinococcosis sera specifically recognize antigens with molecular sizes below 30 kDa. The presence of one band at 7 kDa and/or one band at 26 to 28 kDa is indicative of the presence of Echinococcus-specific IgG in serum. Bands at 7, 12, 15, 24, and 26 to 28 kDa are shared by both Echinococcus species. Cystic echinococcosis sera specifically bind to a component of 16 to 18 kDa as a diffuse band. Alveolar echinococcosis sera specifically bind to antigens of 16, 17, 18, and 20 kDa, as sharp bands (Fig. 1). Five patterns (P) are described. P1, including one band at 7 kDa, and P2, including at least bands at 7 and 16 to 18 kDa, are considered specific for cystic echinococcosis. P3, including at least one band at 26 to 28 kDa plus two narrow bands at 16 and 18 kDa, and P4, which only includes one band of 26 to 28 kDa, are considered specific for alveolar echinococcosis. P5, which includes the bands at 7 and 26 to 28 kDa, without additional intermediate bands, cannot lead to discrimination between the two species.
FIG. 1.
Echinococcus Western Blot IgG. Five immunoblot patterns (P1 through P5) are obtained with cystic and alveolar echinococcosis sera. Most of the significant bands are indicated by arrows. Molecular sizes (in kilodaltons) are indicated on the left. Neg, results for negative-control serum. Reprinted by courtesy of LDBIO Diagnostics.
RESULTS
Diagnosis sensitivity.
Table 1 compares the results obtained with the Echinococcus Western Blot IgG and screening tests. The higher overall sensitivity was matched by the immunoblot, which scored 97.3% for Echinococcus genus diagnosis. The patterns obtained with alveolar echinococcosis sera usually appeared more complex than those obtained with cystic echinococcosis sera (mean numbers of bands, 6.5 versus 2.5). This result paralleled higher mean antibody levels measured by IHA and ELISAs in alveolar echinococcosis sera (data not shown). However, there was no individual relationship between antibody levels and the number of bands, whatever the patient group. For instance, two alveolar echinococcosis sera with negative ELISA results harbored three and four bands, respectively. The number of bands visualized with sera from asymptomatic alveolar echinococcosis patients with calcified and/or small lesions was lower than that obtained with sera from patients with advanced lesions (mean, 3.6 versus 5.6 bands). However, there was no clear relationship among the severity and duration of disease, the localization of lesions, the degree of larval maturation (presence or lack of protoscolices), and the complexity of the immunoblot patterns. Moreover, quite similar immunoblot patterns were observed with cystic echinococcosis sera, whatever the localization of cysts (liver or lung).
TABLE 1.
Sensitivity rates of the Echinococcus Western Blot IgG and of serological screening assays using E. granulosus or E. multilocularis antigens for patient groups with cystic or alveolar echinococcosis
| Patient group | Sensitivity (%) of:
|
|||||
|---|---|---|---|---|---|---|
| E. multilocularis WBa |
E. granulosus IHA
|
ELISA
|
E. granulosus IFA | |||
| Titer, ≥80 | Titer, ≥320 | E. granulosus | E. multilocularis | |||
| Cystic echinococcosis (n = 50) | 98.0 | 94.3 | 80.0 | 79.4 | 55.9 | 100 |
| Alveolar echinococcosis (n = 61) | 96.7 | 94.0 | 74.0 | 92.0 | 86.0 | NDb |
WB, Echinococcus Western Blot IgG.
ND, not determined.
Differential diagnosis.
Table 2 summarizes the number of serum samples showing the different immunoblot patterns described by the manufacturer. The band at 7 kDa was visualized with 96% of cystic as well as alveolar echinococcosis sera. The band at 26 to 28 kDa was visualized with 64 and 96.7% of cystic and alveolar echinococcosis sera, respectively. However, with 82 out of the 108 (76%) positive sera, a correct Echinococcus species diagnosis was made. It should be noted that pattern 4 was visualized with one cystic echinococcosis serum sample. The specificity of this pattern for serological differentiation of an E. multilocularis infection is therefore 88%.
TABLE 2.
Distribution of sera from patient groups with cystic or alveolar echinococcosis according to the immunoblot results
| Serum samples | No. of serum samples with the following Echinococcus Western Blot IgG pattern:
|
|||||
|---|---|---|---|---|---|---|
| Negativea | P1b | P2c | P3d | P4e | P5f | |
| Cystic echinococcosis (n = 50) | 1 | 12 | 22 | 0 | 1 | 14 |
| Alveolar echinococcosis (n = 61) | 2 | 0 | 0 | 41 | 7 | 11 |
| Total (n = 111) | 3 | 12 | 22 | 41 | 8 | 25 |
No bands in the range of 7 through 28 kDa.
Bands at 7 and 24 kDa.
Bands at 7 and 16 to 18 kDa.
Bands at 26 to 28 kDa and at 16 and/or 18 kDa; possibly others.
Band at 26 to 28 kDa only.
Bands at 7 and 26 to 28 kDa.
Cross-reactivity.
The serum sample from the patient with E. vogeli infection harbored pattern 3. Seven of 20 neurocysticercosis serum samples gave rise to cross-reactions. Six of these recognized the antigen of 26 to 28 kDa (P4). The seventh recognized the antigen of 26 to 28 kDa and gave rise to the two sharp bands at 16 and 18 kDa (P3) considered characteristic of alveolar echinococcosis. Cross-reactions also occurred with 3 of 18 serum samples from S. mansoni-infected patients (showing the 7-kDa band exclusively). No false-positive results were obtained with any other serum sample.
DISCUSSION
Echinococcus infections are among the more dangerous helminthic diseases in humans. Efforts have been made to identify and purify Echinococcus species-specific antigens for application in ELISAs and immunoblots. However, it is difficult to obtain sufficient amounts of these antigens for extensive use (8, 11, 12). The present study was therefore carried out in order to evaluate a commercially available immunoblot for routine serological diagnosis of Echinococcus infections.
The high sensitivity of the Echinococcus Western Blot IgG for Echinococcus genus diagnosis, compared to those of the screening tests used in the present study, should first be emphasized. It equals, on average, that of the available Em2plus ELISA, used for immunodiagnosis of alveolar echinococcosis (6). This sensitivity appears slightly higher than that of the combined immunoblots with E. granulosus- and E. multilocularis-rich antigen fractions described by Ito et al. (7). Indeed, it is higher than that of the immunoblot with E. granulosus-rich antigen for cystic echinococcosis, and it equals, on average, that of the immunoblot with E. multilocularis-rich antigen for alveolar echinococcosis. However, this result remains to be confirmed with sera from patients from different geographic foci, since biochemical strain diversity may occur, especially within the species E. granulosus (3). On the basis of the present results and of the simplicity of the method, it can be proposed as a general strategy for immunodiagnosis of both echinococcoses that the Echinococcus Western Blot IgG be performed either as a confirmatory test for a positive screening test result or as a first-line diagnostic test when suggestive clinical manifestations or imaging data are available.
E. multilocularis antigenic components of 16 and 18 kDa appear to be good candidates for differentiation between cystic and alveolar echinococcoses, as previously shown with a crude antigen of E. multilocularis of Japanese origin (8). To our knowledge, the Echinococcus Western Blot IgG is the first standardized assay developed from an antigenic extract from a single species, E. multilocularis, which not only allows the diagnosis of both echinococcoses but also allows differentiation between cystic and alveolar echinococcoses in most cases. Indeed, the Em2plus ELISA, which uses two purified E. multilocularis-derived antigens, is not sensitive enough for the screening of E. granulosus infections.
Although the 18-kDa antigen was designated a good marker for detection of active E. multilocularis lesions (9), we failed to distinguish active from inactive forms of either alveolar or cystic echinococcosis. This can be explained by the greater number of serum samples used in the present study. In contrast, our data parallel those of Dreweck et al., who showed that alveolar echinococcosis sera uniformly recognize E. multilocularis components of low molecular weight, whatever the clinical status of patients (2).
As observed with an immunoblot using a homologous antigen (4), the reactivities of patients with E. vogeli disease seem to resemble those of patients with alveolar echinococcosis more than those of patients with cystic echinococcosis. Further evaluation is required to assess the usefulness of the Echinococcus Western Blot IgG for the diagnosis of E. vogeli infection, since this species, but not E. multilocularis, occurs in Latin American countries.
As previously observed with E. multilocularis-derived antigens (6), cross-reactions with neurocysticercosis and schistosomosis sera occurred. Blotting patterns observed with neurocysticercosis sera also resembled those of alveolar echinococcosis sera, whereas these two infections are usually clinically quite different. In those rare patients presenting with neurologic disorders and tumor-like lesions in the brain, who could harbor a pattern resembling that of echinococcosis, the problem can be ruled out by retesting the sera with an available T. solium immunoblot or assays using recombinant E. multilocularis-derived antigens. Differential diagnosis between echinococcosis and S. mansoni infections may be irrelevant, since they are usually allopatric in distribution and since human infections occur in quite different ways.
In conclusion, this study reveals the considerable potential of the standardized Echinococcus Western Blot IgG for the diagnosis of both cystic and alveolar echinococcoses.
ACKNOWLEDGMENTS
We thank G. Mougeot and J. P. Nozais for gifts of serum, and M. A. Dronde for excellent technical assistance.
REFERENCES
- 1.Bresson-Hadni S, Laplantes J J, Lenys D, Rohmer P, Gottstein B, Jacquier P, Mercet P, Meyer J P, Miguet J P, Vuitton D A. Seroepidemiologic screening of Echinococcus multilocularis infection in a European area endemic for alveolar echinococcosis. Am J Trop Med Hyg. 1994;51:837–846. doi: 10.4269/ajtmh.1994.51.837. [DOI] [PubMed] [Google Scholar]
- 2.Dreweck C M, Lüder C G K, Sobaslay P T, Kern P. Subclass-specific serological reactivity and IgG4-specific antigen recognition in human echinococcosis. Trop Med Int Health. 1997;2:779–787. doi: 10.1046/j.1365-3156.1997.d01-385.x. [DOI] [PubMed] [Google Scholar]
- 3.Eckert J, Thompson R C A. Intraspecific variation of Echinococcus granulosus and related species with emphasis on their infectivity to humans. Acta Trop. 1997;64:19–34. doi: 10.1016/s0001-706x(96)00635-3. [DOI] [PubMed] [Google Scholar]
- 4.Gottstein B, D'Alessandro A, Rausch R L. Immunodiagnosis of polycystic hydatid disease/polycystic echinococcosis due to Echinococcus vogeli. Am J Trop Med Hyg. 1995;53:558–563. doi: 10.4269/ajtmh.1995.53.558. [DOI] [PubMed] [Google Scholar]
- 5.Gottstein B, Eckert J, Fey H. Serological differentiation between Echinococcus granulosus and E. multilocularis infections in man. Z Parasitenkd. 1983;69:347–356. doi: 10.1007/BF00927876. [DOI] [PubMed] [Google Scholar]
- 6.Gottstein B, Jacquier P, Bresson-Hadni S, Eckert J. Improved primary immunodiagnosis of alveolar echinococcosis in humans by an enzyme-linked immunosorbent assay using the Em2+ antigen. J Clin Microbiol. 1993;31:373–376. doi: 10.1128/jcm.31.2.373-376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito A, Ma L, Schantz P M, Gottstein B, Liu Y-H, Chai J-J, Abdel-Hafez S K, Altintas N, Joshi D D, Lightowlers M W, Pawlowski Z S. Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and E. multilocularis protoscolex (EM18) Am J Trop Med Hyg. 1999;60:188–192. doi: 10.4269/ajtmh.1999.60.188. [DOI] [PubMed] [Google Scholar]
- 8.Ito A, Nakao M, Kutsumi H, Lightowlers M W, Itoh M, Sato S. Serodiagnosis of alveolar hydatid disease by Western blotting. Trans R Soc Trop Med Hyg. 1993;87:170–172. doi: 10.1016/0035-9203(93)90476-7. [DOI] [PubMed] [Google Scholar]
- 9.Ito A, Schantz P M, Wilson J F. EM18, a new serodiagnostic marker for differentiation of active and inactive cases of alveolar hydatid disease. Am J Trop Med Hyg. 1995;52:41–44. doi: 10.4269/ajtmh.1995.52.41. [DOI] [PubMed] [Google Scholar]
- 10.Kern P, Frosch P, Helbig M, Wechsler J G, Usadel S, Beckh K, Kunz R, Lucius R, Frosch M. Diagnosis of Echinococcus multilocularis infection by reverse-transcription polymerase chain reaction. Gastroenterology. 1995;109:596–600. doi: 10.1016/0016-5085(95)90350-x. [DOI] [PubMed] [Google Scholar]
- 11.Müller N, Gottstein B, Vogel M, Flury K, Seebeck T. Application of a recombinant Echinococcus multilocularis antigen in an enzyme-linked immunosorbent assay for immunodiagnosis of human alveolar echinococcosis. Mol Biochem Parasitol. 1989;36:151–160. doi: 10.1016/0166-6851(89)90187-4. [DOI] [PubMed] [Google Scholar]
- 12.Vogel M, Gottstein B, Müller N, Seebeck T. Production of a recombinant antigen of Echinococcus multilocularis with high immunodiagnostic sensitivity and specificity. Mol Biochem Parasitol. 1988;31:117–126. doi: 10.1016/0166-6851(88)90162-4. [DOI] [PubMed] [Google Scholar]
- 13.Wen H, New R R C, Craig P S. Diagnosis and treatment of human hydatidosis. Br J Clin Pharmacol. 1993;35:565–574. doi: 10.1111/j.1365-2125.1993.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]