Abstract
We report an efficient and practical iron-catalyzed hydrogen atom transfer protocol for assembling acetylenic motifs into functional alkenes. Diversities of internal alkynes could be obtained from readily available alkenes and acetylenic sulfones with excellent Markovnikov selectivity. An iron hydride hydrogen atom transfer catalytic cycle was described to clarify the mechanism of this reaction.
Keywords: alkynylation, iron-catalysis, alkenes, hydrogen atom transfer, radical
1. Introduction
Alkyne and its derivatives are important structural cores in diversities of bioactive compounds from natural products to pharmaceuticals and functional materials [1,2,3], which also serve as versatile synthetic building blocks in organic synthesis [4,5,6,7,8,9]. As a result, remarkable attention has been paid to the synthesis of these prime frameworks from versatile feedstocks. Straightforward nucleophilic or electrophilic alkynylation of nucleophilic acetylides generated utilizing strong bases relying on their intrinsic acidity or electrophilic acetylide variants prepared through complex routes were considered as traditional strategies to assemble the alkyne moieties onto the organic skeletons for the construction of C (sp3)–C (sp) bonds. Additionally, C (sp3)–C (sp) bond coupling reactions by the catalysis of transition metals serve as powerful methods for the construction of alkynes, wherein some appropriate ligands were employed to restrict the β-elimination of alkyl–metal complexes [10,11,12,13]. Recently, radical-mediated SOMOphilic alkynylation has made remarkable progress depending on the flourish development of radical chemistry, which also provides reliable approaches for the formation of C (sp3)–C (sp) bond. Moreover, diversities of alkyne reagents were designed and synthesized, providing alternative alkyne precursors to enable alkynyl functionalization [14,15,16,17,18,19,20,21,22,23,24,25,26]. Among these, acetylenic sulfones [22,23] exhibited vigorous synthetic abilities in organic transformations, especially forming C (sp3)–C (sp) bonds via a radical-induced process. Generally, acetylenic sulfones are usually treated as efficient radical acceptors, attached by the generated carbon radicals with excellent anti-Michael selectivity to afford enyl radical intermediates, achieving alkynyl functionalization with the realization of a sulfonyl radical via a sequential radical-mediated β-scission process (Scheme 1a). These reactions were amply explored by the efforts of organic chemical scientists (Scheme 1b). Chen [27] and König [28] developed photo-induced decarboxylative alkynylations of redox-active esters using acetylenic sulfones as alkynyl sources under reductive photochemical conditions, respectively. In 2016, Zhu and coworkers reported that the ring opening alkynylation of strained cyclobutanols could be enabled by oxygen radical-induced C–C bond cleavage by the catalysis of manganese salts [29]. In addition, another visible light-promoted oxygen radical-induced ring opening alkynylation via C–C bond cleavage was disclosed by the group of Wang [30]. Meanwhile, Fu and coworkers demonstrated that the alkynyl motifs from acetylenic sulfones could be introduced onto the aliphatic alcohol-derived redox-active esters, affording alkynes bearing quaternary carbons via a photo-induced C–O bond cleavage [31]. Additionally, aliphatic amine-derived Katritzky salts were employed by Gryko and coworkers to realize C–N bond alkynylation with acetylenic sulfones under metal-free photoredox catalytic conditions [32]. In 2019, Studer and coworkers utilized alkyl allyl sulfones as alkyl radical precursors to accomplish desulfonylative C (sp3)–C (sp) bond coupling initiated by 2, 2′-azobis (2-methylpropionitrile) (AIBN) [33]. Moreover, some alkanes or functionalized alkanes could be directly converted into internal alkynes with acetylenic sulfones via the diversity of the radical-mediated C–H bond alkynylation process [34,35,36,37,38,39]. Although remarkable achievements have been made in this research area, some of the reactions suffer from several limitations, such as the utilization of expensive catalysts or peroxides and the narrow scope of substrates and prolix procedures for the preparation of the radical precursors. It is highly desirable to establish a practical and efficient platform to afford C (sp3)–C (sp) bond coupling products from readily available substates in the presence of earth-abundant metal catalysts.
Scheme 1.
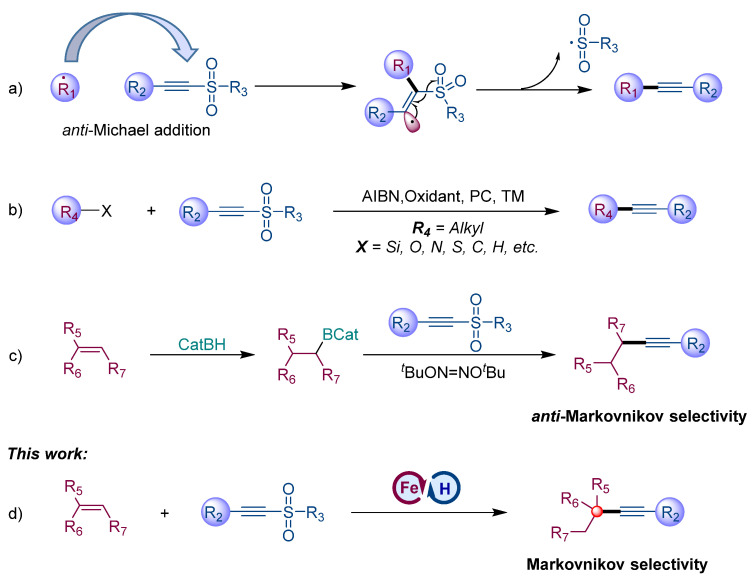
Strategies towards SOMOphilic alkynylation. (a) Radical-mediated alkynylation utilizing acetylenic sulfones as alkynyl sources; (b) SOMOphilic alkynylation via varieties of radical-mediated strategies; (c) anti-Markovnikov selective alkynalation of alkenes with acetylenic sulfones; (d) Iron-catalysed Markovnikov selective alkynalation.
Recently, metal (Fe, Co, Mn)-catalyzed hydrofunctionalization of alkenes has been established as an attractive and robust strategy for the construction of structural skeletons via the metal hydride hydrogen atom transfer (MHAT) process [40,41]. The alkenes interact with the metal hydride in situ generated from the metal catalyst with hydrogen sources to form carbon radical species, which were involved in varieties of chemical bonds formation such as C–H [42,43,44], C–C [45,46,47,48,49,50,51,52,53], C–O [54,55,56,57,58], C–S [59,60], C–N [61,62,63,64,65], and C–F [66,67] bond coupling. However, hydrogen atom transfer-triggered the hydrofunctionalization of alkenes, leading to internal alkynes using acetylenic sulfones as alkyne source was less explored. In 2006, Renaud and coworkers disclosed a radical-mediated alkynylation of alkenes to yield internal alkynes under the initiation of di-tert-butylhyponitrite, wherein the in situ hydroboration of the alkenes contributed to the excellent anti-Markovnikov selectivity (Scheme 1c) [68]. Herein, we developed an iron-catalyzed strategy to synthesize the internal alkynes with Markovnikov selectivity from readily available alkenes via a MHAT process (Scheme 1d).
2. Results
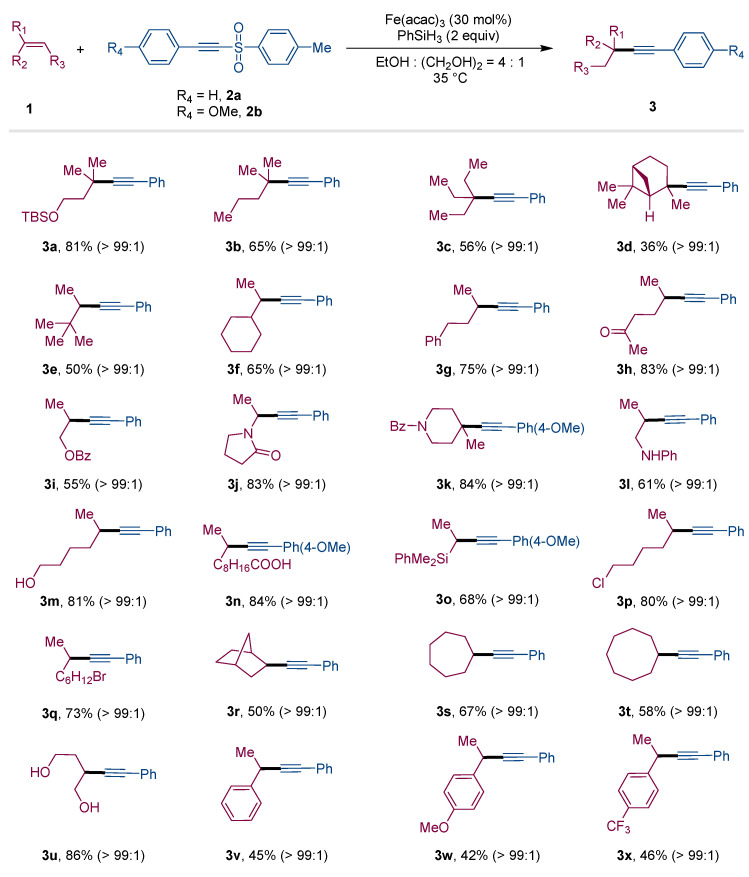
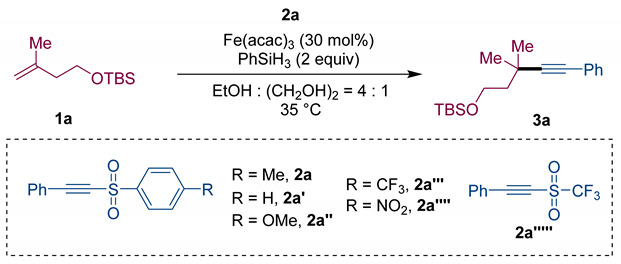
To start our investigation, we probed the reaction employing alkene 1a (0.3 mmol) and acetylenic sulfone 2a (0.2 mmol) as model substrates in the presence of Fe(acac)3 (30 mol%), PhSiH3 (2.0 equiv) in a mixed solvent. As expected, the desired internal alkyne 3a bearing a quaternary carbon center could be obtained with 81% yield (Table 1, Entry 1). Some other acetylenic sulfones 2a′–2a′′′′′ were investigated, and worse results were obtained (Table 1, Entries 2–Entries 6). Additionally, only 62% yield of 3a was generated if the reaction was operated in EtOH without the addition of (CH2OH)2, which showed that (CH2OH)2 played an irreplaceable role contributing to the high efficiency of the transformation (Table 1, Entry 7), because it could suppress the formation of PhSi(OEt)3 [46]. Additionally, the yield of desired product 3a was reduced to 60% with the amount of Fe(acac)3 decreasing to 20 mol% (Table 1, Entry 8). After screening of other catalysts including In(acac)3, Co(acac)3 and FeCl3, it was shown that In(acac)3 and Co(acac)3 were completely ineffective and FeCl3 was of modest efficiency, resulting in the alkyne product 3a with a 45% yield (Table 1, Entries 9–Entries 11). Notably, an apparent decrease in the yield was observed when alkene 1a (0.2 mmol) and acetylenic sulfone 2a (0.3 mmol) participated in the reaction (Table 1, Entry 12).
Table 1.
Optimization of SOMOphilic alkynylation of alkenes a.
| Entry | Variation from the “Standard Conditions” | Yield (%) b |
|---|---|---|
| Entry 1 | none | 85 (81) c |
| Entry 2 | 2a′ instead of 2a | 80 (72) c |
| Entry 3 | 2a″ instead of 2a | 71 |
| Entry 4 | 2a‴ instead of 2a | 49 |
| Entry 5 | 2a⁗ instead of 2a | ND |
| Entry 6 | 2a′′′′′ instead of 2a | ND |
| Entry 7 | Only EtOH instead of EtOH and (CH2OH)2 | 62 |
| Entry 8 | Fe(acac)3 (20 mol%) instead of Fe(acac)3 (30 mol%) | 60 |
| Entry 9 | In(acac)3 instead of Fe(acac)3 | ND |
| Entry 10 | Co(acac)3 instead of Fe(acac)3 | ND |
| Entry 11 | FeCl3 instead of Fe(acac)3 | 45 |
| Entry 12 | 1a (0.2 mmol), 2a (0.3 mmol) | 65 |
a Standard conditions: 1a (0.3 mmol, 1.5 equiv), 2a (0.2 mmol, 1.0 equiv), Fe(acac)3 (30 mol%), PhSiH3 (0.4 mmol, 2.0 equiv), in EtOH (0.8 mL) and (CH2OH)2 (0.2 mL) at 35 °C for 12 h. b Determined by GC-MS using dodecane as the internal standard. c Isolated yield in parentheses.
With the optimal conditions in hand, we then examined the scope of iron-catalyzed SOMOphilic alkynylation, keeping 2a and 2b as radical acceptors, which is presented in Figure 1. These simple and mild conditions turned out to be compatible with a wide range of alkenes with exquisite functional group tolerance. β-methyl alkenes were investigated as suitable substrates to react with 2a, affording the substituted alkynes 3a–3d bearing quaternary carbons in modest to good yields. Moreover, alkenes bearing bulky groups also worked well to provide the corresponding alkynes 3e–3f in satisfactory yields. Since this reaction’s conditions were gentle, alkenes bearing a wide of functional groups such as phenyl (1g), carbonyl (1h), ester (1i), amide (1j, 1k), amine (1l), hydroxyl (1m), carboxyl (1n), silicon (1o) groups underwent the MHAT-promoted alkynylation in 55% to 84% yields. Notably, although the reactions were operated in mixed alcohols, the alkenes bearing halide atoms performed well, generating desired alkynes 3p–3q in good yields, which could be applied for the further transformations. In addition, the reactions of internal alkenes with alkyne reagent 2a were operated smoothly, leading to the formation of the alkynylation products 3r–3u in 50% to 86% yields. The styrene derivatives could also be treated as suitable candidates under the optimized conditions to provide the alkynes 3v–3x in medium yields with excellent selectivity.
Figure 1.
Substrate scope of alkenes a. a Standard conditions: 1 (0.3 mmol, 1.5 equiv), 2a or 2b (0.2 mmol, 1.0 equiv), Fe(acac)3 (30 mol%), PhSiH3 (0.4 mmol, 2.0 equiv), in EtOH (0.8 mL) and (CH2OH)2 (0.2 mL) at 35 °C for 12 h, isolated yields.
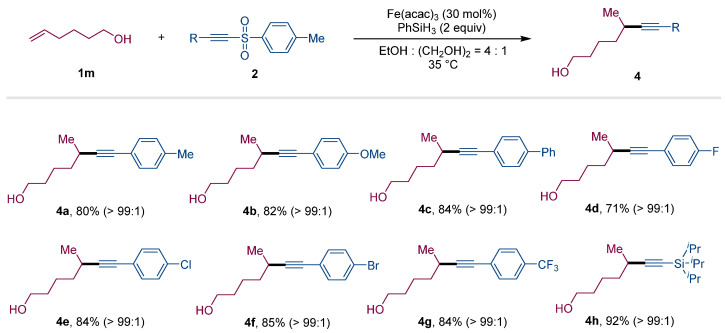
Encouraged by the results of variable alkenes, we continued to investigate the scope of alkyne sources utilizing alkene 1m as a radical precursor under the optimal conditions. Diversities of acetylenic sulfones were prepared and participated in the reaction system. As shown in Figure 2, the electron-donating groups, electron-withdrawing groups and halide atoms on the phenyl rings were tolerated. As examples, acetylenic sulfones with methyl, methoxyl, phenyl, fluoro, chloro, bromo and trifluoromethyl groups engage in the reactions, yielding the corresponding products 4a–4g in 71% to 85% yields. Importantly, triisopropylsilacetylene-derived sulfone demonstrated an excellent performance, yielding the product 4h with a 92% yield, which could be converted into the terminal alkyne under desiliconization conditions.
Figure 2.
Substrate scope of acetylenic sulfones a. a Standard conditions: 1m (0.3 mmol, 1.5 equiv), 2 (0.2 mmol, 1.0 equiv), Fe(acac)3 (30 mol%), PhSiH3 (0.4 mmol, 2.0 equiv), in EtOH (0.8 mL) and (CH2OH)2 (0.2 mL) at 35 °C for 12 h, isolated yields.
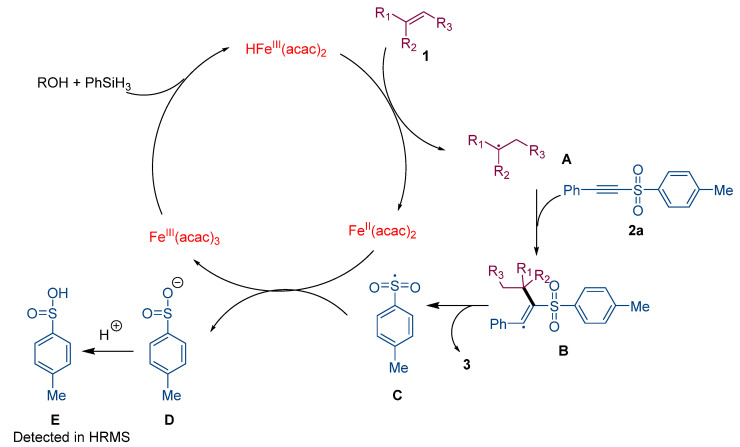
A tentative mechanism of this SOMOphilic alkynylation of alkenes is depicted in Scheme 2 according to the reported iron-catalyzed hydrofunctionalizations of alkenes via the MHAT process [40] and radical-mediated alkynylation [68]. Initially, FeIII(acac)3 was converted into the HFeIII(acac)2 species with the interaction of PhSiH3 in alcohol. Then, MHAT occurred between HFeIII(acac)2 and non-activated alkenes 1, acting as a rate-determining step [69], affording carbon-centered radical A with excellent Markovnikov selectivity as well as FeII(acac)2. Subsequently, anti-Michael addition of A onto acetylenic sulfone 2a generated enyl radical intermediates B, followed by the radical-mediated desulfonation to afford the desired alkyne products 3 with a release of sulfonyl radical, achieving the alkynyl functionalization with the realization of sulfonyl radical C. Finally, the sulfonyl radical C oxidized FeII(acac)2 to FeIII(acac)3 to fulfill the catalytic cycle, generating a sulfinic acid E [70], which was detected in HRMS (Figure S1 in Supplementary Materials).
Scheme 2.
Plausible mechanism.
3. Discussion
In conclusion, we developed an iron-catalyzed SOMOphilic alkynylation of non-activated alkenes with acetylenic sulfone with Markovnikov selectivity. A wide range of secondary and tertiary alkynes bearing variable functional and sensitive groups could be obtained from readily available and easily prepared starting materials by this efficient and mild MHAT strategy. Additional applications in the synthesis and modification of complex molecules or bioactive compounds are under investigation in our laboratory.
4. Materials and Methods
4.1. General Information
Unless otherwise noted, all reactions were performed under an argon atmosphere using flame-dried glassware. All new compounds were fully characterized. NMR-spectra were recorded on Bruker ARX-400 MHz or ARX-600 Associated. 1H NMR spectra data were reported as δ values in ppm relative to chloroform (δ 7.26) if collected in CDCl3. 13C NMR spectra data were reported as δ values in ppm relative to chloroform (δ 77.00). 1H NMR coupling constants were reported in Hz, and multiplicity was indicated as follows: s (singlet); d (doublet); t (triplet); q (quartet); quint (quintet); m (multiplet); dd (doublet of doublets); ddd (doublet of doublet of doublets); dddd (doublet of doublet of doublet of doublets); dt (doublet of triplets); td (triplet of doublets); ddt (doublet of doublet of triplets); dq (doublet of quartets); app (apparent); and br (broad). Mass spectra were obtained using a Micromass Q-Tof instrument (ESI) and Agilent Technologies 5973N (EI). All reactions were carried out in flame-dried 25 mL Schlenk tubes with Teflon screw caps under an argon atmosphere. Unless otherwise noted, materials obtained from commercial suppliers were used without further purification. Acetylenic sulfones 2 were prepared according to the reported procedures [29,71].
4.2. General Procedures of Iron-Catalyzed SOMOphilic Alkynylation
Flame-dried 10 mL Schlenk tube filled with N2, acetylenic sulfones 2 (0.2 mmol, 1.0 equiv) and Fe(acac)3 (21.2 mg, 0.06 mmol, 30 mol%) were added under N2, evacuated and purged with N2 three times. Afterwards, PhSiH3 (43.2 mg, 0.4 mmol, 2 equiv), non-activted alkenes 1 (33.1 mg, 0.3 mmol, 1.5 equiv) and ethanol (0.8 mL) and ethylene glycol (0.2 mL) were added via syringe. The formed mixture was stirred at 35 °C under N2 for 12 h, as monitored by TLC. The solution was then cooled to room temperature, and the solution was diluted with ethyl acetate and transferred to a round bottom flask. The concentrated residue was purified by column chromatography using ethyl acetate / petroleum ether as an eluent to afford the corresponding products and 3 and 4.
4.3. Characterization Data for Products
tert-Butyl((3,3-dimethyl-5-phenylpent-4-yn-1-yl)oxy)dimethylsilane (3a). Colorless oil (48.9 mg, 81%): 1H NMR (500 MHz, CDCl3) δ 7.39–7.36 (m, 2H), 7.29–7.25 (m, 3H), 3.90 (t, J = 7.4 Hz, 2H), 1.77 (t, J = 7.4 Hz, 2H), 1.31 (s, 6H), 0.91 (s, 9H), 0.09 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 131.5, 128.1, 127.4, 123.9, 96.6, 80.6, 61.0, 45.6, 30.3, 29.8, 26.0, 18.3, −5.2; HRMS m/z (ESI) calcd for C19H30NaOSi (M + Na)+ 325.1958, found 325.1960.
(3, 3-Dimethylhex-1-yn-1-yl)benzene (3b). Colorless oil (24.2 mg, 65%): 1H NMR (400 MHz, CDCl3) δ 7.39–7.36 (m, 2H), 7.27–7.24 (m, 3H), 1.55–1.42 (m, 2H), 1.47–1.42 (m, 2H), 1.27 (s, 6H), 0.95 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 131.5, 128.1, 127.3, 124.2, 97.6, 80.2, 45.9, 31.7, 29.3, 18.7, 14.6; HRMS m/z (ESI) calcd for C14H18Na (M + Na)+ 209.1301, found 209.1305.
(3,3-Diethylpent-1-yn-1-yl)benzene (3c). Colorless oil (22.4 mg, 56%): 1H NMR (400 MHz, CDCl3) δ 7.41–7.38 (m, 2H), 7.29–7.26 (m, 3H), 1.53 (q, J = 7.5 Hz, 6H), 0.97 (t, J = 7.5 Hz, 9H); 13C NMR (101 MHz, CDCl3) δ 131.6, 128.1, 127.2, 124.4, 96.0, 82.23, 39.9, 29.8, 8.8; HRMS m/z (ESI) calcd for C15H21 (M + H)+ 201.1638, found 201.1639.
(1S,5R)-2,6,6-Trimethyl-2-(phenylethynyl)bicyclo[3.1.1]heptane (3d). Colorless oil (17.1 mg, 36%): 1H NMR (400 MHz, CDCl3) δ 7.39–7.36 (m, 2H), 7.26–7.23 (m, 3H), 5.41 (d, J = 4.5 Hz, 1H), 2.17 (d, J = 18.8 Hz, 1H), 2.03–1.96 (m, 4H), 1.66 (d, J = 1.9 Hz, 3H), 1.48–1.42 (m, 2H), 1.30 (s, 3H), 1.26 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 131.6, 128.1, 127.3, 120.8, 96.6, 81.0, 43.9, 34.7, 31.0, 29.7, 27.5, 27.3, 27.0, 24.8, 23.3; HRMS m/z (ESI) calcd for C18H23 (M + H)+ 239.1794, found 239.1795.
(3,4,4-Trimethylpent-1-yn-1-yl)benzene (3e). Colorless oil (18.5 mg, 50%): 1H NMR (400 MHz, CDCl3) δ 7.41–7.38 (m, 2H), 7.28–7.26 (s, 3H), 2.45 (q, J = 7.1 Hz, 1H), 1.20 (d, J = 7.1 Hz, 3H), 1.02 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 131.5, 128.1, 127.3, 124.3, 93.9, 91.8, 37.8, 33.6, 27.3, 15.9; HRMS m/z (ESI) calcd for C14H18 Na (M + Na)+ 209.1301, found 209.1303.
(3-Cyclohexylbut-1-yn-1-yl)benzene (3f). Colorless oil (27.7 mg, 65%): 1H NMR (400 MHz, CDCl3) δ 7.41–7.39 (m, 2H), 7.29–7.26 (m, 3H), 2.53–2.50 (m, 1H), 1.95–1.90 (m, 1H), 1.81–1.74 (m, 3H), 1.69–1.65 (m, 1H), 1.36–1.13 (m, 9H); 13C NMR (101 MHz, CDCl3) δ 131.5, 128.1, 127.3, 124.2, 93.9, 81.5, 42.9, 32.5, 31.1, 29.5, 26.48, 26.45, 26.4, 18.3; HRMS m/z (ESI) calcd for C16H21 (M + H)+ 213.1638, found 213.1643.
(3-Methylpent-1-yne-1,5-diyl)dibenzene (3g). Colorless oil (35.3 mg, 75%): 1H NMR (400 MHz, CDCl3) δ 7.46– 7.43 (m, 2H), 7.32– 7.29 (m, 5H), 7.26–7.18 (m, 3H), 2.94–2.87 (m, 1H), 2.84–2.76 (m, 1H), 2.71–2.63 (m, 1H), 1.90–1.79 (m, 2H), 1.30 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 142.1, 131.6, 128.5, 128.3, 128.2, 127.5, 125.8, 124.0, 94.2, 81.3, 38.7, 33.7, 26.0, 21.1; HRMS m/z (ESI) calcd for C18H19 (M + H)+ 235.1481, found 235.1482.
5-Methyl-7-phenylhept-6-yn-2-one (3h). Colorless oil (33.0 mg, 83%): 1H NMR (400 MHz, CDCl3) δ 7.41–7.36 (m, 2H), 7.30–7.27 (m, 3H), 2.74–2.60 (m, 3H), 2.18 (s, 3H), 1.87–1.83 (m, 1H), 1.75–1.68 (m, 1H), 1.27 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.7, 131.5, 128.2, 127.7, 123.7, 93.4, 81.5, 41.5, 30.6, 30.1, 26.0, 21.1; HRMS m/z (ESI) calcd for C14H17O (M + H)+ 201.1274, found 201.1275.
2-Methyl-4-phenylbut-3-yn-1-yl benzoate (3i). Colorless oil (28.9 mg, 55%): 1H NMR (400 MHz, CDCl3) δ 8.11–8.09 (m, 2H), 7.59–7.56 (m, 1H), 7.46 (t, J = 7.6 Hz, 2H), 7.42–7.39 (m, 2H), 7.28 (q, J = 3.2, 2.7 Hz, 3H), 4.46–4.43 (m, 1H), 4.35–4.32 (m, 1H), 3.17 (q, J = 6.8 Hz, 1H), 1.39 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 166.4, 133.0, 131.6, 130.1, 129.6, 128.3, 128.2, 127.9, 123.3, 90.3, 81.9, 67.9, 26.7, 17.7; HRMS m/z (ESI) calcd for C18H17O2 (M + H)+ 265.1223, found 265.1225.
1-(4-Phenylbut-3-yn-2-yl)pyrrolidin-2-one (3j). Yellow oil (30.2 mg, 71%): 1H NMR (400 MHz, CDCl3) δ 7.42–7.39 (m, 2H), 7.31–7.29 (m, 3H), 5.30 (q, J = 7.1 Hz, 1H), 3.66–3.60 (m, 1H),3.49–3.43 (m, 1H), 2.44–2.40 (m,2H), 2.08–2.04 (m, 2H), 1.44 (d, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 173.9, 131.7, 128.3, 128.2, 122.6, 87.3, 83.5, 42.8, 39.3, 31.2, 19.8, 17.7; HRMS m/z (ESI) calcd for C14H16NO (M + H)+ 214.1226, found 214.1230.
(4-((4-Methoxyphenyl)ethynyl)-4-methylpiperidin-1-yl)(phenyl)methanone (3k). Colorless oil (56.1 mg, 84%): 1H NMR (400 MHz, CDCl3) δ 7.41–7.38 (m, 5H), 7.36–7.32 (m, 2H), 6.84–6.81 (m, 2H), 4.65 (d, J = 13.4 Hz, 1H), 3.80 (s, 1H), 3.67–3.65 (m, 1H), 3.45 (t, J = 13.1 Hz,1H), 3.24 (d, J = 13.3 Hz, 1H), 1.89 (d, J = 13.2 Hz,1H), 1.70–1.69 (m, 1H), 1.55 (s, 1H), 1.43–1.39 (m, 1H), 1.35 (s,3H); 13C NMR (101 MHz, CDCl3) δ 170.3, 159.3, 136.3, 132.9, 129.5, 128.4, 126.9, 115.4, 113.9, 92.0, 83.2, 55.3, 45.5, 39.8, 39.3, 38.4, 32.3, 29.6; HRMS m/z (ESI) calcd for C22H24NO2 (M + H)+ 334.1802, found 334.1803.
N-(2-methyl-4-phenylbut-3-yn-1-yl)aniline (3l). Colorless oil (28.7 mg, 61%): 1H NMR (400 MHz, CDCl3) δ 7.44–7.29 (m, 5H), 7.23–7.18 (m, 2H), 6.74–6.67 (m, 3H), 4.05 (br s, 1H), 3.32–3.22 (m, 2H), 3.05–3.00 (m, 1H), 1.34 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 147.9, 131.6, 129.3, 128.2, 127.8, 123.4, 117.6, 113.1, 92.0, 82.2, 49.5, 26.8, 18.7; HRMS m/z (ESI) calcd for C17H18N (M + H)+ 236.1434, found 236.1435.
5-Methyl-7-phenylhept-6-yn-1-ol (3m). Colorless oil (32.9 mg, 81%): 1H NMR (400 MHz, CDCl3) δ 7.40–7.38 (m, 2H), 7.29–7.26 (m, 3H), 3.69–3.66 (m, 2H), 2.68–2.63 (m, 1H), 1.63–1.61 (m, 2H), 1.59–1.50 (m, 4H), 1.26 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 131.5, 128.1, 127.5, 124.0, 94.5, 80.8, 62.9, 36.7, 32.6, 26.5, 23.62, 21.1; HRMS m/z (ESI) calcd for C14H19O (M + H)+ 203.1430, found 203.1433.
12-(4-Methoxyphenyl)-10-methyldodec-11-ynoic acid (3n). Colorless oil (52.4 mg, 84%): 1H NMR (400 MHz, CDCl3) δ 7.34–7.30 (m, 2H), 6.82–6.79 (m, 2H), 3.79 (s, 3H), 2.65–2.57 (m, 1H), 2.34 (t, J = 7.5 Hz, 2H), 1.65–1.59 (m, 2H), 1.53–1.41 (m, 4H), 1.32 (s, 8H), 1.23 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 179.6, 158.9, 132.8, 116.3, 113.7, 93.2, 80.3, 55.2, 37.1, 34.0, 29.4, 29.3, 29.2, 29.0, 27.4, 26.5, 24.7, 21.2; HRMS m/z (ESI) calcd for C20H29O3 (M + H)+ 317.2111, found 317.2115.
(4-(4-Methoxyphenyl)but-3-yn-2-yl)dimethyl(phenyl)silane (3o). Colorless oil (40.1 mg, 68%): 1H NMR (400 MHz, CDCl3) δ 7.63–7.60 (m, 2H), 7.40–7.36 (m, 3H), 7.30–7.27 (m, 2H), 6.81 (d, J = 8.9 Hz, 2H), 3.80 (s, 3H), 2.10 (q, J = 7.2 Hz, 1H), 1.22 (d, J = 7.3 Hz, 3H), 0.43 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 158.7, 136.8, 134.1, 132.7, 129.3, 127.7, 116.9, 113.7, 91.9, 80.3, 55.2, 15.0, 13.5, -4.7, -5.4; HRMS m/z (ESI) calcd for C19H22NaOSi (M + Na)+ 317.1332, found 317.1332.
(7-Chloro-3-methylhept-1-yn-1-yl)benzene (3p). Colorless oil (35.1 mg, 80%): 1H NMR (400 MHz, CDCl3) δ 7.40–7.38 (m, 2H), 7.28–7.26 (m, 3H), 3.57 (t, J = 6.7 Hz, 2H), 2.7–2.62 (m, 1H), 1.86– 1.79 (m, 2H), 1.72–1.59 (m, 2H), 1.56–1.51 (m, 2H), 1.26 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 131.6, 128.2, 127.5, 123.9, 94.2, 81.0, 45.0, 36.2, 32.4, 26.4, 24.8, 21.1; HRMS m/z (ESI) calcd for C14H18Cl (M + H)+ 221.1092, found 221.1093.
(9-Bromo-3-methylnon-1-yn-1-yl)benzene (3q). Colorless oil (42.5 mg, 73%): 1H NMR (400 MHz, CDCl3) δ 7.41–7.39 (m, 2H), 7.29–7.27 (m, 3H), 3.42 (t, J = 6.8 Hz, 2H), 2.69–2.60 (m, 1H), 2.91–2.84 (m, 2H), 1.54–1.44 (m, 6H), 1.42–1.34 (m, 2H), 1.26 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 131.5, 128.1, 127.4, 124.0, 94.6, 80.8, 36.8, 34.0, 32.8, 28.6, 28.1, 27.2, 26.5, 21.1; HRMS m/z (ESI) calcd for C16H22Br (M + H)+ 293.0899, found 293.0902.
(1S,4R)-2-(Phenylethynyl)bicyclo[2.2.1]heptane (3r). Colorless oil (19.4 mg, 50%): 1H NMR (400 MHz, CDCl3) δ 7.42–7.35 (m, 2H), 7.29–7.23 (m, 3H), 2.47–2.45 (m, 1H), 2.41 (d, J = 3.7 Hz, 1H), 2.31 (d, J = 4.3 Hz, 1H), 1.72–1.65 (m, 2H), 1.56–1.47 (m, 2H), 1.27–1.16 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 131.5, 128.1, 127.3, 124.2, 95.7, 80.1, 43.7, 39.4, 36.7, 36.2, 33.6, 28.81, 28.79; HRMS m/z (ESI) calcd for C15H17 (M + H)+ 197.1325, found 197.1328.
(Phenylethynyl)cycloheptane (3s). Colorless oil (26.7 mg, 67%): 1H NMR (400 MHz, CDCl3) δ 7.41–1.38 (m, 2H), 7.30–7.24 (m, 3H), 2.84–2.78 (m, 1H), 1.95–1.87 (m, 2H), 1.81–1.72 (m, 4H), 1.64–1.48 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 131.5, 128.1, 127.3, 124.2, 95.2, 80.8, 34.7, 31.7, 27.9, 25.6; HRMS m/z (ESI) calcd for C15H18Na (M + Na)+ 221.1301, found 221.1302.
(Phenylethynyl)cyclooctane (3t). Colorless oil (24.5 mg, 58%): 1H NMR (400 MHz, CDCl3) δ 7.40–7.37 (m, 2H), 7.27–7.26 (m, 3H), 2.82–2.76 (m, 1H), 1.98–1.91 (m, 2H), 1.81– 1.72 (m, 4H), 1.56–1.53 (m, 8H); 13C NMR (101 MHz, CDCl3) δ 131.5, 128.1, 127.3, 124.2, 31.6, 30.7, 29.7, 27.4, 25.4, 24.5; HRMS m/z (ESI) calcd for C16H21 (M + H)+ 213.1638, found 213.1641.
2-(Phenylethynyl)butane-1,4-diol (3u). Colorless oil (32.7 mg, 86%): 1H NMR (400 MHz, CDCl3) δ 7.42–7.40 (m, 2H), 7.30–7.28 (m, 3H), 3.95–3.90 (m, 1H), 3.87–3.84 (m, 1H), 3.77–3.72 (m, 2H), 3.03–3.00 (m, 1H), 2.47 (br s, 2H), 1.94–1.84 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 131.7, 128.3, 128.1, 123.0, 89.1, 83.8, 65.4, 60.6, 34.5, 33.2; HRMS m/z (ESI) calcd for C12H15O2 (M + H)+ 191.1067, found 191.1068.
But-1-yne-1,3-diyldibenzene (3v). Colorless oil (18.5 mg, 45%): 1H NMR (400 MHz, CDCl3) δ 7.47–7.44 (m, 5H), 7.36–7.33 (m, 2H), 7.30–7.28 (m, 3H), 3.99 (q, J = 7.2 Hz, 1H), 1.59 (d, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 131.6, 130.2, 128.5, 128.2, 127.7, 126.9, 126.6, 123.7, 92.6, 32.5, 24.5; HRMS m/z (ESI) calcd for C16H15 (M + H)+ 207.1168, found 207.1171.
1-Methoxy-4-(4-phenylbut-3-yn-2-yl)benzene (3w). Colorless oil (19.8 mg, 42%): 1H NMR (400 MHz, CDCl3) δ 7.47–7.43 (m, 2H), 7.38–7.36 (m, 2H), 7.32–7.28 (m, 3H), 6.91–6.87 (m, 2H), 3.95 (q, J = 7.1 Hz, 1H), 3.81 (s, 3H), 1.56 (d, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 158.3, 135.5, 131.6, 128.2, 127.9, 127.7, 123.8, 113.9, 92.9, 82.2, 55.3, 31.6, 24.6; HRMS m/z (ESI) calcd for C17H17O (M + H)+ 237.1274, found 237.1276.
1-(4-Phenylbut-3-yn-2-yl)-4-(trifluoromethyl)benzene (3x). Colorless oil (25.3 mg, 46%): 1H NMR (400 MHz, CDCl3) δ 7.62–7.56 (m, 4H), 7.46–7.43 (m, 2H), 7.33–7.29 (m, 2H), 4.04 (q, J = 7.1 Hz, 1H), 1.60 (d, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 147.3, 131.6, 128.3, 128.0, 127.3, 125.5 (q, J = 3.9 Hz), 123.3, 91.4, 83.1, 32.4, 24.3; 19F NMR (376 MHz, Chloroform-d) δ -62.4; HRMS m/z (ESI) calcd for C17H14F3 (M + H)+ 275.1042, found 275.1045.
5-Methyl-7-(p-tolyl)hept-6-yn-1-ol (4a). Colorless oil (34.6 mg, 80%): 1H NMR (400 MHz, CDCl3) δ 7.29–7.27 (m, 2H), 7.10–7.07 (m, 2H), 3.69–3.65 (m, 2H), 2.69–2.60 (m, 1H), 2.33 (s, 3H), 1.66–1.52 (m, 6H), 1.25 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 137.4, 131.4, 128.9, 120.9, 93.6, 80.9, 62.9, 36.78, 32.6, 26.5, 23.6, 21.4, 21.1; HRMS m/z (ESI) calcd for C15H21O (M + H)+ 217.1587, found 217.1589.
7-(4-Methoxyphenyl)-5-methylhept-6-yn-1-ol (4b). Colorless oil (38.2 mg, 82%): 1H NMR (400 MHz, CDCl3) δ 7.33–7.31 (m, 2H), 6.82–6.79 (m, 2H), 3.79 (s, 3H), 3.69–3.65 (m, 2H), 2.68–2.59 (m, 1H), 1.63–1.51 (m, 6H), 1.24 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.0, 132.9, 116.1, 113.8, 92.8, 80.5, 62.9, 55.2, 36.8, 32.6, 26.5, 23.6, 21.2; HRMS m/z (ESI) calcd for C15H20NaO2 (M + Na)+ 255.1356, found 255.1359.
7-([1,1′-Biphenyl]-4-yl)-5-methylhept-6-yn-1-ol (4c). Colorless oil (47.0 mg, 84%): 1H NMR (400 MHz, CDCl3) δ 7.60–7.57 (m, 2H), 7.54–7.51 (m, 2H), 7.48–7.42 (m, 2H), 7.37–7.34 (m, 1H), 3.71–3.67 (m, 2H), 2.73–2.65 (m, 1H), 1.66–1.56 (m, 6H), 1.28 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 140.5, 140.2, 131.9, 128.8, 127.4, 126.9, 126.8, 122.9, 95.2, 80.7, 62.9, 36.7, 32.6, 26.6, 23.7, 21.1; HRMS m/z (ESI) calcd for C20H23 (M + H)+ 279.1743, found 279.1745.
7-(4-Fluorophenyl)-5-methylhept-6-yn-1-ol (4d). Colorless oil (31.5 mg, 71%): 1H NMR (400 MHz, CDCl3) δ 7.37–7.34 (m, 2H), 6.98–6.95 (m, 2H), 3.67 (t, J = 6.0 Hz, 2H), 2.66–2.61 (m, 1H), 1.65–1.48 (m, 6H), 1.25 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 162.0 (d, J = 248.1 Hz), 133.3 (d, J = 8.1 Hz), 115.3 (d, J = 22.4 Hz), 94.1, 79.8, 62.9, 36.7, 32.5, 26.5, 23.6, 21.0; 19F NMR (376 MHz, CDCl3) δ -112.6; HRMS m/z (ESI) calcd for C14H18FO (M + H)+ 221.1336, found 221.1339.
7-(4-Chlorophenyl)-5-methylhept-6-yn-1-ol (4e). Colorless oil (39.9 mg, 84%): 1H NMR (400 MHz, CDCl3) δ 7.32–7.29 (m, 2H), 7.26–7.23 (m, 2H), 3.68–3.65 (m, 2H), 2.62–2.67 (m, 1H), 1.63–1.50 (m, 6H), 1.25 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 133.4, 132.8, 128.4, 122.5, 95.5, 79.8, 62.9, 36.6, 32.5, 26.5, 23.6, 21.0; HRMS m/z (ESI) calcd for C14H18ClO (M + H)+ 237.1041, found 237.1045.
7-(4-Bromophenyl)-5-methylhept-6-yn-1-ol (4f). Colorless oil (47.8 mg, 85%): 1H NMR (400 MHz, CDCl3) δ 7.41–7.39 (m, 2H), 7.26–7.23 (m, 2H), 3.68–3.65 (m, 2H), 2.68–2.59 (m, 1H), 1.65–1.52 (m, 6H), 1.24 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 133.0, 131.3, 122.9, 121.5, 95.7, 79.9, 62.9, 36.6, 32.5, 26.6, 23.6, 20.9; HRMS m/z (ESI) calcd for C14H18BrO (M + H)+ 281.0536, found 281.0538.
Methyl-7-(4-(trifluoromethyl)phenyl)hept-6-yn-1-ol (4g). Colorless oil (45.4 mg, 84%): 1H NMR (400 MHz, CDCl3) δ 7.29–7.27 (m, 2H), 7.10–7.07 (m, 2H), 3.69–3.65 (m, 2H), 2.69–2.60 (m, 1H), 2.33 (s, 3H), 1.66–1.52 (m,6H), 1.25 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 139.4, 131.8, 129.2 (q, J = 32.6 Hz), 125.1 (q, J = 3.7 Hz), 124.0 (q, J = 273.5 Hz), 97.3, 79.8, 62.9, 36.5, 32.5, 26.6, 23.6, 20.9; 19F NMR (376 MHz, CDCl3) δ -62.7; HRMS m/z (ESI) calcd for C15H17F3NaO (M + Na)+ 293.1124, found 293.1125.
5-Methyl-7-(triisopropylsilyl)hept-6-yn-1-ol (4h). Colorless oil (51.9 mg, 92%): 1H NMR (400 MHz, CDCl3) δ 3.64 (t, J = 6.3 Hz, 2H), 2.51–2.43 (m, 1H), 1.65–1.42 (m, 6H), 1.17 (d, J = 6.9 Hz, 3H), 1.09–1.00 (m, 21H); 13C NMR (101 MHz, CDCl3) δ 113.7, 79.8, 63.0, 36.7, 32.5, 26.9, 23.5, 21.3, 18.6, 11.3; HRMS m/z (ESI) calcd for C17H35OSi (M + H)+ 283.2452, found 283.2455.
Acknowledgments
We gratefully acknowledge the support of advanced analysis and testing centre of Nanjing Forestry University for NMR, GC-MS and HRMS.
Supplementary Materials
The following are available online. Figure S1: HRMS spectra of sulfinic acid E; 1H NMR, 13C NMR and 19F NMR spectra of starting materials and products.
Author Contributions
B.Z. and Z.S. conceived the concept, B.Z. directed the project, performed the experiments and wrote the paper, T.Z. performed the experiments, analyzed results, M.M. discussed the results. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (Grants 22102072, 22171137, 22025104, 21972064), Natural Science Foundation of Jiangsu Province (Grants BK20170632, BK20200765).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Diederich F., Stang P.J., Tykwinski R.R. Acetylene Chemistry. Wiley; New York, NY, USA: 2005. [Google Scholar]
- 2.Trost B.M., Li C.-J. Modern Alkyne Chemistry: Catalytic and Atom Economic Transformations. Wiley; New York, NY, USA: 2014. [Google Scholar]
- 3.Talele T.T. Acetylene group, friend or foe in medicinal chemistry. J. Med. Chem. 2020;63:5625. doi: 10.1021/acs.jmedchem.9b01617. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z., Jiang G., Xu Z., Zhao S., Liu W. Advances in alkynyl gold complexes for use as potential anticancer agents. Coord. Chem. Rev. 2020;423:213492. doi: 10.1016/j.ccr.2020.213492. [DOI] [Google Scholar]
- 5.Biyani S.A., Qi Q., Wu J., Moriuchi Y., Larocque E.A., Sintim H.O., Thompson D.H. Use of High-Throughput Tools for Telescoped Continuous Flow Synthesis of an Alkynylnaphthyridine Anticancer Agent, HSN608. Org. Process Res. Dev. 2020;24:2240. doi: 10.1021/acs.oprd.0c00289. [DOI] [Google Scholar]
- 6.Trotuş I.-T., Zimmermann T., Schüth F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 2014;114:1761–1782. doi: 10.1021/cr400357r. [DOI] [PubMed] [Google Scholar]
- 7.Boyarskiy V.P., Ryabukhin D.S., Bokach N.A., Vasilyev A.V. Alkenylation of Arenes and Heteroarenes with Alkynes. Chem. Rev. 2016;116:5894–5986. doi: 10.1021/acs.chemrev.5b00514. [DOI] [PubMed] [Google Scholar]
- 8.Haydl A.M., Breit B., Liang T., Krische M.J. Alkynes as Electrophilic or Nucleophilic Allylmetal Precursors in Transition Metal Catalysis. Angew. Chem. Int. Ed. 2017;56:11312–11325. doi: 10.1002/anie.201704248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iha R.K., Wooley K.L., Nystrom A.M., Burke D.J., Kade M.J., Hawker C.J. Applications of orthogonal “Click” chemistries in the synthesis of functional soft materials. Chem. Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckhardt M., Fu G.C. The First Applications of Carbene Ligands in Cross-Couplings of Alkyl Electrophiles: Sonogashira Reactions of Unactivated Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2003;125:13642–13643. doi: 10.1021/ja038177r. [DOI] [PubMed] [Google Scholar]
- 11.Altenhoff G., Wurtz S., Glorius F. The first palladium-catalyzed Sonogashira coupling of unactivated secondary alkyl bromides. Tetrahedron Lett. 2006;47:2925–2928. doi: 10.1016/j.tetlet.2006.02.111. [DOI] [Google Scholar]
- 12.Vechorkin O., Barmaz D., Proust V., Hu X.L. Ni-Catalyzed Sonogashira Coupling of Nonactivated Alkyl Halides: Orthogonal Functionalization of Alkyl Iodides, Bromides, and Chlorides. J. Am. Chem. Soc. 2009;131:12078–12079. doi: 10.1021/ja906040t. [DOI] [PubMed] [Google Scholar]
- 13.Yi J., Lu X., Sun Y.Y., Xiao B., Liu L. Nickel-Catalyzed Sonogashira Reactions of Non-activated Secondary Alkyl Bromides and Iodides. Angew. Chem. Int. Ed. 2013;52:12409–12413. doi: 10.1002/anie.201307069. [DOI] [PubMed] [Google Scholar]
- 14.Hari D.P., Caramenti P., Waser J. Cyclic hypervalent iodine reagents: Enabling tools for bond disconnection via reactivity umpolung. Acc. Chem. Res. 2018;51:3212–3225. doi: 10.1021/acs.accounts.8b00468. [DOI] [PubMed] [Google Scholar]
- 15.Huang H., Zhang G., Gong L., Zhang S., Chen Y. Visible-Light-Induced Chemoselective Deboronative Alkynylation under Biomolecule-Compatible Conditions. J. Am. Chem. Soc. 2014;136:2280–2283. doi: 10.1021/ja413208y. [DOI] [PubMed] [Google Scholar]
- 16.Le Vaillant F., Courant T., Waser J. Room-Temperature Decarboxylative Alkynylation of Carboxylic Acids Using Photoredox Catalysis and EBX Reagents. Angew. Chem. Int. Ed. 2015;54:11200–11204. doi: 10.1002/anie.201505111. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q.-Q., Guo W., Ding W., Wu X., Chen X., Lu L.-Q., Xiao W.-J. Decarboxylative Alkynylation and Carbonylative Alkynylation of Carboxylic Acids Enabled by Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2015;54:11196–11199. doi: 10.1002/anie.201504559. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Liu X., Jiang H., Liu B., Chen Z., Zhou P. Palladium-Catalyzed Bromoalkynylation of C-C Double Bonds: Ring-Structure-Dependent Synthesis of 7-Alkynyl Norbornanes and Cyclobutenyl Halides. Angew. Chem. Int. Ed. 2011;50:6341–6345. doi: 10.1002/anie.201100002. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y.-S., Xu Z.-Q., Mao L., Zhang F.-F., Xu H.-J. Copper Catalyzed Decarboxylative Alkynylation of Quaternary α-Cyano Acetate Salts. Org. Lett. 2013;15:1472–1475. doi: 10.1021/ol400197y. [DOI] [PubMed] [Google Scholar]
- 20.Sun F., Gu Z. Decarboxylative alkynyl termination of palladium-catalyzed catellani reaction: A facile synthesis of α–alkynyl anilines via ortho C–H amination and alkynylation. Org. Lett. 2015;17:2222–2225. doi: 10.1021/acs.orglett.5b00830. [DOI] [PubMed] [Google Scholar]
- 21.Jayaraman A., Lee S. Selective monoand dialkynylation of 1–fluoro-2, 2- diiodovinylarenes using Pd-catalyzed decarboxylative coupling reactions. Org. Lett. 2019;21:7923–7927. doi: 10.1021/acs.orglett.9b02907. [DOI] [PubMed] [Google Scholar]
- 22.Back T.G. The chemistry of acetylenic and allenic sulfones, Tetrahedron 2001, 57, 5263–5301. Tetrahedron. 2001;57:5263–5301. doi: 10.1016/S0040-4020(01)00299-X. [DOI] [Google Scholar]
- 23.Vaillant F.L., Waser J. Alkynylation of radicals: Spotlight on the “third way” to transfer triple bonds. Chem. Sci. 2019;10:8909–8923. doi: 10.1039/C9SC03033F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerrero-Robles M.A., Vilchis-Reyes M.A., Ramos-Rivera E.M., Alvarado C. Synthesis of alkynyl sulfones. ChemistrySelect. 2019;4:13698–13708. doi: 10.1002/slct.201903728. [DOI] [Google Scholar]
- 25.Ge D., Wang X., Chu X.-Q. SOMOphilic alkynylation using acetylenic sulfones as functional reagents. Org. Chem. Front. 2021;8:5145–5164. doi: 10.1039/D1QO00798J. [DOI] [Google Scholar]
- 26.Wu Z., Xu Y., Zhang H., Wu X., Zhu C. Radical-mediated sulfonyl alkynylation, allylation, and cyanation of propellane. Chem. Commun. 2021;57:6066–6069. doi: 10.1039/D1CC02249K. [DOI] [PubMed] [Google Scholar]
- 27.Yang J., Zhang J., Qi L., Hu C., Chen Y. Visible-light-induced chemoselective reductive decarboxylative alkynylation under biomolecule-compatible conditions. Chem. Commun. 2015;51:5275–5278. doi: 10.1039/C4CC06344A. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz J., König B. Decarboxylative Alkynylation of Biomass-Derived Compounds by Metal-Free Visible Light Photocatalysis. ChemPhotoChem. 2017;1:237–242. doi: 10.1002/cptc.201700034. [DOI] [Google Scholar]
- 29.Ren R., Wu Z., Xu Y., Zhu C. C–C Bond-Forming Strategy by Manganese-Catalyzed Oxidative Ring-Opening Cyanation and Ethynylation of Cyclobutanol Derivatives. Angew. Chem. Int. Ed. 2016;55:2866–2869. doi: 10.1002/anie.201510973. [DOI] [PubMed] [Google Scholar]
- 30.Shi J.-L., Wang Z., Zhang R., Wang Y., Wang J. Visible-Light-Promoted Ring-Opening Alkynylation, Alkenylation, and Allylation of Cyclic Hemiacetals through β-Scission of Alkoxy Radicals. Chem. Eur. J. 2019;25:8992–8995. doi: 10.1002/chem.201901762. [DOI] [PubMed] [Google Scholar]
- 31.Gao C., Li J., Yu J., Yang H., Fu H. Visible-light photoredox synthesis of internal alkynes containing quaternary carbons. Chem. Commun. 2016;52:7292–7294. doi: 10.1039/C6CC01632D. [DOI] [PubMed] [Google Scholar]
- 32.Ociepa M., Turkowska J., Gryko D. Redox-Activated Amines in C(sp3)−C(sp) and C(sp3)−C(sp2) Bond Formation Enabled by Metal-Free Photoredox Catalysis. ACS Catal. 2018;8:11362–11367. doi: 10.1021/acscatal.8b03437. [DOI] [Google Scholar]
- 33.Xia Y., Studer A. Diversity-Oriented Desulfonylative Functionalization of Alkyl Allyl Sulfones. Angew. Chem. Int. Ed. 2019;58:9836–9840. doi: 10.1002/anie.201903668. [DOI] [PubMed] [Google Scholar]
- 34.Guan H., Sun S., Mao Y., Chen L., Lu R., Huang J., Liu L. Iron(II)-Catalyzed Site-Selective Functionalization of Unactivated C(sp3)−H Bonds Guided by Alkoxyl Radicals. Angew. Chem. Int. Ed. 2018;57:11413–11417. doi: 10.1002/anie.201806434. [DOI] [PubMed] [Google Scholar]
- 35.Paul S., Guin J. Radical C(sp3)–H alkenylation, alkynylation and allylation of ethers and amides enabled by photocatalysis. Green Chem. 2017;19:2530–2534. doi: 10.1039/C7GC00840F. [DOI] [Google Scholar]
- 36.Guo A., Han J.-B., Zhu L., Wei Y., Tang X.-Y. Site-Selective α-Alkoxyl Alkynation of Alkyl Esters Mediated by Boryl Radicals. Org. Lett. 2019;21:2927–2931. doi: 10.1021/acs.orglett.9b00985. [DOI] [PubMed] [Google Scholar]
- 37.Yin Z., Zhang Y., Zhang S., Wu X.-F. Copper-Catalyzed Alkynylation of C(sp3)–H Bonds in N-Fluorosulfonamides. Adv. Synth. Catal. 2019;361:5478–5482. doi: 10.1002/adsc.201900992. [DOI] [Google Scholar]
- 38.Han J.-B., San H.H., Guo A., Wang L., Tang X.-Y. Boryl Radical-Mediated C–H Activation of Inactivated Alkanes for the Synthesis of Internal Alkynes. Adv. Synth. Catal. 2021;363:2366–2370. doi: 10.1002/adsc.202000772. [DOI] [Google Scholar]
- 39.Capaldo L., Ravelli D. Decatungstate as Direct Hydrogen Atom Transfer Photocatalyst for SOMOphilic Alkynylation. Org. Lett. 2021;23:2243–2247. doi: 10.1021/acs.orglett.1c00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crossley S.W.M., Obradors C., Martinez R.M., Shenvi R.A. Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chem. Rev. 2016;116:8912–9000. doi: 10.1021/acs.chemrev.6b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green S.A., Crossley S.W.M., Matos J.L.M., Vásquez-Céspedes S., Shevick S.L., Shenvi R.A. The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer. Acc. Chem. Res. 2018;51:2628–2640. doi: 10.1021/acs.accounts.8b00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obradors C., Martinez R.M., Shenvi R.A. Ph(i-PrO)SiH2: An Exceptional Reductant for Metal-Catalyzed Hydrogen Atom Transfers. J. Am. Chem. Soc. 2016;138:4962–4971. doi: 10.1021/jacs.6b02032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crossley S.W.M., Barabé F., Shenvi R.A. Simple, Chemoselective, Catalytic Olefin Isomerization. J. Am. Chem. Soc. 2014;136:16788–16791. doi: 10.1021/ja5105602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma X., Herzon S.B. Non-classical selectivities in the reduction of alkenes by cobalt-mediated hydrogen atom transfer. Chem. Sci. 2015;6:6250–6255. doi: 10.1039/C5SC02476E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo J.C., Gui J., Yabe Y., Pan C.-M., Baran P.S. Functionalized olefin cross-coupling to construct carbon–carbon bonds. Nature. 2014;516:343–348. doi: 10.1038/nature14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo J., Yabe Y., Baran P.S. A Practical and Catalytic Reductive Olefin Coupling. J. Am. Chem. Soc. 2014;136:1304–1307. doi: 10.1021/ja4117632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaspar B., Carreira E.M. Mild Cobalt-Catalyzed Hydrocyanation of Olefins with Tosyl Cyanide. Angew. Chem. Int. Ed. 2007;46:4519–4522. doi: 10.1002/anie.200700575. [DOI] [PubMed] [Google Scholar]
- 48.Lo J.C., Kim D., Pan C.-M., Edwards J.T., Yabe Y., Gui J., Qin T., Gutiérrez S., Giacoboni J., Smith M.W., et al. Fe-Catalyzed C−C Bond Construction from Olefins via Radicals. J. Am. Chem. Soc. 2017;139:2484–2503. doi: 10.1021/jacs.6b13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matos J.L.M., Green S.A., Chun Y., Dang V.Q., Dushin R.G., Richardson P., Chen J.S., Piotrowski D.W., Paegel B.M., Shenvi R.A. Cycloisomerization of Olefins in Water. Angew. Chem. Int. Ed. 2020;59:12998–13003. doi: 10.1002/anie.202003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y.-Y., Bode J.W. Olefin Amine (OLA) Reagents for the Synthesis of Bridged Bicyclic and Spirocyclic Saturated N-Heterocycles by Catalytic Hydrogen Atom Transfer (HAT) Reactions. J. Am. Chem. Soc. 2019;141:9739–9745. doi: 10.1021/jacs.9b05074. [DOI] [PubMed] [Google Scholar]
- 51.He S.-J., Wang J.-W., Li Y., Xu Z.-Y., Wang X.-X., Lu X., Fu Y. Nickel-Catalyzed Enantioconvergent Reductive Hydroalkylation of Olefins with α-Heteroatom Phosphorus or Sulfur Alkyl Electrophiles. J. Am. Chem. Soc. 2020;142:214–221. doi: 10.1021/jacs.9b09415. [DOI] [PubMed] [Google Scholar]
- 52.Wang J.-W., Li Y., Nie W., Chang Z., Yu Z.-A., Zhao Y.-F., Lu X., Fu Y. Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines. Nat. Commun. 2021;12:1313. doi: 10.1038/s41467-021-21600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Nie W., Chang Z., Wang J.-W., Lu X., Fu Y. Cobalt-catalysed enantioselective C(sp3)–C(sp3) coupling. Nat. Catal. 2021;4:901–911. doi: 10.1038/s41929-021-00688-w. [DOI] [Google Scholar]
- 54.Shigehisa H., Aoki T., Yamaguchi S., Shimizu N., Hiroya K. Hydroalkoxylation of Unactivated Olefins with Carbon Radicals and Carbocation Species as Key Intermediates. J. Am. Chem. Soc. 2013;135:10306–10309. doi: 10.1021/ja405219f. [DOI] [PubMed] [Google Scholar]
- 55.Liu B., Jin F., Wang T., Yuan X., Han W. Wacker-Type Oxidation Using an Iron Catalyst and Ambient Air: Application to Late-Stage Oxidation of Complex Molecules. Angew. Chem. Int. Ed. 2017;56:12712–12717. doi: 10.1002/anie.201707006. [DOI] [PubMed] [Google Scholar]
- 56.Touney E.E., Foy N.J., Pronin S.V. Catalytic Radical−Polar Crossover Reactions of Allylic Alcohols. J. Am. Chem. Soc. 2018;140:16982–16987. doi: 10.1021/jacs.8b12075. [DOI] [PubMed] [Google Scholar]
- 57.Ebisawa K., Izumi K., Ooka Y., Kato H., Kanazawa S., Komatsu S., Nishi E., Shigehisa H. Catalyst- and Silane-Controlled Enantioselective Hydrofunctionalization of Alkenes by Cobalt-Catalyzed Hydrogen Atom Transfer and Radical-Polar Crossover. J. Am. Chem. Soc. 2020;142:13481–13490. doi: 10.1021/jacs.0c05017. [DOI] [PubMed] [Google Scholar]
- 58.Puls F., Knölker H.-J. Conversion of Olefins into Ketones by an Iron-Catalyzed Wacker-type Oxidation Using Oxygen as the Sole Oxidant. Angew. Chem. Int. Ed. 2018;57:1222–1226. doi: 10.1002/anie.201710370. [DOI] [PubMed] [Google Scholar]
- 59.Girijavallabhan V., Alvarez C., Njoroge F.G. Regioselective Cobalt-Catalyzed Addition of Sulfides to Unactivated Alkenes. J. Org. Chem. 2011;76:6442–6446. doi: 10.1021/jo201016z. [DOI] [PubMed] [Google Scholar]
- 60.Date S., Hamasaki K., Sunagawa K., Koyama H., Sebe C., Hiroya K., Shigehisa H. Catalytic Direct Cyclization of Alkenyl Thioester. ACS Catal. 2020;10:2039–2045. doi: 10.1021/acscatal.9b05045. [DOI] [Google Scholar]
- 61.Gui J., Pan C.-M., Jin Y., Qin T., Lo J.C., Lee B.J., Spergel S.H., Mertzman M.E., Pitts W.J., La Cruz T.E., et al. Practical olefin hydroamination with nitroarenes. Science. 2015;348:886–891. doi: 10.1126/science.aab0245. [DOI] [PubMed] [Google Scholar]
- 62.Shigehisa H., Koseki N., Shimizu N., Fujisawa M., Niitsu M., Hiroya K. Catalytic Hydroamination of Unactivated Olefins Using a Co Catalyst for Complex Molecule Synthesis. J. Am. Chem. Soc. 2014;136:13534–13537. doi: 10.1021/ja507295u. [DOI] [PubMed] [Google Scholar]
- 63.Yin Y.-N., Ding R.-Q., Ouyang D.-C., Zhang Q., Zhu R. Highly chemoselective synthesis of hindered amides via cobalt-catalyzed intermolecular oxidative hydroamidation. Nat. Commun. 2021;12:2552. doi: 10.1038/s41467-021-22373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagai T., Mimata N., Terada Y., Sebe C., Shigehisa H. Catalytic Dealkylative Synthesis of Cyclic Carbamates and Ureas via Hydrogen Atom Transfer and Radical-Polar Crossover. Org. Lett. 2020;22:5522–5527. doi: 10.1021/acs.orglett.0c01872. [DOI] [PubMed] [Google Scholar]
- 65.Leggans E.K., Barker T.J., Duncan K.K., Boger D.L. Iron(III)/NaBH4-Mediated Additions to Unactivated Alkenes: Synthesis of Novel 200-Vinblastine Analogues. Org. Lett. 2012;14:1428–1431. doi: 10.1021/ol300173v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barker T.J., Boger D.L. Fe(III)/NaBH4-Mediated Free Radical Hydrofluorination of Unactivated Alkenes. J. Am. Chem. Soc. 2012;134:13588. doi: 10.1021/ja3063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie Y., Sun P.-W., Li Y., Wang S., Ye M., Li Z. Ligand-Promoted Iron(III)-Catalyzed Hydrofluorination of Alkenes. Angew. Chem. Int. Ed. 2019;58:7097–7101. doi: 10.1002/anie.201902607. [DOI] [PubMed] [Google Scholar]
- 68.Schaffner A.-P., Darmency V., Renaud P. Radical-Mediated Alkenylation, Alkynylation, Methanimination, and Cyanation of B-Alkylcatecholboranes. Angew. Chem. Int. Ed. 2006;45:5847–5849. doi: 10.1002/anie.200601206. [DOI] [PubMed] [Google Scholar]
- 69.Saladrigas M., Bosch C., Saborit G.V., Bonjoch J., Bradshaw B. Radical Cyclization of Alkene-Tethered Ketones Initiated by Hydrogen-Atom Transfer. Angew. Chem. Int. Ed. 2018;57:182–186. doi: 10.1002/anie.201709659. [DOI] [PubMed] [Google Scholar]
- 70.Dao H.T., Li C., Michaudel Q., Maxwell B.D., Baran P.S. Hydromethylation of Unactivated Olefins. J. Am. Chem. Soc. 2015;137:8046–8049. doi: 10.1021/jacs.5b05144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meesin J., Katrun P., Pareseecharoen C., Pohmakotr M., Reutrakul V., Soorukram D., Kuhakarn C. Iodine-catalyzed Sulfonylation of Arylacetylenic Acids and Arylacetylenes with Sodium Sulfinates: Synthesis of Arylacetylenic Sulfones. J. Org. Chem. 2016;81:2744–2752. doi: 10.1021/acs.joc.5b02810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.