Abstract
Plumeria rubra (L.) is a traditional folkloric medicinal herb used to treat cardiovascular disorders. The present investigation was methodically planned to investigate the pharmacological foundations for the therapeutic effectiveness of P. rubra in cardiovascular illnesses and its underlying mechanisms. Ex vivo vaso-relaxant effects of crude leaf extract of P. rubra were observed in rabbit aorta ring preparations. Hypotensive effects were measured using pressure and force transducers connected to the Power Lab data acquisition system. Furthermore, P. rubra displayed cardioprotective properties in rabbits when they were exposed to adrenaline-induced myocardial infarction. In comparison to the intoxicated group, the myocardial infarction model showed decreased troponin levels, CK-MB, LDH, ALT, ALP, AST, and CRP, as well as necrosis, apoptosis, oedema, and inflammatory cell enrollment. P. rubra has revealed good antioxidant properties and prolonged the noradrenaline intoxicated platelet adhesion. Its anticoagulant, vasorelaxant, and cardioprotective effects in both in vivo and ex vivo investigations are enabled by blocking L-type calcium channels, lowering adrenaline, induced oxidative stress, and tissue tear, justifying its therapeutic utility in cardiovascular disorders.
Keywords: Plumeria rbra, cardioprotective, adrenaline, troponin, CRP
1. Introduction
Cardiovascular disease (CVD) is defined as a set of heart and blood vessel abnormalities that comprise congestive heart failure (CHF), myocardial infarction (MI), peripheral arterial disease (PAD), angina of different types, and coronary heart disease (CHD) [1]. Globally, hypertension is the primary cause of death in humans [2]. Many developing countries, such as India, Iran, Bangladesh, Afghanistan, and Pakistan, are fast catching up with this epidemic, due to drastically changing lifestyle habits [2]. Fast foods, additives, preservatives, biologically engineered food, and the sedentary lifestyle multiply the misery [3]. Although synthetic drugs are incredibly effective in treating cardiovascular diseases, their usage is restricted due to their side effects [4]. MI is a primary ischemic condition, characterized by the severe destruction of myocardial tissue. It occurs due to a mismatch between oxygen demand and blood supply in the myocardium, without atherosclerotic plaque or with atherosclerotic plaque [5]. Exaggerated production of reactive oxygen species (ROS) is accountable for lipid peroxidation of the endocardium. This damage results in the deletion of cardiac antioxidants, the elevation of oxidative stress, and apoptosis [5]. Similarly, catecholamine affects the myocardium in both inotropic and chronotropic ways. Surplus catecholamines cause coronary vasoconstriction, which increases myocardial oxygen demand and decreases myocardial blood delivery, resulting in MI [6,7]. Adrenaline (ADR) is a catecholamine produced by the adrenal glands’ medullary potion. ADR is an approved drug for cardiopulmonary resuscitation in humans. It also has therapeutic uses in treating allergic reactions, glaucoma, asthma, and cardiac arrest [8]. It causes MI above the therapeutic dose (2 mg/kg body weight) [5]. This method was used to assess the cardioprotective effect of test medications to induce MI in laboratory animals. Lipid peroxidation (LPO), which results in the loss of intracellular antioxidants, causes MI [6]. It leads to an increased production of nitrosative derivatives, which cause the overproduction of ROS and, as a result, the myocardium faces immense oxidative stress [9]. It increases Ca++ upsurge by opening L-type calcium channels on cardiomyocytes, and raises oxidative stress by escalating workload [1]. It causes aortic and coronary vasoconstriction at higher doses, and enhances blood coagulation by increasing clotting factor VIII, fibrinolysis, and platelet count [5].
Herbal remedies are highly beneficial in treating a variety of ailments all over the world; herbal medicines continue to provide vital curative agents in conventional and modern medicine, and they are also less toxic than synthetic drugs [10].
Plumeria rubra, Linn. (Family: Apocynaceae) is native to Mexico, Central America, Colombia, and Venezuela but cultivated in tropical and subtropical countries. In South Asian native languages, it is known as ‘Lal Champa’, and Frangipani in English [11]. It is regarded as a great ornamental plant, and is frequently seen in graveyards, gardens, and parks. It is extensively cultivated in tropical and subtropical regions all over the world. Diarrhoea and emesis have traditionally been treated using a decoction of P. rubra bark and roots [12]. In Mexican traditional medicine, the latex and flower of P. rubra are used to treat tooth and earache [11]. High-performance liquid chromatography (HPLC) analysis revealed many vital phytoconstituents, such as α-allamcidin, α-amyrin, β-allamcidin, β-amyrin acetate, β-sitosterol, 13-O-p-Coumaroylplumieride, 15-Demethylplumieride, 2,4,6-trimethoxyaniline, 2,5-dimethoxy-p-benzoquinone, 3-O-caffeoylquinic acid, allamandin, allamcin, arjunolic acid, benzyl salicylate, betulinic acid, citric acid, fulvoplumierin, gaertneroside, isoplumericin, kaempferol, kaempferol-3-rutinoside, kaempferol-3-O-glucoside, liriodendrin, lupeol, lupeol acetate, lupeol carboxylic acid, maslinic acid, methyl salicylate, naphthalene, narcissin, nerolidol, oleanolic acid, oleic acid, P-(E)-coumaric acid, plumericidine, plumericin, plumerinine, plumerubroside, plumieride, plumieride-E-p-coumarate, quercetin 3-O-α-L-arabinopyranoside, quercitrin, quercetin, quinic acid, rubradoid, rubrajaleelol, rubrajaleelic acid, rubranonoside, rutin, scopoletin, stigmasterol, stigmast-7-enol, sweroside, taraxasteryl acetate, and ursolic acid [13]. The decoction of the bark has been used to cure venereal disease, as well as rheumatism, leprosy and fever in the indigenous system of medicine [11]. The root decoction treats bacterial infections, rheumatic pains, and cancers [12]. Pharmacological investigations reported analgesic, antidiabetic, antipyretic, anti-obesity, antimicrobial, lipid-lowering, anticancer, antioxidant, insecticidal, and gastroprotective effects [14]. It was written to treat cardiovascular ailments in folk medicines [15]. However, there is no recorded pharmacological validation about its utility in cardiovascular disorder. The current investigation evaluated the anticoagulant, hypotensive and cardioprotective effect of the aqueous-methanolic leaf extract of P. rubra in rabbits, using in vivo, in vitro and ex vivo experiments. The rabbit animal model was chosen over rats because rabbits have a more similar profile to humans. The calcium channels of rabbits’ aorta are more diversified than that of rats [6].
2. Results
2.1. Phytochemical Evaluation
Phytochemical investigation validated the presence and high presence of flavonoids, phenols, tannins, anthraquinones, saponins, coumarins, and alkaloids in the aqueous-methanolic leaf extract of P. rubra by visually observing the prescribed colour change and precipitate formation of the extract (Table 1).
Table 1.
Phytochemical investigation of P. rubra aqueous-methanolic leaf extract.
| Tests | Observation | Results |
|---|---|---|
| Alkaloid | PPT | Present |
| Phenols | Light purple colour | Present |
| Tannins | Light purple colour | Present |
| Coumarins | Yellow fluorescence | Present |
| Saponins | 1 cm froth | Present |
| Anthraquinones | Pink colour | Present |
| Flavonoid | Light yellow colour | Present |
PPT = precipitate formation; froth = presence of froth in test tube.
2.2. HPLC Analysis
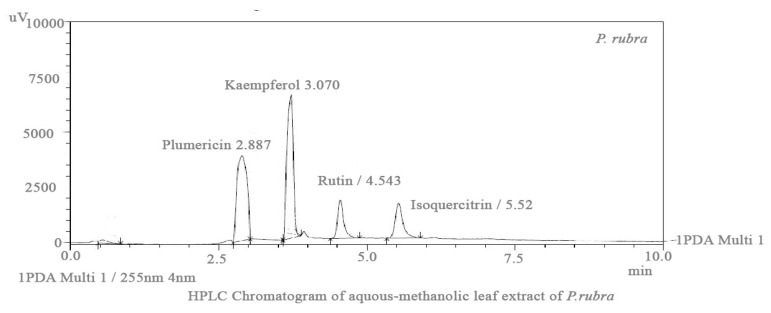
HPLC analysis validated many phytoconstituents in varying concentrations, among them the essential phytochemicals rutin, isoquercetin, kaempferol, and plumericin were identified based on retention time (Figure 1).
Figure 1.
HPLC chromatogram of aqueous-methanolic leaf extract of P. rubra showing the rutin, isoquercetin, kaempferol and plumericin with reference to retention time of standard.
2.3. DPPH Assay
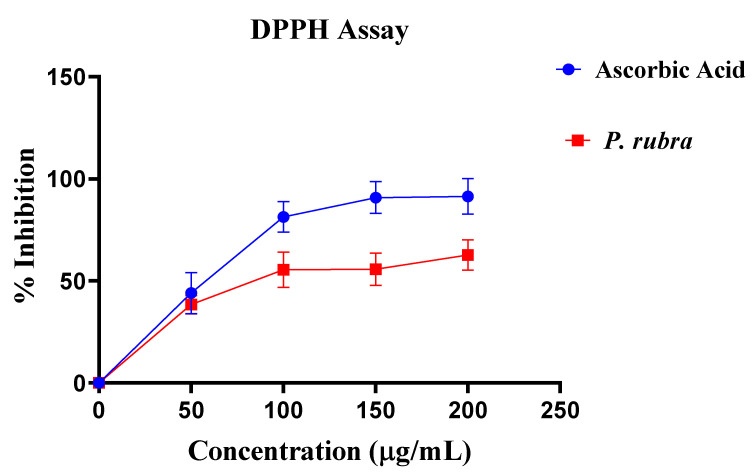
Aqueous-methanolic leaf extract of P. rubra showed good antioxidant potential with the inhibitory concentration of 138 µg/mL about ascorbic acid in DPPH assay (Figure 2).
Figure 2.
Antioxidant potential of P. rubra with respect to ascorbic acid by DPPH assay (n = 5).
2.4. Evaluation of Myocardial Infarction
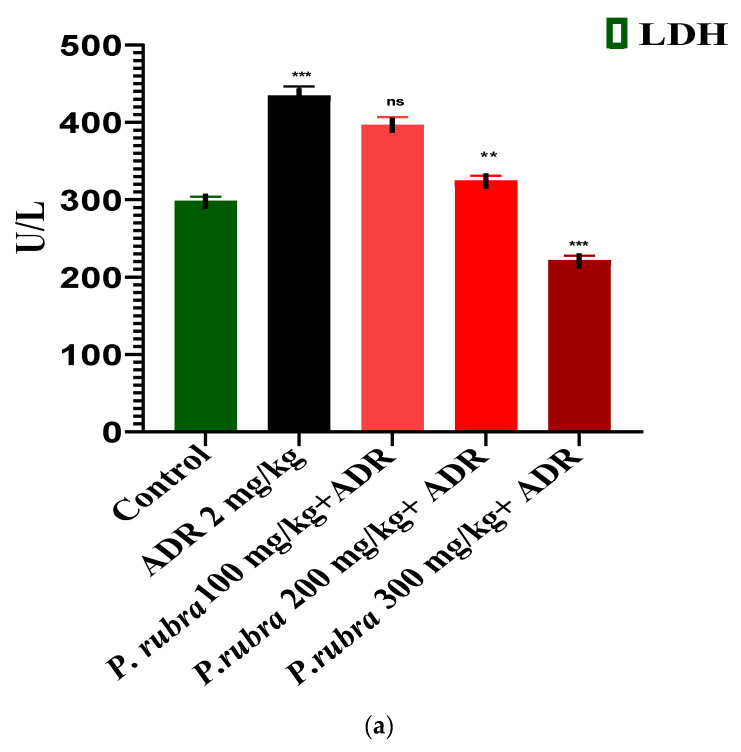
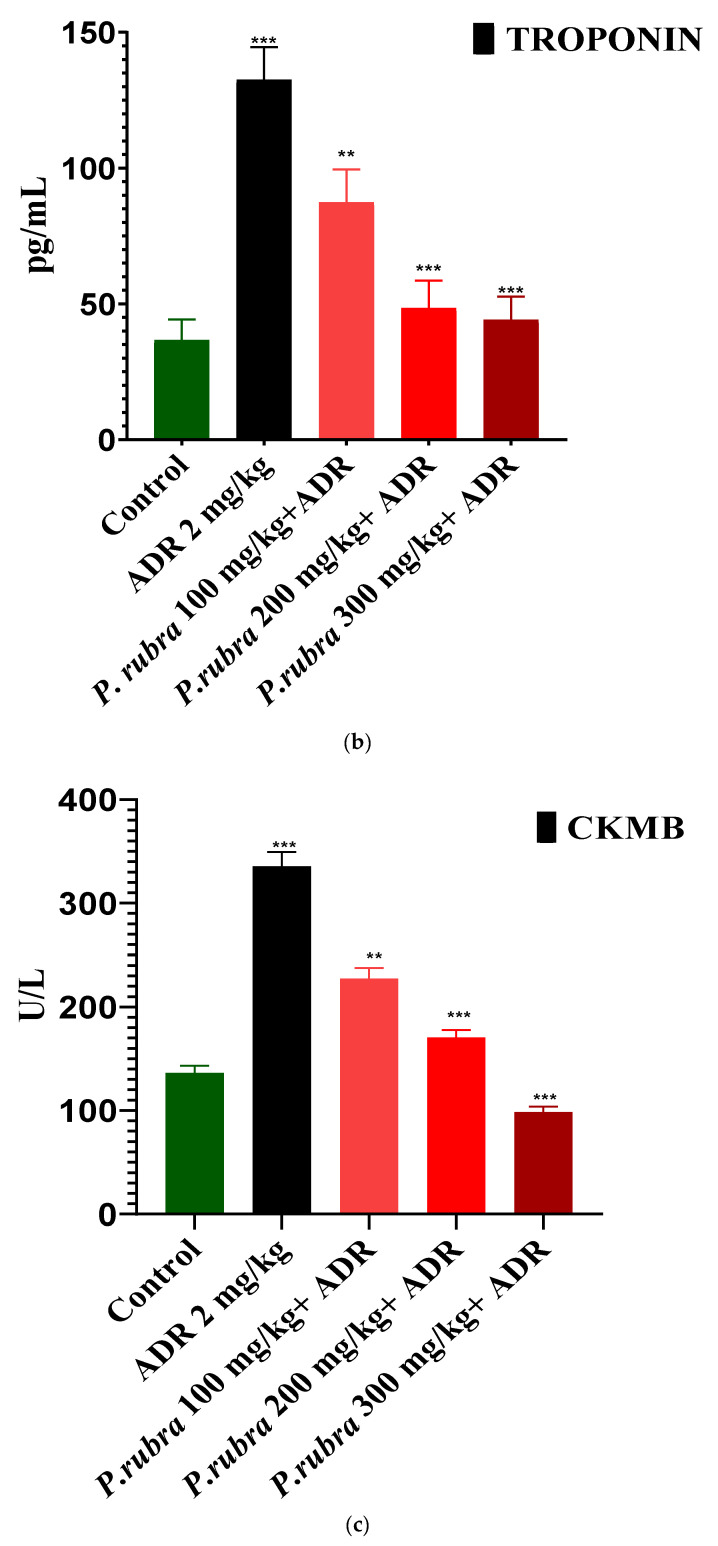
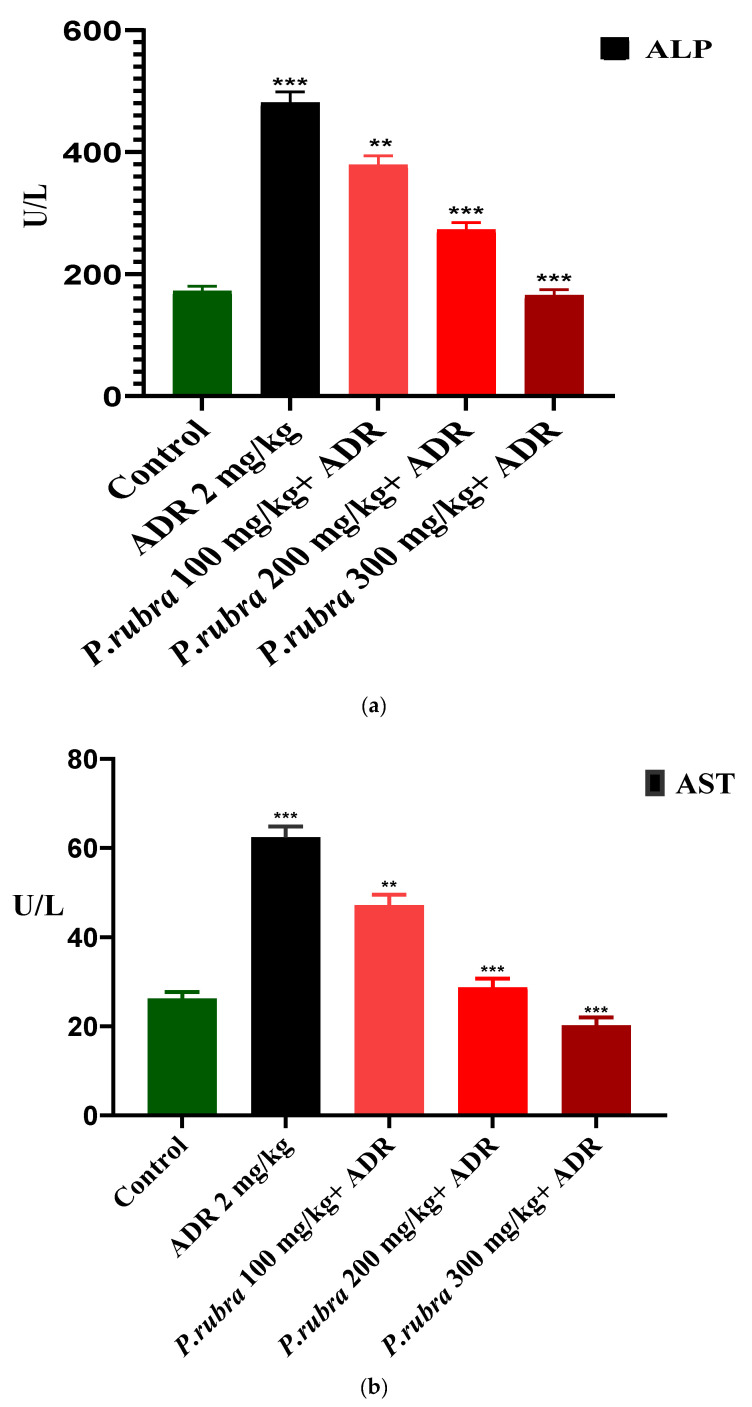
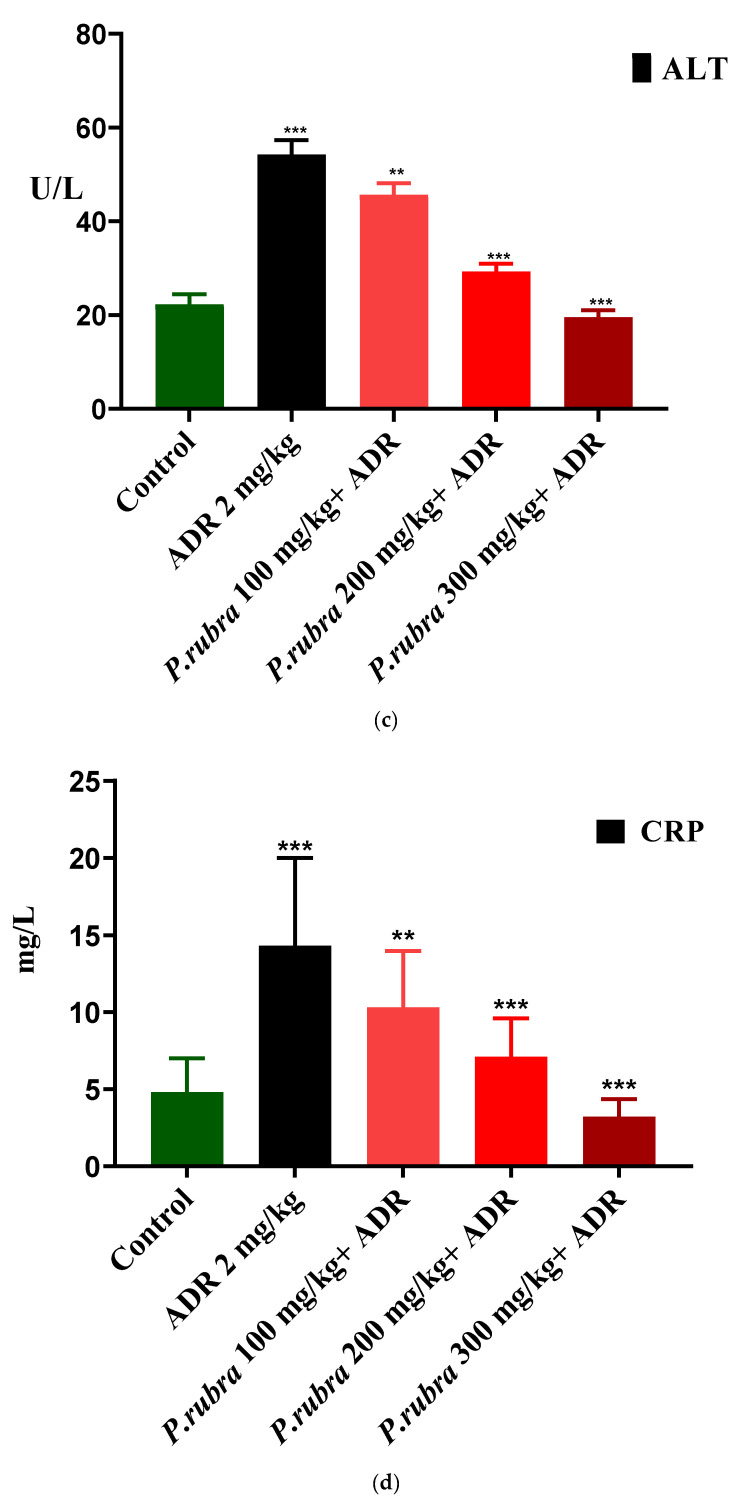
ADR considerably elevates the level of cardiac biomarkers (troponin, CK-MB, LDH) and cardiohepatic biomarkers (ALT, CRP, ALP, AST) (p < 0.05) regarding control. The rabbits in the ADR-intoxicated group (group 2) had significantly higher cardiac markers, whereas the three groups treated with P. rubra at different doses (100, 200, and 300 mg/kg body weight) demonstrated a dose-dependent resistance to the ADR-intoxicated cardiac damage. In contrast, the three groups receiving P. rubra leaf extract had lower average levels of troponin, CK-MB, LDH and AST, ALT, CRP, ALP (p < 0.05) than the ADR-intoxicated group (Figure 3 and Figure 4).
Figure 3.
Cardioprotective effect of three doses of aqueous-methanolic leaf extract of P. rubra on the cardiac biomarkers, LDH (a), CK-MB (b), and troponin (c) against ADR-intoxicated MI. One-way ANOVA and Dunnett’s multiple comparison test were performed (** p < 0.005 & *** p < 0.001 & ns p > 0.005, n = 5).
Figure 4.
Cardioprotective effect of three doses of aqueous-methanolic leaf extract of P. rubra on the cardiohepatic biomarkers, ALP (a), AST (b), ALT (c) and CRP (d), against ADR-induced MI. One-way ANOVA and Dunnett’s multiple comparison test were performed (** p < 0.005, *** p < 0.001 & ns p > 0.005, n = 5).
Effect on Heart to Bodyweight Ratio
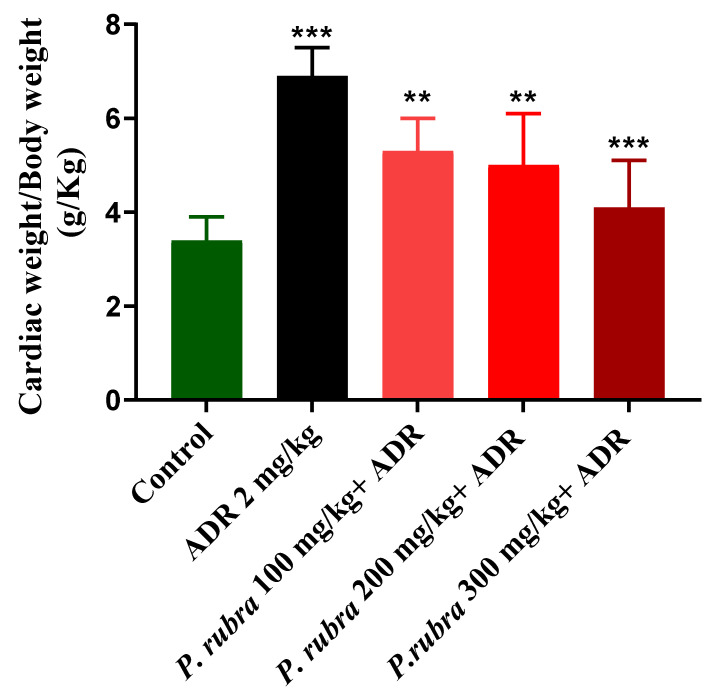
ADR noticeably increased the ratios regarding the control group. All three groups treated with P. rubra reduced their heart to bodyweight ratios less than the ADR-intoxicated group (Figure 5).
Figure 5.
Cardioprotective effect of three doses of aqueous-methanolic leaf extract of P. rubra on heart weight to bodyweight ratio in ADR-intoxicated left ventricular hypertrophy and control. One-way ANOVA and Dunnett’s multiple comparison test were performed (** p < 0.005 & *** p < 0.001, n = 5).
2.5. Histopathology
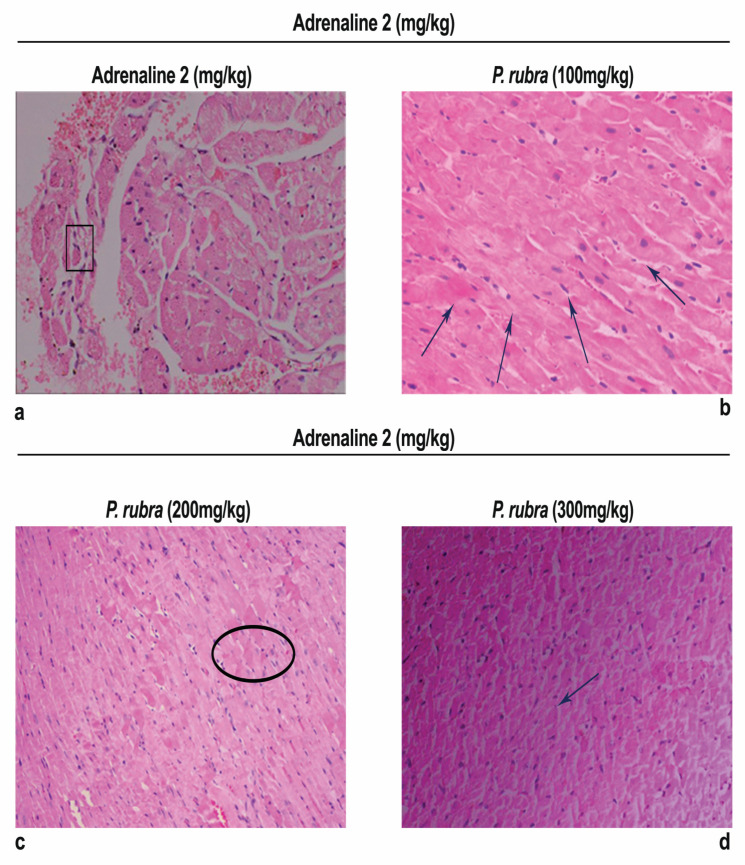
Examination of heart tissue fragments from the ADR-intoxicated group revealed a notable variation in cardiac cell architecture. Histological changes in the ADR-intoxicated group included interstitial oedema, the shred of muscular fibers, cellular infiltration, vacuolar disintegration, disintegration, mottled staining, capillary enlargement, hemorrhage, and myocardium obstruction. All of the groups had brutal necrotic lesions, but the P. rubra-treated groups had less myocardial degradation (Figure 6).
Figure 6.
Photomicrograph showing histopathological variations of ventricle section of rabbit heart: (a) ADR-intoxicated group; (b) P.rubra 100 mg/kg + ADR; (c) P. rubra 200 mg/kg; (d) P. rubra 300 mg/kg + ADR. In comparison to the ADR-intoxicated group, less inflammatory cells, cardiomyocyte deterioration, infiltration and fibrosis were observed in a dose-dependent manner. Rectangular shape indicates enlarged cardiomyocytes, upward arrows indicate cellular infiltration, oval shape indicates cardiac fibrosis and downward arrows indicate dense normal cluster of cardiomyocytes. Magnification is ×100.
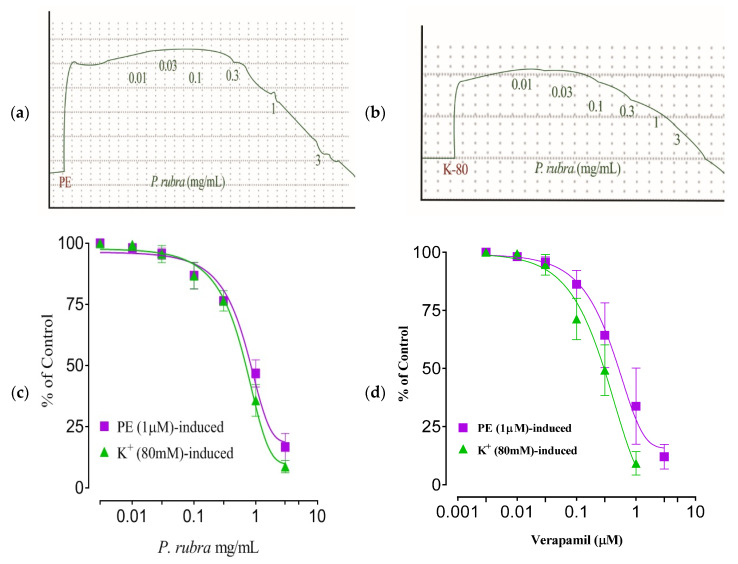
2.6. Aortic Tissue Preparation and Vasorelaxant Activity
The isolated rabbit aorta rings showed a continuous contractile activity when exposed to phenylephrine (PE) (1 M) and K+ (80 mM). P. rubra elicited concentration-dependent relaxation of PE-induced spastic contractions (EC50 = 0.67 mg/mL; Cl 95%: 0.3849–1.188). Likewise, P. rubra elicited concentration-dependent relaxation in the aorta ring preparation against K+ (80 mM)-induced spastic contractions (EC50 = 1.30 mg/mL; Cl 95%: 0.9142–1.848). Similarly, verapamilat at a concentration of 1–3 μM, relaxed the PE (1 M) and K+ (80 mM)-induced contractions having EC50 = 0.11 μM (95% CI: 0.03–0.18 μM; n = 5) and EC50 = 0.03 μM (95% CI: 0.01–0.16 μM; n = 5), respectively (Figure 7a–d).
Figure 7.
Tracings of aqueous-methanolic leaf extract of P. rubra against PE (1 μM)-induced contraction in rabbit aorta strip (a); K+ (80 mM) induced contraction (b); vasorelaxing dose–response curve of P. rubra (c); and verapamil (d) against PE (1 μM) and K+ (80 mM)-induced contractions). All the values (n = 5) are depicted as mean ± SEM.
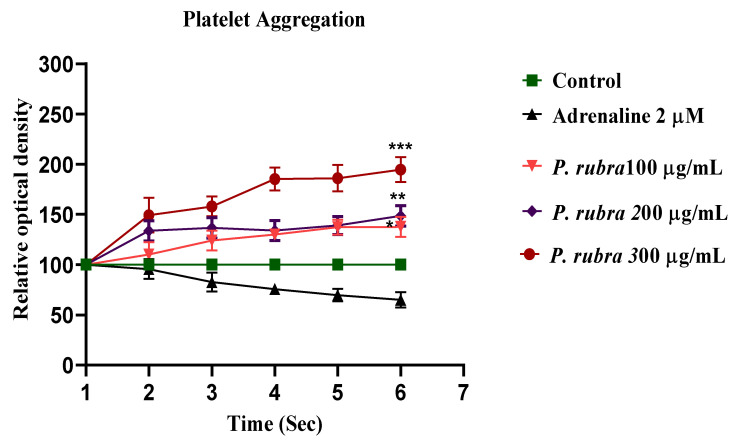
2.7. Antiplatelet Aggregatory Effect
The addition of ADR (2 µM) to a suspension of rinsed human platelets resulted in a significant decrease in optical density at 600 nm, indicating platelet aggregation. At 37 °C, the aggregatory effect was observed. P. rubra (100, 200, and 300 µg/mL) reduced platelet aggregation in a dose-dependent pattern (* p < 0.05, ** p < 0.001, *** p< 0.0001) (Figure 8).
Figure 8.
Anti-aggregatory effect of aqueous-methanolic leaf extract of P. rubra against ADR-induced aggregation on human platelet. All three doses of P. rubra showed significant anti-aggregatory potential in a dose-dependent fashion (n = 5).
2.8. Acute Oral Toxicity Dose Test
In the acute oral toxicity test, P. rubra was found safe up to 3000 mg/kg body weight. No death or morbidity was recorded at any dose, in any animal exposed to P. rubra aqueous-methanolic leaf extract.
3. Discussion
CVDs, particularly ischemic heart disease, MI, and cardiac hypertrophy, are the leading causes of death and morbidity globally [2]. Plants are widely used to cure minor to fatal cardiovascular diseases, and the present investigation was planned in order to screen a plant preparation (P. rubra) which is widely used to treat cardiovascular ailments in Pakistan, without sound pharmacological-documented evidence. Generally, flowers and leaves possess different phytochemical constituents; most of the research on the biological activities of P. rubra’s essential oils and flowers is available [14]. Since ancient times, numerous medicinal plant treatments have been utilized to treat circulatory diseases. However, in terms of cellular and molecular approaches, no scientific evidence has been examined and published on the molecular mechanism of the cardioprotective potential of P. rubra. P. rubra, the medicinal plant in this article which has appeared to show pharmacotherapeutic potential in vitro, in vivo, and ex vivo in animal studies, and therefore may influence cardiovascular ailments. This indigenous medicinal herb has a preventive, therapeutic impact by blocking, modifying, and regulating the production of specific regulatory proteins such as contractile, structural, and glycoproteins. These proteins are involved in calcium levels and promote the function of mitochondria. The cardioprotective action of medicinal plants has been established by decreasing the damage in cardiomyocytes, endothelial cells, vascular smooth muscle cells, and monocytes [16]. The cardioprotective action in cardiomyocytes has been demonstrated by the opening of the KATP channel, increased secretion of atrial natriuretic peptides, apoptosis, inflammation suppression, cardiac hypertrophy, oxidative stress, and endothelial nitric oxide synthase–nitric oxide (NOS–NO) signaling pathways, have all been found to be positive effects of the medicinal plant [17]. An HPLC study of the aqueous-methanolic leaf extract of P. rubra confirmed the presence of rutin, isoquercitrin, kaempferol, and plumerciin (Figure 1). Rutin is well documented for the cellular and molecular remodeling of cardiovascular pathogenesis by inhibiting the ERK1/2 pathway [18], and isoquercitrin attenuates cardiac dysfunction via the AMPKα-dependent pathway [19]. Similarly, kaempferol is reported for cardioprotection via downregulating the ASK1/MAPK signaling pathways [20].
ADR is a non-selective adrenoreceptor agonist, well-reported for the development of AMI and LVH in experimental animals at various doses [7]. Studies have verified that ADR at lower doses increases the weight of the heart (LVH) due to oxidative stress, via an increase in blood pressure through positive ionotropic and chronotropic responses [8]. In comparison, ADR at much higher doses for two consecutive days causes cardiac oedema, apoptosis, necrosis, and endocardial damage resulting in AMI [8]. ADR noticeably elevated the level of cardiac biomarkers, troponin, LDH, CK-MB, and cardiohepatic biomarkers, ALP, AST, CRP, ALT (p < 0.001). The three groups receiving the P. rubra leaf extract exhibited that the average amount of cardiac and cardiohepatic biomarkers was less in comparison with the ADR-intoxicated group, which advocates its cardioprotective character (Figure 3 and Figure 4).
The cardiac weight to body weight ratio regarding the ADR-intoxicated group was significantly less in all three groups treated with P. rubra (Figure 5), which indicated protection against LVH in a dose-dependent manner. A high quantity of flavonoids in plants was reported to be responsible for their cardioprotective effect [21,22], and P. rubra leaf extract was observed to be affluent in flavonoid content (Table 1), [13]. As a result, it stands to reason that the cardioprotective effect of P. rubra could be influenced by the presence of these flavonoids. Reduced CRP levels were recorded in all three groups treated with P. rubra aqueous-methanolic leaf extract, in comparison with the ADR-intoxicated group; that may be a result of the ascorbic acid or flavonoid-dependent antioxidant potential of P. rubra (Figure 2). Our study endorses the previous antioxidant studies of P. rubra [23,24]; saponins have been reported to lower lipid peroxidation levels dose-dependently [25]. The outcome of this investigation further strengthens the claim of already published studies on saponins or saponin-containing plant extracts’ cardioprotective effects in animals [26,27]. P. rubra was found to be rich in saponins (Table 1).
Cardiac weight to body weight ratio regarding the ADR-intoxicated group was significantly less in all three groups treated with P. rubra (Figure 5), which indicated protection against LVH in a dose-dependent manner. A high quantity of flavonoids in plants was reported to be responsible for their cardioprotective effect [21,22], and P. rubra leaf extract was observed to be affluent in flavonoid content (Table 1), [13]. As a result, it stands to reason that the early research [28] found that the aqueous-methanolic leaf extract of P. rubra had a significant LPO inhibitory effect, which supports its potential as a cardioprotective agent, because LPO is a main contributory factor in ADR-intoxicated MI [1,5,6].
Histopathology of the ventricular portion of the ADR-intoxicated group revealed tremendous damage in cardiac cellular architecture, such as the disintegration and tearing of muscular fibers, capillary distention, vacuolar disintegration, mottled staining, interstitial oedema, mononucleate cellular infiltration, hemorrhage, and myocardium obstruction (Figure 6a). Less inflammatory cells and myocardial deterioration were observed in all three groups treated with P. rubra, while ruthless necrotic lesions were observed in the ADR-intoxicated group (Figure 6), which endorses the results of the biochemical investigation (Figure 3 and Figure 4), and justifies its use as a cardioprotective agent.
ADR increases the Ca++ influx by opening the L-type voltage-gated calcium channels located on the myocardium, or increasing the release from intracellular stores of cardiomyocytes [5,7]. In conclusion, this exaggerated Ca++ increases the workload, resulting in oxidative stress [29]. The higher dose of ADR (2 mg/kg body weight) causes constriction of the aorta and coronary [7]. PE (1 μM) and K+ (80 mM) cause hypertension because of vasoconstriction, predominately by activating the alpha 1-adrenergic receptor and/or opening the L-type calcium channels [30], further building up oxidative stress (Figure 7). The vasodilator effect of P. rubra may depend on blocking these voltage-gated L-type calcium channels (Figure 7), as vasorelaxation was observed at a lesser concentration against K+ (80 mM)-induced contraction than PE (1 μM)-induced contraction. The compounds relaxed by the K+ (80 mM)-caused contraction are believed to be calcium channel blockers [1,5,6]. The negative ionotropic and chronotropic response was evident during the ex vivo investigation, similar to verapamil (Figure 7). Flavonoid-dependent vasodilatation was reported in the plants in numerous previous studies [21,22]. High flavonoid content was detected in aqueous-methanolic leaf extract of P. rubra, during the phytochemical investigation (Table 1, Figure 1), which provides logical grounds to believe its cardioprotective effect. Anticoagulant and antithrombotic medicines are integral parts of prescriptions of cardiovascular patients considered vital sources of cardioprotection in present-day pharmacology [31], whereas ADR is a well-documented procoagulant [7]. P. rubra noticeably decreased the platelet aggregatory effect of ADR (Figure 8), which provides another chapter on its use in cardiovascular disorders.
4. Materials and Methods
4.1. Plant Materials
P. rubra (leaves) were collected fresh from the botanical garden of the Muhammad Institute of Medical and Allied Sciences, Multan. An expert taxonomist validated it at the IPAB, BZ, University, Multan. For future reference, the voucher specimen (P.Fl.565-1) has been deposited.
4.2. Extract Preparation
The fresh leaves were dried in the shade, and the vegetative waste/adulterants were separated by handpicking. The dried leaves were converted to coarse powder with a special herbal grinder. P. rubra (250 g) leaf powder was set for soaking in an aqueous-methanolic solvent (70:30 v/v) for nine days, in air-tight laboratory jars. The socked material was filtered through muslin cloth and Whatman-1 filter paper and with the help of a rotary evaporator. This filtrate was evaporated at 37 °C under reduced pressure [32]. The percent yield of the aqueous-methanolic leaf extract was calculated using this formula;
| % age yield = Theoretical yield (g)/Actual yield (g) × 100 | (1) |
4.3. Animals
Male albino rabbits of local bread, with an average weight of 1.5 kg, were acquired from the animal house of the Department of Pharmacology, the Islamia University of Bahawalpur. Animals were fed on standard food and tap water ad libitum. The temperature was maintained at 25 °C. All the experiments were carried out following NIH’s guidelines [33], and were approved by the Department of Pharmacology’s concerned committee (AS and RB/10/8/20).
4.4. Chemicals
Methanol was purchased from Lahore Laboratory Chemicals, Lahore, PB, Pakistan. Verapamil and phenylephrine were purchased from Abbott Laboratories, KPK, Pakistan (PVT) Ltd. Adrenaline was purchased from Ameer Pharmaceuticals, Lahore, PB, Pakistan. LDH, troponin, CK-MB and ALT, ALP, CRP, AST kits were obtained from Pakistan Scientific traders, Lahore, PB, Pakistan. All other chemicals/reagents and solutions utilized in the experiments were of analytical grade.
4.5. Preliminary Phytochemical Evaluation
Phytochemical screening was carried out to screen various phytochemical classes; glycosides, anthraquinones, alkaloids, tannins, flavonoids, and saponins in the P. rubra aqueous-methanolic leaf extract by using the protocol described earlier [34].
4.6. HPLC Analysis
HPLC was used to estimate phenolic acids in aqueous-methanolic leaf extract of P. rubra [13,35]. A binary gradient solvent system was used in HPLC, paired with a C-18 column with dimensions (250 × 4.6 mm), capable of separating 8–9 phenolics in 36 min at a flow-rate speed of 0.0008 µL/min, and a film thickness of 5 µm, with an oven set at 30 °C. The replicability for the separation of components was good with (run-to-run), rutin, kaempferol, plumericin, isoplumericin, and quercetin were prepared as reference (purity > 99 percent), obtained from Aldrich (St. Louis, MO, USA), and the dilutions were prepared with methanol to achieve 50 µg/mL. Samples were distinguished by comparing the P. rubra sample retention times to standards. The separation factor and resolution were used to evaluate the efficiency of separated components using HPLC, as shown in Figure 1.
4.7. Acute Oral Toxicity Dose Test
In 15 rabbits, the acute oral dose toxicity of P. rubra was tested. They were divided into 3 groups of 5 rabbits each; they were fasted for 24 h before receiving doses of 1000, 2000, and 3000 mg/kg orally. The rabbits were observed for 14 days after dosage for jerkiness, exhaustion, and mortality [36].
4.8. Determination of DPPH Assay
DPPH assay was carried out, as outlined in our previous study [5]. To summarize, different amounts of aqueous-methanolic leaf extract of P. rubra (4 mL) were appended to DPPH solution and prepared with up to 5 mL of methanol, then incubated in the dark for 40 min. At 517 nm, the absorbance of the incubated solution was measured using a spectrophotometer. All of the experiments were performed three times, and the percent inhibition was calculated in vitamin C equivalents. The DPPH scavenging outcome is calculated as:
| 1% = A (blank) − B (sample)/A (blank) × 100 | (2) |
4.9. Acute Myocardial Infarction Study
The animals were split into 5 groups, with 6 animals in each. The rabbits in Group 1 were given normal saline, whereas those in Group 2 were given ADR (2 mg/kg body weight subcutaneously) every 24 h, with a gap for 2 successive days. Group-3 rabbits were pre-treated with 100 mg/kg extract for 14 days in a row, before receiving ADR 2 mg/kg on the 14th and 15th days, staggered by 24 h. The rabbits of Group 4 were pre-treated with 200 mg/kg extract for fourteen days in a row and then given ADR 2 mg/kg SC. Every 24 h on the 14th and 15th days. Group-5 rabbits were pre-treated with extract at 300 mg/kg for fourteen days, and then ADR 2 mg/kg was administered SC at 24-h intervals on the 14th and 15th days, while P. rubra dosages were given by oral gavages [33]. The 3 doses were chosen based on previously published research on the P. rubra’s hepatoprotective property [36]. Rabbits were anaesthetized on the 16th day. Blood samples were collected from the rabbits’ marginal ear veins to analyze biochemical markers such as troponin, CK-MB, LDH and ALP, ALT, AST, and CRP levels in serum, measured using standard kits.
Screening of Cardiac Weight to Bodyweight Ratio
Cardiac weight to bodyweight ratio helps key out cardiac weight index and necroses [1,6].
4.10. Histopathology
Rabbits were sacrificed under general anaesthesia, the heart was removed for histological analysis, and the ventricular component of the heart was quickly transferred to a 10% formalin solution. After that, the tissue was immersed in paraffin. A 5 μm segment was cut and stained with hematoxylin-eosin dye, before being mounted in xylene. [37]. Microscopic observation of the ventricular region of the cardiac tissue from different groups was utilized to evaluate the ADR effect alone, and in combination with the three groups of P. rubra on the cardiac cell structure. A compound microscope attached with a camera LCD was used to take micro-images.
4.11. Isolated Aortic Tissue Preparation and Vasorelaxant Activity
The thorax cavity was excavated after the rabbits were slaughtered, and the aorta was carefully dissected. In a Petri dish containing Krebs’ solution, the fat and connective tissues were carefully removed from the aorta. Aortic rings (3–4 mm in length) were cut and placed in a tissue organ bath (10 mL) that already contained the Krebs–Henseleit solution, and was constantly bubbled with carbenogen gas (95 percent O2 and 5 percent CO2). The bottom hook was fixed, while the other was coupled to a force-displacement transducer connected to a Power Lab data collecting system, to record the isometric contractions. During the 40 min stabilization period at 1.0 g resting tension, Kreb’s solution was replaced every 15 min to prevent the accumulation of metabolites. After equilibration, the rings were pre-constructed with 1 × 10−6 M phenylephrine (PE) until the steady contractile curve (5–8 min) was reached, and the vasodilator effect of P. rubra was measured using a cumulative dosing method [38,39].
4.12. Calcium Channel Blocking Activity
P. rubra was tested against sustained spastic contractions caused by high K+ (80 mM) in the isolated rabbit aorta, which were conciliated by the opening of voltage-dependent Ca++ channels, and produced a contractile response by originating extracellular Ca++ influx. Chemicals that block Ca++ ions influx through these channels can reduce high K+ caused contractions. The administration of test material in an additive way against the persistent contractions is required to achieve a concentration-dependent inhibitory response [40]. The relaxant effect of the test drug against the simulated contractions is shown as a percentage (%) of the control contraction response.
4.13. Adrenaline-Induced Platelet Activation and Aggregation
The blood sample was taken from the rabbit’s marginal ear vein and centrifuged (3000 rpm for 15 min) to obtain platelet-rich plasma. ADR (2 µM) was added to the samples as a supplement. Platelet aggregation was investigated using impedance aggregometry, whereas platelet activation was analyzed using flow cytometry before and after supplementation [41]. The effect of the 3 different administered doses of ADR on platelet aggregation (n = 5) was first analyzed. Platelet aggregation was next assessed after administering ADR in conjunction with P. rubra at doses of 100, 200, and 300 µg/mL (n = 5).
4.14. Statistical Analysis
The in vitro results for vasorelaxant activities were expressed as the mean ± SEM. EC50 values (with 95% CI) were calculated using the software Graph Pad Prism version-8 (Graph Pad Software, San Diego, CA, USA), and the dose–response curves were analyzed by the nonlinear regression sigmoidal response curve (variable slope). Similarly, in vivo results were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. * p < 0.05, ** p < 0.001, *** p = 0.0001, was considered to be statistically significant.
5. Conclusions
Plumeria rubra was observed to produce vasorelaxant, hypotensive, anticoagulant, antioxidant, and calcium-channel-blocking effects. The cardioprotective effect of the P. rubra aqueous-methanolic leaf extract may be due to its diversity of phytoconstituents. Treatment with P. rubra may replenish antioxidants in cardiomyocytes, which are needed for resistance to oxidative damage generated by ADR. However, the actual molecular mechanism of cardioprotection is yet to be discovered. For further characterization, an LC-MS/MS spectrum is recommended. Furthermore, tannins, flavonoids, and cardiac glycosides were detected during the phytochemical screening, which may impact cardiovascular illnesses, particularly hypertension-induced LVH and MI. In closing, the therapeutic potential of P. rubra in cardiovascular disorders has been demonstrated in both ex vivo and in vivo studies; this will pave the way for new drug development to treat cardiovascular ailments modulating multiple pathways.
Acknowledgments
Authors are grateful to Qaiser Jabeen, Chairperson Department of Pharmacologyfor her technical support and suggestions.
Author Contributions
Conceptualization, M.H. and L.-H.Z.; methodology, I.A.K.; software, T.A.; validation, A.M.A.; formal analysis, M.S.; writing—original draft preparation, S.A. and A.A.A. investigation, I.A.K.; supervision, M.H.; writing—review and editing, S.K.S. and L.-H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Deanship of Scientific Research at King Khalid University for funding this study through the Small Research Group Project, under grant number GRP:1/275/1442.
Institutional Review Board Statement
All the experiments were carried out in accordance with the NIH guidelines and were approved by the Department of Pharmacology’s concerned Committee (AS & RB/10/8/20).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saqib F., Ali A., Ahmedah H.A., Irimie C.A., Toma S.I., Popovici B.E., Moga M., Irimie M. Cardioprotective, hypotensive and toxicological studies of Populusciliata (Wall. ex Royle) Biomed. Pharmacother. 2021;142:1–16. doi: 10.1016/j.biopha.2021.112065. [DOI] [PubMed] [Google Scholar]
- 2.Khan I.A. Pharmacotherapeutic modifications in cardiopulmonary patients during COVID-19 outbreak. J. Coll. Phys. Surg. Pak. 2020;30:15–17. doi: 10.29271/jcpsp.2020.supp2.132. [DOI] [PubMed] [Google Scholar]
- 3.Ashraf N., Rashid A., Naz U., Ashraf M.M., Khaliq S., Khan I.A., Nazir A., Sarwar A., Asif A. Detection of antibiotics residues in protein containing diets (meat and eggs) of human through different methods. J. Univ. Med. Coll. 2018;9:1–11. [Google Scholar]
- 4.Quiñones M., Miguel M., Aleixandre G. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013;68:125–131. doi: 10.1016/j.phrs.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Khan I.A., Hussain M., Munawar S.H., Iqbal M.O., Arshad S., Manzoor A., Shah M.A., Abbas K., Shakeel W., Syed S.K. Jasminum sambac: A Potential candidate for drug development to cure cardiovascular ailments. Molecules. 2021;26:5664. doi: 10.3390/molecules26185664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saqib F., Arif Aslam M., Mujahid K., Marceanu L., Moga M., Ahmedah H.T., Chicea L. Studies to elucidate the mechanism of cardio protective and hypotensive activities of Anogeissusacuminata (Roxb. ex DC.) in rodents. Molecules. 2020;25:3471. doi: 10.3390/molecules25153471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin M.M., El-Gazayerly O.N., Gawad N.A., Halim S.M. Effect of formulation variables on design, in vitro evaluation of valsartan SNEDDS and estimation of its antioxidant effect in adrenaline-induced acute myocardial infarction in rats. Pharm. Dev. Technol. 2016;21:909–920. doi: 10.3109/10837450.2015.1078354. [DOI] [PubMed] [Google Scholar]
- 8.Fathiazad F., Matlobi S., Khorrami N. Phytochemical screening and evaluation of cardioprotective activity of ethanolic extract of Ocimumbasilicum L. (basil) against isoproterenol induced myocardial infarction in rats. DARU J. Pharm. Sci. 2012;20:198–203. doi: 10.1186/2008-2231-20-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsoupras A., Lordan R., Zabetakis I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients. 2018;10:604. doi: 10.3390/nu10050604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaito A., Thuan D.T.B., Nguyen T.H.D., Hasan H., Halabi S., Abdelhady S., Nasrallah G.K., Pintus G. Herbal medicine for cardiovascular diseases: Efficacy, mechanisms, and safety. Front. Pharmacol. 2020;11:422–428. doi: 10.3389/fphar.2020.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan I.A., Aziz A., Raza M.A., Saleem M., Bashir S., Alvi A. Study pertaining to the hypothermic activity of Plumeria rubra, Linn. in prostaglandin 1 and typhoid vaccine-induced pyrexia models in rabbits. West Indian Med. J. 2015;12:1. doi: 10.7727/wimj.2015.172. [DOI] [Google Scholar]
- 12.Khan I.A., Lodhi A.H., Munawar S.H., Manzoor A., Manzoor Z., Raza M.A. Dermatological evaluation of anti-Irritant and anti-Inflammatory effect of Plumerin-R isolated from the latex of Plumeria rubra Linn. Lat. Am. J. Pharm. 2018;37:317–320. [Google Scholar]
- 13.Bihani T. Plumeria rubra L.–A review on its ethnopharmacological, morphological, phytochemical, pharmacological and toxicological studies. J. Ethnopharmacol. 2021;264:113291. doi: 10.1016/j.jep.2020.113291. [DOI] [PubMed] [Google Scholar]
- 14.Aziz A., Khan I.A., Munawar S.H., Shaheed S. Antipyretic activity of methanolic bark extract of Plumeria rubra Linn. in various pyrexia induced models. Int. J. Res. Dev. Pharm. Life Sci. 2013;2:680–685. [Google Scholar]
- 15.Khare C.P. Encyclopedia of Indian Medicinal Plants, Rational Western Therapy. Ayurvedic and Other Traditional Usage, Botany. Springer; Berlin/Heidelberg, Germany: 2004. pp. 314–315. [Google Scholar]
- 16.Omer M.I., Khan I.A., Manzoor M., Arshad S., Sial A.S. Cardioprotective effect of hydroalcoholic leaf extract of Jatropha mollissima on isoproterenol-induced myocardial infarction in rats. Pharm. Mag. 2021;17:251–254. [Google Scholar]
- 17.Omer M.I., Akhtar I., Rizvi A.A., Arshad S., Sial A.S. The nephroprotective effects of Daucus carota and Eclipta prostrata against cisplatin-induced nephrotoxicity in rats. Metabolism. 2021;12:12702–12721. doi: 10.1080/21655979.2021.2009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawa N.S., Juriyati J.A., Yusof A., Yusof K. Roles of rutin in cardiac remodeling. J. Funct. Foods. 2020;64:606–619. [Google Scholar]
- 19.Huang S.H., Xu M., Wu H.M., Wan C.X., Wang H.B., Wu Q.Q., Liao H.H., Deng W., Tang Q.Z. Isoquercitrin attenuated cardiac dysfunction via AMPKα-dependent pathways in LPS-treated mice. Mol. Nutr. Food Res. 2018;62:303–321. doi: 10.1002/mnfr.201800955. [DOI] [PubMed] [Google Scholar]
- 20.Feng H., Cao J., Zhang G., Wang Y. Kaempferol attenuates cardiac hypertrophy via regulation of ASK1/MAPK signaling pathway and oxidative stress. Planta Med. 2017;83:837–845. doi: 10.1055/s-0043-103415. [DOI] [PubMed] [Google Scholar]
- 21.Bucki R., Pastore J.J., Giraud F., Sulpice J.C., Janmey P.A. Flavonoid inhibition of platelet procoagulant activity and phosphoinositide synthesis. J. Thromb. Haemost. 2003;1:1820–1828. doi: 10.1046/j.1538-7836.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 22.Khan I.A., Aziz A., Saqib F., Munawar S.H., Manzoor A., Manzoor Z., Raza M.A. Pharmacological evaluation of Rumex vesicarius Linn leaf extract and fractions in rabbit gastrointestinal ailment. Afr. J. Pharm. Pharmacol. 2014;8:333–341. [Google Scholar]
- 23.Panchal S.K., Brown L. Cholesterol versus Inflammation as Cause of Chronic Diseases. Nutrients. 2019;11:2332. doi: 10.3390/nu11102332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salma A., El-Marasy S.A., Awdan E., Hassan H., Heba M.I. Cardioprotective effect of thymol against adrenaline-induced myocardial injury in rats. Heliyon. 2020;7:35–39. doi: 10.1016/j.heliyon.2020.e04431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Y., Hu Z., Li A., Zhu Z., Yang N., Ying Z. Recent advances in biotransformation of saponins. Molecules. 2019;24:2365. doi: 10.3390/molecules24132365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y., Duan L., Chen H.W., Liu Y.M., Zhang Y. Network pharmacology-based prediction and verification of the targets and mechanism for Panax notoginseng saponins against coronary heart disease. Evid.-Based Complement. Altern. Med. 2019;65:37–52. doi: 10.1155/2019/6503752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mundugaru R., Udaykumar P., Senthilkumar S., Bhat S. Cardioprotective activity of fruit of Garcinia pedunculata on isoprenaline induced myocardial infarction in rat. J. Pharmacol. 2018;11:231–235. doi: 10.3329/bjp.v11i1.24941. [DOI] [Google Scholar]
- 28.Prince P.S., Rajakumar S., Dhanasekar K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur. J. Pharmacol. 2011;668:233–240. doi: 10.1016/j.ejphar.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 29.Sun J., Yu X., Huangpu H., Yao F. Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 2019;109:254–261. doi: 10.1016/j.biopha.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Satoh S., Nishida S. Electropharmacological actions of Ginkgo biloba extract on vascular smooth and heart muscles. Clin. Chim. Acta. 2004;342:13–22. doi: 10.1016/j.cccn.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Wang X.L. Potential herb-drug interaction in the prevention of cardiovascular diseases during integrated traditional and western medicine treatment. Chin. J. Integr. Med. 2015;21:3–9. doi: 10.1007/s11655-014-1892-5. [DOI] [PubMed] [Google Scholar]
- 32.Khan I.A., Lodhi A.H., Munawar S.H., Manzoor A., Manzoor Z., Raza M.A. Formulation and evaluation of allicin and curcumin gel improves normal and diabetic ulcers in rabbits. Lat. Am. J. Pharm. 2018;37:1602–1607. [Google Scholar]
- 33.National Institute of Health . Guide for the Care and Use of Laboratory Animals. NIH Publication; Bethesda, MD, USA: 1985. No. 85–23. [Google Scholar]
- 34.Trease G.E., Evans W.C. Trease and Evans’ Pharmacognosy. 16th ed. Saunders/Elsevier; Edinburgh, Australia: 2009. [Google Scholar]
- 35.Khan I.A., Lodhi A.H., Munawar S.H., Manzoor A., Manzoor Z., Raza M.A., Iqbal O. Assessment of ameliorative effect ofAab-e-Shifa polyherbal formulation in experimentally-induced wound in rabbits. Trop. J. Pharm. Res. 2020;19:2357–2362. [Google Scholar]
- 36.Chowdhur A., Dasgupta B., Kalita G. Hepatoprotective activity of Plumeria alba extract against paracetamol induced-hepatotoxicity in rats. Int. J. Pharm. Pharm. Sci. 2012;4:618–620. [Google Scholar]
- 37.Khan I.A., Lodhi A.H., Munawar S.H., Manzoor A., Manzoor Z., Raza M.A. Formulation and evaluation of rutin-allicin gelagainst diabetic foot ulcer. Lat. Am. J. Pharm. 2020;39:725–729. [Google Scholar]
- 38.Imtiaz S.M., Aleem A., Saqib F., Ormenisan A.N., Elena Neculau A., Anastasiu C.V. The Potential involvement of an ATP-dependent potassium channel-opening mechanism in the smooth muscle relaxant properties of Tamarixdioica Roxb. Biomolecules. 2019;9:722. doi: 10.3390/biom9110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isik B.S., Altay F., Capanoglu E. The uniaxial and coaxial encapsulations of sour cherry (Prunus cerasus L.) concentrate byelectro spinning and their in vitro bioaccessibility. Food Chem. 2018;265:260–273. doi: 10.1016/j.foodchem.2018.05.064. [DOI] [PubMed] [Google Scholar]
- 40.Gilani A.H., Janbaz K.H., Zaman H., Lateef A., Suria A. Possible presence of calcium channel blocker(s) in Rubiacordifolia: An indigenous medicinal plant. JPMA. 1994;44:84–89. [PubMed] [Google Scholar]
- 41.Singh S., Malm C.J., Ramström S., Hesse C., Jeppsson A. Adrenaline enhances in vitro platelet activation and aggregation inblood samples from ticagrelor-treated patients. Res. Pract. Thromb. Haemost. 2018;2:718–725. doi: 10.1002/rth2.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.