Abstract
Salvadora persica L. (S. persica, Siwak) is an ethnic plant that is widely used for improving oral hygiene. This study aimed to provide a phytochemical profiling of S. persica ethyl acetate fraction (SPEAF) and to evaluate the healing activity of a muco-adhesive formula of the fraction against acetic acid-induced oral ulcers in rats. HPLC-ESI-QTOF-MS-MS analysis of SPEAF resulted in the tentative identification of 56 metabolites containing fatty acids (23%), urea derivatives (10.5%) and sulphur compounds (10%), in addition to several amides, polyphenols and organic acids (6.5%, 5% and 2%, respectively). For the first time, 19 compounds were identified from S. persica. In vitro and in vivo experiments indicated that the extract is non-toxic. SPEAF exhibited superior healing activities compared to both the negative and positive control groups on days 7 and 14 of tongue ulcer induction. This was confirmed by histopathological examinations of haematoxylin and eosin-stained (H&E) and Masson’s trichrome-stained tongue sections. Moreover, SPEAF showed potent anti-inflammatory activities, as evidenced by the inhibited expression of interleukin-6 (IL-6) and tumour necrosis alpha (TNF-α). Moreover, SPEAF exhibited potent antioxidant activity, as it prevented malondialdehyde (MDA) accumulation, reduced glutathione (GSH) depletion and superoxide dismutase (SOD) exhaustion. SPEAF significantly enhanced hydroxyproline tongue content and upregulated collagen type I alpha 1 (Col1A1) mRNA expression. SPEAF also improved angiogenesis, as shown by the increased mRNA expression of the angiopoietin-1 (Ang-1). In conclusion, S. persica has a wide range of secondary metabolites and ameliorates acetic acid-induced tongue ulcers in rats. This can be attributed, at least partly, to its anti-inflammatory, antioxidant, procollagen and angiogenic activities. These findings provide support and validity for the use of S. persica as a traditional and conventional treatment for oral disorders.
Keywords: Salvadora persica L., HPLC-ESI-QTOF-MS-MS analysis, anti-inflammatory, antioxidant, angiogenesis, oral ulcer, wound healing
1. Introduction
Several diseases of known and unknown aetiology can cause recurrent intraoral mucosal ulceration. Oral ulceration can be benign conditions, such as aphthae, herpetiform, erosive lichen planus, benign mucous membrane pemphigoid, allergy, infection, candidiasis, streptococcal stomatitis, histoplasmosis and acute necrotising ulcerative gingivitis, or conditions with a potential for a severe course, such as pemphigus vulgaris, lupus erythematosus, cyclic neutropenia neoplasm, Behcet’s disease and erythema multiforme [1,2].
Oral ulceration has been linked to a wide range of systemic medicines, with clinical manifestations ranging from superficial, nonspecific ulcerations to aphthous-like lesions or widespread mucosal erosions [3]. Nicorandil [4,5], captopril [6] and some nonsteroidal anti-inflammatory medicines can produce aphthous-like and nonspecific oral ulcers [7]. Oral ulcers can be classified as acute or chronic depending on their presentation and progress, with chronic ulcers lasting more than 14 days. [2].
Therapeutic options for the treatment of recurrent aphthous stomatitis include topical medications, antiseptics, analgesics, corticosteroids and antibiotics. In addition to systemic medication, immunomodulators have been reported [3]. Thalidomide is a tumour necrosis alpha (TNF-α) inhibitor with anti-inflammatory activity. However, due to teratogenic and other side effects (e.g., irreversible polyneuropathy), its use has been restricted [8]. The possible use of biological agents (e.g., infliximab) as anti-TNF-α therapy for recurrent aphthae in Behcet’s illness has also been documented [9].
Wound healing is a complex process involving haemostasis, inflammation, proliferation and tissue remodelling [10]. Many phyto-therapeutic studies have been conducted on oral antiulcerogenic activity based on haemostasis [11]; antioxidant [12,13,14], antibacterial [15] and anti-inflammatory activities [11,16,17,18,19]; proliferation/angiogenesis [20]; and remodelling phase/re-epithelialisation [11].
Salvadora persica L. Salvadoraceae (Siwak, S. persica) is a plant that has been used for many centuries as an oral hygiene tool, particularly in Saudi Arabia. Using Siwak (tooth stick) to clean the mouth is a well-established Islamic belief [21]. S. persica has been shown to have anti-gingivitis, anti-plaque and anti-cariogenic properties; gingival wound healing property; whitening capability; orthodontic chain preservation capability; and biocompatibility with oral cells in many investigations. S. persica in various forms has helped manage and treat oral health [22]. Comprehensive phytochemical investigations have revealed the presence of carbohydrates, flavonoids, terpenes, sterols, alkaloids, glycosides, organic sulphur compounds, elemental sulphur and small amounts of fluoride, calcium, phosphorus, silica and ascorbic acid [21,23].
Oral ulcers heal through the same phases as cutaneous wounds, but there are some differences, such as the wet external environment, the presence of saliva and various cell morphologies [24]. The environment of oral ulcers directs drug delivery to the application of muco-adhesive formulation [25,26]. In light of the research cited, this study prepared a fraction of S. persica, which has potential wound healing activity, in chemically induced tongue ulcers in rats and explored its components by using HPLC/MS/MS analysis.
2. Materials and Methods
2.1. Plant Material
S. persica sticks were bought from Riyadh, Saudi Arabia. The plant was identified by Dr. Talal Dahan, assistant professor of plant classification at Bisha University, Saudi Arabia. A voucher specimen (No. PG005) was deposited in properly labelled polythene bags for future reference at Department of Pharmacognosy, Faculty of Pharmacy, The British University in Egypt, Egypt. The authors followed the IUCN Policy Statement on Research Involving Species at Risk of Extinction.
2.2. Animals
Fifty male Wistar rats (200–225 g) were used for the study, courtesy of the Animal Facility, Faculty of Pharmacy, King Abdulaziz University (KAU), Saudi Arabia. The animals were kept on a 12 h light–dark cycle and a controlled temperature of 22 ± 2 °C. The research ethics committee of the Faculty of Pharmacy, King Abdulaziz University (KAU), officially approved experimental protocols (Reference # PH-1443-22).
2.3. Chemicals
All solvents were of analytical grade, while those used in UPLC/PDA/ESI/MS assays were of HPLC grade. Carboxymethyl cellulose (CMC) was obtained from Sigma-Aldrich (Taufkirchen, Germany).
2.4. Preparation of S. persica Ethyl Acetate Fraction (SPEAF)
Fresh plant sticks of S. persica were freeze-dried (laboratory freeze dryer VaCo 5, ZIRBUS, Bad Grund, Germany) and then ground to a fine powder using a commercially available food blender. A ground sample (500 g) was used to prepare the fraction. Ethyl acetate fraction was prepared by cold-percolating 500 g of dried powder of the plant sticks in 1 L of ethyl acetate for 72 h, and fresh solvent was used every 24 h. This fraction was prepared after isolating petroleum ether fraction using the same method described above. The solvent was removed and recovered in a rotary evaporator (Büchi Rotavapor RII; Büchi Labortechnik, Flawil, Switzerland) at 40 °C using a Büchi vacuum pump to yield 5.4 g of yellowish-brown powder, which was kept in a brown screw-capped tube in a −20 °C freezer until further analysis.
2.5. High-Resolution HPLC-ESI-QTOF-MS-MS Analysis
A 6530 QTOF LC/MS (Agilent Technologies) equipped with an autosampler (G7129A), a quat pump (G7104C) and a column comp (G7116A) was used for chromatographic separation. The injection volume was 6 μL. The analytes were separated in a Zorbax RP-18 column (Agilent Technologies; dimensions: 150 mm × 3 mm, dp = 2.7 μm) at a flow rate of 0.230 mL/min. The mobile phase consisted of a combination of solvent A (0.1% formic acid) and solvent B (acetonitrile + 0.1% formic acid). The gradient elution was as follows: 0–20 min (98–90% A), 20–50 min (90–80% A), 50–70 min (80–50% A), 70–90 min (50–30% A), 90–110 min (30–10% A) and 110–120 min (10–0% A) [27]. Mass spectra were simultaneously acquired using ESI in (+,−) ionisation modes, with a capillary voltage of 5500 V. The mass spectra were recorded in the m/z range of 100–1000 m/z. The gas temperature and drying gas flow were 190 °C and 6 L·/min, respectively. The skimmer and fragmentator voltages were set to 65 V and 130 V, respectively, and collision energy was 10 V. The nebulisation pressure was 25 psi g.
Tentative Identification of Metabolites
The tentative identification and analysis of LC-MS-MS of the metabolites of S. persica were carried out using Sirius® software version 4.7.4 to predict fragmentation and molecular formulae [28]. The chemical structures were predicted using CSI: FingerID® [29], while compound classes were predicted directly from MS/MS using CANOPUS® [30].
2.6. Preparation of Plain and Ethyl Acetate Fraction Muco-Adhesive Formulae
In order to prepare the adhesive sponge formula, 2% CMC (w/v) was sprinkled in distilled water and stirred until homogenous gels formed, as prescribed by [25]. SPEAF was dissolved in distilled water to a final concentration of 5.0% (w/v) and stirred using a magnetic stirrer until homogeneous dispersions were achieved. Then, using a magnetic stirrer, CMC was sprinkled in the previously prepared dispersions until a consistent gel was achieved. Muco-adhesive property was achieved by the determination of muco-adhesive time of prepared formulae [25].
2.7. Cytotoxicity Assay
Oral epithelial cells (OEC) were obtained from Nawah Scientific Inc., (Mokatam, Cairo, Egypt). Cell viability was assessed by SRB assay. Aliquots of 100 μL cell suspension (5 × 103 cells) were pipetted in 96-well plates and incubated in complete media for 24 h. Cells were treated with another aliquot of 100 μL media containing SPEAF at various concentrations. After 72 h of exposure, cells were fixed by replacing media with 150 μL of 10% TCA and incubated at 4 °C for 1 h. The TCA solution was removed, and the cells were washed 5 times with distilled water. Aliquots of 70 μL SRB solution (0.4% w/v) were added and incubated in a dark place at room temperature for 10 min. Plates were washed 3 times with 1% acetic acid and allowed to air-dry overnight. Then, 150 μL of TRIS (10mM) was added to dissolve protein-bound SRB stains; the absorbance was measured at 540 nm by using a BMGLABTECH®-FLUOstar Omega microplate reader (Ortenberg, Germany) [31].
2.8. Acute Oral Toxicity Study
Acute oral toxicity of SPEAF was evaluated in male Wistar rats according to OECD guideline No. 423. Based on previous pilot studies in our laboratories, a limit test was performed. Animals were fasted overnight, and SPEAF was administered orally using gastric feeding needle at a dose of 2000 mg/kg [32].
2.9. Experimental Design
The animals were randomly divided into five groups, with 10 in each group. With the exception for negative control animals (10 rats), the rats were anaesthetized using ketamine (50 mg/kg) and xylazine (5 mg/kg). Round filter papers with a diameter of 5.0 mm were soaked in 15 mL of 50% acetic acid. The acid-soaked filter paper was pressed onto the inferior surface of the tongue for 60 s [33]. The animals were assigned into five groups, with 10 in each group, as follows.
Group 1 (negative control): normal rats with no exposure to acetic acid or any treatment.
Group 2 (acetic acid ulcer): acetic acid-challenged animals with no treatment.
Group 3 (ulcer + vehicle): acetic acid ulcer group treated topically once daily with plain muco-adhesive formulae in the ulcer area.
Group 4 (ulcer + SPEAF): acetic acid ulcer group treated topically once daily with SPEAF (5% in muco-adhesive formulae) in the ulcer area.
Group 5 (positive control): acetic acid ulcer group treated topically once daily with Jogel® (Sedico, Giza, Egypt; 10% jojoba oil and 0.5% lidocaine hydrochloride) in the ulcer area.
All treatments continued for 14 days. On day seven, four animals from each group were sacrificed by decapitation, and their tongues were dissected and kept in 10% neutral formalin. On day 14, the rest of the animals in all groups were sacrificed by decapitation, and their tongues were dissected. One part of the tongues from each animal was kept in 10% neutral formalin, and the other part was flash frozen in liquid nitrogen and kept at −80 °C for further analysis.
2.10. Histopathological Examination
The excised tongue tissues on days 7 and 14 were fixed in 10% neutral buffered formalin. They were dehydrated in serial dilutions of ethyl alcohol, immersed in xylene and embedded in paraffin. On the glass slides, 5-micrometre-thick sections were made. After dewaxing, the tissue sections were rehydrated. Some sections were stained using haematoxylin and eosin (H&E), and others were stained with Masson’s trichrome stains.
2.11. Immunohistochemical Staining
The tissue sections were de-paraffinised, rehydrated and boiled in 0.1 M citrate buffer (pH 6.0) for 10 min. The sections were then kept in 5% bovine serum albumin in Tris-buffered saline (TBS) for 2 h. The tissue sections were then incubated with primary antibodies to interleukin-6 (IL-6), (Cat. No.: ab9324, Abcam®, Cambridge, UK) and TNF-α (Cat. No.: ab220210, Abcam®, Cambridge, UK) at 4 °C for 12 h. Following flushing with TBS, the tissue sections were incubated with either anti-mouse or anti-rabbit biotinylated secondary antibody according to the primary antibody reactivity (Cell & Tissue Staining Kit, Cat. No.: CTS002, CTS006, R&D Systems, Minneapolis, MN, USA). Image evaluations were obtained using Image J (1.52a, National Institutes of Health NIH, Rockville, Maryland, USA) with a minimum of three sections per rat.
2.12. Biochemical Analysis
The tongue tissues were homogenised in a 10-fold volume of ice-cooled phosphate-buffered saline (50 mM potassium phosphate, pH 7.4). The homogenates were centrifuged for 15 min at 10,000× g and 4 °C, followed by the collection of the supernatant, which was used for oxidative stress analysis. Commercially available kits were used to assess the liver content of malondialdehyde (MDA; Cat. No. MD 2529; Biodiagnostic, Giza, Egypt) and reduced glutathione (GSH; Cat. No. GR 2511; Biodiagnostic, Giza, Egypt) and the enzyme activities of superoxide dismutase (SOD; Cat. No. SD 2521; Biodiagnostic, Giza, Egypt). Hydroxyproline was determined using ELISA kits (Cat. No. Ab22294, Abcam, Cambridge, UK), according to the manufacturer’s instructions.
2.13. RT-qPCR for Collagen Type I Alpha 1 (Col1A1) and Angiopoietin-1 (Ang-1)
Tongue homogenates were subjected to RNA extraction by using a commercially available kit (NucleoSpin, Macherey-Nagel GmbH & Co. KG, Duerin, Germany), followed by spectrophotometric determination of purity and concentration (dual-wavelength Beckman, Spectrophotometer, Foster City, California, USA). cDNA was performed using a cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Then, a PCR Master Mix Kit (Qiagen, Valencia, CA, USA) was used to perform PCR amplification reactions. The Col1A1 (NM_053304.1) primer and GAPDH (NM_017008.4) as housekeeping genes were used. The forward/reverse nucleotide sequences of Col1A1, Ang-1 and GAPDH were 5′-ATCAGCCCAAACCCCAAGGAGA-3/5′-CGCAGGAAGGTCAGCTGGATAG-3, 5′-AGGCCCCTCTGAACCCTAAG-3/5′-AGAGGCATACAGGGACAACACA-3 and 5′-CCATTCTTCCACCTTTGATGCT-3/5′-TGTTGCTGTAGCCATATTCATTGT-3, respectively. The data were expressed in the cycle threshold (Ct). The expression of Col1A1 relative to GAPDH was calculated based on the delta–delta Ct (ΔΔCt) values.
2.14. Statistical Analysis
All data were expressed as mean ± SD. The data were analysed using a one-way analysis of variance and Tukey test. All analyses were performed using GraphPad Prism software version 8.00 (GraphPad Software, La Jolla, CA, USA). A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Identification of SPEAF
For the complete phytochemical profiling of the ethyl acetate fraction, a high-resolution ESI-QTOF-LC-MS-MS analysis was carried out. A total of 56 compounds were detected and tentatively identified (Table 1), 19 of which were identified in S. persica for the first time. The majority of the compounds identified were fatty acids (23%), followed by urea derivatives (10.5%) and sulphur compounds (10%). Several amides, phenolic compounds and organic acids were also detected at 6.5%, 5% and 2%, respectively. Other individual minor compounds were detected at 1.5%.
Table 1.
LC–MS–MS data of the tentatively identified compounds in SPEAF.
| # | Retention Time | Compound | Area% | MS1 (−ve) | MS1 (+ve) | MS2 | Molecular Formula | Error |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.02 | Gallic acid | 1.5 | 169.01417 | 125 | C7H6O5 | 6.04 | |
| 2 | 10.6 | Glutaric acid | 0.43 | 131.042 | --- | C5H8O4 | −1.53 | |
| 3 | 11.16 | Deoxy ellagic acid | 0.67 | 287.0191 | 241, 181, 151 | C14H8O7 | −2.08 | |
| 4 | 13.97 | Hydroxy stachrydine | tr | 158.0822 | 141, 131, 115 | C7H13O3N | −0.6 | |
| 5 | 16.17 | Sugar derivative | 0.43 | 267.1081 | 221, 153 | C10H20O8 | −0.41 | |
| 6 | 21.2 | Methoxy ellagic acid | 0.3 | 315.01459 | 241, 181, 151 | C15H8O8 | −0.16 | |
| 7 | 22.38 | Benzamide | 1.13 | 122.0599 | 105 | C7H7NO | −1.15 | |
| 8 | 23.95 | Salicylic acid | 0.73 | 137.0231 | --- | C7H6O3 | −1.28 | |
| 9 | 24.69 | Diethyl malate | 0.7 | 189.0764 | 145, 100 | C8H14O5 | −0.46 | |
| 10 | 25.4 | Sulfated hexosyl phenolic derivative | 0.22 | 395.0676 | 315, 241, 153 | C14H20O11S | 2.22 | |
| 11 | 27.067 | Methylbenzamide | 0.32 | 136.0747 | --- | C8H9NO | −7.27 | |
| 12 | 27.41 | Unknown | 1.43 | 194.0809 | 164, 134 | C10H13NO3 | −1.31 | |
| 13 | 28.111 | Benzyl urea | 2.52 | 151.0857 | --- | C8H10N2O | 5.89 | |
| 14 | 31.31 | O-benzyl hexosyl sulfate | 0.42 | 349.0585 | 269, 241, 193 | C13H18O9S | −3.94 | |
| 15 | 32.83 | Unknown | 0.35 | 521.2331 (2M − H) | 260 | C11H19NO6 | −2.97 | |
| 16 | 34.32 | Phenolic acid derivative | 0.14 | 281.13909 | 151 | C15H22O5 | −1.27 | |
| 17 | 34.36 | Di-O-methyl ellagic acid | 0.98 | 329.032 | 315, 241, 181, 151 | C16H10O8 | 5.19 | |
| 18 | 35.902 | Benzyl isothiocyanate | 8.7 | 150.0374 | --- | C8H7NS | −5.95 | |
| 19 | 37.29 | Coumaric acid | tr | 163.03897 | 119 | C9H8O3 | −1.54 | |
| 20 | 38.88 | Acetyl Phenyl alanine | tr | 413.16868 (2M − H) | 415.1810 (2M + H) | 206, 188 | C11H13NO3 | −7.58 |
| 21 | 47.645 | Unknown | 1.53 | 123.0431 | --- | C5H4N3O | 3.15 | |
| 22 | 56.8 | Ferulic acid | 0.39 | 193.0492 | 179, 149 | C10H10O4 | −1.74 | |
| 23 | 57.55 | Methoxy flavanone hexosyl rhamnoside | 0.58 | 609.18129 | 463, 301 | C28H34O15 | −1.98 | |
| 24 | 59.78 | Caffeic acid conjugate | 0.13 | 387.0352 | 341, 193 | C18H12O10 | −3.41 | |
| 25 | 60.399 | N-benzyl-N′ hydroxy benzyl urea | 0.09 | 257.1269 | 241, 198, 181, 163 | C8H10N2O2 | −6.03 | |
| 26 | 66.42 | Caffeic acid conjugate | 0.07 | 377.18179 | 341, 161 | C17H30O9 | 1.67 | |
| 27 | 67.61 | Syringin | 0.15 | 371.1344 | 209 | C17H24O9 | 0.51 | |
| 28 | 70.88 | N-benzyl benzamide | 0.15 | 212.1057 | --- | C14H13NO | −6.08 | |
| 29 | 71.663 | N-benzyl 2-phenyl acetamide | 0.2 | 226.1215 | --- | C15H15NO | −5.04 | |
| 30 | 71.7 | N,N′ dibenzyl urea | 7.73 | 241.1326 | 181, 163, 108 | C15H18N2O | −3.9 | |
| 31 | 74.819 | Unknown | 0.06 | 353.1957 | --- | C19H28O6 | −1.94 | |
| 32 | 75.37 | Sulfur compound derivative | 0.61 | 281.0402 | 186 | C12H12N2O2S2 | −7.8 | |
| 33 | 92.482 | Hydroxy tetradecanoic acid | 3.94 | 487.4005 (2M − H) | 243 | C14H28O3 | 1.31 | |
| 34 | 102.79 | Hydroxy hexadecanoic acid | 0.69 | 543.4565 (2M − H) | 271.2266 | C16H32O3 | −0.63 | |
| 35 | 106.09 | Linolenic acid | 1.49 | 555.4408 (2M − H) | 557.4496 (2M + H) | 277 | C18H30O2 | −2.01 |
| 36 | 107.327 | Myristic acid | 0.45 | 455.4111 (2M − H) | 227 | C14H28O2 | 1.19 | |
| 37 | 108.559 | Hydroxy octadecenoic acid | 0.99 | 595.4890 (2M − H) | 297 | C18H34O3 | −8.92 | |
| 38 | 109.176 | Hexadecenoic acid | 0.95 | 507.4416 (2M − H) | 509.4512 (2M + H) | 253 | C16H30O2 | −0.64 |
| 39 | 109.35 | Unknown | 3.09 | 339.2299 | 253, 113 | C23H32O2 | −3.05 | |
| 40 | 111.332 | Arachidic acid | 0.12 | 313.2727 | 285, 267 | C20H42O2 | 4.52 | |
| 41 | 111.527 | Linoleic acid | 2.43 | 559.4781 (2M − H) | 561.4821 (2M + H) | 279 | C18H32O2 | −5.52 |
| 42 | 111.552 | Fatty acid amide derivative | 0.45 | 635.5489 (2M + H) | 318 | C21H35NO | −1.89 | |
| 43 | 113.309 | Fatty acid amide derivative | 0.88 | 687.5803 (2M + H) | 344 | C23H37NO | −4.5 | |
| 44 | 113.598 | Heptadecenoic acid | 0.29 | 535.4730 (2M − H) | 537.4845 (2M + H) | 267 | C17H32O2 | −0.36 |
| 45 | 115.622 | Hydroxy octadecanoic acid | 0.54 | 599.5241 (2M − H) | 299 | C18H36O3 | −2.59 | |
| 46 | 116.188 | Palmitic acid | 4.98 | 511.4714 (2M − H) | 513.4861 (2M + H) | 255 | C16H32O2 | −2.42 |
| 47 | 116.23 | Cholesterol derivative | tr | 663.4529 | 607, 551 | C39H58N4O5 | 5.74 | |
| 48 | 117.307 | Oleic acid | 4.71 | 563.5026 (2M − H) | 565.5175 (2M + H) | 281 | C18H34O2 | −2.38 |
| 49 | 118.394 | N-benzylpalmitamide | 0.06 | 691.6144 (2M + H) | 346 | C23H39NO | −0.42 | |
| 50 | 120.34 | N-benzyl octadecenamide | 1.79 | 743.6433 (2M + H) | 372 | C25H41NO | −0.05 | |
| 51 | 121.418 | Nonadecenoic acid | 0.26 | 591.5330 (2M − H) | 295 | C19H36O2 | −3.79 | |
| 52 | 122.592 | N-benzyl heptadecanamide | 0.1 | 719.6405 (2M + H) | 360 | C24H41NO | 3.63 | |
| 53 | 123.163 | Hydroxy eicosanoic acid | 0.73 | 655.5859 (2M − H) | 327 | C20H40O3 | −2.7 | |
| 54 | 123.505 | Stearic acid | 0.31 | 567.5334 (2M − H) | 283 | C18H36O2 | −3.24 | |
| 55 | 123.666 | Diisooctyl phthalate | 1.2 | 391.2832 | 167, 149 | C24H38O4 | −5.58 | |
| 56 | 125.314 | 13-Docosenamide | 1.44 | 338.3416 | 321 | C22H43NO | −0.13 |
Fifteen different fatty acids were tentatively identified. Palmitic and oleic acids were the major fatty acids detected; both were identified previously in the stems and roots of S. persica [34]. Myristic acid (36), hydroxy octadecenoic acid (37), hexadecenoic acid (3), arachidic acid (40), linoleic acid (41), heptadecenoic acid (44) and stearic acid (54) were detected previously in the stems and roots of S. persica [23,34], while linolenic acid (35) was detected previously in the seed oil of S. persica [35]. Hydroxytetradecanoic acid (33), hydroxyhexadecanoic acid (34), hydroxyoctadecanoic acid (45), nonadecenoic acid (51) and hydroxyeicosanoic acid (53) were detected in this plant for the first time.
Three urea derivatives were detected in the fraction, and the majority of them consisted of N,N′-dibenzyl urea (30, 7.7%), followed by benzyl urea (13, 2.5%) and n-benzyl-N′ hydroxy benzyl urea (25, 0.09%). These compounds were detected and isolated previously in this species [23,36].
Four sulphur-containing compounds were also detected. Benzyl isothiocyanate (18) was the major compound found in the fraction (8.7%); it was detected previously with O-benzyl hexosyl sulphate (14) in S. persica [23,34]. The molecular formulae of the sulphated hexosyl phenolic derivative (10) and sulphur compound derivative (32) are C14H20O11S and C12H12N2O2S2, respectively. Both compounds were not detected before in genus Salvadora.
Ten amide derivatives were also observed, most of which were amide derivatives of fatty acids. Compounds n-benzyl benzamide (28), n-benzyl 2-phenyl acetamide (29), n-benzylpalmitamide (49) and n-benzyl heptadecanamide (52) were identified previously in S. persica [23,36], while benzamide (7), methylbenzamide (11), fatty acid amide derivatives (42, 43), n-benzyl octadecenamide (50) and 13-docosenamide (56) were detected in this plant for the first time.
Regarding phenolic compounds, 11 compounds were detected. All were phenolic acids and their derivatives, except compound 23 (methoxy flavanone hexosyl rhamnoside), which is a flavonoid glycoside. With a molecular formula of C15H22O5, compound 16 was detected for the first time in S. persica, and it was tentatively identified as a phenolic acid derivative. All other phenolic compounds were previously reported in the same plant [23,37,38,39]. Three organic acids and their derivatives were identified. Salicylic and glutaric acids were detected previously in the plant [37,40], whereas compound 9 (diethyl malate) was detected for the first time in S. persica.
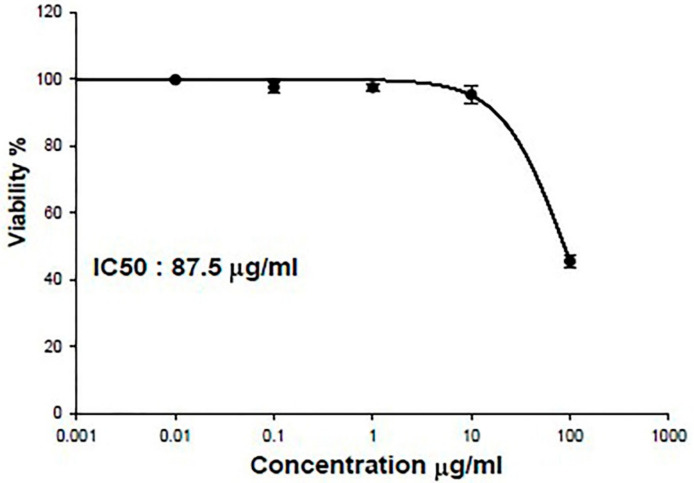
3.2. IC50 of SPEAF on OEC
As shown in Figure 1, IC50 of SPEAF in oral epithelial cells (OEC) was relatively high (87.5 μg/mL) indicating very weak cytotoxicity of the extract.
Figure 1.
IC50 of SPEAF in oral epithelial cells (OEC).
3.3. Acute Oral Toxicity Study
No mortality was observed in the tested animals at 24 h after oral administration of 2000 mg/kg of SPEAF. The same result was obtained when the test was repeated using three additional animals at the same dose. According to the Acute Toxic Class Method reported in OECD guidelines No.423, SPEAF is considered to be Category 5 with LD50 > 2000 mg/kg.
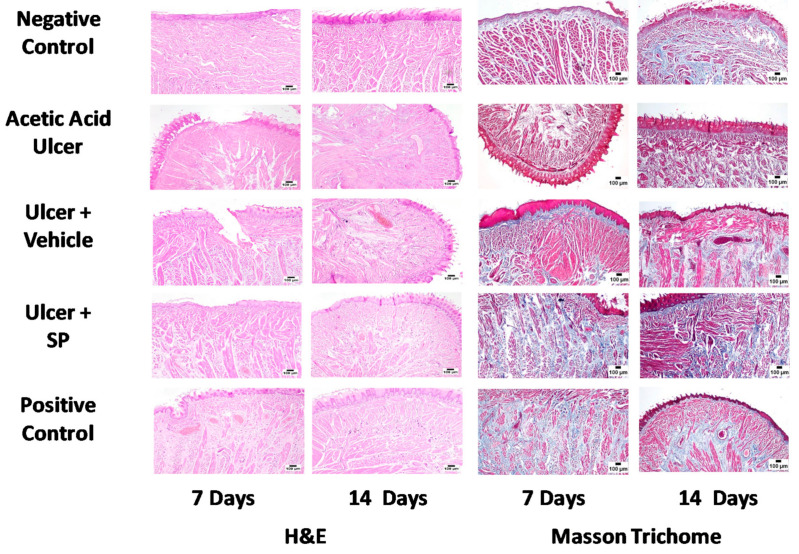
3.4. Histopathological Examination
Microscopic examination of H&E-stained tongue sections from negative control animals on days 7 and 14 showed skeletal muscle arranged in longitudinal and transverse bundles with a normal histological structure. The tongues were covered by papillae and stratified squamous epithelium that appeared keratinised on the dorsal surface. The acetic acid group showed several ulcerative mucosal surfaces, with necrotic areas that extended to the underlying muscle bundles. Severely dilated blood spaces were commonly observed. Vehicle-treated animals exhibited ulceration of the epithelial covering layer accompanied by variable haemorrhages and the accumulation of eosinophilic tissue debris. Dispersions of muscle bundles with oedema, inflammatory cells and increased number of mast cells were also observed. SPEAF-treated animals showed obvious protection against acetic acid ulcers. The mucosal surface appeared normal in several examined sections. A few sections showed ulcerative epithelial layers, while other sections revealed an aggregation of perivascular inflammatory cell infiltration. Positive control animals exhibited noticeable protection, as numerous sections showed marked healing of the ulcerative area, but congested blood vessels with ulceration were observed in some sections. In order to substantiate these observations, collagen deposition was assessed by staining sections from all groups using Mason’s trichrome stain. The negative control animals showed normal histological architecture. The acetic acid group showed tongue sections with a relatively decreased deposition of collagen fibers. The tongue sections from the SPEAF and positive control groups exhibited a higher degree of collagen deposition (Figure 2).
Figure 2.
Histopathological effects of SPEAF on acetic acid-induced tongue ulcer of rats. SPEAF: S. persica ethyl acetate fraction. Haematoxylin and Eosin (H&E).
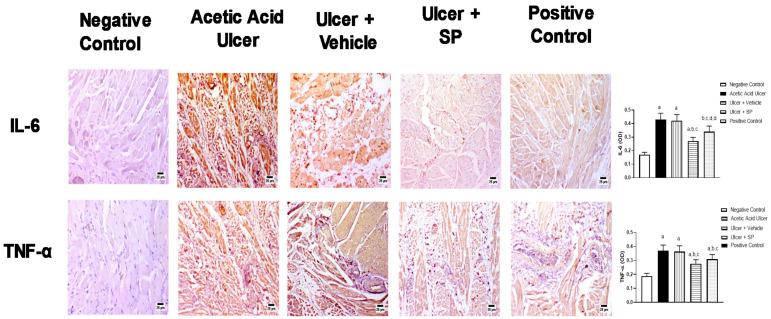
3.5. Immunohistochemical Assessment of the Expression of Inflammation Markers
The potential of SPEAF as an anti-inflammatory agent was assessed in stressed tongue tissues. Figure 3 shows that the challenge in tongues with acetic acid was associated with the enhanced expressions of IL-6 and TNF-α by 176% and 84%, respectively, compared to corresponding control values. The application of SPEAF significantly reduced IL-6 and TNF-α expressions by 36% and 24%, respectively, compared to the acetic acid-alone group. Similarly, the treatment of tongues with the positive control preparation significantly inhibited IL-6 and TNF-α by 20% and 15%, respectively, compared to the acetic acid ulcer group.
Figure 3.
Effect of SPEAF on expression of inflammation markers in acetic acid-induced tongue ulcer in rats. Statistical analysis was performed by one-way ANOVA followed by Tukey’s test. a Significant difference from negative control group at p < 0.05. b Significant difference from acetic acid group at p < 0.05. c Significant difference from ulcer + vehicle group at p < 0.05. d Significant difference from Ulcer + SPEAF group at p < 0.05. SPEAF: S. persica ethyl acetate fraction.
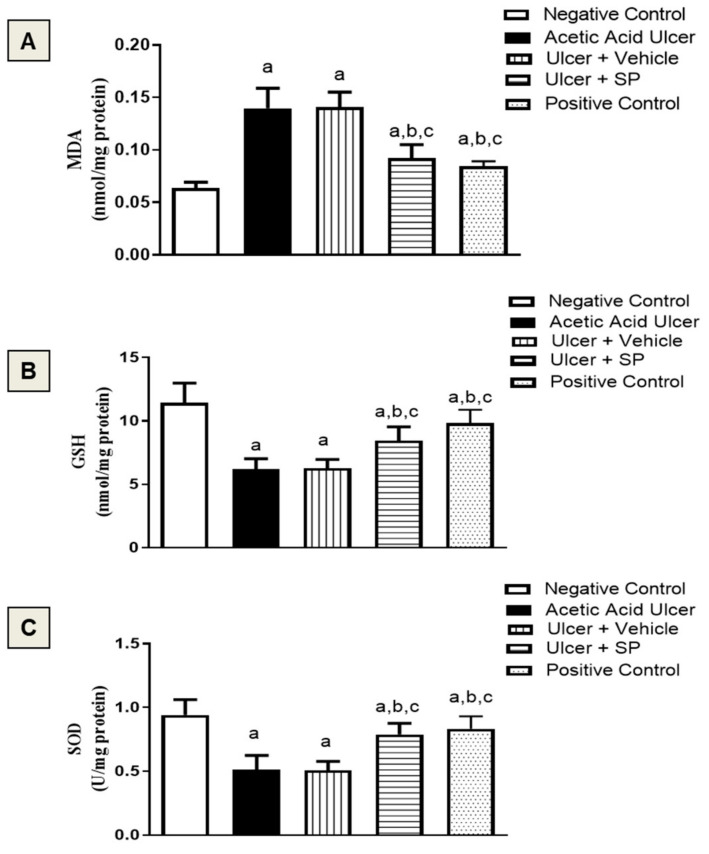
3.6. Assessment of Oxidative Status
Figure 4A shows that MDA, a product of polyunsaturated fatty acid peroxidation, was increased by an acetic acid challenge by 133% of the control value. SPEAF ameliorated MDA accumulation by 36%. Moreover, acetic acid resulted in GSH depletion compared to the negative control values. SPEAF and the positive control preparation significantly ameliorated GSH decrease by 34% and 56%, respectively, compared to the acetic acid group (Figure 4B). Acetic acid insult was associated with decreased SOD activity. Both SPEAF and the positive control preparation inhibited SOD exhaustion by 60% and 62%, respectively (Figure 4C).
Figure 4.
Effect of SPEAF on MDA (A), GSH (B) and SOD (C) in acetic acid-induced tongue ulcer in rats. Data are presented as mean ± SD (n = 6). Statistical analysis was performed by one-way ANOVA followed by Tukey’s test. a Significant difference from negative control group at p < 0.05. b Significant difference from acetic acid group at p < 0.05. c Significant difference from ulcer + vehicle group at p < 0.05. SPEAF: S. persica ethyl acetate fraction. MDA: malondialdehyde. GSH: glutathione. SOD: superoxide dismutase.
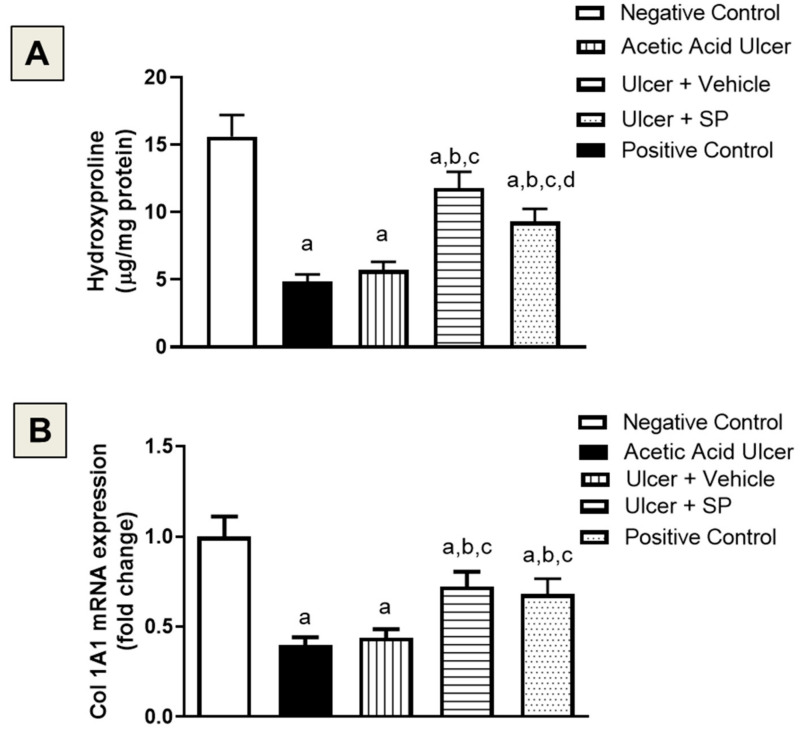
3.7. Assessment of Collagen Content
Acetic acid-induced tongue ulcers were associated with a significant decrease in hydroxyproline by 57% compared to the negative control group. The SPEAF group showed the highest hydroxyproline content, as it was significantly enhanced by 76% compared to the acetic acid group (Figure 5A). These results were confirmed by assessing the mRNA expression of Col1A1. Both SPEAF and the positive control preparation significantly enhanced Col1A1 expression by 64% and 55%, respectively, compared to the acetic acid group (Figure 5B).
Figure 5.
Effect of SPEAF on hydroxyproline content (A) and Col1A1 expression (B) in acetic acid-induced tongue ulcer in rats. Data are presented as Mean ± SD (n = 6). Statistical analysis was performed by one-way ANOVA followed by Tukey’s test. a Significant difference from negative control group at p < 0.05. b Significant difference from acetic acid group at p < 0.05. c Significant difference from ulcer + vehicle group at p < 0.05. d Significant difference from ulcer + SPEAF group at p < 0.05. SPEAF: S. persica ethyl acetate fraction.
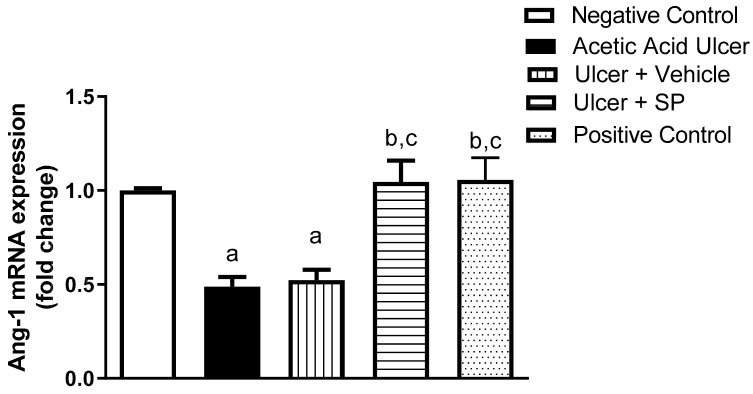
3.8. Assessment of Ang-1 mRNA Expression
The potential angiogenic activity of SPEAF was assessed in the tongue tissues. Figure 6 shows that SPEAF, and positive control preparation significantly enhanced Ang-1 mRNA expression by an approximately one-fold increase compared to the acetic acid group.
Figure 6.
Effect of SPEAF on Ang-1 mRNA expression in acetic acid-induced tongue ulcer in rats. Data are presented as mean ± SD (n = 6). Statistical analysis was performed by one-way ANOVA followed by Tukey’s test. a Significant difference from negative control group at p < 0.05. b Significant difference from acetic acid group at p < 0.05. c Significant difference from ulcer + Vehicle group at p < 0.05. SPEAF: S. persica ethyl acetate fraction.
4. Discussion and Conclusions
Oral ulcers are a common disorder of the oral mucosa. There are many predisposing conditions, including systemic diseases [41]. The primary treatment of oral ulcers includes the use of local corticosteroids in addition to antiseptic/anti-inflammatory agents and local anaesthetics [42]. However, these may have intolerable adverse effects [42,43]. For this reason, natural products have attracted great attention, as they constituted 16% of FDA-approved drugs in 2018 [44]. In particular, S. persica is considered to be nature’s gift for oral health [45,46]. The current study presents a phytochemical profile of SPEAF using HPLC-ESI-QTOF-MS-MS analysis. Nineteen compounds were detected for the first time in this plant, 12 of which were tentatively identified as benzamide (7); diethyl malate (9); methylbenzamide (11); acetyl phenylalanine (20); hydroxytetradecanoic acid (37); hydroxyhexadecanoic acid (34); hydroxyoctadecanoic acid (45); n-benzyl octadecenamide (50); nonadecanoic acid (51); hydroxyeicosanoic acid (53); diisooctyl phthalate (55); and 13-docosenamide (56). According to Sirius, compounds 5 and 16 were a sugar derivative and a phenolic acid derivative, with molecular formulae of C10H20O8 and C15H22O5, respectively. Compounds 10 and 32 were sulphated compound derivatives, with molecular formulae of C14H20O11S and C12H12N2O2S2, respectively. Compounds 42 (C21H35NO) and 43 (C23H37NO) were fatty acid amide derivatives, while compound 47 was a cholesterol derivative, with the molecular formula C39H58N4O5. Moreover, the healing activity of a muco-adhesive formula of S. persica against acetic acid-induced oral ulcers in rats was evaluated for the first time. Our data highlighted the safety of the extract. In addition, it expedited healing rates in wounded animals. This was confirmed histologically, as microscopic examinations indicated an almost intact epithelial covering, with fewer signs of inflammation and enhanced collagen deposition. This is consistent with previously published experimental data on the wound-healing potential of the plant in the skin or the tongue [47,48].
The transition from the inflammatory phase to the proliferative phase is a key step during wound healing [49]. Consequently, if the inflammatory response is extended or exacerbated, it results in a delay in the subsequent phases of proper wound healing [49,50]. Our results showed that the observed healing activity of S. persica was accompanied by a shortening of the inflammation phase. This was highlighted by the ability of S. persica in animal models of peptic ulcer [51] and paw oedema [52]. The effectiveness of S. persica gel application in the treatment of moderate and deep pockets in patients with periodontitis was associated with the inhibition of the crevicular fluid IL-6 and TNF-α [52,53]. Flavonoids have been reported to possess potent anti-inflammatory activity [54]. Based on our phytochemical profiling study, it can be suggested that the S. persica content of gallic acid (10), deoxy ellagic acid (3), methoxy ellagic acid (6), sulphated hexosyl polyphenols (10), O-benzyl hexosyl sulphate (14), phenolic acid derivatives (16, C15H22O5), di-O-methyl ellagic acid (17), coumaric acid (19), ferulic acid (22), methoxy flavanone hexosyl rhamnoside (23) and caffeic acid conjugate (24), representing 5% of the identified compounds of SPEAF, may contribute to observed anti-inflammatory activities. In addition, the major fatty acids identified were palmitic acid (4.98%), which has anti-inflammatory effects on endothelial cells (ECs) with TNF-α and counter endothelial dysfunction [55] and oleic acids (4.71%), which is an anti-inflammatory fatty acid that plays a role in the activation of different pathways of immune-competent cells [56]. The identified urea derivatives (10.5%, with N,N′-dibenzyl urea; 7.7%) showed promising anti-inflammatory activity (62–84% TNF-α and 73–92% IL-6 inhibitory activity) at a concentration of 10 μM, with reference to standard dexamethasone [57]. Moreover, in addition from having a reported potent bactericidal activity against oral pathogens and MRSA [58], the detected sulphur compound, benzyl isothiocyanate (8.7%), significantly attenuated TPA-enhanced hydrogen peroxide levels and acted as an inhibitor of O2 generation in mouse skin [59].
Oxidative stress contributes to the pathology of ulcerogenesis. Conversely, antioxidants reduce cellular damages that arise as a consequence of the increased generation of oxidative species [60]. The present study highlights the antioxidant activity of S. persica in tongue tissues and is supported by several studies [61,62,63]. Based on our phytochemical analyses of S. persica extract, antioxidant activity can be attributed to its polyphenol (5%) [64] and fatty acid (23%) content [65]. Farag et al. (2018) examined the antioxidant activities of S. persica ethanol extract and some isolated compounds called persicaline [66]. Essentially, antioxidants have been reported to play an important role in expediting wound healing by reducing oral mucosa inflammation, thus decreasing the risk of developing precancerous lesions [67]. This provides further insight into the healing properties of S. persica. This also supports observed anti-inflammation, as oxidative stress and inflammation are interconnected. The generated ROS induces the activation of transcription factors, which drive the expression of pro-inflammatory mediators, such as IL-6 and TGF-β [68].
Collagen, a key protein of the extracellular matrix produced by fibroblasts, is involved in the healing of connective tissues [69]. The final stages of wound healing involve remodelling, which should result in the closure of wounds or ulcers. In this context, collagen and hydroxyproline play an important role [70]. The results from this study indicated increased hydroxyproline formation and Col1A1 mRNA expression in the S. persica-treated group in comparison to untreated animals. In accordance with these findings, S. persica extract has been reported to inhibit collagen degradation in demineralised dentin [71]. Moreover, S. persica extract-laden collagen hybrid constructs showed enhanced periodontal tissue regeneration [72], consistent with the ability of S. persica to enhance fibroblast proliferation and viability [73]. This also supports the observed ability of S. persica to enhance angiogenesis, as evidenced by increased Ang-1 mRNA expression. In other words, collagen deposition in wound tissues is associated with enhanced angiogenesis [74]. Angiogenesis plays a key role in the wound-healing process and is involved in the migration, growth and differentiation of ECs [75]. The ability of naturally occurring compounds has been reviewed [76]. Our data are supported by the ability of flavonoids to boost angiogenesis in non-cancerous tissues [77]. In conclusion, S. persica has a wide range of secondary metabolites ameliorate acetic acid-induced tongue ulcers in rats. This can be attributed, at least partly, to its anti-inflammatory, antioxidant, procollagen and angiogenic activities. These findings provide support and validity for the use of S. persica as a traditional and conventional treatment for oral disorders and validate its application in the treatment of oral ulcers, which warrants further clinical studies.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research DSR at Umm Al-Qura University for supporting this work by Grant Code: 19-MED-1-03-0010.
Author Contributions
Conceptualization, N.A., N.B. and S.S.A.-G.; methodology, N.S., K.A.N., S.A., A.R.A. and A.B.A.-N.; software, K.A.N. and N.S.; validation, N.A. and S.S.A.-G.; formal analysis, N.S.; investigation, N.S. and K.A.N.; resources, N.A. and S.S.A.-G.; data curation, N.S. and K.A.N.; writing—original draft preparation, N.S., K.A.N., S.A. and A.R.A.; writing—review and editing, N.A. and A.B.A.-N.; visualization, N.S.; supervision, N.A. and A.B.A.-N.; project administration, N.A.; funding acquisition, N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research is fully funded by DSR at UQU, KSA (Grant Code: 19-MED-1-03-0010).
Institutional Review Board Statement
The research ethics committee of the Faculty of Pharmacy, King Abdulaziz University (KAU), officially approved experimental protocols (Reference # PH-1443-22).
Data Availability Statement
The data that support the findings of this study are openly available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burgess J.A., Johnson B.D., Sommers E. Pharmacological management of recurrent oral mucosal ulceration. Drugs. 1990;39:54–65. doi: 10.2165/00003495-199039010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Chen P., Yao H., Su W., He Y., Cheng K., Wang Y., Peng W., Li P. Sleep deprivation worsened oral ulcers and delayed healing process in an experimental rat model. Life Sci. 2019;232:116594. doi: 10.1016/j.lfs.2019.116594. [DOI] [PubMed] [Google Scholar]
- 3.Field E.A., Allan R.B. Review article: Oral ulceration–aetiopathogenesis, clinical diagnosis and management in the gastrointestinal clinic. Aliment. Pharm. Ther. 2003;18:949–962. doi: 10.1046/j.1365-2036.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- 4.McGettigan P., Ferner R.E. Painful perianal ulcers with nicorandil. BMJ. 2020;370:m3351. doi: 10.1136/bmj.m3351. [DOI] [PubMed] [Google Scholar]
- 5.Critchlow D. Part 3: Impact of systemic conditions and medications on oral health. Br. J. Community Nurs. 2017;22:181–190. doi: 10.12968/bjcn.2017.22.4.181. [DOI] [PubMed] [Google Scholar]
- 6.Adara A., Onalana O., Aktasb H., Ertugrulc S., Cakana F. A very rare complication of sublingual captopril. J. Exp. Clin. Med. 2019;36:91–93. doi: 10.5835/jecm.omu.36.03.005. [DOI] [Google Scholar]
- 7.Hasan A.A., Ciancio S. Association between ingestion of nonsteroidal anti-inflammatory drugs and the emergence of aphthous-like ulcers. J. Int. Acad. Periodontol. 2009;11:155–159. [PubMed] [Google Scholar]
- 8.Shetty K. Thalidomide in the concurrent management of recurrent aphthous ulcerations and Kaposi sarcoma in HIV patients with severe immunosuppression. Oral Oncol. Extra. 2006;42:26–31. doi: 10.1016/j.ooe.2005.08.005. [DOI] [Google Scholar]
- 9.Adler S., Baumgartner I., Villiger P.M. Behcet’s disease: Successful treatment with infliximab in 7 patients with severe vascular manifestations. A retrospective analysis. Arthritis Care Res. 2012;64:607–611. doi: 10.1002/acr.21557. [DOI] [PubMed] [Google Scholar]
- 10.Guo S., Dipietro L.A. Factors affecting wound healing. J. Dent. Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazarlu O., Iranshahi M., Kashani H.R.K., Reshadat S., Habtemariam S., Iranshahy M., Hasanpour M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharm. Res. 2021;174:105841. doi: 10.1016/j.phrs.2021.105841. [DOI] [PubMed] [Google Scholar]
- 12.Gomes M.S., Lins R.D.A.U., Langassner S.M.Z., da Silveira E.J.D., de Carvalho T.G., de Sousa Lopes M.L.D., de Souza Araujo L., de Medeiros C.A.C.X., de Carvalho Leitao R.F., Guerra G.C.B., et al. Anti-inflammatory and antioxidant activity of hydroethanolic extract of Spondias mombin leaf in an oral mucositis experimental model. Arch. Oral Biol. 2020;111:104664. doi: 10.1016/j.archoralbio.2020.104664. [DOI] [PubMed] [Google Scholar]
- 13.Yu Z., Jin C., Xin M., He J. Effect of Aloe vera polysaccharides on immunity and antioxidant activities in oral ulcer animal models. Carbohydr. Polym. 2009;75:307–311. doi: 10.1016/j.carbpol.2008.07.029. [DOI] [Google Scholar]
- 14.Sakarcan A., Sehirli O., Velioglu-Ovunc A., Ercan F., Erkanl G., Gedik N., Sener G. Ginkgo biloba extract improves oxidative organ damage in a rat model of thermal trauma. J. Burn Care Rehabil. 2005;26:515–524. doi: 10.1097/01.bcr.0000185115.17261.50. [DOI] [PubMed] [Google Scholar]
- 15.Tatke P. Antioxidant, antimicrobial and wound healing activity of Salvadora persica twig extracts. J. Complement. Med. Altern. Healthc. 2018;7:555720. doi: 10.19080/JCMAH.2018.07.555720. [DOI] [Google Scholar]
- 16.Tewtrakul S., Tungcharoen P., Sudsai T., Karalai C., Ponglimanont C., Yodsaoue O. Antiinflammatory and Wound Healing Effects of Caesalpinia sappan L. Phytother. Res. 2015;29:850–856. doi: 10.1002/ptr.5321. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen V.L., Truong C.T., Nguyen B.C.Q., Vo T.V., Dao T.T., Nguyen V.D., Trinh D.T., Huynh H.K., Bui C.B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS ONE. 2017;12:e0185674. doi: 10.1371/journal.pone.0185674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z.C., Wu S.S., Su W.Y., Lin Y.C., Lee Y.H., Wu W.H., Chen C.H., Wen Z.H. Anti-inflammatory and burn injury wound healing properties of the shell of Haliotis diversicolor. BMC Complement. Altern. Med. 2016;16:487. doi: 10.1186/s12906-016-1473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deveci M., Eski M., Sengezer M., Kisa U. Effects of cerium nitrate bathing and prompt burn wound excision on IL-6 and TNF-a levels in burned rats. Burns. 1999;26:41–45. doi: 10.1016/S0305-4179(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 20.Upadhyay N.K., Kumar R., Siddiqui M.S., Gupta A. Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid. Based Complement. Altern. Med. 2011;2011:659705. doi: 10.1093/ecam/nep189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farag M., Abdel-Mageed W.M., El Gamal A.A., Basudan O.A. Salvadora persica L.: Toothbrush tree with health benefits and industrial applications—An updated evidence-based review. Saudi Pharm. J. 2021;29:751–763. doi: 10.1016/j.jsps.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordin A., Bin Saim A., Ramli R., Abdul Hamid A., Mohd Nasri N.W., Bt Hj Idrus R. Miswak and oral health: An evidence-based review. Saudi J. Biol. Sci. 2020;27:1801–1810. doi: 10.1016/j.sjbs.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farag M., Shakour Z.T., Lubken T., Frolov A., Wessjohann L.A., Mahrous E. Unraveling the metabolome composition and its implication for Salvadora persica L. use as dental brush via a multiplex approach of NMR and LC-MS metabolomics. J. Pharm. Biomed. Anal. 2021;193:113727. doi: 10.1016/j.jpba.2020.113727. [DOI] [PubMed] [Google Scholar]
- 24.Ahangar P., Mills S.J., Smith L.E., Gronthos S., Cowin A.J. Human gingival fibroblast secretome accelerates wound healing through anti-inflammatory and pro-angiogenic mechanisms. NPJ Regen. Med. 2020;5:24. doi: 10.1038/s41536-020-00109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ossama M., Lamie C., Tarek M., Wagdy H.A., Attia D.A., Elmazar M.M. Management of recurrent aphthous ulcers exploiting polymer-based Muco-adhesive sponges: In-vitro and in-vivo evaluation. Drug Deliv. 2021;28:87–99. doi: 10.1080/10717544.2020.1858999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y., Liu W., Lei Y., Wu T., Zhang S., Guo Y., Liu Y., Chen D., Yuan Q., Wang Y. Effect of gelatin sponge with colloid silver on bone healing in infected cranial defects. Pt 1Mater. Sci. Eng. C Mater. Biol. Appl. 2017;70:371–377. doi: 10.1016/j.msec.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Swilam N., Nematallah K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020;10:14851. doi: 10.1038/s41598-020-71847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duhrkop K., Fleischauer M., Ludwig M., Aksenov A.A., Melnik A.V., Meusel M., Dorrestein P.C., Rousu J., Bocker S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods. 2019;16:299–302. doi: 10.1038/s41592-019-0344-8. [DOI] [PubMed] [Google Scholar]
- 29.Dührkop K., Shen H., Meusel M., Rousu J., Böcker S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc. Natl. Acad. Sci. USA. 2015;112:12580–12585. doi: 10.1073/pnas.1509788112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duhrkop K., Nothias L.F., Fleischauer M., Reher R., Ludwig M., Hoffmann M.A., Petras D., Gerwick W.H., Rousu J., Dorrestein P.C., et al. Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat. Biotechnol. 2021;39:462–471. doi: 10.1038/s41587-020-0740-8. [DOI] [PubMed] [Google Scholar]
- 31.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J., Bokesch H., Kenney S., Boyd M. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 32.OECD . Test No. 423: Acute Oral Toxicity—Acute Toxic Class Method. OECD Publishing; Paris, France: 2002. [DOI] [Google Scholar]
- 33.Majumder R., Adhikari L., Dhara M., Sahu J. Evaluation of anti-inflammatory, analgesic and TNF-alpha inhibition (upon RAW 264.7 cell line) followed by the selection of extract (leaf and stem) with respect to potency to introduce anti-oral-ulcer model obtained from Olax psittacorum (Lam.) Vahl in addition to GC-MS illustration. J. Ethnopharmacol. 2020;263:113146. doi: 10.1016/j.jep.2020.113146. [DOI] [PubMed] [Google Scholar]
- 34.Farag M.A., Fahmy S., Choucry M.A., Wahdan M.O., Elsebai M.F. Metabolites profiling reveals for antimicrobial compositional differences and action mechanism in the toothbrushing stick “miswak” Salvadora persica. J. Pharm. Biomed. Anal. 2017;133:32–40. doi: 10.1016/j.jpba.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Ali M., Naqvi B., Watson I.A. Possibility of converting indigenous Salvadora persica L. seed oil into biodiesel in Pakistan. Int. J. Green Energy. 2018;15:427–435. doi: 10.1080/15435075.2018.1472603. [DOI] [Google Scholar]
- 36.Khalil A.T. Benzylamides from Salvadora persica. Arch. Pharm. Res. 2006;29:952–956. doi: 10.1007/BF02969277. [DOI] [PubMed] [Google Scholar]
- 37.Noumi E., Hajlaoui H., Trabelsi N., Ksouri R., Bakhrouf A., Snoussi M. Antioxidant activities and RP-HPLC identification of polyphenols in the acetone extract of Salvadora persica. Afr. J. Pharm. Pharmacol. 2011;5:966–971. doi: 10.5897/AJPP11.245. [DOI] [Google Scholar]
- 38.Ibrahim M.M., Al Sahli A.A., Alaraidh I.A., Al-Homaidan A.A., Mostafa E.M., El-Gaaly G.A. Assessment of antioxidant activities in roots of Miswak (Salvadora persica) plants grown at two different locations in Saudi Arabia. Saudi J. Biol. Sci. 2015;22:168–175. doi: 10.1016/j.sjbs.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtani K., Kasai R., Yamasaki K., Tanaka O., Kamel M.S., Assaf M.H., El-Shanawani M.A., Ali A.A. Lignan glycosides from stems of Salvadora persica. Phytochemistry. 1992;31:2469–2471. doi: 10.1016/0031-9422(92)83301-E. [DOI] [Google Scholar]
- 40.Kumari A., Parida A.K. Metabolite profiling of the leaf extract reveals the antioxidant and nutraceuticals potential of the halophyte Salvadora persica. RSC Adv. 2016;6:51629–51641. doi: 10.1039/C6RA08415J. [DOI] [Google Scholar]
- 41.Porter S.R., Leao J.C. Review article: Oral ulcers and its relevance to systemic disorders. Aliment. Pharm. Ther. 2005;21:295–306. doi: 10.1111/j.1365-2036.2005.02333.x. [DOI] [PubMed] [Google Scholar]
- 42.Altenburg A., El-Haj N., Micheli C., Puttkammer M., Abdel-Naser M.B., Zouboulis C.C. The treatment of chronic recurrent oral aphthous ulcers. Dtsch. Arztebl. Int. 2014;111:665–673. doi: 10.3238/arztebl.2014.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher D.A. Adverse effects of topical corticosteroid use. West. J. Med. 1995;162:123–126. [PMC free article] [PubMed] [Google Scholar]
- 44.De la Torre B.G., Albericio F. The Pharmaceutical Industry in 2019. An analysis of FDA drug approvals from the perspective of molecules. Molecules. 2020;25:745. doi: 10.3390/molecules25030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jassoma E., Baeesa L., Sabbagh H. The antiplaque/anticariogenic efficacy of Salvadora persica (Miswak) mouthrinse in comparison to that of chlorhexidine: A systematic review and meta-analysis. BMC Oral Health. 2019;19:64. doi: 10.1186/s12903-019-0741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mekhemar M., Geib M., Kumar M., Hassan Y., Dorfer C. Salvadora persica: Nature’s gift for periodontal health. Antioxidants. 2021;10:712. doi: 10.3390/antiox10050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fatima N., Iqbal W., Yaqeen S.S. Evaluation of wound healing effects between Salvadora persica ointment and Solcoseryl jelly in animal model. Pak. J. Pharm. Sci. 2015;28:1777–1780. [PubMed] [Google Scholar]
- 48.Faruk E.M., Nafea O.E., Fouad H., Ebrahim U.F.A., Hasan R.A.A. Possible healing effects of Salvadora persica extract (MISWAK) and laser therapy in a rabbit model of a caustic-induced tongue ulcers: Histological, immunohistochemical and biochemical study. J. Mol. Histol. 2020;51:341–352. doi: 10.1007/s10735-020-09884-7. [DOI] [PubMed] [Google Scholar]
- 49.Landen N.X., Li D., Stahle M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016;73:3861–3885. doi: 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandi J., Cheri S., Manfredi M., Di Carlo C., Vita Vanella V., Federici F., Bombiero E., Bazaj A., Rizzi E., Manna L., et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020;10:11572. doi: 10.1038/s41598-020-68483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lebda M.A., El-Far A.H., Noreldin A.E., Elewa Y.H.A., Al Jaouni S.K., Mousa S.A. Protective effects of Miswak (Salvadora persica) against experimentally induced gastric ulcers in rats. Oxidative Med. Cell. Longev. 2018;2018:6703296. doi: 10.1155/2018/6703296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibrahim A.Y., El-Gengahi S.E., Motawea H.M., Sleem A.M. Anti-Inflammatory activity of Salvadora persica L. against carrageenan induced paw oedema in rat relevant to inflammatory cytokinese. Not. Sci. Biol. 2011;3:22–28. doi: 10.15835/nsb346378. [DOI] [Google Scholar]
- 53.Niazi F.H., Noushad M., Tanvir S.B., Ali S., Al-Khalifa K.S., Qamar Z., Al-Sheikh R. Antimicrobial efficacy of indocyanine green-mediated photodynamic therapy compared with Salvadora persica gel application in the treatment of moderate and deep pockets in periodontitis. Photodiagn. Photodyn. Ther. 2020;29:101665. doi: 10.1016/j.pdpdt.2020.101665. [DOI] [PubMed] [Google Scholar]
- 54.Maleki S.J., Crespo J.F., Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- 55.de Souza C.O., Valenzuela C.A., Baker E.J., Miles E.A., Rosa Neto J.C., Calder P.C. Palmitoleic acid has stronger anti-Inflammatory potential in human endothelial cells compared to oleic and palmitic acids. Mol. Nutr. Food Res. 2018;62:e1800322. doi: 10.1002/mnfr.201800322. [DOI] [PubMed] [Google Scholar]
- 56.Carrillo C., Cavia Mdel M., Alonso-Torre S. Role of oleic acid in immune system; mechanism of action; a review. Nutr. Hosp. 2012;27:978–990. doi: 10.3305/nh.2012.27.4.5783. [DOI] [PubMed] [Google Scholar]
- 57.Keche A.P., Kamble V.M. Synthesis and anti-inflammatory and antimicrobial activities of some novel 2-methylquinazolin-4(3H)-one derivatives bearing urea, thiourea and sulphonamide functionalities. Arab. J. Chem. 2019;12:1522–1531. doi: 10.1016/j.arabjc.2014.10.025. [DOI] [Google Scholar]
- 58.Sofrata A., Santangelo E.M., Azeem M., Borg-Karlson A.K., Gustafsson A., Putsep K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PLoS ONE. 2011;6:e23045. doi: 10.1371/journal.pone.0023045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyoshi N., Takabayashi S., Osawa T., Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: Implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004;25:567–575. doi: 10.1093/carcin/bgh051. [DOI] [PubMed] [Google Scholar]
- 60.Kesarwala A.H., Krishna M.C., Mitchell J.B. Oxidative stress in oral diseases. Oral Dis. 2016;22:9–18. doi: 10.1111/odi.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibrahim I.I., Moussa A.A., Chen Z., Zhang J., Cao W.G., Yu C. Bioactive phenolic components and antioxidant activities of water-based extracts and flavonoid-rich fractions from Salvadora persica L. leaves. Nat. Prod. Res. 2021 doi: 10.1080/14786419.2021.1908281. [DOI] [PubMed] [Google Scholar]
- 62.Abd El-Naby A.S., El Asely A.M., Amin A.A., Samir F., El-Ashram A., Dawood M.A.O. Miswak (Salvadora persica) modulated the growth performance, antioxidative response, and histopathological damage induced by zinc toxicity in Nile tilapia (Oreochromis niloticus) Environ. Sci. Pollut. Res. Int. 2020;27:31918–31932. doi: 10.1007/s11356-020-09429-1. [DOI] [PubMed] [Google Scholar]
- 63.Lebda M.A., El-Hawarry W.N., Shourbela R.M., El-Far A.H., Shewita R.S., Mousa S.A. Miswak (Salvadora persica) dietary supplementation improves antioxidant status and nonspecific immunity in Nile tilapia (Oreochromis niloticus) Fish. Shellfish Immunol. 2019;88:619–626. doi: 10.1016/j.fsi.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 64.Kharouf N., Haikel Y., Ball V. Polyphenols in dental applications. Bioengineering. 2020;7:72. doi: 10.3390/bioengineering7030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elagbar Z.A., Naik R.R., Shakya A.K., Bardaweel S.K. Fatty acids analysis, antioxidant and biological activity of fixed oil of Annona muricata L. seeds. J. Chem. 2016;2016:6948098. doi: 10.1155/2016/6948098. [DOI] [Google Scholar]
- 66.Farag M., Abdel-Mageed W.M., Basudan O., El-Gamal A. Persicaline, a new antioxidant sulphur-containing imidazoline alkaloid from Salvadora persica roots. Molecules. 2018;23:483. doi: 10.3390/molecules23020483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Comino-Sanz I.M., Lopez-Franco M.D., Castro B., Pancorbo-Hidalgo P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021;10:3558. doi: 10.3390/jcm10163558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schinella G.R., Tournier H.A., Prieto J.M., De Buschiazzo P.M., Rıos J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002;70:1023–1033. doi: 10.1016/S0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 69.Narayanan A.S., Page R.C., Swanso J. Collagen synthesis by human fibroblasts. Regulation by transforming growth factor-fl in the presence of other inflammatory mediators. Biochem. J. 1989;260:463–469. doi: 10.1042/bj2600463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jansen R.G., van Kuppevelt T.H., Daamen W.F., Kuijpers-Jagtman A.M., Von den Hoff J.W. Tissue reactions to collagen scaffolds in the oral mucosa and skin of rats: Environmental and mechanical factors. Arch. Oral Biol. 2008;53:376–387. doi: 10.1016/j.archoralbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Khunkar S., Hariri I., Alsayed E., Linjawi A., Khunkar S., Islam S., Bakhsh T.A., Nakashima S. Inhibitory effect of Salvadora persica extract (Miswak) on collagen degradation in demineralized dentin: In vitro study. J. Dent. Sci. 2021;16:208–213. doi: 10.1016/j.jds.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arslan Y.E., Kantarcıoğlu İ. Salvadora persica extract-laden jellyfish collagen hybrid constructs for periodontal tissue regeneration. J. Turk. Chem. Soc. 2019;6:51–62. doi: 10.18596/jotcsa.484936. [DOI] [Google Scholar]
- 73.Balto H.A., Halawany H.S., Jacob V., Abraham N.B. The efficacy of Salvadora persica extracts in preserving the viability of human foreskin fibroblasts. Saudi Dent. J. 2015;27:137–140. doi: 10.1016/j.sdentj.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao C., Markowicz M., Pallua N., Noah E.M., Steffens G. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials. 2008;29:66–74. doi: 10.1016/j.biomaterials.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 75.Cucci L.M., Satriano C., Marzo T., La Mendola D. Angiogenin and copper crossing in wound healing. Int. J. Mol. Sci. 2021;22:10704. doi: 10.3390/ijms221910704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan D., Gong X., Wang X., Li M. Role of active components of medicinal food in the regulation of angiogenesis. Front. Pharm. 2020;11:594050. doi: 10.3389/fphar.2020.594050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He Q., Li S., Li L., Hu F., Weng N., Fan X., Kuang S. Total favonoids in Caragana (TFC) promotes angiogenesis and enhances cerebral perfusion in a rat model of ischemic stroke. Front. Neurosci. 2018;12:635. doi: 10.3389/fnins.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available from the corresponding author upon reasonable request.