Abstract
Recognition of a previously experienced item or object depends upon the successful retrieval of memory for the object. The neural mechanisms that support object recognition memory in the mammalian brain are not well understood. The rodent hippocampus plays a well-established role in spatial memory, and we previously demonstrated that temporary inactivation of the mouse hippocampus impairs object memory, as assessed with a novel object preference (NOP) test. The present studies were designed to test some remaining issues regarding the contribution of the CA1 sub-region of the mouse dorsal hippocampus to long-term object memory. Specifically, we examined whether the retrieval of spatial memory (as assessed by the Morris water maze; MWM) and object recognition memory are differentially sensitive to inactivation of the CA1 region. The current study used pre-test local microinfusion of muscimol directly into the CA1 region of dorsal hippocampus to temporarily interrupt its function during the respective retrieval phases of both behavioral tasks, in order to compare the contribution of the CA1 to object memory and spatial memory. Histological analyses revealed that local intra-CA1 injection of muscimol diffused within, and not beyond, the CA1 region of dorsal hippocampus. The degree of memory retrieval impairment induced by muscimol was comparable in the two tasks, supporting the view that object memory and spatial memory depend similarly on the CA1 region of rodent hippocampus. Further, we confirmed that the muscimol-induced impairment of CA1 function is temporary. First, mice that exhibited impaired object memory retrieval immediately after intra-CA1 muscimol, subsequently exhibited unimpaired retrieval of object memory when tested 24 h later. Secondly, a cohort of mice that exhibited impaired object memory retrieval after intra-CA1 muscimol later acquired spatial memory in the MWM comparable to that of control mice. Together, these results offer further support for the involvement of the CA1 region of mouse hippocampus in object recognition memory, and provide evidence to suggest that the NOP task is as much a test of hippocampal function as the classic MWM test.
Keywords: Object recognition, Morris water maze, Novel object preference, Hippocampus, Muscimol, Spatial memory
1. Introduction
In primates, episodic memory, memory for the what, when and where details of events (Tulving, 2002), is known to depend on the hippocampus (Eichenbaum, 2001; Nadel & Peterson, 2013; Squire, Wixted, & Clark, 2007). Likewise, there is behavioral evidence (Broadbent, Squire, & Clark, 2004; Clark, Zola, & Squire, 2000; Cohen et al., 2013; de Lima, Luft, Roesler, & Schroder, 2006;Gould et al., 2002) and physiological evidence (Beer, Chwiesko, Kitsukawa, & Sauvage, 2013; Cohen et al., 2013; Hampson, Simeral, & Deadwyler, 1999; Manns & Eichenbaum, 2009; Sauvage, Nakamura, & Beer, 2013; Wood, Dudchenko, & Eichenbaum, 1999) that the rodent hippocampus encodes and consolidates memory for information that is both spatial and non-spatial. Despite these findings, the role of the rodent hippocampus in object recognition memory remains unclear.
The spontaneous object recognition task, or novel object preference (NOP) task (Ennaceur & Delacour, 1988) has emerged as a principal tool for assessing rodent object memory (Leger et al., 2013). In the traditional version of the NOP task, a rodent is presented with two identical novel objects in a familiar arena during the sample session. Spontaneous exploration of the sample session objects presumably enables the rodent to encode a memory for the objects. After a delay, the rodent is returned to the arena, now containing one novel and one familiar object, each one occupying the same location where the previous objects had been, for the test session. Rodents’ affinity for novelty (Berlyne, 1950) results in preferential exploration of the novel object over the familiar object, indicating that a memory for the familiar object was successfully encoded and consolidated after the sample session. Among other advantages, the spontaneous nature of the NOP task enables testing without extensive training or manipulation of internal states to motivate performance.
Although there are many advantages to the NOP task, its use in studies investigating the contribution of the rodent hippocampus to object memory has frequently yielded conflicting findings – many have reported that NOP performance is spared after hippocampal lesion (Barker & Warburton, 2011; Forwood, Bartko, Saksida, & Bussey, 2007; Forwood, Winters, & Bussey, 2005; Mumby, Gaskin, Glenn, Schramek, & Lehmann, 2002; Winters, Forwood, Cowell, Saksida, & Bussey, 2004), while others report that NOP performance is impaired after temporary or permanent hippocampal lesion (Broadbent, Gaskin, Squire, & Clark, 2010; Broadbent et al., 2004; Clark et al., 2000; Cohen et al., 2013; Wood, Mumby, Pinel, & Phillips, 1993). Further, in cases where NOP performance is impaired following hippocampal lesion or inactivation, the effect appears to be delay-dependent. That is, NOP performance was impaired when delays longer than 10 min were imposed between encoding and retrieval, but was intact when shorter delays were imposed (Clark et al., 2000; Hammond, Tull, & Stackman, 2004). These results suggest that the hippocampus may be engaged in long-term object memory, while other regions may support a working memory-like process that facilitates NOP performance after short delays. Furthermore, Gaskin and colleagues (Gaskin, Tremblay, & Mumby, 2003) reported that hippocampal-lesioned rats were impaired in remembering objects explored preoperatively, but those same rats exhibited intact memory for objects explored postoperatively – even when delays of 24 h, 1 week or 3 weeks were imposed (Mumby, Tremblay, Lecluse, & Lehmann, 2005). This pattern of results suggests that in the absence of a functional hippocampus, lesion-induced compensation enables extra-hippocampal structures to support the encoding and storage of object representations (Mumby et al., 2005). Differences in the pattern of results from studies of NOP performance and its sensitivity to hippocampal manipulations likely relate to many factors: lesion size, lesion specificity, delay between sample and test, presence or absence of spatial or contextual components, and specific memory process(es) interrupted (for a review of these issues, see Cohen & Stackman, 2015). In contrast to the mixed reports of NOP performance after hippocampal lesion or inactivation, there is extensive evidence that permanent lesion, or temporary inactivation, of the perirhinal cortex consistently impairs object memory in rats (Winters, Bartko, Saksida, & Bussey, 2010; Winters & Bussey, 2005; Winters et al., 2004). The lasting impairment of NOP performance following permanent lesion of the perirhinal cortex indicates that any lesion-induced circuit plasticity is insufficient to recover the behavior, suggesting the prominent position of the perirhinal cortex in the neural circuit supporting object memory. In summarizing a controversial literature regarding the role of the hippocampus in object memory based on studies using the NOP task, the hippocampus appears to contribute to long-term memory for objects and object-in-context memory.
Moving beyond questions about the selective involvement of the hippocampus in object memory, it is of interest to define the contribution of select hippocampal sub-regions in discrete object memory processes. Mapping regional activation by expression of the immediate early gene Arc revealed that performance by mice in spatial and non-spatial object recognition tasks induced comparable activation of the CA1 region, and that the CA3 region was more strongly activated by the spatial task, while activation of the perirhinal cortex appeared to be selectively activated for features of the non-spatial task (i.e., stimulus or item type) (Beer et al., 2013). The distinct pattern of CA1 versus CA3 activation is consistent with evidence that the CA1 and CA3 contribute distinctly to memories for the sequence of non-spatial events (Farovik, Dupont, & Eichenbaum, 2010). We previously reported that temporary inactivation of the CA1 region of the dorsal hippocampus in mice via bilateral muscimol infusion before the sample session, or immediately after the sample session, abolished novel object preference during the NOP test session 24 h later (Cohen et al., 2013). These results are consistent with the view that the CA1 region of the mouse hippocampus is critical for the encoding and consolidation of memory for objects explored in a familiar context (i.e., object-in-context memory). Inactivation of the CA1 region also impaired the retrieval of a memory of objects independent of context (Cohen et al., 2013). Our findings were consistent with prior reports that rodent object recognition memory processes are impaired after a pre-sample session intra-CA1 infusion of APV (Baker & Kim, 2002), or lidocaine (Hammond et al., 2004); in particular when delays of 24 h are imposed between the sample and test sessions. Taken together, these results indicate that CA1 neuronal activity is essential for long-term object memory, and this perhaps suggests that the NOP task permits the assessment of a form of event memory that depends upon output from the hippocampus by way of the CA1 region. The consistency with which object memory impairments are observed after temporary CA1 inactivation is noteworthy given the conflicting results reported after permanent partial or complete lesions of the hippocampus. In contrast to the reported variability in effects of hippocampal compromise on object memory, spatial memory impairments are consistently observed in rodents after permanent or reversible lesions of the hippocampus (Brioni, Decker, Gamboa, Izquierdo, & McGaugh, 1990; Chrobak, Stackman, & Walsh, 1989; Lee & Kesner, 2004; Morris, Garrud, Rawlins, & O’Keefe, 1982; Morris, Schenk, Tweedie, & Jarrard, 1990; Moser & Moser, 1998; Riedel et al., 1999). Therefore, it is possible that, in rodents, object memory may be less sensitive to perturbation of the CA1 region of hippocampus than that of spatial memory.
The current study tested this hypothesis by comparing the effects of pre-test inactivation of the CA1 region on object memory retrieval and spatial memory retrieval using a common cohort of male C57BL/6J mice for both the NOP and the Morris water maze (MWM) tasks. Given that several days of training are typically required for mice to acquire spatial memory in the MWM, the NOP sample session was repeated so that each mouse was presented with one sample session each day for 3 days. This extended training procedure was conducted so the respective tests of memory retrieval were conducted at a more similar post-training time point for the respective tasks. The results indicate that bilateral inactivation of the CA1 region of the dorsal hippocampus impaired object memory and spatial memory to a comparable degree. Thus, our findings provide the first behavioral evidence that object recognition and spatial memory rely equally on the CA1 region of rodent hippocampus. We also addressed the concern that muscimol-induced inactivation of the CA1 may lead to permanent functional changes in this sub-region. Specifically, we confirmed that CA1 functionality recovers completely after intra-CA1 muscimol, by demonstrating that mice can successfully retrieve object memory 24 h after a pre-test inactivation of CA1. Moreover, mice that failed to retrieve object memory while under the influence of intra-CA1 muscimol, subsequently acquired spatial memory in the MWM equivalent to that of control-treated mice. The results of these three experiments are consistent with the view that the CA1 region of the rodent hippocampus is a critical component of the medial temporal lobe network that supports non-spatial and spatial memory.
2. Materials and methods
2.1. General materials and methods
2.1.1. Subjects
Male 9–16 week old C57BL/6J mice (The Jackson Laboratory, BarHarbor, ME) were group housed in polycarbonate cages with ad libitum access to food and water. The vivarium was maintained at 22 ± 4 °C and 50 ± 5% humidity and was set on a 12 h light/12 h dark cycle. All behavioral testing was completed during the light phase of the cycle. All animal use procedures were conducted in accordance with the guidelines required by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Florida Atlantic University’s Institutional Animal Care and Use Committee approved all procedures prior to the start of the experiments.
2.1.2. Surgery
Mice were anesthetized with isoflurane (3–5% for induction, 1.5–2% for maintenance) and secured in a stereotaxic apparatus (Model 1900, David Kopf Instruments, Tujunga, CA). Sterile bilateral stainless steel 26 gauge guide cannulae (Plastics One, Roanoke, VA), were implanted directly above the CA1 region of the dorsal hippocampus, as described previously (Cohen et al., 2013). The coordinates for placement were: 2.0 mm posterior, ±1.5 mm lateral, and 1.6 mm ventral, relative to bregma (Franklin & Paxinos, 2008).
2.1.3. Microinfusions
Immediately prior to beginning the microinfusion session, 1 mg of muscimol (Tocris, Ellisville, MO) was dissolved in 1 mL of sterile 0.9% saline. Solutions were vortexed immediately prior to being drawn into the infusion tubing. Bilateral intrahippocampal microinfusions were administered 40 min prior to the beginning of testing sessions. Bilateral 32-gauge infusion cannulae (Plastics One, Inc.) were connected to 10 μL Hamilton syringes (Hamilton Company, Reno, NV) mounted in a CMA 400 Microinfusion Syringe Pump (CMA Microdialysis, Solna, Sweden). A total volume of 0.70 μL of sterile saline or muscimol (0.35 μg of muscimol per side, simultaneously) was administered to each mouse at a flow rate of 0.334 μL/min. Microinfusion cannulae remained in place for an additional 2 min to allow for diffusion of the perfusate, then dummy cannulae and dust caps were replaced and mice were placed in holding cages (2 per cage for NOP, 4 per cage for MWM) until the commencement of behavioral testing.
2.1.4. Histology
Upon completion of behavioral testing, mice were euthanized by isoflurane overdose followed by decapitation to dissect brains. The brains were fixed, cryoprotected, frozen at −19 °C, and 50-μm coronal sections were prepared. Sections were then mounted, stained with Cresyl violet and coverslipped. A subset of the mice received bilateral intra-CA1 infusions of fluorophore-conjugated muscimol (BODIPY TMR-X (Molecular Probes) in sterile saline, 0.35 μL per side), 15 min before euthanasia and brain dissection in order to visualize the distribution of this volume of muscimol infused into the CA1 region. These sections were mounted and cover slipped with ProLong Gold antifade reagent with DAPI (Molecular Probes) to improve the visualization of the diffusion of fluorophore-conjugated muscimol within the brain. Analyses revealed that fluorophore-conjugated muscimol was distributed only within the CA1 region of dorsal hippocampus after intra-CA1 infusion (Fig. 1A), and verfied the position of the infusion cannulae tips to be within the CA1 for all mice (Fig. 1B). Only data from mice with accurate cannulae placement (n = 40) were included in the analyses. Data were excluded for three mice that experienced motor impairments following intra-CA1 muscimol infusion.
Fig. 1.
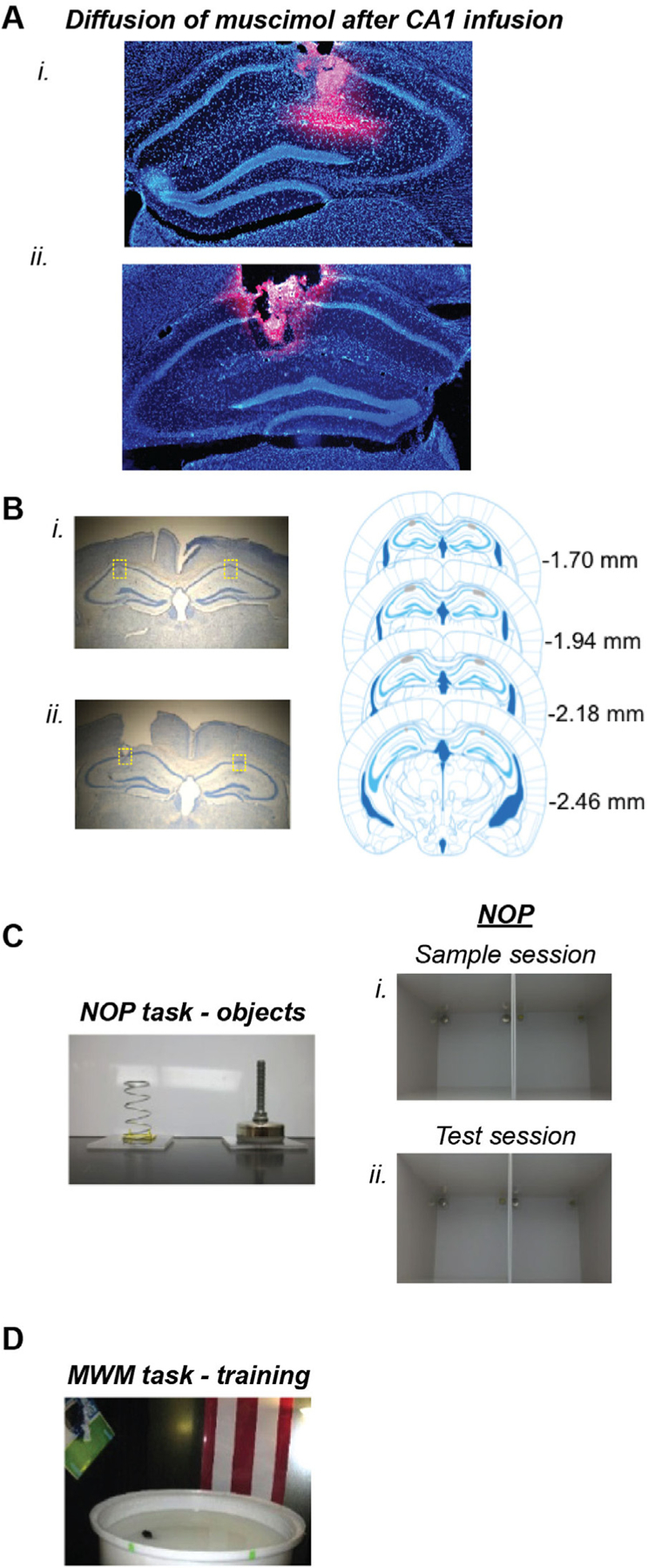
Diffusion of muscimol, histological verification, objects and testing arenas.A. (i and ii), Representative photomicrographs of coronal sections through the dorsal hippocampus from three mice that received bilateral infusions of fluorophore-conjugated muscimol (BODIPY TMR-X, Molecular Probes). Sections were mounted using DAPI fluoromount to improve visualization of the locally infused fluorophore-conjugated muscimol. Images reveal that the red fluorescence (i.e., the fluorescent conjugated muscimol) diffused within the hippocampus, but remained within the CA1 region of the dorsal hippocampus. B. left, Representative photomicrographs of Cresyl violet-stained sections from two mice indicating accurate placement of guide cannulae above the CA1 region of the dorsal hippocampus and the infusion cannulae tracks into the CA1. right, Composite montage depicting the distribution of bilateral microinfusion placements into the CA1 region of the dorsal hippocampus from all mice included in the data analyses from both Experiments 1 and 2. All infusions were confirmed to be within the region depicted by gray. Numbers refer to distance (in mm) from bregma; plates are modified from the respective coronal plates of the Franklin and Paxinos (2008) mouse stereotaxic atlas. C. Left, Photographs of the objects used in the NOP experiments. Right, Photograph depicting the configuration of objects within both arenas during the sample sessions (i), and the test session (ii). These independent dual chamber configurations permitted two mice to be simultaneously tested during each sample or test session. D. Photograph of the MWM room, depicting approximate locations of a subset of the extra-maze cues and the size of pool. In the image, a mouse is standing on the platform submerged within the SW quadrant of the pool.
2.2. Novel object preference (NOP) task procedures
To acquire data in a time-efficient manner, two mice were tested simultaneously in separate acrylonitrile butadiene styrene (ABS) arenas of equal size (38 × 38 × 64 cm high). Mouse behavior was captured with Ethovision XT (Noldus Information Technology, Leesburg, VA) via a video camera suspended 150 cm above the arena floor directly above the border walls separating the two arenas; the live feed was displayed on a computer screen approximately 4.7 m from the testing arena, where the video file was automatically saved (see Fig. 1C for a diagram of the NOP arena set-ups and objects). The pair of objects used had been previously determined to elicit equivalent exploration by naïve male C57BL/6J mice. Therefore, the preferential exploration of the novel object presented during the test session is not simply a reflection of the NOP task being conducted in a biased manner.
Beginning 7 days postoperative, all mice were habituated to the arenas and to the handling necessary for the intrahippocampal infusions. On Days 1–3, mock infusions were conducted (1/day) during which each mouse was restrained, and dummy infusion cannulae were inserted into the guide cannula, which did not project beyond the guide cannulae. This procedure was designed to habituate the mice to all microinfusion procedures. After the mock infusions, mice were placed into holding cages. After 40 min, the mice were transported into the testing room and placed, individually, in one of two NOP arenas for a sample session. During each sample session, each arena contained a pair of identical objects. A mouse was placed into each arena and allowed to freely explore the objects for 10 min. The same pair of objects was used for each of the three sample sessions. To ensure all mice were matched for significant object exploration, mice who did not explore the objects for a total of at least 100 s across the three 10-min NOP sample sessions (n = 3) were excluded from all analyses. On each day, after the 10-min sample session, mice were returned to polycarbonate holding cages, transported back into the procedure room and returned to their home cages. The arenas and objects were wiped down with a 10% ethanol solution between each trial.
For Experiment 1, the test session was presented 24 h after the third sample session. Mice received bilateral intrahippocampal microinfusions of sterile saline (n = 9) or muscimol (n = 12) and were then placed in pairs into holding cages. After 40 min, the mice were transported into the testing room and placed into the same testing arenas in which they had received the sample sessions. During the test session, the arena was configured identically to the sample session, except that one of the objects in each arena had been replaced with a novel object. The novel and familiar object designations, as well as the location of the object that was replaced with the novel object, was counterbalanced across mice. Each mouse freely explored the objects and arena for exactly 5 min. The arena and objects were wiped down with a 10% ethanol solution between each trial (see Fig. 2A Experiment 1, for schematic of protocol).
Fig. 2.
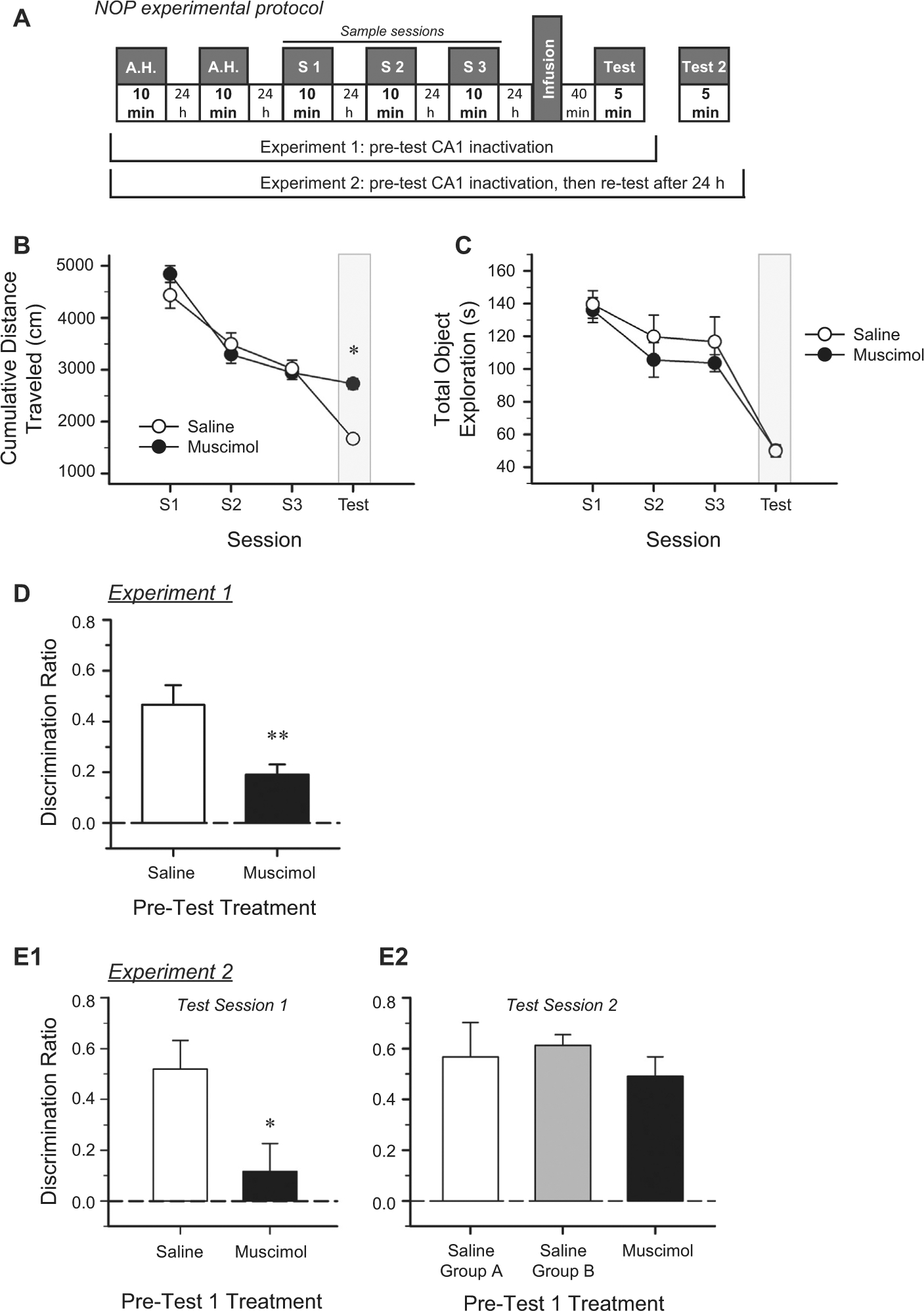
Pre-test intra-CA1 muscimol impairs retrieval of long-term object memory. A. Schematic of the experimental protocols used for the NOP task in Experiments 1 and 2. A.H.: Arena Habituation (exposure to empty arena); S1-S3: Sample Sessions (each of three 10-min exposures (1/day) to a pair of identical objects in the familiar arena; the same objects were used for each sample session). B. Cumulative distance traveled decreased significantly across the 10-min sample sessions (P < 0.001) indicating habituation to the arena; however, there was no difference between the future treatment groups (n.s.). Mice that received pre-test intra-CA1 muscimol traveled a significantly greater distance during the 5-min test session than did the pre-test saline mice, possibly due to impaired retrieval of the memory of the arena. C. There was no significant difference between pre-test treatment groups in total time spent exploring objects over the three 10-min sample sessions or during the 5-min test session. Shaded regions in B and C highlight the session conducted under the influence of the microinfusion. D. Mice that received pre-test intra-CA1 saline exhibited a significantly stronger preference for the novel object than did the pre-test muscimol-treated mice. Dashed line indicates chance performance. All error bars indicate ± S.E.M. * = P < 0.01; ** = P < 0.005. E. In Experiment 2, mice received three sample sessions (1/day) as in Experiment 1. Mice received intra-CA1 saline or muscimol before NOP test session 1. E1. Mice that received intra-hippocampal saline prior to NOP Test session 1 exhibited significant discrimination between the familiar and novel objects. However, the mice treated with muscimol were impaired, consistent with results in D. Dashed line indicates chance performance. All error bars indicate ± S.E.M. * = P < 0.03. E2. Mice that received intra-CA1 saline prior to NOP test session 1 were assigned to either saline group A (exposed to Test session 1 and Test session 2), or saline group B (exposed to only Test session 2). During Test session 2, saline group A again demonstrated significant object discrimination and performed comparably to saline group B. Additionally, the muscimol-treated mice that exhibited impaired discrimination during Test session 1, exhibited significant object discrimination during Test session 2, performing similar to both saline groups. Dashed line indicates chance performance. All error bars indicate ± S.E.M.
2.2.1. Testing possible long-term effects of muscimol-induced inactivation of CA1
A naïve cohort of mice was tested in an experimental protocol comprising three sample sessions (Days 1–3), followed on Day 4 by intra-CA1 microinfusions and then a test session. On Day 5 all mice were returned to the arena for a second test session, this time drug-free, to determine whether those mice that had received intra-CA1 muscimol 24 h earlier would continue to exhibit impaired object memory. The sample sessions were conducted in the same manner as described above for Days 1–3 (Experiment 1). Day 4 was designated Test Day 1. Eleven mice received bilateral intra-CA1 microinfusions of saline 40 min prior to the start of the test session, while the remaining 8 mice were administered muscimol. The saline-treated mice were then randomly divided into 2 groups, saline group A (verify successful object discrimination) and saline group B. Subsequently, saline group A and the muscimol group were behaviorally tested for object recognition, while saline group B was returned to their home cages. The test session was conducted in the same fashion as described above for Experiment 1. On day 5, all three groups of mice were mock infused and then exposed to test session 2. Again, the testing procedure was the same as described above (see Fig. 2A Experiment 2, for schematic of protocol). During test session 2, saline group B represented object discrimination following a 48 h retention interval. Presumably, during test session 1, muscimol-treated mice experienced a “black-out”, simulating a test of 48 h object memory retention during test session 2. In this manner, the most appropriate test session 2 comparison is between muscimol-treated mice prior to test session 1 and saline group B.
2.2.2. NOP analyses
Total object exploration, cumulative distance traveled (CDT) and velocity while moving were all recorded by Ethovision and averaged for each session. A researcher, blind to treatment, scored object exploration using a manual event encoder while reviewing the digital videorecording of each mouse’s sample and test session exploration of each object using computer display XNote Stop-watches (dnSoft Research Group, www.dnsoft.swrus.com). A mouse was considered to be exploring the object if it was facing the object and its nose was within 2.0 cm of the object. Time spent grooming beside the object or using the object merely to climb higher while facing away from the object was not considered exploration time. Measures of time spent exploring the objects during each sample session were determined for each mouse, and then analyzed by two-factor (pre-test treatment condition, sample session) repeated measures ANOVA to determine whether the sample session performance for mice was different between the pre-test intrahippocampal treatment groups. Repeated measures ANOVA were followed by post hoc multiple comparisons Holm-Sidak tests or a two-tailed Student’s t-test, where appropriate. Object memory was inferred from analyses of the discrimination ratio, computed for each mouse by dividing the difference in time spent exploring the novel and familiar test session objects by the total time spent exploring both test session objects. A discrimination ratio score >0 indicates that the mouse successfully retrieved the memory of the sample object and preferentially explored the novel object. Mean discrimination ratio scores were computed for each treatment condition and analyzed by independent groups t-test.
2.3. Morris water maze (MWM) task procedures
A 62 cm tall cylindrical pool, measuring 1.06 m in diameter, was filled with water and made opaque by the addition of non-toxic white tempra paint (Sargent Art, Inc., Hazleton, PA). The water was maintained at a temperature of 23.0 ± 1 °C for the duration of the experiment. The escape platform (46 cm tall × 8 cm dia clear plastic cylinder) was submerged 1 cm below the surface of the water. The pool was surrounded by black curtains positioned approximately 45 cm from the edge of the pool. Ethovision acquired mouse behavior via a video camera centered above the pool.
Habituation to the MWM testing environment began approximately 1 wk after the completion of NOP testing (Experiment 1). On Days 1 and 2, the curtains were closed around the pool to eliminate access to polarizing visual cues, and the submerged escape platform was placed in the center of the pool. Each mouse was placed on the platform, where it was to remain for a total of 60 s. If the mouse left the platform before 60 s elapsed, it was encouraged to climb immediately back onto the platform. After 60 s elapsed, the mouse was placed under a warm air stream for approximately 20 min before being returned to its home cage.
On Days 3–6, the curtains were opened and visual cues were suspended from the fabric of the curtains, and the platform was placed in the center of the SE quadrant of the pool. Each mouse received four trials per day, one from each of the four release points (N, S, E, W). Upon escaping onto the platform, or after 60 s, the mouse remained on the platform for 30 s before being returned to the holding cage located behind the curtain. Mice were trained in a squad, with the inter-trial interval varying between 2 and 5 min. Once all mice had received the four training trials, the holding cage was carried out of the testing room and placed under the warm air stream before the mice were returned to their home cage. On Days 5 and 6, each mouse received mock infusions 40 min prior to the onset of training (see Fig. 1D for a photographic representation of the MWM training set-up).
On Day 7, each mouse received bilateral intrahippocampal microinfusion of either sterile saline or muscimol, and 40 min later received a platform-less probe test of spatial memory. To control for incidental carryover effects, drug assignments for the MWM experiment were conducted as follows: intra-CA1 treatment conditions were counterbalanced so that half of the mice received the same infusion as before NOP testing (i.e., saline-saline or muscimol-muscimol) and the other half received a different infusion (i.e., saline-muscimol or muscimol-saline). Each mouse was released into the pool at the arbitrarily selected north starting point and allowed to swim for 60 s before being removed from the pool.
2.3.1. MWM analyses
Training trial measures of latency to escape (s), mean cumulative distance from the platform border (cm), and mean velocity (cm/s) were averaged for each 4-trial block of training and then analyzed with a two-factor (future pre-test treatment, trial block) repeated measures ANOVA followed by post hoc multiple comparisons Tukey’s Honestly Significant Difference (HSD) tests or a two-tailed Student’s t-test, where appropriate. Probe test data were analyzed to determine respective treatment group means for measures of percent dwell in each of the four pool quadrants, mean distance from the platform center, and the percent dwell in a specified 10 cm wide annulus adjacent to the pool wall. The accuracy of platform search behavior was determined by calculating the time spent in the exact target location (the platform zone) as a percentage of the total time spent in the pool during the 60 s probe test. The relative sizes of the arena and the platform indicate that the chance percentage of time spent in the platform zone is 0.005696%. Each treatment group’s average time spent in the platform zone was compared to chance in order to determine whether search accuracy was significantly different. Heat maps of probe test search behavior of each intra-CA1 treatment group were constructed using Ethovision XT 11 tracking software by calculating the total dwell times across the pool surface; cooler colors indicate low dwell time, while warmer colors indicate higher dwell time in a given location. Regional dwell times were averaged over all mice within each treatment group, and color codes were adjusted in scale so the same color denotes the same amount of time exploring that area.
3. Results
3.1. Experiment 1: Pre-test inactivation of the CA1 region of dorsal hippocampus impairs retrieval of object memory
To test whether inactivation of the CA1 differentially affects the retrieval of object memory and spatial memory, we infused the GABA-A agonist, muscimol, or saline into the CA1 region of the hippocampus prior to testing mice for object memory in a 3-sample session version of the NOP task. The same mice were then trained for 4 days in the MWM task, and then the mice again received intra-CA1 muscimol or saline prior to a probe test for spatial memory. Two factor repeated measures ANOVA on the cumulative distance traveled, velocity, and total object exploration measures from the sample sessions yielded a significant main effect of session (Fig. 2B; F2,38 = 194.2, P < 0.001), (F2,38 = 91.6, P < 0.001), and (Fig. 2C; F2,38 = 14.9, P < 0.001), respectively. There were no significant effects of future treatment on cumulative distance traveled (F1,19 < 1.00, n.s.; Fig. 2B), mean velocity (F1,19 < 1.00, n.s.), or total object exploration (F1,19 < 1.00, n.s.; Fig. 2C). There was a significant interaction between future treatment and session for cumulative distance traveled, (F2,38 = 6.7, P < 0.01), and for velocity, (F2,38 = 3.7, P < 0.05), but not for total object exploration (F2,38 = 0.5, n.s.). These results indicate that prior to test day microinfusions, the two groups of mice exhibited matched sample session performance. The significant differences across sample sessions in the measured behaviors indicate equivalent habituation of the mice to the arena and objects.
On Day 4, mice received intra-CA1 muscimol or saline and 40 min later, a NOP test session. Saline-treated mice preferentially explored the novel object, while the muscimol-treated mice failed to do so. Accordingly, the mean discrimination ratio of the pre-test saline group was significantly greater than that of the pre-test muscimol group (t19 = −3.38, P = 0.003; Fig. 2D). The two treatment groups spent similar amounts of time exploring the objects during the test session (t19 = 0.82, n.s.; Fig. 2C Test); however, the distance traveled by the muscimol-treated mice was significantly greater than that of the saline-treated mice (t19 = 7.61, P < 0.001; Fig. 2B Test). The increased exploration of the arena by the pre-test muscimol treatment group suggests that the CA1 inactivation may have impaired retrieval of the memory of the objects and the memory of the arena itself.
3.2. Experiment 2: Object memory impairing effects of intra-CA1 muscimol are temporary
A naïve cohort of mice was used in a separate experiment to verify that the memory-impairing effects of intra-CA1 muscimol were transient; that is, that upon recovery from the drug, the previously blocked memory should be intact and retrievable. Thus, while this experiment was designed to confirm the temporary nature of the muscimol inactivation technique, this precise control measure assessing memory retrieval during inactivation, and again after inactivation, to our knowledge, has not been previously reported. During the first of the 3 sample sessions of the NOP task, there were no significant exploration differences between mice of future treatment groups (F2,18 = 0.25, n.s.; data not shown). Test session 1 was conducted 24 h later in which a subset of the mice (n = 4) received bilateral intra-CA1 microinfusions of saline (saline group A) and another subset (n = 8) received bilateral intra-CA1 muscimol. The remaining mice (n = 7) were administered intra-CA1 saline microinfusions and then returned to their home cages without exposure to the test arena or the objects (saline group B). As expected, the saline group A mice demonstrated significant discrimination between test session stimuli, preferring the novel object, while the muscimol mice performed near chance (t9 = 2.28, P = 0.046; Fig. 2E1). After a 24 h delay, all three groups of mice received mock infusions and were then tested in the behavioral task (i.e., test session 2). Saline group A continued to demonstrate strong discrimination between objects, as did saline group B. Interestingly, the mice that had received intra-CA1 muscimol prior to test session 1 were fully recovered by test session 2 as demonstrated by a strong discrimination between familiar and novel objects (F2,18 = 0.73, n.s.; Fig. 2E2). The recovery of memory retrieval by the muscimol group indicates that the inactivation of the CA1 region of dorsal hippocampus transiently interrupts memory retrieval, and the object memory is not otherwise affected. These results suggest that intra-CA1 muscimol does not cause long-lasting impairments in hippocampal function.
3.3. Experiment 3: Pre-test inactivation of the CA1 region impairs retrieval of spatial memory in the Morris water maze
As described above, following completion of Experiment 1, the same mice then underwent training in the hidden platform MWM task. After acquiring the spatial task, the contribution of CA1 neuronal activity to the retrieval of spatial memory was determined (see Fig. 3A, for schematic of protocol).
Fig. 3.
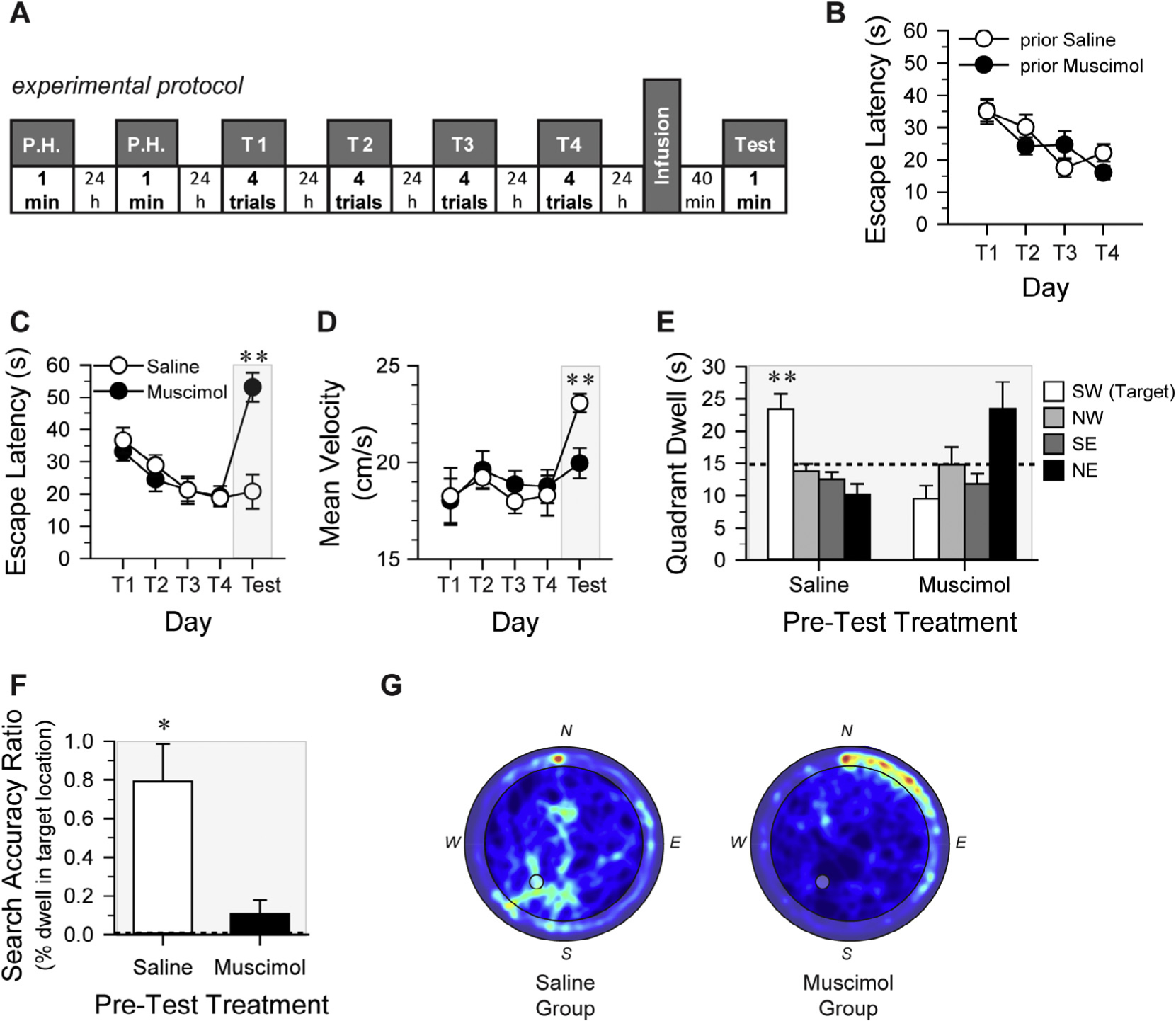
Pre-test intra-CA1 muscimol impairs retrieval of long-term spatial memory and memory for task performance. A. Schematic of the experimental protocol used for the MWM task. P.H.: Pool Habituation (exposure to the pool without extra-maze visual cues, platform in center of pool); T1-T4: Training sessions comprising 4 trials/day (spatial training with extra-maze visual cues and platform in SW quadrant); Test: platform-less probe test of spatial memory retention. Mice received intra-CA1 saline or muscimol 10–11 days prior to the onset of MWM training (see Fig. 2D). B. Escape latency across the four training sessions plotted according to the previous intra-CA1 treatment (prior Saline, prior Muscimol). Prior intra-CA1 treatment did not affect spatial training in the MWM, indicating that the effects of muscimol on CA1 were temporary. Treatment assignments for pre-MWM probe test microinfusions were reversed from that of pre-NOP test session. Escape latency (C) and mean velocity (D) plotted according to MWM pre-probe test treatment. There was no effect of future treatment on either dependent measure across training trial blocks. However, pre-test muscimol-treated mice took a significantly longer latency to reach the platform location than did the pre-test saline-treated mice (P < 0.001), indicating impaired retrieval of spatial memory. The mean velocity of pre-test saline-treated mice was significantly greater than that of the pre-test muscimol-treated mice. E. Mice that received pre-probe test intra-CA1 saline spent significantly more time in the target SW quadrant than in any other quadrant, and more time in the target quadrant than did the muscimol-treated mice. Although pre-test intra-CA1 muscimol-treated mice spent more time in the NE quadrant, they failed to exhibit a statistically significant preference for any quadrant. Dashed line indicates chance performance: 15 s dwell in a given quadrant. F. Specific search accuracy was calculated by finding the percentage of time during the 60-s probe test that each mouse spent in the exact location of the pool where the platform had been during training. Pre-test saline-treated mice exhibited a significantly greater search accuracy ratio than would be expected by chance (see dashed line at 0.005696; P = 0.002); however, muscimol-treated mice did not perform better than chance. G. Composite map of all swim paths during the probe test for each pre-test treatment condition. Plots present quadrant dwell as a “heat map” representing mean dwell times according to a color code with darker, cooler colors indicating low dwell times and warmer colors indicating high dwell times. The pre-test saline-treated mice appropriately concentrated their search in the SW quadrant, while the pre-test muscimol-treated mice failed to concentrate their search in the SW quadrant and instead demonstrate predominant thigmotaxis. The gray circle in center of the SW quadrant of each composite image indicates the location where the platform had been submerged during training. All error bars indicate ± S.E.M. *, P < 0.01; **, P ≤ 0.001. The gray shaded boxes in plots C–F indicate behavioral testing that occurred under the influence of the respective intra-CA1 treatment.
3.3.1. Training results
The purpose of analyzing the MWM training data was two-fold. First, in order to further assess whether the muscimol infusions administered before the NOP task 10–11 days earlier had any long-term effects, MWM training data was analyzed according to previous intra-CA1 treatment condition. Secondly, to confirm that groups formed for the MWM probe test were behaviorally equivalent; training data was analyzed based on future treatment group. Escape latencies across the four training days for the mice that had previously received saline or muscimol before NOP testing are represented in Fig. 3B, while Fig. 3C depicts the escape latencies for the mice according to their future treatment group.
Across training days, there was a statistically significant difference in overall latency to escape onto the platform (F1,19 = 40.91, P < 0.001) and mean distance to platform center (MDtP; F1,19 = 45.16, P < 0.001); however, there was not a significant difference between previous treatment groups’ latencies (F1,19 < 1.00, n.s.; Fig. 3B) or MDtP scores (F1,19 = 1.029, n.s.), or between future treatment groups latencies (F1,19 < 1.00, n.s.; Fig. 3C) or MDtP scores (F1,19 < 1.00, n.s.) across training. Additionally, there were no significant differences in swim velocity between previous treatment groups (F1,19 < 1.00, n.s.) or future treatment groups(F1,19 < 1.00, n.s.; Fig. 3D). These results indicate that the bilateral infusion of muscimol into the CA1 region prior to the NOP test session 10–11 days earlier had no lasting effects on hippocampal function, extending the findings of the NOP test Experiment 2. Mice in each treatment group behaved similarly regardless of the previous treatment; therefore, no further distinction in MWM acquisition was be made between mice that previously received intra-CA1 muscimol or saline. The lack of difference in MWM acquisition according to future treatment condition suggests that mice were appropriately matched for task acquisition prior to the test-day infusions.
3.3.2. Probe test results
Quadrant dwell times were significantly different between intra-CA1 treatment groups in the NE quadrant (F1,19 = 10.62, P = 0.004; Fig. 3E) and in the SW target quadrant (F1,19 = 18.15, P < 0.001). The amount of time that saline-treated mice spent in the target SW quadrant (the location of the platform during the previous training trials) was significantly greater than that spent in the other quadrants (SW v. NW: P = 0.001, SW v. NE = P < 0.001, and SW v. SE: P < 0.001), while the time the muscimol-treated mice spent in the SW quadrant was either significantly less (SW v. NE: P = 0.007) or not significantly different (SW v. NW: n.s., SW v. SE: n.s.) than the time spent in the other quadrants. Furthermore, the control groups spent significantly more time in the target SW quadrant than did the muscimol group (t19 = −4.26, P < 0.001). Lastly, the saline-treated mice spent significantly more time in the SW quadrant than would be expected by chance (t11 = 3.56, P = 0.004), but the muscimol-treated mice spent significantly less time than would be expected by chance (t8 = −2.67, P = 0.028). These results revealed that spatial memory and navigation were intact in the saline-treated group, but were impaired in the muscimol-treated group. This conclusion is further supported by the finding that, during the probe test, the pre-test muscimol mice experienced a significantly longer latency to reach the location in the pool where the platform had been during training than did the pre-test saline-treated mice (t19 = −4.44, P = 0.0003; Fig. 3C).
Additional analyses were conducted to further examine the nature of the behavioral impairment induced by intra-CA1 muscimol. Spatial search accuracy was calculated for each mouse by determining the time spent during the probe test in the exact target location (the platform zone) as a percentage of total time spent in the pool (Fig. 3F). Saline-treated mice spent significantly more time (0.792% of the probe test) in the platform zone than would be predicted by chance (chance = 0.006%; t11 = 4.06, P = 0.002), but the muscimol-treated mice spent only 0.108% of their time in the platform zone; performance not significantly greater than chance (t8 = 1.40, P = 0.198). The mean spatial search accuracy was significantly different between groups (t19 = −2.93, P = 0.009). Visualization of the swim paths of mice from the respective treatment conditions suggested that muscimol-treated mice were thigmotaxic (see Fig. 3G for representative composite “heat” maps illustrating the location × dwell time distribution of each treatment group across the entire pool surface during the probe test). The composite search pattern map of the saline-treated group indicates search behavior concentrated in the SW quadrant of the pool after release from the N start point (see left plot of Fig. 3G). In contrast, the composite map of the muscimol-treated group lacks evidence of a spatially constrained search, and further suggests that the muscimol-treated mice remained close to the N start point, searching adjacent to the pool wall in the NE quadrant. It is not clear why the intra-CA1 muscimol-treated mice concentrated their search in this region of the pool, since the experimenter was out of view of the mice during all probe tests (i.e., standing behind opaque curtain panels during the test). In light of the significant differences between the platform search behaviors expressed by saline- and muscimol-treated mice revealed by the composite maps, we further analyzed thigmotaxic behavior during the probe test. Intra-CA1 muscimol-treated mice spent significantly more time (47.25% of the probe test) in the thigmotaxic zone (a virtual 10 cm wide annulus along the pool wall), as compared to saline-treated mice (18.72% of the probe test; t19 = −3.938, P < 0.001).
The mean swim velocity of the muscimol-treated mice during the MWM probe test was not significantly different from their mean swim velocity during the last day of drug-free training (t8 = −0.83, n.s.; Fig. 3D). This result suggests that intra-CA1 muscimol did not alter mobility of mice in the MWM; a conclusion also supported by the NOP data. Interestingly, the mean swim velocity of the saline-treated mice was significantly higher during the probe test, compared to that during the last training session (t11 = −4.47, P = 0.001). The increased swim velocity observed in the saline-treated mice during the probe test may reflect the difference in trial type – the unexpected lack of the platform may have increased the anxiety or stress level in the mice. That this increase in swim speed during the probe test was not observed in the muscimol-treated mice perhaps further serves to indicate that inactivation of the CA1 impairs recognition of the lack of the platform in addition to impaired retrieval of the spatial memory for the platform location. Together, these results indicate that bilateral dorsal CA1 microinfusions of muscimol effectively impaired performance in the MWM, a well-established hippocampaldependent task (Morris, Garrud, Rawlins, & O’Keefe, 1982; Riedel et al., 1999).
3.4. NOP and MWM impairment comparison
A primary objective of this study was to compare the magnitude of memory impairment induced by intra CA1 muscimol-treatment across the two distinct tasks. To do so, the DR scores (NOP) and the MDtP scores (MWM) were normalized to their respective best individual score for each test (see Fig. 4). A two factor (treatment and task) ANOVA on normalized memory scores revealed a significant main effect of treatment (F1,38 = 36.81, P < 0.001), but no significant main effect of task (F1,38 = 1.12, P > 0.2), and no significant treatment × task interaction (F1,38 = 0.54, P > 0.4). The lack of significant interaction suggests a comparable impairing effect of muscimol on both tasks. To enable statistical analysis of our a priori hypothesis that retrieval of object memory is less sensitive to CA1 inactivation than that of spatial memory, the normalized memory scores were further analyzed using a one-way ANOVA, which yielded a significant main effect of group (F3,38 = 13.71, P < 0.001). Pairwise multiple comparisons Holm-Sidak tests of the normalized memory scores found no significant difference between pre-NOP muscimol and pre-MWM muscimol (t19 = 0.23, P > 0.8), and no significant difference between pre-NOP saline and pre-MWM saline (t19 = 1.27,P > 0.35). The comparable scores of the saline-treated mice across both conditions indicate that the normalized scores appropriately compare performance across the two different behavioral tests. The comparable scores of the muscimol-treated mice across the two tests reveal that the muscimol-induced impairment is equally severe, suggesting that retrieval of object memory in the NOP task and spatial memory in the MWM are equally reliant on CA1 neuronal activity.
Fig. 4.

Pre-test inactivation of the CA1 region impairs retrieval of object memory and spatial memory equivalently. To compare the degree of memory impairment produced by intra-CA1 muscimol in the two distinct tasks, discrimination ratio (DR) scores from the NOP experiment and MDtP scores from the MWM experiment were normalized to the best actual score for each experiment. The scatter plot depicts the distribution of scores of individual mice for the respective task according to the intra-CA1 treatment condition; the treatment mean ± SEM normalized scores are also indicated (open circles, saline; filled squares, muscimol). Note: overlap in data points prevents all individual scores from being visible. In addition to being significantly different from the respective muscimol condition, the scores of the saline-treated mice were consistent across the two experiments (all comparisons, P > 0.05). The scores of the muscimol-treated mice were also consistent across the two experiments (all comparisons, P > 0.05), indicating that intra-CA1 muscimol impaired object memory and spatial memory retrieval to a comparable degree. All error bars indicate ± SEM.
4. Discussion
The current study used a temporary neuronal inactivation technique in mice to test whether the retrieval of object memory and spatial memory are differentially dependent on neuronal activity in the CA1 region of the dorsal hippocampus. Our histological analyses confirmed the placement of infusion cannulae within the CA1 region of dorsal hippocampus, and in a subset of mice the distribution of fluorophore-conjugated muscimol after intra-CA1 infusion revealed that the fluorophore diffused from the site of initial infusion but remained within the CA1 region of the dorsal hippocampus (see Fig. 1A). These results provide support that the muscimol-induced inactivation of the present studies was confined to the CA1 neurons of the dorsal hippocampus. We previously reported that 30 min after intra-CA1 infusion, fluorophore-conjugated muscimol spreads to approximately a 300 μm radius, while remaining confined to the CA1 region of the dorsal hip pocampus, thereby affecting less than 1% of the total hippocampal volume (Cohen et al., 2013). We have also reported that the behavioral impairments observed in mice after intra-CA1 infusion of fluorophore-conjugated muscimol are identical to those observed after intra-CA1 muscimol (Cohen et al., 2013; Stackman, Lora, & Williams, 2012). Intra-CA1 muscimol has been shown to silence the activity of rat CA1 pyramidal neurons beginning almost immediately post-infusion and lasting for at least 140 min (Bonnevie et al., 2013). Assuming a similar time course of intra-CA1 muscimol for mice, the inactivation would have begun 40 min before the retrieval of object or spatial memory, and the inactivation of CA1 neurons would have been maintained throughout the duration of the respective test sessions. Based on these results, we interpret the behavioral effects reported in the present study as revealing the selective influence of CA1 neurons on the retrieval of object recognition memory and of spatial memory.
Our study yielded two main novel findings. First, we determined that pre-test inactivation of CA1 produced comparable impairments of object memory retrieval in a modified version of the NOP task, and of spatial memory retrieval in the MWM task. The NOP task was modified for the current study to repeat the sample or acquisition phase of the task in appreciation of the fact that the acquisition phase of the MWM task typically requires multiple sessions. The impairments exhibited by the mice that received intra-CA1 muscimol microinfusion prior to the NOP test were comparable to the impairments exhibited by mice that received muscimol prior to the MWM test, indicating that retrieval of long-term memory for objects or places relies equally on the function of the CA1 region of the hippocampus. Second, the demonstration that pre-test inactivation of the CA1 region impairs novel object preference 24 h after the last sample session in the NOP task, extends prior reports of the involvement of the dorsal hippocampus in distinct phases of long-term memory for objects. Given that the present study was conducted in a commonly used inbred strain of mice, and the popularity of the NOP task for the testing of mutant mouse lines, the present findings may improve understanding of the contribution of the CA1 region to memory processes in various genetically engineered murine models. In addition to these main findings, we also confirmed that intra-CA1 muscimol reversibly blocks the retrieval of object memory by showing that mice, impaired after intra-CA1 infusion of muscimol, successfully performed the NOP test session one day later. Together, these findings are consistent with the view that intact CA1 neuronal function is critical for the successful retrieval of object memory required to support novel object preference during the test session.
The results support and extend findings presented in our prior report (Cohen et al., 2013), and other reports demonstrating the contribution of the CA1 region of the dorsal hippocampus to object memory processes in rodents (Broadbent et al., 2010; Clark et al.,2000; Cohen & Stackman, 2015; Hammond et al., 2004). It has been argued that the hippocampal contribution to the NOP test reflects the contextual aspects of the task. Specifically, given the configuration of object placement in the arena, the mice likely encode a memory of object-in-context – a form of memory one might suspect to be dependent upon the CA1. While this is certainly likely, we have previously reported that CA1 inactivation also impaired memory for objects independent of context (Cohen et al., 2013). Further, if the nature of the impairment observed in the mice in the NOP task after CA1 inactivation only reflected impaired retrieval of contextual information, one might have expected the mice to have preferentially explored the familiar object (Mumby et al.,2002), or to have also spent significant time exploring the arena, indicating the muscimol-treated mice perceive it to be relatively novel. It is important to note that analyses of NOP test session behavior did indicate a significant increase in distance traveled by the muscimol-treated mice compared to the saline-treated mice, perhaps due to impaired contextual memory. However, the muscimol-treated mice did not exhibit a preference for exploring the familiar object, as is generally seen by rodents encountering familiar objects in unfamiliar contexts (Mumby et al., 2002). Thus, the pattern of results observed is consistent with the view that the contribution of CA1 neuronal activity to object recognition memory reflects more than simply the processing of contextual information.
It is interesting to note that temporary or permanent lesion of the perirhinal cortex has been shown to impair object recognition memory, but permanent lesions of perirhinal cortex spare spatial memory in the MWM in rats (Winters & Bussey, 2005; Winters et al., 2004). These results suggest that the perirhinal cortex participates selectively in object recognition memory, while the findings of the current study suggest that the CA1 region of the dorsal hippocampus participates in both object memory and spatial memory to a similar degree, a conclusion supported by the recent work of others as well (Beer et al., 2013; Farovik et al., 2010). Further, there is extensive evidence supporting the significant contribution of the perirhinal cortex to object recognition memory when the delay between the encoding session and the retrieval session is relatively short (Barker & Warburton, 2011; Barker et al., 2006; Brown & Aggleton, 2001; Brown, Warburton, & Aggleton, 2010). In an attempt to integrate our current findings with the broader literature, we suggest that object recognition memory depends upon a functional interaction between the perirhinal cortex and the hippocampus, which supports the encoding, consolidation, and retrieval of long-term object memory (Cohen & Stackman, 2015). By this view, the essential role of the hippocampus becomes apparent when a significant duration of time between encoding and retrieval must be bridged. Thus, in modifying the standard NOP task procedure for the present study – which extended the delay between the encoding and eventual retrieval of object memory, we may have increased the dependence of the behavior on the dorsal hippocampus, or CA1 specifically. It is worth noting that if the hippocampus selectively participates in long-term, but not short-term, object memory processes (i.e., in a delay-dependent manner), then the duration of time that must be bridged would have to be known to the animal or the hippocampus a priori at the time of encoding. An alternative view is that the perirhinal cortex participates in encoding object information and in time engages the hippocampus when the memory load becomes sufficiently significant, as proposed in our recent review (Cohen & Stackman, 2015) or in order to bind object information together with contextual information.
Finally, our results demonstrating that inactivation of the CA1 region of mouse hippocampus impaired retrieval of spatial memory in the MWM are consistent with a broad literature confirming the significant contribution of the rodent hippocampus to spatial memory and navigation.
4.1. Intra-CA1 muscimol impairs spatial memory and appropriate search behavior
Riedel et al. (1999) reported that rats tested under AMPA receptor blockade-mediated hippocampal inactivation exhibited search behavior that was restricted to an incorrect location of the pool. Similarly, after muscimol inactivation of the CA1 region, our mice exhibited a focused search in an inappropriate location of the pool. Specifically, as illustrated by the composite heat map images of search behavior during the probe test (Fig. 3G), mice that had received intra-CA1 muscimol spent much of the probe test swimming along the edge of the pool in the NE quadrant. Thus, the quality of the search behavior exhibited by intra-CA1 muscimol-treated mice appeared to be very different than that reported after intrahippocampal AMPA receptor blockade by Riedel et al. (1999). As suggested by the composite map, analyses confirmed that pre-test muscimol-treated mice exhibited significantly greater thigmotaxic swimming behavior during the probe test than did saline-treated controls. These results suggest that silencing CA1 neuronal activity also disturbed the selection of an appropriate platform search strategy, which is characteristically observed in well-trained mice during the probe test. Mice tend to exhibit thigmotaxis on the first days of pool habituation and hidden platform training, but quickly learn it is not an ineffective escape strategy. Although the thigmotaxic behavior we observed during the probe test could be considered an escape strategy, the predominant thigmotaxis by all of the mice after inactivation of CA1 suggests rather that muscimol temporarily erased the memory of the spatial task, instead of simply blocking retrieval of the memory for the precise spatial location of the hidden platform. As exhibited by their decreased escape latencies across training days, the future muscimol-treatment group successfully learned that a thigmotaxic strategy was not effective, but the pre-test inactivation of CA1 appears to have impaired the retrieval of this procedural (i.e., response requirements) memory for strategy, along with the retrieval of the precise spatial memory. Pre-test muscimol-treated mice appear to have reverted back to a thigmotaxic-based search strategy during the probe test. The deficit in strategy or the lack of appropriate platform search response is particularly interesting given the confined distribution of muscimol to within the CA1 region of the dorsal hippocampus. These results suggest that muscimol inactivation of CA1 produces a robust impairment of appropriate water maze motor behavior in mice, which is distinct from that reported following a more extensive AMPA receptor blockade-mediated impairment of the dorsal hippocampus (Riedel et al., 1999).
5. Conclusions
Inactivation of the CA1 region during the NOP and MWM test sessions impaired memory retrieval to the same degree, as assessed by the respective tasks. These results indicate that the rodent CA1 plays a comparable role in the retrieval of object memory and of spatial memory. Lesion size has been presented as a determining factor in whether permanent hippocampal damage affects object recognition memory. Specifically, it has been argued that hippocampal lesions affecting less than 75% of the hippocampus are insufficient to produce an object recognition deficit (Broadbent et al., 2004). However, the inactivation technique used in the present study likely affected much less than 75% of hippocampal volume yet still elicited robust impairments. We previously reported that 30 min after intra-CA1 infusion, fluorophore-conjugated muscimol spreads to approximately a 300 lm radius, while remaining confined to the CA1 region of the dorsal hippocampus, thereby affecting less than 1% of total hippocampal volume (Cohen et al., 2013). The current study used an identical infusion technique and volume of muscimol, and our analyses indicate a similar pattern of distribution within the CA1 region (see Fig. 1A). Thus, the current study provides evidence that selective inactivation of the CA1 region, comprising a small percentage of the dorsal hippocampus is sufficient to produce dramatic deficits in the retrieval of both spatial memory and object memory.
For the present study, we opted to first examine the effects of intra-CA1 muscimol on the retrieval of memory in the NOP task, and then follow with an analysis of the same manipulation on the retrieval of memory in the MWM task. While task order may have influenced the behavioral results, we chose to expose the mice to the less stressful NOP task first before proceeding with the more aversive MWM task. We interpret the remarkable consistency in degree of memory retrieval impairment induced by intra-CA1 muscimol in both tasks as evidence that task order had limited influence on the main effects of the manipulation. Furthermore, the rate of acquisition of the MWM task by mice previously trained and tested in the NOP task appears to be consistent with MWM task acquisition data we previously reported (Stackman et al., 2012), suggesting that prior NOP training did not affect subsequent MWM training. Finally, there appeared to be no carryover effects of intra-CA1 muscimol on MWM task acquisition as NOP pre-test muscimol-treated mice acquired the MWM task similarly to that of NOP pre-test saline-treated mice.
In summary, the findings support the view that the CA1 region of the dorsal hippocampus is necessary for object recognition memory retrieval. More importantly, the reported results demonstrate that the impairments exhibited in NOP and MWM after bilateral intra-CA1 muscimol were comparable, indicating for the first time that the CA1 region of dorsal hippocampus contributes equally to the retrieval of both object recognition memory and spatial memory. These novel findings suggest a potential division of resources within the CA1 region of the hippocampus to support both spatial and non-spatial object memory. Additionally, the results provide further evidence that intracranial microinfusion of muscimol is an effective and temporary method for selectively inactivating a given brain region. Finally, the current findings support the view that the NOP task represents a compelling non-aversive alternative to the MWM as test of hippocampal function for rodents; which, while being non-spatial in nature, is equally dependent on CA1 neuronal activity.
Acknowledgements
This research was supported by a grant from the NIH/NIMH (MH086591) to RWS.
References
- Baker KB, & Kim JJ (2002). Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning & Memory, 9, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, & Warburton EC (2011). When is the hippocampus involved in recognition memory? Journal of Neuroscience, 31, 10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC, Koder T, Dolman NP, More JC, Aggleton JP, … Brown MW (2006). The different effects on recognition memory of perirhinal kainate and NMDA glutamate receptor antagonism: Implications for underlying plasticity mechanisms. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26, 3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer Z, Chwiesko C, Kitsukawa T, & Sauvage MM (2013). Spatial and stimulus-type tuning in the LEC, MEC, POR, PrC, CA1, and CA3 during spontaneous item recognition memory. Hippocampus, 23, 1425–1438. [DOI] [PubMed] [Google Scholar]
- Berlyne DE (1950). Novelty and curiosity as determinants of exploratory behaviour. British Journal of Psychology. General Section, 41, 68–80. [Google Scholar]
- Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, … Moser MB (2013). Grid cells require excitatory drive from the hippocampus. Nature Neuroscience, 16, 309–317. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Decker MW, Gamboa LP, Izquierdo I, & McGaugh JL (1990). Muscimol injections in the medial septum impair spatial learning. Brain Research, 522, 227–234. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, & Clark RE (2010). Object recognition memory and the rodent hippocampus. Learning & Memory, 17, 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, & Clark RE (2004). Spatial memory, recognition memory, and the hippocampus. Proceedings of the National academy of Sciences of the United States of America, 101, 14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, & Aggleton JP (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2, 51–61. [DOI] [PubMed] [Google Scholar]
- Brown MW, Warburton EC, & Aggleton JP (2010). Recognition memory: Material, processes, and substrates. Hippocampus, 20, 1228–1244. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Stackman RW, & Walsh TJ (1989). Intraseptal administration of muscimol produces dose-dependent memory impairments in the rat. Behavioral and Neural Biology, 52, 357–369. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, & Squire LR (2000). Impaired recognition memory in rats after damage to the hippocampus. Journal of Neuroscience, 20, 8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Ásgeirsdóttir HN, & Stackman RW Jr., (2013). The rodent hippocampus is essential for nonspatial object memory. Current Biology, 23, 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, & Stackman RW Jr., (2015). Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behavioural Brain Research, 285, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima MN, Luft T, Roesler R, & Schroder N (2006). Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neuroscience Letters, 405, 142–146. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2001). The hippocampus and declarative memory: Cognitive mechanisms and neural codes. Behavioural Brain Research, 127, 199–207. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, & Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research, 31, 47–59. [DOI] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, & Eichenbaum H (2010). Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learning & Memory, 17, 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood SE, Bartko SJ, Saksida LM, & Bussey TJ (2007). Rats spontaneously discriminate purely visual, two-dimensional stimuli in tests of recognition memory and perceptual oddity. Behavioral Neuroscience, 121, 1032–1042. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, & Bussey TJ (2005). Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus, 15, 347–355. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, & Paxinos G (2008). The mouse brain in stereotaxic coordinates (3rd ed.). San Diego, CA: Academic Press. [Google Scholar]
- Gaskin S, Tremblay A, & Mumby DG (2003). Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus, 13, 962–969. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Rowe WB, Heman KL, Mesches MH, Young DA, Rose GM, &Bickford PC (2002). Effects of hippocampal lesions on patterned motor learning in the rat. Brain Research Bulletin, 58, 581–586. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, & Stackman RW (2004). On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiology of Learning and Memory, 82, 26–34. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, & Deadwyler SA (1999). Distribution of spatial and nonspatial information in dorsal hippocampus. Nature, 402, 610–614. [DOI] [PubMed] [Google Scholar]
- Lee I, & Kesner RP (2004). Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus, 14, 301–310. [DOI] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, & Freret T (2013). Object recognition test in mice. Nature Protocols, 8, 2531–2537. [DOI] [PubMed] [Google Scholar]
- Manns JR, & Eichenbaum H (2009). A cognitive map for object memory in the hippocampus. Learning & Memory, 16, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, & O’Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Schenk F, Tweedie F, & Jarrard LE (1990). Ibotenate lesions of hippocampus and/or subiculum: Dissociating components of allocentric spatial learning. European Journal of Neuroscience, 2, 1016–1028. [DOI] [PubMed] [Google Scholar]
- Moser MB, & Moser EI (1998). Distributed encoding and retrieval of spatial memory in the hippocampus. Journal of Neuroscience, 18, 7535–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, & Lehmann H (2002). Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learning & Memory, 9, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Tremblay A, Lecluse V, & Lehmann H (2005). Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus, 15, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Nadel L, & Peterson MA (2013). The hippocampus: Part of an interactive posterior representational system spanning perceptual and memorial systems. Journal of Experimental Psychology: General, 142, 1242–1254. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff E, Martin SJ, Bridge H, … Morris RG (1999). Reversible neural inactivation reveals hippocampal participation in several memory processes. Nature Neuroscience, 2, 898–905. [DOI] [PubMed] [Google Scholar]
- Sauvage MM, Nakamura NH, & Beer Z (2013). Mapping memory function in the medial temporal lobe with the immediate-early gene Arc. Behavioural Brain Research, 254, 22–33. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, & Clark RE (2007). Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience, 8, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW Jr., Lora JC, & Williams SB (2012). Directional responding of C57BL/6J mice in the Morris water maze is influenced by visual and vestibular cues and is dependent on the anterior thalamic nuclei. Journal of Neuroscience, 32, 10211–10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53, 1–25. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bartko SJ, Saksida LM, & Bussey TJ (2010). Muscimol, AP5, or scopolamine infused into perirhinal cortex impairs two-choice visual discrimination learning in rats. Neurobiology of Learning and Memory, 93, 221–228. [DOI] [PubMed] [Google Scholar]
- Winters BD, & Bussey TJ (2005). Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. Journal of Neuroscience, 25, 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, & Bussey TJ (2004). Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. Journal of Neuroscience, 24, 5901–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, & Eichenbaum H (1999). The global record of memory in hippocampal neuronal activity. Nature, 397, 613–616. [DOI] [PubMed] [Google Scholar]
- Wood ER, Mumby DG, Pinel JP, & Phillips AG (1993). Impaired object recognition memory in rats following ischemia-induced damage to the hippocampus. Behavioral Neuroscience, 107, 51–62. [DOI] [PubMed] [Google Scholar]


