Abstract
(1) Background: Postpartum weight may increase compared to pre-pregnancy due to weight retention or decrease due to weight loss. Both changes could pose deleterious effects on maternal health and subsequent pregnancy outcomes. Therefore, this study aimed to assess postpartum weight change and its associated factors. (2) Methods: A total of 585 women from the KIlte-Awlaelo Tigray Ethiopia (KITE) cohort were included in the analysis. (3) Results: The mean pre-pregnancy body mass index and weight gain during pregnancy were 19.7 kg/m2 and 10.8 kg, respectively. At 18 to 24 months postpartum, the weight change ranged from −3.2 to 5.5 kg (mean = 0.42 kg [SD = 1.5]). In addition, 17.8% of women shifted to normal weight and 5.1% to underweight compared to the pre-pregnancy period. A unit increase in weight during pregnancy was associated with higher weight change (β = 0.56 kg, 95% CI [0.52, 0.60]) and increased probability to achieve normal weight (AOR = 1.65, 95% CI [1.37, 2.00]). Food insecurity (AOR = 5.26, 95% CI [1.68, 16.50]), however, was associated with a shift to underweight postpartum. Interestingly, high symptoms of distress (AOR = 0.13, 95% CI [0.03, 0.48]) also negatively impacted a change in weight category. (4) Conclusions: In low-income settings such as northern Ethiopia, higher weight gain and better mental health during pregnancy may help women achieve a better nutritional status after pregnancy and before a possible subsequent pregnancy.
Keywords: postpartum weight change, postpartum maternal nutrition, postpartum weight retention, pre-pregnancy weight, gestational weight gain
1. Introduction
Women gain weight during pregnancy, driven mainly by normal physiological changes and tissue remodeling. The optimal weight increase depends on pre-pregnancy body mass index (BMI). To this end, specific weight gain ranges according to pre-pregnancy BMI categories are recommended by the Institute of Medicine (IOM) guidelines [1]. After pregnancy, women are supposed to return to their pre-pregnancy weight, usually by the sixth postpartum week. However, many studies show that 20 to 50% of women retain a substantial part of the weight gained during pregnancy at six months to two years postpartum [2,3,4,5,6,7,8]. High postpartum weight retention may contribute to obesity and adverse health outcomes, including chronic non-communicable diseases [9,10,11]. Postpartum weight retention or weight gain may also complicate subsequent pregnancies [11,12,13,14]. On the other hand, in low-income countries where undernutrition is widespread, retaining some weight after pregnancy may be translated into better nutritional status (a change from underweight to normal weight category). Therefore, it may have an advantageous effect on maternal and child health outcomes [15].
Postpartum weight change, defined as the difference between postpartum weight and weight before pregnancy, may be predicted by different factors. These include parity [16], pre-pregnancy body mass index [2,4], gestational weight gain (GWG) [2,3,6,17,18,19,20,21,22,23,24], physical activity [4], breastfeeding [4,24,25,26,27], psychosocial characteristics [28,29], and dietary factors [30]. However, most studies focused solely on assessing the association between pre-pregnancy body mass index, gestational weight gain, and postpartum weight, overlooking potentially important effects of socioeconomic characteristics, reproductive and obstetric conditions, psychosocial factors, and food and dietary habits [2,3,18,19,20,21,22,23]. In addition, previous studies were mainly conducted in developed countries, where pre-pregnancy overweight and excessive gestational weight gain are major public health problems. For instance, approximately 40%, 45%, and 30% of women in the USA, UK, and Europe, respectively, enter pregnancy being overweight [1,22,31,32,33]. At the same time, nearly 50% of women in the USA, UK, and Europe gain more weight during pregnancy than recommended [3,22,32,33].
Unlike the situation in developed countries, the foremost concern in low-income countries is pre-pregnancy undernutrition and gaining inadequate weight during pregnancy [34]. Therefore, studies on postpartum weight change in low-income countries may have to be aimed at different outcomes than in developed countries. However, no study has assessed postpartum weight change in low-income countries where it may manifest as excess weight retention or excess weight loss. A change to both sides of the spectrum could pose significant public health concerns because of their deleterious effects on maternal health and subsequent birth outcomes. In addition, most previous studies used self-reported weight(s) to compute postpartum weight change [18], instead of actual weight measurements before and after pregnancy. As a result, previous findings may be biased, and a knowledge gap concerning actual postpartum weight change and its determinants remains for low-income countries. Therefore, our study aimed to assess measured instead of self-reported postpartum weight change in relation to pre-pregnancy weight and gestational weight gain. We investigated a wide range of factors with respect to their association with postpartum weight change in a population of pregnant women in the northern part of Ethiopia.
2. Methods
2.1. Study Design, Setting, and Population
The present study was part of the KIlte-Awlaelo Tigray Ethiopia (KITE) cohort study designed to assess maternal nutrition, adverse birth outcomes, and child growth, as reported previously [35]. The KITE cohort, a population-based prospective study, was conducted between February 2018 and September 2020 in the Kilte-Awlaelo Health and Demographic Surveillance Site (KA-HDSS) in the eastern zone of the Tigray region in northern Ethiopia. The KA-HDSS comprises thirteen kebeles (the smallest administrative unit). The total population of KA-HDSS is about 110,000. Most of the population lives in rural conditions, where agriculture is the primary source of income.
The study population and measurements were described in detail previously [35]. The study population included pregnant women whose delivery date lay before February 2019. To be eligible for the study, pregnant women needed to be married, aged 18 or older, had pre-pregnancy weight measurements, and completed ≤20 weeks of gestation at inclusion. The sample size was calculated to address the objectives of the KITE cohort study, which was to demonstrate a difference of 8% in low birth weight, depending on mid-upper arm circumference (MUAC) (above vs. below 23.0 cm). The additional parameters used were alpha = 0.05 and beta = 0.20. Including an estimated 10% drop-out rate, the total sample size was calculated at 1100.
The weight of non-pregnant women (n = 17,500) living in the study area was measured using a SECA scale to the nearest 100 g between August and October 2017. Subsequently, eligible women were identified by applying different methods including a community-based survey by Health Extension Workers through the “Women’s Development Army”, a network of health information workers reaching individual households through their health posts. Further, the records of the nearby antenatal clinics and the KA-HDSS database were used. All eligible pregnant women identified between February and September 2018 were included consecutively. In total, 991 women were recruited for the KITE cohort study. Out of the 991 women, 585 were followed until 18 to 24 months postpartum. After the index pregnancy, women who became pregnant again within the follow-up period were excluded from the present analysis.
2.2. Data Collection and Measurements
Qualified Health Extension Workers collected the data via an interviewer-administered questionnaire and anthropometric measurements. The data collection was supplemented by extracting data available in the KA-DHSS database and prenatal records. As the details of the measures are accessible elsewhere [35,36], only brief descriptions are provided below.
Anthropometric data: Weight (to the nearest 100 g) using a SECA scale, height (to the nearest 0.1 cm) using a height-measuring board, and MUAC (to the nearest 0.1 cm) using a non-stretchable tape were measured at inclusion. Also, weight and MUAC were measured during pregnancy at 32 to 36 weeks of gestation, and 18 to 24 months postpartum. All measurements were obtained twice and averaged. Similarly, pre-pregnancy BMI, BMI at inclusion, and postpartum BMI were computed from height measured at inclusion and weight measured before pregnancy, at inclusion, and 18 to 24 months postpartum, respectively, as BMI = weight (kg)/[height (m)]2. Then, based on the BMI (in kg/m2), women were classified as underweight (BMI < 18.5 kg/m2), normal (BMI = 18.5 to 24.9 kg/m2), and overweight (BMI ≥ 25.0 kg/m2). Gestational weight gain calculated as the difference between weights at 32 to 36 weeks of gestation and before pregnancy was classified based on the IOM guidelines [1]. Moreover, postpartum weight change was calculated by subtracting pre-pregnancy weight from the weight measured 18 to 24 months postpartum.
Sociodemographic data: Age, residence, education, occupation, household size, and economic status were extracted from the KA-DHSS database. KA-DHSS updates the database every six months. However, the regular biannual update of the KA-DHSS database does not include economic status. Hence, adjustments for a change in the socioeconomic proxy indicator variables, since the last updates were made at inclusion. Wealth index quintiles designating the lowest to the highest economic statuses were generated from the proxy indicators. These indicators included housing characteristics, access to improved water and sanitation facilities, and ownership of household assets, land, and livestock [37].
Access to improved drinking water sources refers to access to piped water on-premises, public taps or standpipes, tube wells or boreholes, protected dug wells, protected springs, and/or rainwater collection. Similarly, access to an improved sanitation facility was defined as access to an unshared toilet facility, pit latrine with a slab, ventilated improved pit latrine, or flush toilet [38].
Health extension package implementation was assessed at inclusion by checking if the women’s respective households were certified as models. A model household was defined as a household trained on a health extension package and that implemented the package afterwards [39,40,41]. Additionally, self-reported physical activity was collected at inclusion using the short form of the International Physical Activity Questionnaire (IPAQ) and summarized according to the scoring protocol [42,43]. Moreover, self-reported history of pre-pregnancy illnesses was collected at inclusion.
Reproductive and obstetric conditions: Gravidity and parity were extracted from the KA-DHSS database. In addition, self-reported intimate partner violence was obtained at inclusion using the four-item HITS (Hurt, Insult, Threaten, and Scream) questions, each rated from 1 to 5. A summed score >10 was used as suggestive of violence [44]. Additionally, women were asked nine questions addressing five domains relating to women’s empowerment at inclusion: earning and control over income, decision-making on household purchases, mobility and healthcare autonomy, attitude towards intimate partner violence, and ownership of assets [45,46,47]. Coding each response as 0 or 1 and summing all the responses, a women’s empowerment score ranging from 0 to 9 was obtained. By assigning the domains an equal weight of 1 point each, to be shared by the indicators within the domains, women who scored ≥80% were considered empowered [48]. Additionally, women were queried at inclusion if they wanted to get pregnant at the time they became pregnant, later, or not at all. Accordingly, an index pregnancy that was wanted later or not wanted at all was considered unplanned.
Moreover, self-reported stressful life events that occurred over the past year, illness during pregnancy, pregnancy complications, prenatal care, and complications at birth were obtained at 32 to 36 weeks or at or immediately after birth, as appropriate [45,49,50]. For women who began prenatal care at ≤16 weeks of gestation, prenatal care was defined as adequate plus (five or more visits), adequate (four visits), or intermediate (two to three visits). Prenatal care was considered inadequate if started at >16 weeks and/or comprising fewer than two visits [51]. Apart from the self-reported history of illness during pregnancy, data on HIV status, urine analysis, stool examination, venereal diseases, hepatitis B, haemoglobin, and other diseases were retrieved from prenatal records when available. Based on the measurement at prenatal care booking, iron deficiency anaemia was defined as haemoglobin <11 g/dL [52]. Self-reported duration of exclusive breastfeeding was also obtained at 18 to 24 months postnatal.
Food and dietary data: At inclusion, 24 h of dietary diversity data were collected by asking women about a list of food groups, with ‘yes’ or ‘no’ response options. Consumed foods were grouped into ten categories: grains, white roots and tubers; pulses; nuts and seeds; dairy; meat, fish, and poultry; egg; dark green leafy vegetables; other vitamin-A rich fruit and vegetables; other fruit; and other vegetables. A score of consuming at least five groups was defined as adequate dietary diversity [53]. Additionally, data on fasting were collected by asking women if they participate in the weekly fast and adhere to the long fast times. Finally, women were categorized as fasting if they fasted both weekly and for the long fasting times.
Food insecurity was assessed using the Household Food Insecurity Access Scale, consisting of nine ‘yes’ or ‘no’ occurrence questions. Each positive response was followed by a frequency-of-occurrence question, asking how often—(1) rarely, (2) sometimes, or (3) often—the reported food insecurity-associated condition happened in the previous month. Then, the sum of the frequency-of-occurrence questions yielded a food insecurity score ranging from 0 to 27. If the responses to all occurrence questions were ‘no’ or if the affirmative response was only to “did you worry that your household would not have enough food” in a rare frequency of occurrence, households were classified as food secure [54].
Psychosocial characteristics data: The ten-item Edinburgh Postnatal Depression Scale (EPDS) [55] and the seven-item anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A) [56] were used to measure depression and anxiety. Each item in both scales was rated from 0 to 3. For stress, the four-item Perceived Stress Scale (PSS-4), scored from 0 to 4, was used [57]. All were measured at 32 to 36 weeks of gestation. The HADS-A score ≥8, EPDS score of ≥13, and PSS-4 ≥ 8 were defined as high depression, anxiety, and stress symptoms, respectively. Total perinatal distress was calculated as the sum of anxiety, stress, and depression scores. To indicate the level of distress, the presence of high symptoms in one, two, or all of the three domains, i.e., anxiety, depression, or stress, was considered.
The five-item Turner Support Scale measured partner support; each scored from 0 to 3, with scores of <10 indicating low support [58]. Support from significant other social sources was also rated using the Oslo-3 Social Support Scale, and a score ≤8 was considered low [59]. Both measures of support at inclusion were summed up as a total support score. Additionally, low total social support was defined as low support from both partner and other social sources.
2.3. Statistical Analysis
Data were entered to Epi-Data (Version 3.1, EpiData Association, Denmark. Http://www.epidata.dk accessed on 30 June 2018), verified by re-entering 20% of the completed questionnaires selected randomly, and analyzed with Stata (Version 14, Stata Corporation, and College Station, Texas, USA). Proportions and means (SD) or medians (IQRs) were used to describe the characteristics of the participants. In addition, baseline characteristics were compared between women who successfully completed the study and those who did not using a Chi-squared test, an independent Student’s t-test, or a Mann–Whitney U-test (as appropriate) to evaluate selective loss to follow-up. Additionally, the unadjusted association of the independent variables with postpartum weight change as a continuous score was estimated using univariable linear regression.
To assess factors independently influencing postpartum weight change, a hierarchical multivariable linear regression modeling was applied. In the first model, all statistically significant independent variables (p < 0.05, tested two-sided) from the univariable analysis, except pre-pregnancy body mass index and gestational weight gain, were included. Further, an interaction between food insecurity and perinatal distress was included as per the likelihood ratio test in the first model. Next, pre-pregnancy body mass index was added. In the third and final model, gestational weight gain was included. A hierarchical modeling approach was selected to evaluate the relevance of the independent variables. Normality of residuals was assessed through the normal probability plot and quantile–quantile plot. Homogeneity of variance was checked using the Breusch–Pagan test. In addition, multicollinearity was tested using variance inflation factor (vif).
Furthermore, logistic regression was used to identify factors associated with a change in BMI category between the prenatal and the postnatal period. In this case, the dependent variables were shifting from pre-pregnancy underweight to normal postnatal weight and shifting from pre-pregnancy normal weight to postnatal underweight. All statistically significant independent variables (p < 0.05, tested two-sided) from the univariable logistic regression analysis were included in the respective multivariable logistic regression analysis.
We performed inverse probability-weighted analyses to examine the possibility of selective loss to follow-up. First, we fitted a probit model with follow-up status (successfully completed the study vs. lost to follow-up) as a dependent variable and baseline characteristics as independent variables. The baseline characteristics included residence, age, parity, education, occupation, being a model household, history of illness, food insecurity, diet diversity, physical activity, pre-pregnancy BMI, and GWG. Then, we calculated the predicted probability of successfully completing the study from the probit model. Finally, the inverse of the predicted probability was used to weight the observations in a re-analysis of factors associated with postpartum weight change [60].
3. Results
From the 991 women included at an average of 14.8 weeks (SD = 1.9) of gestation, 585 were followed until 18 to 24 months postpartum. The women who were lost to follow-up did not differ from women who completed the study and were included in the present analysis for most of the baseline characteristics. However, women who were lost to follow-up were more likely to be urban dwellers, housewives, and have had a history of illness during pregnancy. On average, the women included in the present analysis were 29.6 years (SD = 6.4) old, and the majority (69.2%) lived in rural conditions. Education-wise, about a third (32.7%) had at most a primary school education. By occupation, 353 (59.1%) were farmers and 180 (30.2%) were housewives. Moreover, more than half of the women (51.3%) reported low physical activity. Moreover, their average parity, including the index birth, was 3.8 (SD = 2.3). Regarding the women’s health condition, 111 (18.6%) had a history of illness during the index pregnancy, and 44.4% had high symptoms of distress at least in one of the three domains (Table 1).
Table 1.
Socio-demographic factors, reproductive and obstetric conditions, food and dietary habits, and psychosocial characteristics.
| Characteristics | Baseline n = 991 |
Lost to Follow-Up n = 406 |
Completed the Study, n = 585 |
p-Value 1 |
|---|---|---|---|---|
| Age at inclusion, mean (SD) | 29.3 (6.5) | 28.9 (6.5) | 29.6 (6.5) | 0.111 |
| <25 years | 273 (27.6) | 120 (29.6) | 153 (26.1) | 0.478 |
| 25–34 years | 490 (49.5) | 197 (48.5) | 293 (50.1) | |
| ≥35 years | 228 (23.0) | 89 (21.9) | 139 (23.8) | |
| Rural by residence, n (%) | 647 (65.3) | 244 (60.1) | 403 (68.9) | 0.004 |
| Educational status, n (%) | 0.186 | |||
| No formal education | 362 (36.5) | 136 (33.5) | 226 (38.6) | |
| Primary education | 326 (32.9) | 135 (33.3) | 191 (32.7) | |
| Secondary education and above | 303 (30.6) | 135 (33.3) | 168 (28.7) | |
| Occupational status, n (%) $ | 0.005 | |||
| Farmer | 541 (54.6) | 197 (48.5) | 344 (58.8) | |
| Housewife | 337 (34.0) | 159 (39.2) | 178 (30.4) | |
| Others * | 113 (11.4) | 50 (12.3) | 63 (10.8) | |
| Economic status | 0.845 | |||
| Lowest | 198 (20.0) | 84 (20.7) | 114 (19.5) | |
| Low | 198 (20.0) | 81 (20.0) | 117 (20.0) | |
| Middle | 200 (20.2) | 83 (20.4) | 117 (20.0) | |
| High | 200 (20.2) | 85 (20.9) | 115 (19.7) | |
| Highest | 195 (19.6) | 73 (18.0) | 122 (20.8) | |
| Household size, mean (SD) | 5.5 (2.0) | 5.3 (2.0) | 5.6 (2.0) | 0.027 |
| Model household, n (%) | 242 (24.4) | 106 (26.1) | 136 (23.3) | 0.303 |
| History of pre-pregnancy illness, n (%) | 142 (14.3) | 56 (13.8) | 86 (14.7) | 0.688 |
| Level of physical activity, n (%) | 0.058 | |||
| Low | 527 (53.2) | 226 (55.7) | 301 (51.4) | |
| Moderate | 425 (42.9) | 159 (39.1) | 266 (45.5) | |
| High | 39 (3.9) | 21 (5.2) | 18 (3.1) | |
| Total support score, mean (SD) | 21.3 (3.8) | 21.3 (3.9) | 21.3 (3.7) | 0.944 |
| Low social support, n (%) | 75 (7.6) | 38 (9.4) | 37 (6.3) | 0.413 |
| Improved source of drinking water, n (%) | 888 (89.6) | 367 (90.4) | 521 (89.1) | 0.499 |
| Improved sanitation facility, n (%) | 135 (13.6) | 51 (12.6) | 84 (14.4) | 0.417 |
| Number of stressful life events, mean (SD) | 0.6 (0.9) | 0.7 (1.0) | 0.6 (0.9) | 0.161 |
| Total perinatal distress score (n = 938), mean (SD) | 19.0 (9.2) | 19.0 (9.2) | 19.1 (9.2) | 0.890 |
| Level of perinatal distress (n = 938), n (%) | ||||
| Not distressed at all | 532 (56.7) | 207 (58.6) | 325 (55.6) | 0.792 |
| Distressed in one domain | 201 (21.4) | 71 (20.1) | 130 (22.2) | |
| Distressed in two domains | 118 (12.6) | 42 (11.9) | 76 (13.0) | |
| Distressed in three domains | 87 (9.3) | 33 (9.4) | 54 (9.2) | |
| Total parity, mean (SD) $ | 3.7 (2.3) | 3.4 (2.2) | 3.8 (2.3) | 0.013 |
| Primigravida | 196 (19.8) | 88 (21.7) | 108 (18.5) | 0.038 |
| Two to three | 341 (34.4) | 154 (37.9) | 187 (32.0) | |
| Four to five | 239 (24.1) | 89 (28.9) | 150 (25.6) | |
| More than five | 215 (21.7) | 75 (18.5) | 140 (23.9) | |
| Unplanned pregnancy, n (%) | 405 (40.9) | 161 (39.7) | 244 (41.7) | 0.518 |
| Intimate partner violence score, mean (SD) | 6.9 (3.0) | 6.9 (3.1) | 6.9 (3.0) | 0.933 |
| Experienced intimate partner violence, n(%) | 161 (16.3) | 75 (18.5) | 86 (14.7) | 0.113 |
| Women empowerment score, mean (SD) | 5.6 (1.5) | 5.5 (1.6) | 5.6 (1.4) | 0.202 |
| Low women empowerment, n (%) | 114 (11.5) | 47 (11.6) | 67 (11.5) | 0.952 |
| Adequacy of prenatal care use, n (%) | 0.829 | |||
| Inadequate | 418 (42.2) | 170 (41.9) | 248 (42.4) | |
| Intermediate | 126 (12.7) | 56 (13.8) | 70 (12.0) | |
| Adequate | 389 (39.3) | 158 (38.9) | 231 (39.5) | |
| Adequate plus | 58 (5.9) | 22 (5.4) | 36 (6.1) | |
| History of illness during pregnancy, n (%) | 225 (22.7) | 116 (28.6) | 109 (18.6) | 0.000 |
| Number of pregnancy complications, mean (SD) | 1.2 (1.4) | 1.3 (1.4) | 1.2 (1.5) | 0.270 |
| Number of complications at birth, mean (SD) | 0.4 (0.8) | 0.4 (0.9) | 0.4 (0.8) | 0.259 |
| Dietary diversity score, mean (SD) | 4.6 (1.4) | 4.7 (1.4) | 4.5 (1.4) | 0.051 |
| Adequate dietary diversity score, n (%) | 518 (52.3) | 219 (53.9) | 299 (51.1) | 0.380 |
| Food insecurity score, median (IQR) | 0 (0–8) | 0 (0–8) | 0 (0–8) | 0.604 a |
| Food insecure, n (%) | 392 (39.6) | 155 (38.2) | 237 (40.5) | 0.459 |
| Fasting, n (%) | 694 (70.0) | 290 (71.4) | 404 (69.1) | 0.423 |
| Exclusive breastfeeding in months, mean (SD) | 5.4 (1.2) | — | 5.4 (1.2) | — |
| Exclusive breastfeeding (n = 585), n (%) | 439 (73.5) | — | 439 (73.5) | — |
1 For comparisons between women who were lost to follow-up and those who successfully completed the study, * Students, unemployed, and so on, $ The difference between women who were lost to follow-up and those who successfully completed the study was insignificant after stratification by residence. p-Values written in bold show significant difference.
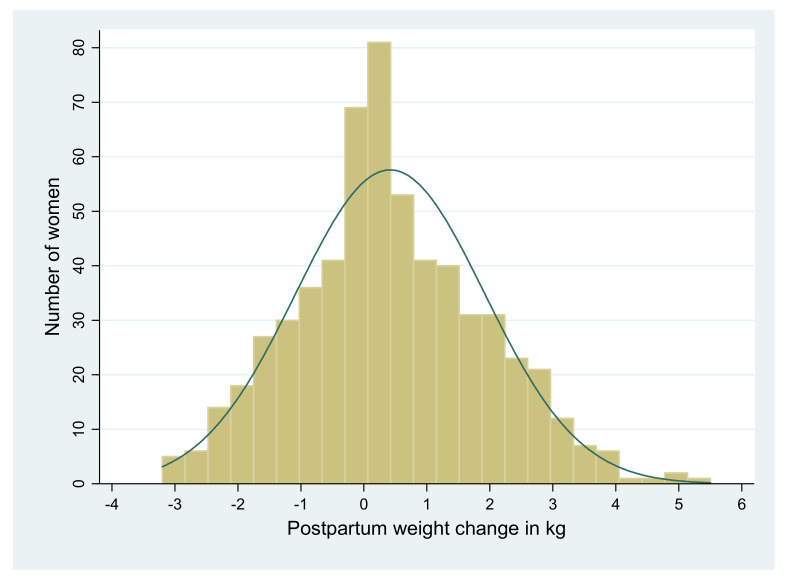
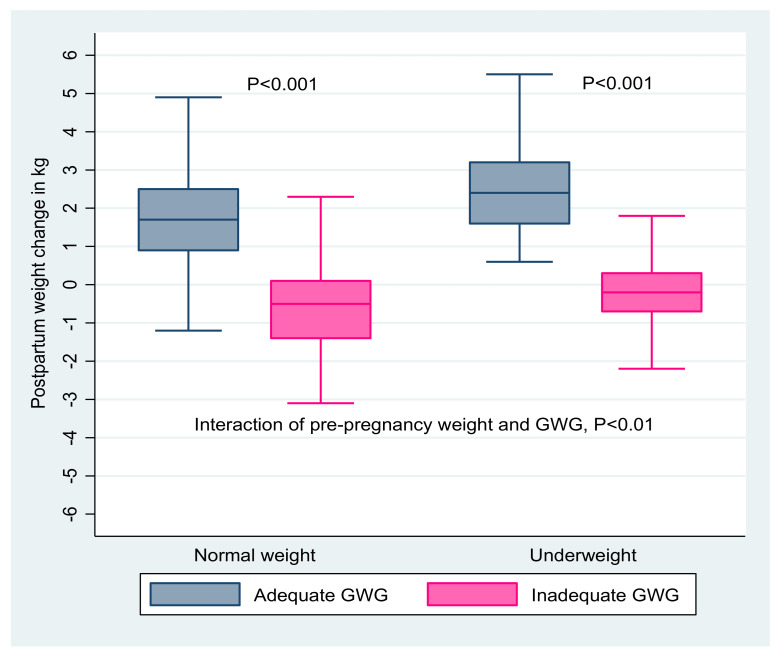
Before pregnancy, the mean body mass index of the 585 women included in the present study was 19.7 kg/m2 (SD = 2.0). On average, the women gained 10.8 kg (SD = 2.3) over the course of pregnancy. At 18 to 24 months postpartum, their pre-pregnancy weight changed from −3.2 to 5.5 kg (Figure 1), with the mean change being 0.42 kg (SD = 1.5). As a result, the average body mass index increased to 19.9 kg/m2 (SD = 2.2). In terms of a change in weight category, 17.8% of women who were underweight before pregnancy achieved normal weight, whereas 5.1% shifted to underweight compared to the pre-pregnancy period. In total, 194 (33.2%) women were underweight at 18 to 24 months postpartum (Supplementary Table S1). The postpartum weight change in kg in relation to pre-pregnancy body mass index and gestational weight gain is shown in Figure 2.
Figure 1.
Distribution of postpartum weight change in kg among 585 women from the Tigray region, northern Ethiopia, 2018.
Figure 2.
Postpartum weight change in kg separated by pre-pregnancy BMI (left panel: normal weight: BMI 18 to 25; right panel: underweight: BMI < 18.5) and gestational weight gain (GWG, adequate in blue, inadequate in pink) in Tigray region, northern Ethiopia, 2018. p-values indicate comparisons of adequate versus inadequate GWG among normal weight and underweight women.
In the univariable analysis, women empowerment, dietary diversity, social support, adequacy of prenatal care, pre-pregnancy BMI, and gestational weight gain were positively associated with postpartum weight change. Additionally, physical activity, food insecurity, and perinatal distress were negatively related with postpartum weight change. In the first multivariable model, women empowerment (β [95% CI] 0.11 [0.01, 0.20]), adequate prenatal care (0.49 [0.22, 0.77]), and high physical activity (−0.52 [−1.04, −0.001]) retained their association. When model 1 was additionally adjusted for pre-pregnancy BMI in model 2, adequate prenatal care (0.48 [0.21, 0.74]) and pre-pregnancy BMI (0.14 [0.07, 0.22]) were associated with postpartum weight change. After adjusting model 1 for both pre-pregnancy weight and weight gain during pregnancy in model 3, an average of 0.56 kg (0.52, 0.60) was retained at 18 to 24 months postpartum for each kilogram of increase in gestational weight gain (Table 2).
Table 2.
Linear regression analysis of factors associated with postpartum weight change in kg.
| Characteristics | Univariable Analysis |
p-Value | Multivariable Model 1 a |
p-Value | Multivariable Model 2 b | p-Value | Multivariable Model 3 c | p-Value |
|---|---|---|---|---|---|---|---|---|
| Unadj. β (95% CI) | Adj. β (95% CI) | Adj. β (95% CI) | Adj. β (95% CI) | |||||
| Women empowerment score | 0.13 (0.04, 0.21) | 0.003 | 0.11 (0.01, 0.20) | 0.033 | 0.07 (−0.03, 0.17) | 0.156 | −0.01 (−0.07, 0.05) | 0.848 |
| Food insecurity score | −0.03 (−0.05, −0.01) | 0.015 | −0.03 (−0.10, 0.04) | 0.399 | −0.003 (−0.03, 0.04) | 0.856 | 0.02 (−0.004, 0.04) | 0.729 |
| Dietary diversity score | 0.11 (0.02, 0.21) | 0.014 | 0.04 (−0.07, 0.15) | 0.451 | 0.02 (−0.09, 0.13) | 0.760 | 0.02 (−0.04, 0.08) | 0.107 |
| Total physical activity | ||||||||
| Low | Reference | - | Reference | - | Reference | - | Reference | - |
| Moderate | −0.26 (−0.51, −0.01) | 0.043 | −0.22 (−0.47, 0.04) | 0.102 | −0.13 (−0.39, 0.15) | 0.340 | 0.01 (−0.15, 0.16) | 0.949 |
| High | −0.65 (−1.13, −0.17) | 0.008 | −0.52 (−1.04,−0.001) | 0.049 | −0.42 (−0.95, 0.10) | 0.115 | 0.02 (−0.36, 0.37) | 0.967 |
| Total social support score | 0.04 (0.003, 0.07) | 0.030 | 0.01 (−0.03, 0.05) | 0.661 | 0.02 (−0.02, 0.06) | 0.350 | −0.000 (−0.02, 0.02) | 0.973 |
| Total perinatal distress score | −0.01 (−0.03,−0.001) | 0.042 | −0.004 (−0.02, 0.02) | 0.683 | 0.03 (−0.05, 0.10) | 0.445 | −0.002 (−0.05, 0.04) | 0.922 |
| Adequacy of prenatal care | ||||||||
| Inadequate | Reference | - | Reference | - | Reference | - | Reference | - |
| Intermediate | 0.32 (−0.08, 0.71) | 0.116 | 0.24 (−0.16, 0.63) | 0.240 | 0.21 (−0.18, 0.61) | 0.293 | 0.08 (−0.18, 0.33) | 0.545 |
| Adequate | 0.54 (0.27, 0.81) | 0.000 | 0.49 (0.22, 0.77) | 0.000 | 0.47 (0.20, 0.74) | 0.001 | 0.04 (−0.13, 0.21) | 0.672 |
| Adequate plus | 0.12 (−0.40, 0.65) | 0.645 | −0.04 (−0.59, 0.51) | 0.88 | −0.14 (−0.67, 0.39) | 0.613 | −0.14 (−0.45, 0.18) | 0.371 |
| Pre-pregnancy BMI in kg/m2 | 0.17 (0.11, 0.23) | 0.000 | Not included | - | 0.15 (0.07, 0.23) | 0.000 | −0.06 (0.52, 0.60) | 0.078 |
| Gestational weight gain in kg | 0.53 (0.50, 0.57) | 0.000 | Not included | - | Not included | - | 0.56 (0.52, 0.60) | 0.000 |
| Adjusted R2 | Not applicable | 5.6% | 8.2% | 66.3% | ||||
a adjusted for interaction between total perinatal distress and food insecurity scores, b Model 1 + pre-pregnancy BMI, and c Model 1 + pre-pregnancy BMI + gestational weight gain. Unadj.; unadjusted, Adj; adjusted and BMI; body mass index. p-Values written in bold show significant association.
Table 3 shows factors associated with a change in weight category at 18 to 24 months postpartum in relation to pre-pregnancy weight. Food insecurity (AOR [95% CI] 5.26 [1.68, 16.50]) was associated with shifting from normal pre-pregnancy weight to underweight 18 to 24 months postpartum. A 1 kg decrease in gestational weight gain (AOR 2.38 [1.64, 3.45]) was associated with higher odds of shifting from normal pre-pregnancy weight to underweight at 18 to 24 months postpartum. Similarly, being distressed in two domains (AOR 0.13 [0.03, 0.48]) was associated with lower odds of shifting to the normal weight category. Conversely, a 1 kg increase in gestational weight gain was associated with shifting from pre-pregnancy underweight to the normal weight category (AOR 1.65 [1.37, 2.00]).
Table 3.
Factors associated with a change in BMI category at 18 to 24 months postpartum.
| Characteristics | Total, n = 372 | Shifted to Underweight 1 | COR (95% CI) | p-Value | AOR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Parity including the index birth, n (%) | ||||||
| Primigravida | 73 (19.6) | 3 (15.8) | 0.37 (0.10, 1.44) | 0.151 | 1.18 (0.21, 6.70) | 0.854 |
| Two to three | 116 (31.2) | 3 (15.8) | 0.23 (0.06, 0.86) | 0.030 | 0.24 (0.04, 1.53) | 0.130 |
| Four to five | 98 (26.3) | 4 (21.1) | 0.36 (0.11, 1.21) | 0.099 | 0.35 (0.08, 1.51) | 0.161 |
| More than five | 85 (22.9) | 9 (47.4) | Reference | - | Reference | - |
| Unplanned index pregnancy, n (%) | 146 (39.3) | 12 (8.2) | 2.80 (1.07, 7.29) | 0.035 | 3.06 (0.89, 10.51) | 0.076 |
| Intimate partner violence, n (%) | 39 (10.5) | 6 (15.4) | 4.43 (1.58, 12.56) | 0.005 | 3.19 (0.77, 13.23) | 0.111 |
| Food insecurity, n (%) | 95 (25.5) | 13 (13.7) | 7.08 (2.61, 19.22) | 0.000 | 5.26 (1.68, 16.50) | 0.004 |
| Inadequate dietary diversity, n (%) | 136 (36.6) | 12 (8.8) | 3.16 (1.21, 8.25) | 0.019 | 3.19 (0.90, 11.34) | 0.074 |
| GWG in kg, mean (SD) | 11.4 (2.1) | 8.6 (1.3) | 2.17 (1.67, 2.78) * | 0.000 | 2.38 (1.64, 3.45) * | 0.000 |
| Characteristics | Total, n = 213 | Shifted to Normal Weight 2 | COR (95% CI) | p-Value | AOR (95% CI) | p-Value |
| Rural in residence, n (%) | 162 (76.1) | 24 (14.8) | 0.46 (0.22, 0.98) | 0.043 | 0.55 (0.19, 1.56) | 0.260 |
| Educational status, n (%) | ||||||
| No formal education | 97 (45.5) | 13 (13.4) | Reference | - | Reference | - |
| Primary education | 67 (31.5) | 19 (28.4) | 2.56 (1.16, 5.64) | 0.020 | 2.38 (0.86, 6.65) | 0.097 |
| Secondary and above | 49 (23.0) | 6 (12.2) | 0.90 (0.32, 2.54) | 0.845 | 0.52 (0.12, 2.33) | 0.394 |
| Perinatal distress, n (%) | ||||||
| Not distressed at all | 76 (35.7) | 21 (27.6) | Reference | - | Reference | - |
| Distressed in one domain | 58 (27.2) | 10 (17.2) | 0.55 (0.23, 1.28) | 0.162 | 0.38 (0.14, 1.08) | 0.068 |
| Distressed in two domains | 40 (18.8) | 3 (7.5) | 0.21 (0.06, 0.77) | 0.018 | 0.13 (0.03, 0.48) | 0.002 |
| Distressed in three domains | 39 (18.3) | 4 (10.3) | 0.30 (0.10, 0.95) | 0.040 | 0.38 (0.10, 1.46) | 0.158 |
| GWG in kg, mean (SD) | 9.7 (2.1) | 11.4 (2.1) | 1.63 (1.37, 1.94) | 0.000 | 1.65 (1.37, 2.00) | 0.000 |
COR; crude odds ratio, AOR; adjusted odds ratio, 1 shifted from normal weight to underweight compared to pre-pregnancy, 2 shifted from underweight to normal compared to pre-pregnancy, and * COR and AOR reversed. p-Values written in bold show significant association.
Because of the loss to follow-up, we re-analyzed the data weighted with the inverse probability of successfully completing the study to examine the effect of selection bias on effect estimates and the observed associations. Of the baseline characteristics considered in the analysis, only a history of illness during pregnancy (AOR = 0.60, 95% CI [0.44, 0.83]) was associated with the probability of successfully completing the study. Furthermore, we did not see any difference between the weighted and unweighted analyses with regard to the effect estimates and observed associations described above (data not shown).
4. Discussion
In the present study, we have assessed postpartum weight change in relation to pre-pregnancy weight and gestational weight gain and its associated factors among women in the northern part of Ethiopia. In our study population, the average weight change at 18 to 24 months postpartum was 0.42 kg (SD = 1.5), much lower than reported in the existing literature [61,62]. The disagreement with the literature is likely explained by the difference in the socioeconomic and nutritional characteristics of the women. In our study, a sizable number of the women were food insecure and had inadequate diet diversity. Moreover, 36.4% of the women were underweight before pregnancy and 62.2% did not achieve adequate gestational weight gain [35], unlike in the previously reported studies. At 18 to 24 months postpartum, about one third (33.2%) of the women were still underweight. This implies that the postpartum period is an underutilized window of opportunity to improve maternal nutritional status, and possibly child health, in our study area.
In terms of a change in weight category, 17.8% of women who were underweight before pregnancy had achieved normal weight at 18 to 24 months postpartum. Gaining more weight during pregnancy within the range recommended by the IOM guideline was associated with a higher postpartum weight change [62,63] and shifting from lower to normal weight category. These results show that adequate gestational weight gain is essential to optimize maternal health in low-income countries where pre-pregnancy under-nutrition is prevalent. In addition, the women who shifted from low to normal weight may have improved maternal reserve after pregnancy. This improved maternal reserve may help women achieve optimal lactation without depleting their nutrient stores and be better prepared for a possible next pregnancy. Thus, adequate gestational weight gain may contribute to enhancing subsequent pregnancy outcomes and child health. As shown in our previous work, interventions that advance women’s empowerment, dietary quality, pre-pregnancy nutritional status, and prenatal care utilization may improve gestational weight gain [36].
With specific reference to shifting to a lower weight category, 5.1% of women moved from normal pre-pregnancy weight to underweight at 18 to 24 months postpartum. In low-income countries, postpartum weight loss compounded with inadequate weight gain during pregnancy, and even more in a situation of pre-pregnancy undernutrition, might negatively impact women’s health. In addition, postpartum weight loss leading to being underweight might also affect outcomes of subsequent pregnancies. Therefore, postpartum weight status deserves the attention of the health care professionals in low-income countries. During pregnancy, gaining less weight was corroborated with postpartum weight loss and shifting from a higher (normal) to a lower weight category. This finding implies that evidence-driven interventions geared to achieve adequate gestational weight gain are needed in low-income countries like Ethiopia. Our findings may also suggest the need for post-pregnancy nutritional interventions as part of the maternal care continuum to optimize maternal health and subsequent pregnancy outcomes.
Furthermore, food insecurity was associated with higher odds of becoming underweight in the postpartum period among normal-weight women before pregnancy. The observed association may be partly explained by inadequate dietary intake due to lack of access to food. The relationship between food insecurity and being underweight postpartum may also show the need for nutritional interventions focusing on food-insecure women/households. Similarly, distress was associated with a lower likelihood of shifting from underweight to normal weight. Clearly, distress was associated with remaining underweight from before pregnancy to about two years postpartum. The effect of distress on appetite, nutrient/food intake, and dietary choices may partly explain the observed association. Although the link between food insecurity and distress can be bidirectional, the impact of food insecurity on distress may also aggravate the situation. Therefore, the relation between distress and remaining underweight from before pregnancy to about two years postpartum may highlight the need to tailor perinatal interventions and better screen and manage maternal mental health.
No previous study assessed factors associated with a shift in weight after pregnancy compared to pre-pregnancy, i.e., from normal to underweight or underweight to normal weight. Previous studies reported a positive association between food insecurity and distress and net postpartum weight change [64,65,66], exactly opposite to our finding. The most likely explanation for the disagreement in the results is the differences in characteristics of the study population. For example, food insecurity and distress may affect predominantly food choices (i.e., high energy low nutrient density foods) in developed countries and instead lead to more postpartum weight retention [67]. In some cases, distress may also be followed by emotional eating and corroborate with more weight retention. On the contrary, food insecurity in low income countries is all about limited access to adequate food that impairs both the quality and quantity of diet/foods/nutrients in our study population. Furthermore, socioeconomic adversity such as food insecurity is high in low-income countries like Ethiopia and may cause psychological distress and worsen its influence on maternal diet and nutrient status.
To our knowledge, this is the first study on this topic in sub-Saharan Africa. The results highlight the need to understand and map the problem to develop more targeted interventions. However, the follow-up time in the present study was short. Future studies with longer follow-up of the women after pregnancy may be needed to gain better insight into long-term risks and benefits. These long-term risks and benefits may be evaluated in terms of maternal health and child growth as well as outcomes of subsequent pregnancies. Of note, our subgroup analyses assessing factors associated with a change in weight category may not be adequately powered. Furthermore, data on potential confounding factors such as physical activity and dietary characteristics were only collected at inclusion, early during pregnancy, and may not adequately reflect the situation during the postnatal period. Therefore, future studies with a higher sample size and including data on potential confounders after pregnancy are recommended.
5. Conclusions
Our findings suggest that higher weight gain and better mental health during pregnancy may help women achieve a better nutritional status after pregnancy and before a possible subsequent pregnancy. Hence, in low-income settings such as northern Ethiopia, where maternal undernutrition is common, adequate support during pregnancy may improve maternal and child health outcomes.
Acknowledgments
The Universities of Groningen, Aksum, and Mekelle are acknowledged for their valuable support. Our gratitude goes to the regional health bureau, district health offices, supervisors, data collectors, and women who participated in this study. We are also thankful to the Central Statistics Agency of Ethiopia and district health bureaus of the study area, as well as Tahtay-Maichew district health bureau, who allowed us to use their scales for anthropometric measurements.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14010131/s1, Table S1. Summary of the nutritional characteristics of the women who successfully completed the study and included in the final analyses
Author Contributions
K.H.M., H.G., A.M.B., H.M.B., and E.M.v.d.B. designed the study. K.H.M., H.G., and A.M.B. were involved in the data collection. K.H.M., H.G., H.M.B., and E.M.v.d.B. analyzed the data, interpreted the findings, and prepared the manuscript. H.G., H.M.B., and E.M.v.d.B. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The Universities of Groningen, Aksum, and Mekelle supported the study in one or in another way.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of College of Health Science, Aksum University [(ref. number: IRB 026/2017 dated 15 August 2017)].
Informed Consent Statement
Informed consent was obtained from all women involved in the study.
Data Availability Statement
Dataset will be available on reasonable request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines . In: Weight Gain during Pregnancy: Reexamining the Guidelines. Rasmussen K.M., Yaktine A.L., editors. National Academies Press; Washington, DC, USA: 2009. [PubMed] [Google Scholar]
- 2.Haugen M., Brantsæter A.L., Winkvist A., Lissner L., Alexander J., Oftedal B., Magnus P., Meltzer H.M. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: A prospective observational cohort study. BMC Pregnancy Childbirth. 2014;14:201. doi: 10.1186/1471-2393-14-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rode L., Kjærgaard H., Ottesen B., Damm P., Hegaard H.K. Association between gestational weight gain according to body mass index and postpartum weight in a large cohort of Danish women. Matern. Child Health J. 2012;16:406–413. doi: 10.1007/s10995-011-0775-z. [DOI] [PubMed] [Google Scholar]
- 4.Endres L.K., Straub H., McKinney C., Plunkett B., Minkovitz C.S., Schetter C.D., Ramey S., Wang C., Hobel C., Raju T., et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet. Gynecol. 2015;125:144–152. doi: 10.1097/AOG.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullaney L., O’Higgins A.C., Cawley S., Daly N., McCartney D., Turner M.J. Maternal weight trajectories between early pregnancy and four and nine months postpartum. Public Health. 2016;135:144–146. doi: 10.1016/j.puhe.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Ma D., Szeto I.M.Y., Yu K., Ning Y., Li W., Wang J., Zheng Y., Zhang Y., Wang P. Association between gestational weight gain according to prepregnancy body mass index and short postpartum weight retention in postpartum women. Clin. Nutr. 2015;34:291–295. doi: 10.1016/j.clnu.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Van Poppel M.N.M., Hartman M.A., Hosper K., Van Eijsden M. Ethnic differences in weight retention after pregnancy: The ABCD study. Eur. J. Public Health. 2012;22:874–879. doi: 10.1093/eurpub/cks001. [DOI] [PubMed] [Google Scholar]
- 8.Nasreddine L., Ayoub J., Abbas N., Abdul Malik M., Naja F. Postpartum Weight Retention and Its Determinants in Lebanon and Qatar: Results of the Mother and Infant Nutrition Assessment (MINA) Cohort. Int. J. Environ. Res. Public Health. 2020;17:7851. doi: 10.3390/ijerph17217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagl M., Linde K., Stepan H., Kersting A. Obesity and anxiety during pregnancy and postpartum: A systematic review. J. Affect. Disord. 2015;186:293–305. doi: 10.1016/j.jad.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 10.Sommer C., Sletner L., Mørkrid K., Jenum A.K., Birkeland K.I. Effects of early pregnancy BMI, mid-gestational weight gain, glucose and lipid levels in pregnancy on offspring’s birth weight and subcutaneous fat: A population-based cohort study. BMC Pregnancy Childbirth. 2015;15:84. doi: 10.1186/s12884-015-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moll U., Olsson H., Landin-Olsson M. Impact of Pregestational Weight and Weight Gain during Pregnancy on Long-Term Risk for Diseases. PLoS ONE. 2017;12:e0168543. doi: 10.1371/journal.pone.0168543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace J.M., Bhattacharya S., Horgan G.W. Weight change across the start of three consecutive pregnancies and the risk of maternal morbidity and SGA birth at the second and third pregnancy. PLoS ONE. 2017;12:e0179589. doi: 10.1371/journal.pone.0179589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cnattingius S., Villamor E. Weight change between successive pregnancies and risks of stillbirth and infant mortality: A nationwide cohort study. Lancet. 2016;387:558–565. doi: 10.1016/S0140-6736(15)00990-3. [DOI] [PubMed] [Google Scholar]
- 14.Villamor E., Cnattingius S. Interpregnancy Weight Change and Risk of Adverse Pregnancy Outcomes: A Population-based Study. Obstet. Gynecol. Surv. 2007;62:156–157. doi: 10.1097/01.ogx.0000256801.97592.d6. [DOI] [PubMed] [Google Scholar]
- 15.Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., De Onis M., Ezzati M., Grantham-Mcgregor S., Katz J., Martorell R., et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 16.Hill B., McPhie S., Skouteris H. The Role of Parity in Gestational Weight Gain and Postpartum Weight Retention. Women’s Health Issues. 2016;26:123–129. doi: 10.1016/j.whi.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Siega-Riz A.M., Viswanathan M., Moos M.K., Deierlein A., Mumford S., Knaack J., Thieda P., Lux L.J., Lohr K.N. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: Birthweight, fetal growth, and postpartum weight retention. Am. J. Obstet. Gynecol. 2009;201:339.e1–339.e14. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Begum F., Colman I., McCargar L.J., Bell R.C. On behalf of the Alberta Pregnancy Outcomes Gestational Weight Gain and Early Postpartum Weight Retention in a Prospective Cohort of Alberta Women. J. Obstet. Gynaecol. Canada. 2012;34:637–647. doi: 10.1016/S1701-2163(16)35316-6. [DOI] [PubMed] [Google Scholar]
- 19.Maddah M., Nikooyeh B. Weight retention from early pregnancy to three years postpartum: A study in Iranian women. Midwifery. 2009;25:731–737. doi: 10.1016/j.midw.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 20.He X., Hu C., Chen L., Wang Q., Qin F. The association between gestational weight gain and substantial weight retention 1-year postpartum. Arch. Gynecol. Obstet. 2014;290:493–499. doi: 10.1007/s00404-014-3235-3. [DOI] [PubMed] [Google Scholar]
- 21.Ashley-Martin J., Woolcott C. Gestational Weight Gain and Postpartum Weight Retention in a Cohort of Nova Scotian Women. Matern. Child Health J. 2014;18:1927–1935. doi: 10.1007/s10995-014-1438-7. [DOI] [PubMed] [Google Scholar]
- 22.Huang T.-T., Wang H.-S., Dai F.-T. Effect of pre-pregnancy body size on postpartum weight retention. Midwifery. 2010;26:222–231. doi: 10.1016/j.midw.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Hollis J.L., Crozier S.R., Inskip H.M., Cooper C., Godfrey K.M., Harvey N.C., Collins C.E., Robinson S.M. Modifiable risk factors of maternal postpartum weight retention: An analysis of their combined impact and potential opportunities for prevention. Int. J. Obes. 2017;41:1091–1098. doi: 10.1038/ijo.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rong K., Yu K., Han X., Szeto I.M.Y., Qin X., Wang J., Ning Y., Wang P., Ma D. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: A meta-analysis of observational studies. Public Health Nutr. 2015;18:2172–2182. doi: 10.1017/S1368980014002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang M., Gao H., Vinyes-Pares G., Yu K., Ma D., Qin X., Wang P. Association between breastfeeding duration and postpartum weight retention of lactating mothers: A meta-analysis of cohort studies. Clin. Nutr. 2018;37:1224–1231. doi: 10.1016/j.clnu.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Jarlenski M.P., Bennett W.L., Bleich S.N., Barry C.L., Stuart E.A. Effects of breastfeeding on postpartum weight loss among U.S. women. Prev. Med. 2014;69:146–150. doi: 10.1016/j.ypmed.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Olmedo N., Hernández-Cordero S., Neufeld L.M., García-Guerra A., Mejía-Rodríguez F., Méndez Gómez-Humarán I. The Associations of Maternal Weight Change with Breastfeeding, Diet and Physical Activity during the Postpartum Period. Matern. Child Health J. 2016;20:270–280. doi: 10.1007/s10995-015-1826-7. [DOI] [PubMed] [Google Scholar]
- 28.Whitaker K., Young-Hyman D., Vernon M., Wilcox S. Maternal Stress Predicts Postpartum Weight Retention. Matern. Child Health J. 2014;18:2209–2217. doi: 10.1007/s10995-014-1470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogaerts A.F.L., Van den Bergh B.R.H., Witters I., Devlieger R. Anxiety during early pregnancy predicts postpartum weight retention in obese mothers. Obesity. 2013;21:1942–1949. doi: 10.1002/oby.20352. [DOI] [PubMed] [Google Scholar]
- 30.Olson C.M., Strawderman M.S., Hinton P.S., Pearson T.A. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int. J. Obes. Relat. Metab. Disord. 2003;27:117–127. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 31.Thomson J.L., Tussing-Humphreys L.M., Goodman M.H., Olender S. Baseline Demographic, Anthropometric, Psychosocial, and Behavioral Characteristics of Rural, Southern Women in Early Pregnancy. Matern. Child Health J. 2016;20:1980–1988. doi: 10.1007/s10995-016-2016-y. [DOI] [PubMed] [Google Scholar]
- 32.Garay S.M., Sumption L.A., Pearson R.M., John R.M. Risk factors for excessive gestational weight gain in a UK population: A biopsychosocial model approach. BMC Pregnancy Childbirth. 2021;21:43. doi: 10.1186/s12884-020-03519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein R.F., Abell S.K., Misso M.L., Ranasinha S., Boyle J., Black M.H., Li N., Hu G., Corrado F., Rode L., et al. Gestational weight gain across continents and ethinicity: Systematic review and meta-analysis of maternal and infant outcomes in over one million women. BMC Med. 2018;16:153. doi: 10.1186/s12916-018-1128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asefa F., Nemomsa D. Gestational weight gain and its associated factors in Harari Regional State: Institution based cross-sectional study, Eastern Ethiopia. Reprod. Health. 2016;13:101. doi: 10.1186/s12978-016-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misgina K.H., Boezen H.M., van der Beek E.M., Mulugeta A., Groen H. What factors are associated with pre-pregnancy nutritional status? Baseline analysis of the KITE cohort: A prospective study in northern Ethiopia. BMJ Open. 2021;11:e043484. doi: 10.1136/bmjopen-2020-043484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misgina K.H., van der Beek E.M., Boezen H.M., Bezabih A.M. Pre-conception and prenatal factors influencing gestational weight gain: A prospective study in Tigray region, northern. BMC Pregnancy Childbirth. 2021;21:718. doi: 10.1186/s12884-021-04171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hjelm L., Mathiassen A., Miller D., Wadhwa A. Creation of a Wealth Index. World Food Programme; Rome, Italy: 2017. [Google Scholar]
- 38.WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation . Progress on Sanitation and Drinking Water: 2010 Update. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 39.Assefa Y., Gelaw Y.A., Hill P.S., Taye B.W., Van Damme W. Community health extension program of Ethiopia, 2003–2018: Successes and challenges toward universal coverage for primary healthcare services. Global. Health. 2019;15:24. doi: 10.1186/s12992-019-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medhanyie A., Spigt M., Kifle Y., Schaay N., Sanders D., Blanco R., GeertJan D., Berhane Y. The role of health extension workers in improving utilization of maternal health services in rural areas in Ethiopia: A cross sectional study. BMC Health Serv. Res. 2012;12:352. doi: 10.1186/1472-6963-12-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yitayal M., Berhane Y., Worku A., Kebede Y. Health extension program factors, frequency of household visits and being model households, improved utilization of basic health services in Ethiopia. BMC Health Serv. Res. 2014;14:156. doi: 10.1186/1472-6963-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.IPAQ Research Committee The International Physical Activity Questionnaire (IPAQ): Short last 7 days self-administered format. 2002. [(accessed on 30 August 2017)]. Available online: https://cdn-links.lww.com/permalink/jcrp/a/jcrp_2016_04_12_kaminsky_jcrp-d-16-00031r1_sdc1.pdf.
- 43.IPAQ Research Committee Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. 2005. [(accessed on 30 August 2017)]. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=5641f4c36143250eac8b45b7&assetKey=AS%3A294237418606593%401447163075131.
- 44.Rabin R.F., Jennings J.M., Campbell J.C., Bair-Merritt M.H. Intimate Partner Violence Screening Tools. Am. J. Prev. Med. 2009;36:439–445.e4. doi: 10.1016/j.amepre.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CSA and ICF International Ethiopia Demographic and Health Survey 2011. Central Statistical Agency and ICF International; Addis Ababa, Ethiopia: Calverton, MD, USA: 2012. [Google Scholar]
- 46.Alaofè H., Zhu M., Burney J., Naylor R., Douglas T. Association Between Women’s Empowerment and Maternal and Child Nutrition in Kalalé District of Northern Benin. Food Nutr. Bull. 2017;38:302–318. doi: 10.1177/0379572117704318. [DOI] [PubMed] [Google Scholar]
- 47.Jennings L., Na M., Cherewick M., Hindin M., Mullany B., Ahmed S. Women’s empowerment and male involvement in antenatal care: Analyses of Demographic and Health Surveys (DHS) in selected African countries. BMC Pregnancy Childbirth. 2014;14:297. doi: 10.1186/1471-2393-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malapit H.J.L., Quisumbing A.R. What dimensions of women’s empowerment in agriculture matter for nutrition in Ghana? Food Policy. 2015;52:54–63. doi: 10.1016/j.foodpol.2015.02.003. [DOI] [Google Scholar]
- 49.Berntson J., Patel J.S., Stewart J.C. Number of recent stressful life events and incident cardiovascular disease: Moderation by lifetime depressive disorder. J. Psychosom. Res. 2017;99:149–154. doi: 10.1016/j.jpsychores.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bitew T., Hanlon C., Kebede E., Honikman S., Fekadu A. Antenatal depressive symptoms and perinatal complications: A prospective study in rural Ethiopia. BMC Psychiatry. 2017;17:301. doi: 10.1186/s12888-017-1462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotelchuck M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am. J. Public Health. 1994;84:1414–1420. doi: 10.2105/AJPH.84.9.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WHO . Vitamin and Mineral Nutrition Information System. World Health Organization; Geneva, Switzerland: 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Google Scholar]
- 53.FAO & FHI 360 . Minimum Dietary Diversity for Women: A Guide to Measurement. FAO; Rome, Italy: 2016. [Google Scholar]
- 54.Coates J., Swindale A., Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v.3) Food and Nutrition Technical Assistance Project, Academy for Educational Development; Washington, DC, USA: 2007. [Google Scholar]
- 55.Cox J.L., Holden J.M., Sagovsky R. Detection of Postnatal Depression. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 56.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 57.Karam F., Bérard A., Sheehy O., Huneau M.C., Briggs G., Chambers C., Einarson A., Johnson D., Kao K., Koren G., et al. Reliability and validity of the 4-item perceived stress scale among pregnant women: Results from the OTIS antidepressants study. Res. Nurs. Health. 2012;35:363–375. doi: 10.1002/nur.21482. [DOI] [PubMed] [Google Scholar]
- 58.Cheng E.R., Rifas-Shiman S.L., Perkins M.E., Rich-Edwards J.W., Gillman M.W., Wright R., Taveras E.M. The Influence of Antenatal Partner Support on Pregnancy Outcomes. J. Women’s Health. 2016;25:672–679. doi: 10.1089/jwh.2015.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kocalevent R.-D., Berg L., Beutel M.E., Hinz A., Zenger M., Härter M., Nater U., Brähler E. Social support in the general population: Standardization of the Oslo social support scale (OSSS-3) BMC Psychol. 2018;6:31. doi: 10.1186/s40359-018-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Widen E.M., Whyatt R.M., Hoepner L.A., Mueller N.T., Ramirez-Carvey J., Oberfield S.E., Hassoun A., Perera F.P., Gallagher D., Rundle A.G. Gestational weight gain and obesity, adiposity and body size in African–American and Dominican children in the Bronx and Northern Manhattan. Matern. Child Nutr. 2016;12:918–928. doi: 10.1111/mcn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin J.E., Hure A.J., Macdonald-Wicks L., Smith R., Collins C.E. Predictors of post-partum weight retention in a prospective longitudinal study. Matern. Child Nutr. 2014;10:496–509. doi: 10.1111/j.1740-8709.2012.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Ha A.V., Zhao Y., Pham N.M., Nguyen C.L., Nguyen P.T.H., Chu T.K., Tang H.K., Binns C.W., Lee A.H. Postpartum weight retention in relation to gestational weight gain and pre-pregnancy body mass index: A prospective cohort study in Vietnam. Obes. Res. Clin. Pract. 2019;13:143–149. doi: 10.1016/j.orcp.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Nehring I., Schmoll S., Beyerlein A., Hauner H., von Kries R. Gestational weight gain and long-term postpartum weight retention: A meta-analysis. Am. J. Clin. Nutr. 2011;94:1225–1231. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 64.Laraia B., Vinikoor-Imler L.C., Siega-Riz A.M. Food insecurity during pregnancy leads to stress, disordered eating, and greater postpartum weight among overweight women. Obesity. 2015;23:1303–1311. doi: 10.1002/oby.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leonard K.S., Adams E.L., Savage J.S., Paul I.M., Kraschnewski J.L., Pattison K.L., Kjerulff K.H., Symons Downs D. Influence of prenatal perceived stress on postpartum weight retention is mediated by high gestational weight gain in women with overweight. Clin. Obes. 2021;11:e12446. doi: 10.1111/cob.12446. [DOI] [PubMed] [Google Scholar]
- 66.Pedersen P., Baker J.L., Henriksen T.B., Lissner L., Heitmann B.L., Sørensen T.I.A., Nohr E.A. Influence of psychosocial factors on postpartum weight retention. Obesity. 2011;19:639–646. doi: 10.1038/oby.2010.175. [DOI] [PubMed] [Google Scholar]
- 67.Mello J.A., Gans K.M., Risica P.M., Kirtania U., Strolla L.O., Fournier L. How is food insecurity associated with dietary behaviors? An analysis with low-income, ethnically diverse participants in a nutrition intervention study. J. Am. Diet. Assoc. 2010;110:1906–1911. doi: 10.1016/j.jada.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Dataset will be available on reasonable request from the authors.