Abstract
In the present study, carboxymethyl cellulose nanofibrils (CMCNFs) with different carboxyl content (0.99–2.01 mmol/g) were prepared via controlling the ratio of monochloroacetic acid (MCA) and sodium hydroxide to Eucalyptus bleached pulp (EBP). CMCFs-PEI aerogels were obtained using the crosslinking reaction of polyethyleneimine (PEI) and CMCNFs with the aid of glutaraldehyde (GA). The effects of pH, contact time, temperature, and initial Cu2+ concentration on the Cu2+ removal performance of CMCNFs-PEI aerogels was highlighted. Experimental data showed that the maximum adsorption capacity of CMCNF30-PEI for Cu2+ was 380.03 ± 23 mg/g, and the adsorption results were consistent with Langmuir isotherm (R2 > 0.99). The theoretical maximum adsorption capacity was 616.48 mg/g. After being treated with 0.05 M EDTA solution, the aerogel retained an 85% removal performance after three adsorption–desorption cycles. X-ray photoelectron spectroscopy (XPS) results demonstrated that complexation was the main Cu2+ adsorption mechanism. The excellent Cu2+ adsorption capacity of CMCNFs-PEI aerogels provided another avenue for the utilization of cellulose nanofibrils in the wastewater treatment field.
Keywords: carboxymethylation, cellulose nanofibrils, aerogel, adsorption
1. Introduction
With the rapid development of the social economy, heavy metal pollution has become a thorny and crucial problem in modern society [1]. Anthropogenic activities such as mining, smelting, oil refining, and the manufacture of paint, release large amounts of these toxic and dangerous chemicals into the environment [2]. Of all kinds, Cu2+ is considered to be a major contaminant in water and can accumulate in the human liver, causing severe hemolysis and anemia. Therefore, the removal of Cu2+ from wastewater in an effective manner has become an important current issue [3].
There are many ways to solve the problem of heavy metal ion pollution in wastewater, mainly including chemical precipitation, ion exchange, ultrafiltration, flocculation, electrodialysis, adsorption, and reverse osmosis, etc. [4]. Among these methods, adsorption is very popular due to its high removal efficiency, flexibility in design, and low cost [5]. The adsorbents usually include activated carbon, clay, biochar, and polymers, etc. [6]. Although these adsorbents have high adsorption capacities for some heavy metal ions, they still present some disadvantages, such as unsatisfactory non-biodegradability, high cost of preparation or renewable energy, and secondary pollution. Therefore, it is necessary to find a kind of adsorbent with high adsorption capacity to solve this problem.
Recently, cellulose, as the most common structural amphiphilic renewable polymer resource in the biosphere, has been proven to have good adsorbent adsorption performance [7,8]. Many carboxylated nanocelluloses have been used in the removal of heavy metal ions, such as TO-CNF obtained by TEMPO oxidation [9], CNC obtained by Fe2+/H2O2 oxidation [10], and carboxymethylated nanocellulose [11]. Among them, carboxymethyl cellulose (CMCs), a cellulose derivative with a high carboxyl group content obtained by the alkalization and etherification of cellulose [8], is one of the most promising substrates for aerogels on account of its economic benefits and non-toxic natural polymers [11]. However, the abundant carboxymethyl groups make them very hydrophilic and thus limit their practical applications in aqueous environments. The separation of CMC-based adsorbents from water has become a difficult problem, and affects the regeneration and recycling of the adsorbent [12]. Therefore, it is an imperative problem to preparena CMC-based adsorbent which is stable in water and has cyclic performance. Assembling individual fibers into aerogel/hydrogel is a better way to easily separate from bulk solutions as aerogel consists of interconnected porous solid materials. Li et al. [11] used Al3+ as a crosslinking agent to obtain CMC-Al beads for removing heavy metal ions. As well, there are a few reports of combining CMC with other biological macromolecules for removing heavy metal ions, such as CMC-chitosan [13] and CMC-sodium alginate [14]. Li et al. [15] obtained the NFC solution through TEMPO oxidation, and then achieved physically crosslinked network NFC/PEI composite hydrogel (NPH) through electrostatic combination with PEI (polyethyleneimine) solutions, which showed good adsorption effect for Cu2+ and Pb2+. However, rare studies have reported on grafted PEI with CMCNFs.
Branched PEI has plenty of amino groups, and usually were selected to fabricate adsorbents [16]. In this article, CMCFs-PEI aerogels were obtained using the crosslinking reaction of polyethyleneimine (PEI) and CMCNFs with different carboxyl content. Furthermore, their Cu2+ removal performance was systematically investigated under different conditions. After that, the removal mechanism of Cu2+ removal was demonstrated using FTIR (Fourier transform infrared spectroscopy) and XPS analysis. The adsorption and desorption characteristics of the aerogel were also detected.
2. Experimental
2.1. Methods and Materials
Materials: Eucalyptus bleached pulp (EBP, Jinan, Shandong Province, China), sodium hydroxide (NaOH, AR grade, Jinan, Shandong Province, China), monochloroacetic acid (MCA, Jinan, Shandong Province, China), anhydrous methanol(Jinan, Shandong Province, China), glutaraldehyde (GA, 50% in water, Aladdin), PEI (polyethylenrimine, 70000 Mw, Macklin), copper nitrate (Cu (NO3)2 (1000 mg/L), Beijing, China), hydrochloric acid (HCl, 37%, Jinan, Shandong Province, China), ethylenediaminetetraacetic acid (EDTA, Jinan, Shandong Province, China).
2.2. Preparation of Carboxymethyl Cellulose Nanofibers (CMCNFs) Dispersions
During the reaction, the quality ratio of eucalyptus bleached pulp (EBP) to NaOH was 1:1 (w/w). In order to obtain CMCNFs dispersions with different carboxylate contents, reagents with different MCA (10, 20, 30 g) were selected.
First, dry weight EBP (20 g) was blended with 100 mL NaOH solution (5 mol/L) in a sealed bag and the mixture was vigorously rubbed at room temperature for one hour. The MCA solution (40% MCA/deionized water w/v) was put in a sealed bag slowly and heated it to 65 °C for 2 h. The treated wood fibers are then repeatedly rinsed with the ethanol mixture until the pH of the filtrate was neutral. After that, the carboxymethylated EBP was treated with a high pressure homogenizer. The samples were denoted as CMCNF10, 20, 30 (10, 20, 30 were the amount of MCA).
2.3. Preparation of CMCNFs-PEI Aerogel
A total of 50 mL CMCNFs suspension (0.5 wt%) reacted with 50 mL GA (0.5%) solution at 50 °C for 0.5 h to crosslinked CMCNFs and GA. Subsequently, 50 mL of PEI solution (2% in water) was added to the mixture. After mixing and stirring for 10 min, the product was centrifuged and washed thoroughly with deionized water 3 times, and then freeze-dried to obtain CMCNFs-PEI aerogel.
2.4. Characterization
Both wood fibers, CMCNFs (freeze-dried aerogel) and CMCNFs-PEI aerogels, were characterized by FTIR (Prestige21, Shimadzu Corporation, Karlsruhe, Germany). The content of carboxylate in CMCNFs was determined by standard conductance titration (DDSJ-319L, Shanghai, China). The morphology of CMCNFs was characterized by atomic force microscope (AFM, Brooker, Karlsruhe, Germany). Scanning electron microscope (SEM, Regulus 8220, Tokyo, Japan) was used to observe the form of CMCNFs-PEI aerogel. The crystalline structure of EBP and CMCNFs was measured by X-ray diffraction (XRD, Bruker, Karlsruhe, Germany). The carboxyl content of the prepared CMCNFs was determined by conductivity meter (DDSJ-318, Shanghai, China). Thermogravimetric analysis (TGA) of CMCNFs was performed using a synchronous thermal analyzer (STA449, Selb, Germany) under inert (N2) atmosphere heated continuously from room temperature (RT) at 10 °C/min to 600 °C. Besides, X-ray photoelectron spectroscopy (XPS, ESCALABXi+, New York, NY, USA) was analyzed for the surface chemical compositions of CMCNF30-PEI aerogel and CMCNF30-PEI loaded Cu2+.
2.5. Adsorption Experiments for Cu2+ Adsorption by Three Aerogels
The adsorption kinetics and isotherms of Cu2+ by CMCFs-PEI were investigated by batch adsorption experiments. Add aerogels (0.05 g) and Cu2+ solution (50 mL) to a 100 mL flask. Then put the flask in Temersionoxcillator registrarion (NoKi) at 25 °C to reach the adsorption equilibrium. When the pH exceeded 6.5, Cu2+ ions could be converted to Cu (OH)2 precipitation [14]. The effect of initial pH values in the range of 2–6 was investigated, in which the pH of the Cu2+ solution was controlled by 1 M NaOH/1 M HCl solution. For the kinetics study, the effect of contact time from 0 to 8 h was investigated at optimal pH. The adsorption isotherm experiments were carried out in the concentration range of 20~400 mg/L metal ions. The residual concentration of Cu2+ were determined by flame atomization atomic absorption spectrometry (AAS, GGX-600, China), the equilibrium adsorption capacity (qe, mg/g) and removal rate were calculated according to (Equation (1)) [17].
| (1) |
| (2) |
where C0 is the initial concentration of Cu2+ (mg/L) and Ce is the concentration of Cu2+ at equilibrium time; V (mL) is the volume of the solution and m (mg) is the aerogel dose.
2.6. Adsorption Kinetics and Isotherms
Pseudo-first-order and second-order kinetic models were used to simulate the adsorption kinetic data of Cu2+ on CMCNFs-PEI, and the expression is found in Equations (3) and (4) [18]:
| (3) |
| (4) |
where qt is the adsorption capacity after time t and qe is the saturated adsorption capacity of Cu2+; k1 (min−1) and k2 (g/(mg min)) are rate constants of pseudo-first- and second-order kinetics, respectively.
The adsorption isotherms of Cu2+ adsorption by CMCNFs-PEI aerogel; the Langmuir and Freundlich adsorption isotherm models were used to analyze the experimental data, which are represented as Equations (5) and (6) [19]:
| (5) |
| (6) |
where Qe (mg/g) is the equilibrium adsorption capacity, Ce (mg/L) is the Cu2+ solution concentration at equilibrium, Qm (mg/g) is the maximum adsorption capacity and b is the Langmuir adsorption constant related to adsorption energy, Kf and n are the Freundlich adsorption constants which indicate the capacity and intensity of the adsorption, respectively.
Thermodynamic parameters such as free energy, enthalpy change and entropy change are determined by thermodynamic equations, as follows (7) and (8) [20]:
| (7) |
| (8) |
where Kd (mL/g) is the equilibrium constant, R is the gas constant (8.314 J/mol/K), and T (K) is the absolute temperature.
2.7. Cycle Testing of CMCNFS-PEI
In order to evaluate the cycling stability of the aerogel, the adsorption-desorption cyclic experiments were carried out. Cu2+ was adsorbed by CMCNF30-PEI aerogel, then immersed in 0.05 M EDTA solution and stirred at 25 °C for 3 h to remove Cu2+. The aerogel was then immersed in distilled water several times to elute all the salt until a pH of about 7 was reached before another adsorption process was carried out.
3. Results and Discussion
3.1. Characterization of the Aerogel
The FTIR spectra of EBP, CMCNFs, and CMCNFs-PEI are presented in Figure 1a. A comparison of the FTIR results reveals that new peaks appeared in CMCNFs after carboxymethylation. For EBP, the peak at 3400 cm−1 corresponds to the stretching vibration of O–H groups, and the peak at 1370 cm−1 and 1322 cm−1 belong to C-H vibration [21], whereas in the FTIR spectra of the CMCNF10 sample, the stretching vibration of the carbonyl (C=O) present in the carboxylate groups (COO−) was observed at 1606 cm−1. However, CMCNF20 and CMCNF30 have a stretching vibration at 1740 cm−1 (−COOH), which may be due to higher carboxyl content. To prove the successful grafting of PEI onto CMCNFs, the FTIR spectra of CMCNFs-PEI aerogels are shown in Figure 1b. Compared to CMCNFs, there are many new adsorption peaks appearing at 1453 cm−1, 1592 cm−1, and 1648 cm−1 associated with the amino groups on CMCNFs-PEI aerogels. Also, the occurrence of -CH2- stretching vibrations at 2923 cm−1 and 2848 cm−1 also can prove it. The adsorption peak at 1740 cm−1 should be contributed from stretching C=O groups, which appeared in three CMCNFs-PEI aerogels.
Figure 1.
FTIR spectra of (a) EBP, CMCNFs, (b) CMCNFs-PEI, (c) XRD spectra of EBP and CMCNFs, (d) illustration of approach used for determination of carboxyl group in CMCNFs, (e) TGA, and (f) DTG curves of all samples.
The XRD was used to analyze the effect of carboxymethylation on the crystal structure of CMCNFs (Figure 1c). Three peaks were observed at 2θ = 15.72°, 22.5°, and 34.5° in EBP. This is representative of EBP which is typical of cellulose I. For CMCNFs, the 2θ = 22.5° diffraction peak disappeared, and the 2θ = 20.5° diffraction peak corresponding to the alkali cellulose became obvious. At 2θ = 15.72°, the diffraction peak became weak and disappeared at 2θ = 34.5°. After treatment with 5 mol/L NaOH solution, the crystal lattice changed greatly, which is called intracellular swelling. There are two kinds of intracellular swelling. One is where the solvent enters the intracellular cell and increases the distance between macromolecular chains of cellulose, which is also called separate swelling. The other involves the formation of a compound with cellulose, which then changes the cellular structure of cellulose, and the cellular structure of primary cellulose is transformed into the crystalline structure of alkali cellulose [22].
The thermal degradation onset temperature (T0) and maximum decomposition temperature (Tmax) are shown in Table 1. During the process from RT to 100 °C, due to the loss of water, the four substances have a slight quality degradation. The T0 and Tmax of the EBP were about 276.5 and 388.78 °C, respectively; thus, the CMCNFs exhibited a lower T0 and Tmax. The NaOH/ chloroacetic acid system introduced a large amount of carboxyl groups, and at neutral pH, the hydrogen bonds were disrupted and the molecular chains became less constrained. A higher carboxyl content lowered the T0 and Tmax, for example, T0 and Tmax of CMCNF30 (232.96 and 330.78 °C) was lower than that of CMCNF10 (267.99 and 353.76 °C). The carboxyl group on the surface of the CNCNFs increased, and the thermal stability gradually decreased because of the more thermally unstable carboxyl groups insertion of the CNF surfaces. The more the carboxyl groups on the nanofibrils, the lower the T0 and Tmax.
Table 1.
Thermal stability parameters of EBP and CMCNFs.
| EBP | CMCNF10 | CMCNF20 | CMCNF30 | CMCNF10-PEI | CMCNF20-PEI | CMCNF30-PEI | |
|---|---|---|---|---|---|---|---|
| Carboxyl content (mmol/g) | 0.99 | 1.52 | 2.01 | ||||
| T0 (°C) | 276.5 | 267.99 | 225.71 | 232.96 | 218.64 | 219.60 | 218.19 |
| Tmax (°C) | 388.78 | 353.76 | 340.49 | 330.78 | 380.09 | 380.36 | 381.50 |
T0, Tmax were calculated from TGA curves.
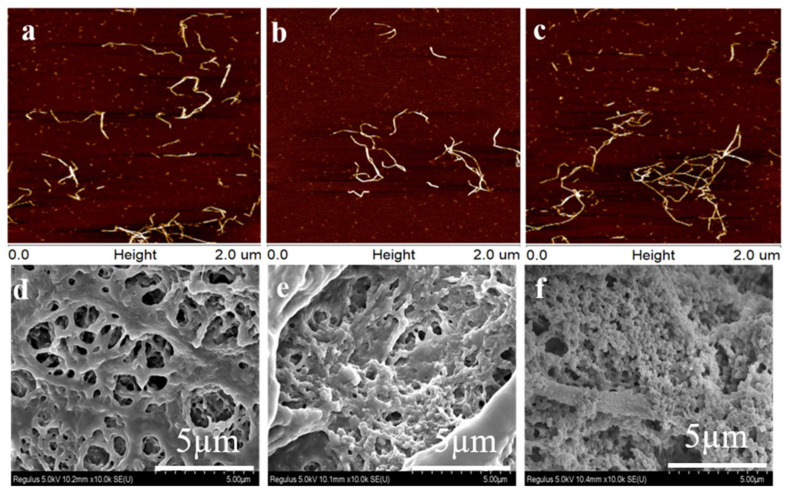
The surface morphology of CMCNFs was examined by AFM (Figure 2a–c) and CMCNFs-PEI was examined by SEM (Figure 2d–f). As a result, it can be clearly seen that there is a larger aspect ratio. From the SEM of CMCNFS-PEI aerogel, it can be seen that there are pore structures in the typical 3D network. This can be used as a transport channel for heavy metal ions from the aqueous medium to enter the interior of the aerogel network through the rich pore structure. At the same time, it can also improve contact chance of Cu2+ with CMCNFs-PEI aerogel. When the adsorbent contacts the wastewater, water molecules can penetrate into the interior as soon as possible to achieve the effect of rapid adsorption. It may be that the water solubility of CMCNF30 increased with the increase of carboxyl group content, leading to the different surface morphology of CMCNF30-PEI. Compared with other heavy metal adsorbents, this aerogel has attracted extensive attention in the field of water treatment due to its unique 3D structure.
Figure 2.
AFM images for dispersions of (a) CMCNF10, (b) CMCNF20, (c) CMCNF30; SEM images for aerogel of (d) CMCNF10-PEI, (e) CMCNF20-PEI, (f) CMCNF30-PEI.
A total of 0.03 g of CMCNF30 aerogel obtained by freeze drying of CMCNF30 suspension was weighed and placed into 20 mL of deionized water for a hydrophilicity test. As shown in Figure 3, from the appearance, the color of CMCNF30 aerogel is white. After PEI modification, the color of CMCNF30/PEI aerogel turned yellow due to Schiff base reaction. CMCNF30/PEI aerogel of the same quality was used for comparison. The volume of both CMCNF30 and CMCNF30/PEI aerogels increased due to water absorption one minute after the aerogels were placed in deionized water. After 12 h, CMCNF30 aerogel dissolved in water, however, CMCNF30/PEI aerogel did not changed. This proves that the hydrophilicity of CMCNF decreases after PEI modification.
Figure 3.
CMCNF30 and CMCNF30/PEI aerogel in deionized water for (a) 0 s, (b) 1 min, (c) 12 h; (d) adsorption–desorption isotherm of CMCNF30-PEI aerogel at 77K.
Figure 3d shows the N2 adsorption–desorption curve of the sample at a temperature of 77 K. The curve of CMCNF30-PEI aerogel shown in the figure is type four, which also verifies that the nanoparticles have a mesoporous structure. As can be seen from Table 2, the specific surface area and average pore size of CMCNF10-PEI increased from 0.82 to 1.52 m2/g, and the average pore size increased from 92.85 to 163.3 Å. The adsorption of P/P0 between 0–0.6 representing micropore (<20 Å) is negligible. The adsorption then increased rapidly, when P/P0 was between 0.6–1.0, representing mesoporous structures (20–500 Å) and indicating the presence of mesopores in CMCNF30-PEI, which provided a positive effect for the adsorption of heavy metals.
Table 2.
Specific surface area and average pore size of CMCNFs-PEI aerogel.
| Sample | Bet Surface Area (m2/g) | Langmuir Surface Area (m2/g) | Pore Size (Å) |
|---|---|---|---|
| CMCNF10-PEI | 0.82 | 0.21 | 92.85 |
| CMCNF20-PEI | 1.16 | 1.55 | 121.8 |
| CMCNF30-PEI | 1.52 | 2.15 | 163.3 |
3.2. Cu2+ Adsorption
3.2.1. Adsorption Kinetics of CMCNFs-PEI Aerogel
We attempted to understand the Cu2+ removal performance of CMCNFs-PEI with pH and time. As presented in Figure 4a–c, due to the presence of amino groups on CMCNFs-PEI, the surface charge changes with the change of pH, thereby affecting the adsorption capacity. With the increase of pH, adsorption capacity also increases. Owing to the protonation of amino groups, the absorbent showed low adsorption capacity to Cu2+ at low pH conditions. When at a pH of six, the adsorption capacity increased to the maximum. It is noticeable that the adsorption capacity of aerogels increases as the carboxyl content of aerogels increases. According to the experimental data, the content of CMCNF10-PEI increased from 15.26 to 18.67 mg/g for CMCNF30-PEI. Therefore, the adsorption performance of CMCNFs-PEI for Cu2+ was studied under the condition of a pH of six.
Figure 4.
Effect of initial pH on Cu2+ adsorption; (a) CMCNF10-PEI (b) CMCNF20-PEI (c) CMCNF30-PEI. (d) Contact time removal capacity for Cu2+ adsorption (pH = 6, C0 = 20 mg/L, T = 25 °C). (e) Plots of pseudo-first-order, and (f) pseudo-second-order of CMCNF10-PEI, CMCNF20-PEI, CMCNF30-PEI. (g) Effect of different initial concentration on Cu2+ adsorption of three CMCNFs-PEI (pH = 6, t = 24 h, T = 25 °C). (h) CMCNF10, (i) CMCNF20-PEI, (j) CMCNF30-PEI isotherm model for Cu2+ adsorption.
To understand the basic kinetics of Cu2+ removal by CMCNFs-PEI, we evaluated the relationship between contact time (t) and removal capacity (Qt) between exposure time 0 to 540 min (Figure 4d,e). The removal rate increased rapidly at the beginning of the experiment and then reached equilibrium after 120 min. Two simplified kinetic models can be used to fit the experimental data of the adsorption process. The fitting results of adsorption kinetics for Cu2+ ion adsorption by CMCNFs-PEI aerogels is exhibited in Table 2. The non-linear correlation coefficient (R2) values for the second-order are higher than first-order, which indicates that the Cu2+ removal by CMCNFs-PEI is a rate-limiting and chemisorption process.
3.2.2. Adsorption Isotherms of CMCNFs-PEI Aerogel
In order to confirm the interaction between CMCNFs-PEI and Cu2+, and that the maximum adsorption capacity of CMCNFs-PEI is the key to evaluate its removal performance, we studied the relationship between the initial Cu2+ concentration and equilibrium removal capacity of CMCNFs-PEI.
The removal results of the CMCNFs-PEI with different initial concentrations of Cu2+ are shown in Figure 4g–i. The adsorption isotherm parameters are displayed in Table 3. For CMCNF10-PEI, R2 > 0.98; for other adsorbents (CMCNF20-PEI, CMCNF30-PEI), R2 > 0.99. The results show that both Langmuir and Freundlich models can fit the adsorption isotherm well. The Langmuir model showed that the active adsorption sites were uniformly distributed on the surface of the aerogel, and the adsorption of Cu2+ ions occurred at the binding sites of functional groups and the surface, forming a monolayer [22]. However, Freundlich isotherm is used in the low to intermediate adsorbate concentration range [23]. The maximum Langmuir adsorption capacity for Cu2+ on CMCNF10-PEI aerogel was determined to be 307.43 mg/g. The adsorption capacity increased with the increase of carboxyl group content. The maximum adsorption capacity of CMCNF30-PEI was 616.48 mg/g, which was considered to be a super high capacity. This may be due to the carboxyl group and the amino group acting as electrostatic attraction and chelation to Cu2+, respectively. In Figure 5b, we provide a comparison between the current CMCNFs-PEI aerogels and other related Cu2+ adsorbents presented in the literatures. Compared with previous reported results, the adsorption ability of CMCNFs-PEI aerogels for Cu2+ are much higher.
Table 3.
Kinetic and isotherm parameters for Cu2+ adsorption onto CMCNFs-PEI aerogels.
| Model | Parameters | Aerogels | ||
|---|---|---|---|---|
| CMCNF10-PEI | CMCNF20-PEI | CMCNF30-PEI | ||
| 1st-order kinetic | k1 (g/mg∙h) | 0.063 | 0.096 | 0.1086 |
| qexp (mg/g) | 15.26 | 16.62 | 18.67 | |
| qcal (mg/g) | 14.50 | 15.31 | 16.99 | |
| R 2 | 0.9470 | 0.9060 | 0.9387 | |
| 2nd-order kinetic | k2 (g/mg∙h) | 0.0059 | 0.0074 | 0.0078 |
| qexp (mg/g) | 15.26 | 16.62 | 18.67 | |
| qcal (mg/g) | 15.51 | 16.48 | 18.18 | |
| R 2 | 0.9874 | 0.9652 | 0.9745 | |
| Langmuir isotherm | KL (L/g) | 0.0036 | 0.0019 | 0.0013 |
| q0 (mg/g) | 307.43 | 528.36 | 616.48 | |
| R 2 | 0.9845 | 0.9915 | 0.9972 | |
| Freundlich isotherm | KF | 82.07 | 62.18 | 114.02 |
| n | 4.13 | 2.74 | 5.74 | |
| R 2 | 0.9843 | 0.9933 | 0.9845 | |
Figure 5.
(a) The maximum adsorption capacity for Cu2+ with different cellulose-based adsorbents was compared with CMCNFs-PEI aerogels: Cu2+ adsorption capacity in this work, pomelo peel carboxylated cellulose nanofibers-PEI (POCNF-PEI) [24]; polydopamine-CNF-PEI (PDA-CNF-PEI) [25]; carboxymethylated CNFs (CMCNF-2.7) [26]; NFC/PEI composite aerogel (NPAs) [15]; NFCs/poly(2-(dimethylamino) ethyl methacrylate) interpenetrating network hydrogels (NPIHs) [27]; cellulose nanowhiskers (CNWs) [28]. (b) Effect of temperature on the Cu2+ adsorption of CMCNF30-PEI.
3.2.3. Thermodynamics of CMCNF30-PEI Adsorption
Thermodynamic parameters associated with the adsorption of Cu2+ using CMCNF30-PEI are presented in Figure 5b and Table 4. In this section, the adsorption capacity and removal efficiency of CMCNF30-PEI at a pH of six and the initial Cu2+ concentration of 20 mg/L at different temperatures (288, 298 and 308 K) were investigated. It can be seen from the figure that with the increase of temperature, both adsorption capacity and removal efficiency increase. ∆G > 0 and decreases with the increase of temperature, indicating the increasing spontaneity of the adsorption. Therefore, the adsorption of Cu2+ using the aerogel appears to be an irreversible and spontaneous endothermic process.
Table 4.
Thermodynamic parameters of CMCNF30-PEI adsorption for Cu2+.
| T(K) | ∆G (KJ/mol) | ∆H (KJ/mol) | ∆S (KJ/mol/K) |
|---|---|---|---|
| 288 | −20.6 | 49.33 | 0.2428 |
| 298 | −23.02 | ||
| 308 | −25.45 |
3.2.4. Adsorption Mechanism of CMCNFs-PEI on Cu2+
XPS was used to study the elemental composition of CMCNF30-PEI and CMCNF30-PEI loaded Cu2+ ions (Figure 6a–h). Obviously, several new peaks appeared, assigned to Cu 2p doublet (Cu 2p1/2 and Cu 2p3/2), Cu 3s, and Cu 3p, which confirms the presence of Cu2+ on CMCNF30-PEI. In addition, the detailed XPS spectra of different elements at high resolution were deconvolved to evaluate the contribution of each component. As shown in Figure 6b, before adsorption, the N 1s peak could be fitted into the three components of 398.15, 399, and 400.15 eV corresponding to -N-, -NH- and -NH2, respectively. The high resolution XPS O 1s core-level spectrum of CMCNF30-PEI (Figure 6c) can be divided into two components at 530.5 eV and 531.8 eV, which were assigned to C-O and C=O, respectively. After Cu2+ ions were adsorbed (Figure 6f), the peaks of N 1s shifted to 398.7 eV, 399.3 eV, and 401.3 eV, and proved that all three amino groups are involved in the binding of Cu2+ ions [29]. At the same time, a new peak of 406.1 eV appeared in the N 1s spectra, which was due to the nitrogen in the amino groups sharing a lone electron pair to form the metal complex. In Figure 6g, for the O 1s spectra of CMCNF30-PEI aerogel, the C-O and C=O components increased to 532.9 eV and 531.5 eV, which is due to the electrons in the oxygen atom giving the oxygen of the carboxylic group a shared bond with the Cu2+ ions. In the expanded spectrum shown in Figure 6h, two characteristic peaks for the energy levels of Cu 2p1/2 (934.25 eV) and Cu 2p3/2 (954.15 eV) are visible [30]. In general, oxygen atoms and nitrogen atoms in CMCNF30-PEI have significant contributions to Cu2+ ion adsorption. The XPS spectra provided direct evidence of Cu2+ adsorption on CMCNF30-PEI via chemisorption.
Figure 6.
(a) Wide-scan XPS spectrum and (b–d) high-resolution core-level spectrum of CMCNF30-PEI, (e) wide-scan XPS spectrum and (f–h) high-resolution core-level spectrum of CMCNF30-PEI after Cu2+ adsorption.
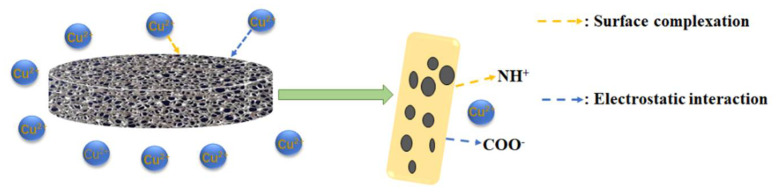
The adsorption mechanism of the CMCNFs-PEI for Cu2+ derived from the data presented in the present study is shown in Figure 7. XPS results show that both N and O atoms have a certain effect on Cu2+. Among them, -COOH plays the role of electrostatic attraction, and functional groups such as -NH and -NH2 serve as a ligand for producing ligand–metal surface complexes, thus achieving the effect of Cu2+ removal.
Figure 7.
Schematic illustration of the adsorption mechanism of the CMCNFs-PEI.
3.2.5. Desorption and Reusability
The ideal aerogel should have high adsorption capacity and have good regeneration and recycling properties, which are an important index of the practical application of water remediation. After adsorbtion with Cu2+ (Figure 8a), as can be clearly seen, the internal structure remained 3D reticulated, but the channels became tense. From FTIR spectra (Figure 8b), CMCNF30-PEI aerogel showed that the positions of characteristic peaks related to amino and carboxyl groups changed after Cu2+ adsorption. In order to explore the recycling ability of CMCNF30-PEI, 0.05 M EDTA solution was used as the desorption agent. Figure 8c shows the performance of CMCNF30-PEI for Cu2+ adsorption during three cycles. Although the regeneration efficiency decreased slightly with the cycle time, it still retained 85% after three adsorption–desorption cycles, indicating the excellent recyclability and reusability of the developed aerogel. The stability test showed the excellent cycling ability of the current test, thus it can be applied to practical wastewater treatment.
Figure 8.
(a) SEM image of CMCNF30-PEI after adsorbed Cu2+. (b) FTIR spectra of CMCNF30-PEI aerogel and CMCNF30-PEI aerogel loaded with Cu2+. (c) Three adsorption cycles of CMCNF30-PEI aerogel for Cu2+ solution.
3.2.6. Comparison with Reported Studies
The final adsorption properties of CMCNF30-PEI were evaluated and presented in Table 5 for comparison with reported data. The maximum adsorption capacity of CMCNF30-PEI for Cu2+ can reach 588.26 mg/g; 3-dialdehyde nano-fibrillated cellulose (DNFCs), TO-CNF/PVA/PEI nanoparticles, Fe3O4@zeolite NaA and composite from cellulose nanocrystals of Almond Prunus dulcis shell (CPCNCs) are much higher. Having excellent cycling performance as well, CMCNF30-PEI may play an important role in practical water treatment.
Table 5.
Comparison of Cu2+ adsorption capacity of different materials.
4. Conclusions
In summary, we demonstrated a facile and novel CMCNFs-PEI adsorbent that has a high adsorption capacity for Cu2+. Compared with pure CMCNF, the hydrophilicity of CMCNF-PEI obviously decreased, and this led to an appearance of a 3D network structure. Remarkably, adsorption isotherm data revealed that the maximum adsorption capacity for Cu2+ was in the order: CMCNC30-PEI (618.48 mg/g) > CMCNF20-PEI (528.36 mg/g) > CMCNF10-PEI (307.43 mg/g). Kinetic studies showed that the adsorption process of Cu2+ on CMCNFs-PEI was more likely to be modeled by pseudo-second-order equation. The adsorption mechanism of Cu2+ might be attributed to the active sites (carboxyl, amino groups) on the surface of CMCNF30-PEI. In addition, EDTA solution can regenerate CMCNF30-PEI, with the adsorption capacity still retained at 85% after three cycles. This work provides a facile and novel method for preparing the CMCNFs-based Cu2+ adsorbent.
Author Contributions
R.S.: Conceptualization, Methodology, Writing—original draft. Y.C.: Software. D.W.: Visualization, D.Y.: Supervision. Q.D.: Data curation, Writing—review & editing. R.L.: Resources. C.W.: Project administration, Funding acquisition, Formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This article was financially supported by the National Key R&D Program of China (2017YFB0307900) and the Foundation (No. ZZ20200107) of State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology, Shandong Academy of Sciences, Natural Science Foundation of Shandong Province, China (ZR2021QC158).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Na Kim H., Ren W.X., Kim J.S., Yoon J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2011;41:3210–3244. doi: 10.1039/C1CS15245A. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q., Zheng J., Wen L., Yang C., Zhang L. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: Performance and quantum chemical mechanism. Chemosphere. 2019;224:509–518. doi: 10.1016/j.chemosphere.2019.02.138. [DOI] [PubMed] [Google Scholar]
- 3.Amarasinghe B., Williams R.A. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007;132:299–309. doi: 10.1016/j.cej.2007.01.016. [DOI] [Google Scholar]
- 4.Fu F., Wang Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011;92:407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Shen C., Zhao Y., Li W., Yang Y., Liu R., Morgen D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019;372:1019–1027. doi: 10.1016/j.cej.2019.04.219. [DOI] [Google Scholar]
- 6.Qin H., Hu T., Zhai Y., Lu N., Aliyeva J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2019;258:113777. doi: 10.1016/j.envpol.2019.113777. [DOI] [PubMed] [Google Scholar]
- 7.Musarurwa H., Tavengwa N.T. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohydr. Polym. 2020;237:116142. doi: 10.1016/j.carbpol.2020.116142. [DOI] [PubMed] [Google Scholar]
- 8.Thomas B., Raj M.C., Athira K.B., Rubiah M.H., Joy J., Moores A., Drisko G.L., Sanchez C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018;118:11575–11625. doi: 10.1021/acs.chemrev.7b00627. [DOI] [PubMed] [Google Scholar]
- 9.Ma H., Hsiao B.S., Chu B. Ultrafine Cellulose Nanofibers as Efficient Adsorbents for Removal of UO22+ in Water. ACS Macro Lett. 2011;1:213–216. doi: 10.1021/mz200047q. [DOI] [PubMed] [Google Scholar]
- 10.Fan X.-M., Yu H.-Y., Wang D.-C., Mao Z.-H., Yao J., Tam K.C. Facile and Green Synthesis of Carboxylated Cellulose Nanocrystals as Efficient Adsorbents in Wastewater Treatments. ACS Sustain. Chem. Eng. 2019;7:18067–18075. doi: 10.1021/acssuschemeng.9b05081. [DOI] [Google Scholar]
- 11.Li S.-S., Song Y.-L., Yang H.-R., An Q.-D., Xiao Z.-Y., Zhai S.-R. Carboxymethyl cellulose-based cryogels for efficient heavy metal capture: Aluminum-mediated assembly process and sorption mechanism. Int. J. Biol. Macromol. 2020;164:3275–3286. doi: 10.1016/j.ijbiomac.2020.08.186. [DOI] [PubMed] [Google Scholar]
- 12.Lian Z., Li Y., Xian H., Ouyang X.-K., Lu Y., Peng X., Hu D. EDTA-functionalized magnetic chitosan oligosaccharide and carboxymethyl cellulose nanocomposite: Synthesis, characterization, and Pb(II) adsorption performance. Int. J. Biol. Macromol. 2020;165:591–600. doi: 10.1016/j.ijbiomac.2020.09.156. [DOI] [PubMed] [Google Scholar]
- 13.Xu X., Ouyang X.-K., Yang L.-Y. Adsorption of Pb(II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J. Mol. Liq. 2020;322:114523. doi: 10.1016/j.molliq.2020.114523. [DOI] [Google Scholar]
- 14.Cheng Y., Lu L., Zhang W., Shi J., Cao Y. Reinforced low density alginate-based aerogels: Preparation, hydrophobic modification and characterization. Carbohydr. Polym. 2012;88:1093–1099. doi: 10.1016/j.carbpol.2012.01.075. [DOI] [Google Scholar]
- 15.Li J., Zuo K., Wu W., Xu Z., Yi Y., Jing Y., Dai H., Fang G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II) Carbohydr. Polym. 2018;196:376–384. doi: 10.1016/j.carbpol.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Si R., Wu C., Yu D., Ding Q., Li R. Novel TEMPO-oxidized cellulose nanofiber/polyvinyl alcohol/polyethyleneimine nanoparticles for Cu2+ removal in water. Cellulose. 2021;28:10999–11011. doi: 10.1007/s10570-021-04236-4. [DOI] [Google Scholar]
- 17.Li H., Wang Y., Ye M., Zhang X., Zhang H., Wang G., Zhang Y. Hierarchically porous poly(amidoxime)/bacterial cellulose composite aerogel for highly efficient scavenging of heavy metals. J. Colloid Interface Sci. 2021;600:752–763. doi: 10.1016/j.jcis.2021.05.071. [DOI] [PubMed] [Google Scholar]
- 18.Xing H.T., Chen J.H., Sun X., Huang Y.H., Su Z.B., Hu S.R., Weng W., Li S.X., Guo H.X., Wu W.B., et al. NH2-rich polymer/graphene oxide use as a novel adsorbent for removal of Cu(II) from aqueous solution. Chem. Eng. J. 2015;263:280–289. doi: 10.1016/j.cej.2014.10.111. [DOI] [Google Scholar]
- 19.Zhang X., Elsayed I., Navarathna C., Schueneman G.T., Hassan E.B. Biohybrid Hydrogel and Aerogel from Self-Assembled Nanocellulose and Nanochitin as a High-Efficiency Adsorbent for Water Purification. ACS Appl. Mater. Interfaces. 2019;11:46714–46725. doi: 10.1021/acsami.9b15139. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z., Wu C., Ding Q., Yu D., Li R. Novel dual modified alkali lignin based adsorbent for the removal of Pb2+ in water. Ind. Crop. Prod. 2021;173:114100. doi: 10.1016/j.indcrop.2021.114100. [DOI] [Google Scholar]
- 21.Zhu Y., Wu C., Yu D., Ding Q., Li R. Tunable micro-structure of dissolving pulp-based cellulose nanofibrils with facile prehydrolysis process. Cellulose. 2021;28:3759–3773. doi: 10.1007/s10570-021-03760-7. [DOI] [Google Scholar]
- 22.Lei Z., Gao W., Zeng J., Wang B., Xu J. The mechanism of Cu (II) adsorption onto 2,3-dialdehyde nano-fibrillated celluloses. Carbohydr. Polym. 2019;230:115631. doi: 10.1016/j.carbpol.2019.115631. [DOI] [PubMed] [Google Scholar]
- 23.Wang N., Ouyang X.-K., Yang L.-Y., Omer A.M. Fabrication of a Magnetic Cellulose Nanocrystal/Metal–Organic Framework Composite for Removal of Pb(II) from Water. ACS Sustain. Chem. Eng. 2017;5:10447–10458. doi: 10.1021/acssuschemeng.7b02472. [DOI] [Google Scholar]
- 24.Tang F., Yu H., Abdalkarim S.Y.H., Sun J., Fan X., Li Y., Zhou Y., Tam K.C. Green acid-free hydrolysis of wasted pomelo peel to produce carboxylated cellulose nanofibers with super absorption/flocculation ability for environmental remediation materials. Chem. Eng. J. 2020;395:125070. doi: 10.1016/j.cej.2020.125070. [DOI] [Google Scholar]
- 25.Tang J., Song Y., Zhao F., Spinney S., da Silva Bernardes J., Tam K.C. Compressible cellulose nanofibril (CNF) based aerogels produced via a bio-inspired strategy for heavy metal ion and dye removal. Carbohydr. Polym. 2019;208:404–412. doi: 10.1016/j.carbpol.2018.12.079. [DOI] [PubMed] [Google Scholar]
- 26.Qin F., Fang Z., Zhou J., Sun C., Chen K., Ding Z., Li G., Qiu X. Efficient Removal of Cu2+ in Water by Carboxymethylated Cellulose Nanofibrils: Performance and Mechanism. Biomacromolecules. 2019;20:4466–4475. doi: 10.1021/acs.biomac.9b01198. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Xu Z., Wu W., Jing Y., Dai H., Fang G. Nanocellulose/Poly(2-(dimethylamino)ethyl methacrylate)Interpenetrating polymer network hydrogels for removal of Pb(II) and Cu(II) ions. Colloids Surfaces A Physicochem. Eng. Asp. 2018;538:474–480. doi: 10.1016/j.colsurfa.2017.11.019. [DOI] [Google Scholar]
- 28.Rodrigues F.H., Magalhaes C.E.D.C., Medina A.L., Fajardo A.R. Hydrogel composites containing nanocellulose as adsorbents for aqueous removal of heavy metals: Design, optimization, and application. Cellulose. 2019;26:9119–9133. doi: 10.1007/s10570-019-02736-y. [DOI] [Google Scholar]
- 29.Mo L. 3D multi-wall perforated nanocellulose-based polyethylenimine aerogels for ultrahigh effcient and reversible removal of Cu(2+) ions from water. Chem. Eng. J. 2019;378:122157. doi: 10.1016/j.cej.2019.122157. [DOI] [Google Scholar]
- 30.Cao Y., Qian X., Zhang Y., Qu G., Xia T., Guo X., Jia H., Wang T. Decomplexation of EDTA-chelated copper and removal of copper ions by non-thermal plasma oxidation/alkaline precipitation. Chem. Eng. J. 2019;362:487–496. doi: 10.1016/j.cej.2019.01.061. [DOI] [Google Scholar]
- 31.Cao J., Wang P., Shen J., Sun Q. Core-shell Fe3O4@zeolite NaA as an Adsorbent for Cu2+ Materials. 2020;13:5047. doi: 10.3390/ma13215047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maaloul N., Oulego P., Rendueles M., Ghorbal A., Díaz M. Biopolymer composite from cellulose nanocrystals of almond (Prunus dulcis) shell as effective adsorbents for Cu2+ ions from aqueous solutions. J. Environ. Chem. Eng. 2021;9:105139. doi: 10.1016/j.jece.2021.105139. [DOI] [Google Scholar]