Key Words: urinary bladder neoplasms, neoadjuvant therapy, prognosis, cystectomy, urinary diversion
Abstract
Purpose:
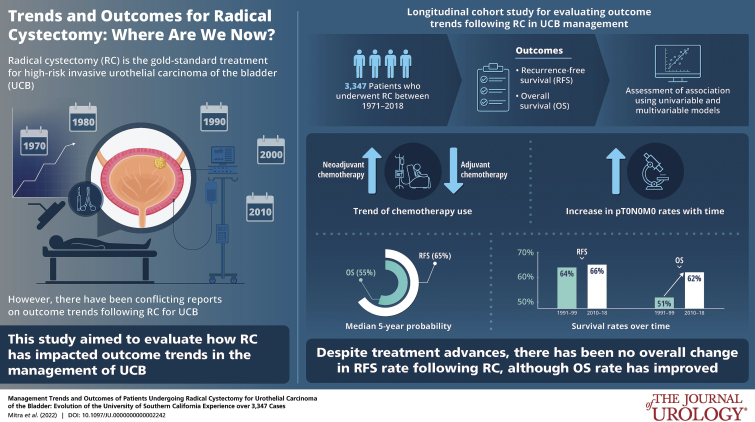
There are conflicting reports on outcome trends following radical cystectomy (RC) for bladder cancer.
Materials and Methods:
Evolution of modern bladder cancer management and its impact on outcomes was analyzed using a longitudinal cohort of 3,347 patients who underwent RC at an academic center between 1971 and 2018. Outcomes included recurrence-free survival (RFS) and overall survival (OS). Associations were assessed using univariable and multivariable models.
Results:
In all, 70.9% of cases underwent open RC in the last decade, although trend for robot-assisted RC rose since 2009. While lymphadenectomy template remained consistent, nodal submission changed to anatomical packets in 2002 with increase in yield (p <0.001). Neoadjuvant chemotherapy (NAC) use increased with time with concomitant decrease in adjuvant chemotherapy; this was notable in the last decade (p <0.001) and coincided with improved pT0N0M0 rate (p=0.013). Median 5-year RFS and OS probabilities were 65% and 55%, respectively. Advanced stage, NAC, delay to RC, lymphovascular invasion and positive margins were associated with worse RFS (all, multivariable p <0.001). RFS remained stable over time (p=0.73) but OS improved (5-year probability, 1990–1999 51%, 2010–2018 62%; p=0.019). Among patients with extravesical and/or node-positive disease, those who received NAC had worse outcomes than those who directly underwent RC (p ≤0.001).
Conclusions:
Despite perioperative and surgical advances, and improved pT0N0M0 rates, there has been no overall change in RFS trend following RC, although OS rates have improved. While patients who are downstaged with NAC derive great benefit, our real-world experience highlights the importance of preemptively identifying NAC nonresponders who may have worse post-RC outcomes.
Abbreviations and Acronyms
- AC
adjuvant chemotherapy
- ERAS
enhanced recovery after surgery
- MSKCC
Memorial Sloan Kettering Cancer Center
- NAC
neoadjuvant chemotherapy
- OS
overall survival
- RC
radical cystectomy
- RFS
recurrence-free survival
- UCB
urothelial carcinoma of the bladder
Radical cystectomy (RC) is considered the gold-standard treatment for high-risk invasive urothelial carcinoma of the bladder (UCB). Population-based studies report conflicting UCB outcome trends over time, although these investigations do not differentiate between patients undergoing RC versus bladder-preserving therapies for less aggressive disease.1,2 Most studies concur that RC performed at high-volume and academic centers are associated with better outcomes.3,4
Advances in perioperative and surgical UCB management including enhanced recovery after surgery (ERAS) pathways and robot-assisted surgery have improved patient experience while maintaining oncological efficacy.5,6 Neoadjuvant chemotherapy (NAC) has been associated with modest survival benefit.7 However, pathological stage remains the most important predictor of outcomes following RC.8–10 This study examined clinicopathological and outcome trends of patients undergoing RC for UCB at the University of Southern California following a consistent surgical philosophy in the face of changing management paradigms over time. The aim was to juxtapose historical and contemporary patient groups to evaluate how temporal evolution of high-risk UCB management has impacted outcomes following RC at a high-volume academic center.
Materials and Methods
Patient Population and Management
A total of 3,957 subjects who underwent RC between 1971 and 2018 were identified through a prospectively maintained database (IRB No. HS01B014). Study criteria, preoperative evaluation and management are detailed in the supplementary methods (https://www.jurology.com). RC and urinary diversion were performed by open or robot-assisted approach per patient and surgeon preference. Per institutional standard, all eligible patients underwent extended pelvic lymphadenectomy.8 Since 2012, patients were started on ERAS pathway.5 Pathological stage was based on tumor-node-metastasis system at RC (supplementary table 1 and supplementary methods, https://www.jurology.com). Postoperative followup was at 4-month intervals in year 1, 6-month intervals in year 2, and annually thereafter with laboratory and imaging studies as indicated.
Data Analysis
Associations between clinicopathological characteristics and outcomes were assessed using univariable and multivariable models (supplementary methods, https://www.jurology.com). Trends were determined annually, or between decades or eras. Directed acyclic graphs were used to identify confounding variables.11 Oncological outcomes included recurrence-free survival (RFS) and overall survival (OS).
Results
Patient Characteristics and Management Trends
A total of 3,347 patients underwent RC for UCB and met study criteria for final analysis. Population included 2,685 (80.2%) males, with median age of 68 (IQR, 61–75) years (supplementary table 2, https://www.jurology.com). Median age and proportion of nonCaucasians treated increased over time (p <0.001; supplementary table 2, https://www.jurology.com; fig. 1). In all, 43.4% of all males had histological evidence of concomitant prostatic adenocarcinoma in their cystoprostatectomy specimen; 58 (1.7%) patients experienced perioperative mortality, ie death, within 30 days of surgery or prior to discharge, whichever was later.
Figure 1.
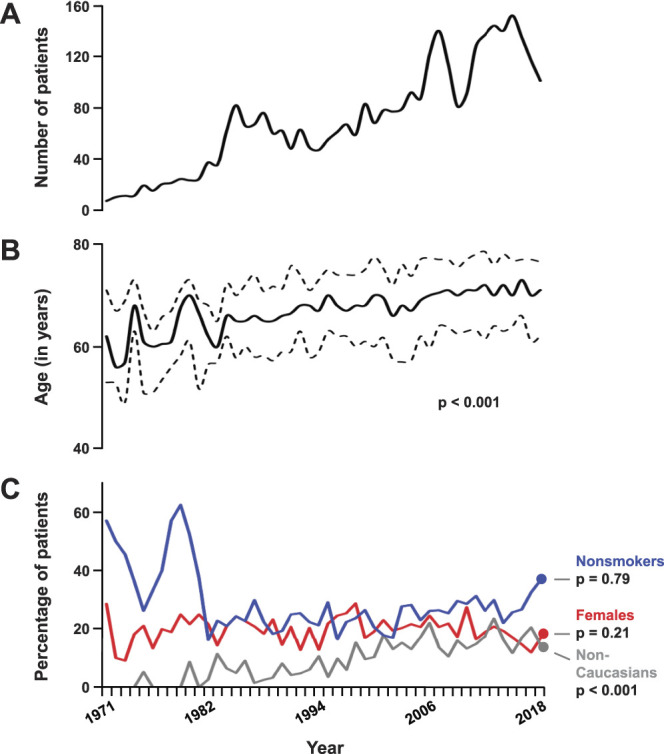
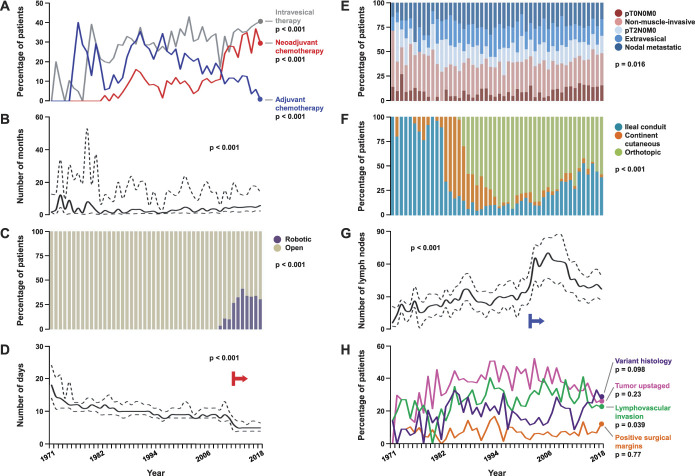
Annualized trends of baseline characteristics of patients with bladder cancer. Line graphs show trends in number of patients (A), median age (solid) with IQR (dotted; B), and proportion of patients who were nonsmokers (blue), females (red) and nonCaucasians (gray; C) across the study duration. Annualized p value calculated by Kruskal-Wallis test for age, and by Mantel-Haenszel test for trend for other categorical measurements.
Intravesical therapy and NAC administration increased over time, with concomitant decrease in adjuvant chemotherapy (AC); this was especially notable in last decade (all, p <0.001; fig. 2 and supplementary table 2, https://www.jurology.com). Cisplatin-based combinations have been the mainstay for NAC (88% patients) and AC (84.4% patients) regimens. Associations between patient and management characteristics are summarized in supplementary figure 1 (https://www.jurology.com). Higher proportions of patients receiving NAC and AC were younger than those who did not receive these treatments (p ≤0.005). Median time from diagnosis to RC was 6.2 (IQR 4.6–13.6) months for patients receiving NAC, and 2.7 (IQR 1.3–14) months for those directly undergoing surgery (p <0.001). NAC administration was not associated with overall surgical margin status (p=0.46); 50.1% and 20.2% of patients receiving NAC achieved tumor downstaging and complete pathological response (pT0N0M0), respectively. The corresponding proportions for patients directly undergoing RC were 27% and 8.7%, respectively (both p <0.001).
Figure 2.
Annualized trends of disease and management characteristics of patients with bladder cancer. Line graphs show trends in proportion of patients who received intravesical therapy (gray), neoadjuvant (red) and AC (blue; A), time from diagnosis to cystectomy (B), postoperative length of hospital stay (D), lymph node yield (G), and proportion of patients with variant histology (indigo), tumor upstaging (magenta), lymphovascular invasion (green) and positive surgical margins (orange; H). Bar graphs show proportions of patients undergoing robotic (purple) and open (tan) radical cystectomy (C), with distribution of pathological stage (E), and type of urinary diversion performed across the study duration (F). Graphs for time to cystectomy, length of stay and nodal yield display median values along solid curve, and IQR between dotted curves; corresponding annualized p values calculated by Kruskal-Wallis test. Annualized p value calculated by Mantel-Haenszel test for trend for all other categorical measurements. Red arrow in panel D indicates institution of enhanced postoperative recovery pathway. Blue arrow in panel G indicates institution of node packeting during pelvic lymphadenectomy.
There has been an increasing trend for robot-assisted RC since 2009, although open approach was still used in 70.9% of patients in the last decade (p <0.001; fig. 2 and supplementary table 2, https://www.jurology.com); 65.8% of patients underwent intracorporeal ileal conduit urinary diversion during robot-assisted RC and 59.6% of patients underwent orthotopic neobladder urinary diversion during open RC (p <0.001). While there was a trend towards decreasing proportion of ileal conduit diversions and increasing proportion of orthotopic neobladders until 2009, the last decade witnessed an increase in ileal conduits associated with higher percentages of older patients with more comorbidities (p <0.001). While our extended pelvic lymphadenectomy template has remained consistent since the 1980s, specimen submission changed from en bloc to anatomically defined nodal packets in 2002 with consequent increase in nodal yield (p <0.001).12 Proportion of patients with pT0N0M0 disease increased in the last decade (p=0.013), with no significant trend differences in clinical stage, variant histology or tumor upstaging rates (supplementary table 2, https://www.jurology.com). Median hospital stay decreased to 5 (IQR 4–7) days since implementation of ERAS in 2012 from 9 (IQR 8–12) days (p <0.001).
Associations and Trends with Outcomes
Median followup was 10.1 years (range, 3 months to 40.4 years), during which 1,038 (31%) patients recurred and 1,900 (56.8%) patients died. Median±SE 5-year RFS and OS probabilities were 65±1% and 55±1%, respectively (fig. 3).
Figure 3.
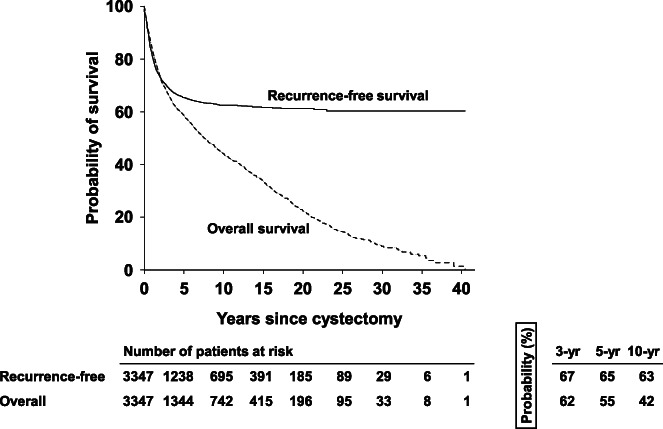
Clinical outcomes of all bladder cancer patients. Kaplan-Meier curves show recurrence-free (solid) and overall (dotted) survival probabilities of patients with urothelial carcinoma of bladder across entire study population.
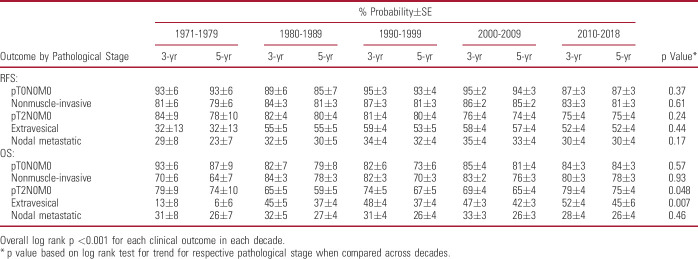
Univariable associations of patient characteristics with outcomes are detailed in supplementary table 3 (https://www.jurology.com). Advanced pathological stage was associated with worse RFS and OS (both p <0.001; fig. 4). This overall trend persisted even after stratification by decade of surgery (table 1). NAC and AC were univariably associated with worse RFS (both p <0.001), consistent with them being administered for more advanced disease (supplementary fig. 1, https://www.jurology.com). Longer delay to RC, variant histology, lymphovascular invasion, tumor upstaging, and positive surgical margins were also associated with worse outcomes (all p <0.001).
Figure 4.
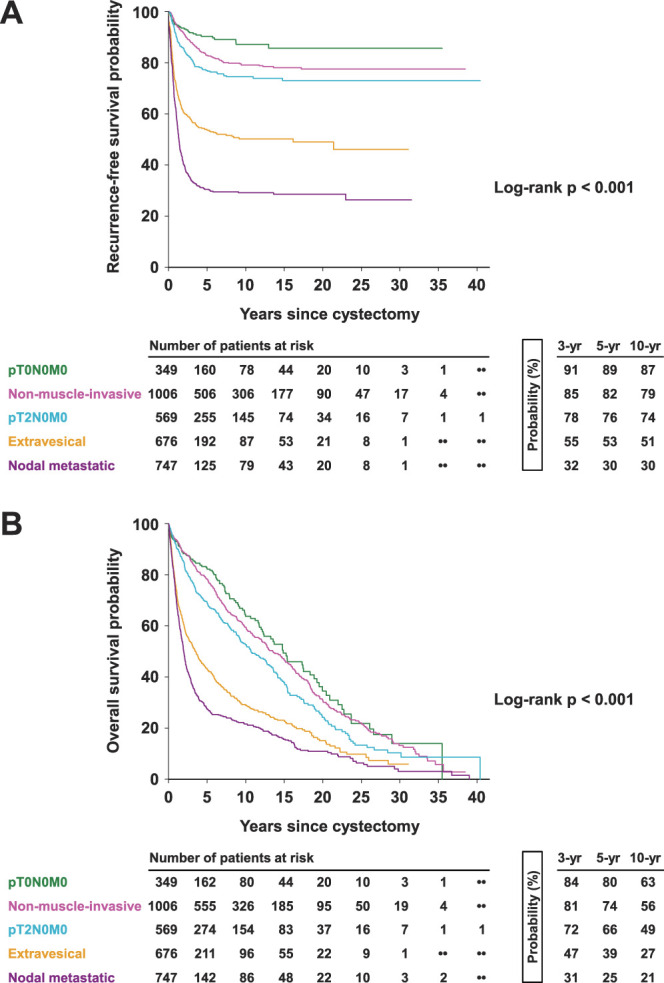
Comparison of clinical outcomes of patients with bladder cancer stratified by pathological stage. Kaplan-Meier curves show recurrence-free (A) and overall survival probabilities (B) of patients with pT0N0M0 (green), nonmuscle-invasive (magenta), pT2N0M0 (aqua), extravesical (orange), and nodal metastatic (purple) disease. Overall p value calculated by log rank test.
Table 1.
Univariable associations of pathological stage with clinical outcomes in patients undergoing radical cystectomy for bladder cancer, stratified across decades
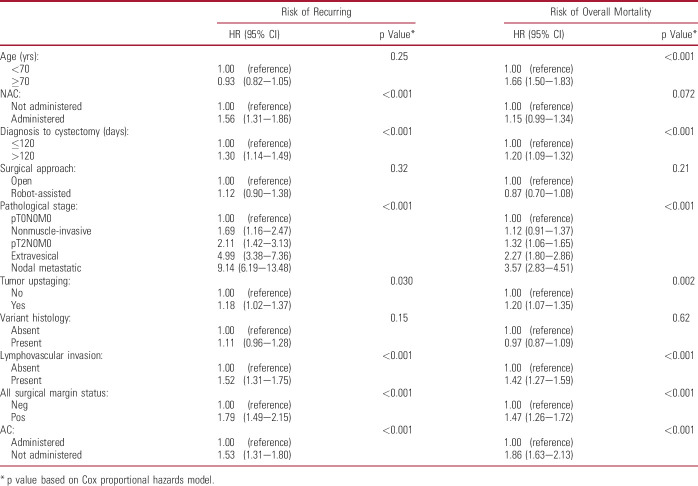
Relevant univariably prognostic variables were included in multivariable models to estimate their independent relationships with outcomes (table 2). This confirmed that advanced pathological stage was associated with worse outcomes (RFS and OS p <0.001). Other independent RFS predictors included NAC and AC administration (both p <0.001). Longer delay to RC, lymphovascular invasion, tumor upstaging, and positive margins remained independently associated with worse outcomes (all p ≤0.030). Given the interplay of various demographic and clinicopathological characteristics in determining outcomes, an exploratory variable analysis using directed acyclic graph was performed to outline causal relationships and eliminate confounders with the hypothesis that pathological stage is a primary outcome predictor following RC (supplementary fig. 2, https://www.jurology.com). This identified biasing paths that could be controlled by adjusting for age, comorbidity measured by American Society of Anesthesiologists® score, NAC administration and lymphovascular invasion (supplementary table 4, https://www.jurology.com), which would be sufficient to estimate the total effect of pathological stage on outcomes. The resulting multivariable model confirmed the independent associations of pathological stage (p <0.001) and the other variables with outcomes (supplementary table 5, https://www.jurology.com). Despite causal relationship-based adjustment, NAC administration remained associated with worse RFS and OS (both, p <0.001).
Table 2.
Multivariable associations of patient, disease and management characteristics with clinical outcomes in patients undergoing radical cystectomy for bladder cancer
There was no change in RFS trend across decades (p=0.73; supplementary table 2, https://www.jurology.com) or annually (p=0.94; fig. 5, and supplementary fig. 3, https://www.jurology.com). For example, median±SE 5-year RFS probability was 64%±2% during 1990–1999 and 66%±2% during 2010–2018. However, OS trend improved across decades (p=0.019) and annually (p=0.009). For instance, median±SE 5-year OS probability improved from 51%±2% during 1990–1999 to 62%±2% during 2010–2018. When stratified by pathological stage, the trend in improved OS was significantly notable in patients with pT2-4N0M0 disease at RC (table 1).
Figure 5.
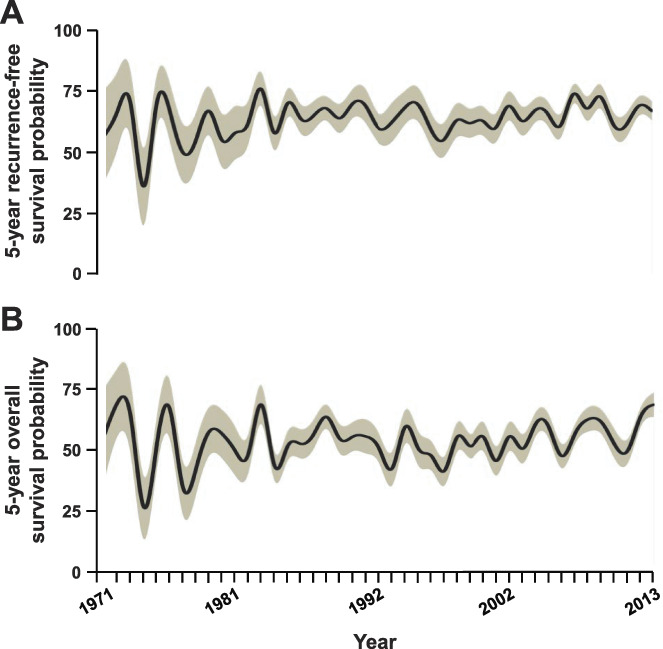
Annualized trends of clinical outcomes following radical cystectomy for bladder cancer. Five-year probability of recurrence-free (A) and overall survival (B) for patients (solid line) with corresponding standard error estimates (shaded area). Estimates determined for patients undergoing cystectomy until 2013 to allow for adequate followup. Univariable log rank test for trend p=0.94 and 0.009, respectively.
Use of NAC
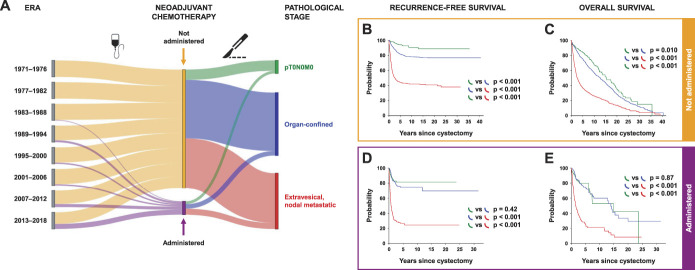
Trends in proportion of patients receiving NAC and associated pathological stages were analyzed (fig. 6, A). NAC administration increased across eras with correspondingly increased pT0N0M0 rate (supplementary table 2, https://www.jurology.com). Among patients who did not receive NAC, advanced pathological stage was associated with worse RFS and OS (overall p <0.001; supplementary table 6, https://www.jurology.com; stratified p <0.001; fig. 6, B,C). While this association with pathological stage was also noted among NAC recipients (overall p <0.001, supplementary table 6, https://www.jurology.com), stratified analysis between pT0N0M0 and organ-confined disease showed no difference in RFS (p=0.42) or OS (p=0.87; fig. 6, D and E). However, RFS and OS for these disease stages were better than for patients with extravesical and/or nodal metastatic disease who received NAC (stratified p <0.001). Among patients with extravesical and/or nodal metastatic disease at RC, those who previously received NAC had worse RFS (log-rank p <0.001) and OS (log-rank p=0.001) than those who directly underwent RC (supplementary table 6, https://www.jurology.com; data not shown).
Figure 6.
NAC administration and association with clinical outcomes. A, flow diagram depicts the relative proportion of patients in each 6-year era who received (purple) or did not receive (orange) NAC, and corresponding proportions who were found to have pT0N0M0 (green), organ-confined (blue), and extravesical and/or nodal metastatic (red) disease on radical cystectomy. Thickness of each colored curve corresponds to relative proportion for respective originating node. Kaplan-Meier curves show probabilities of recurrence-free (B, D), and overall survival (C, E) among patients who did not (B, C) and did (D, E) receive NAC, when stratified by pathological stage. Log rank p values compared across strata shown.
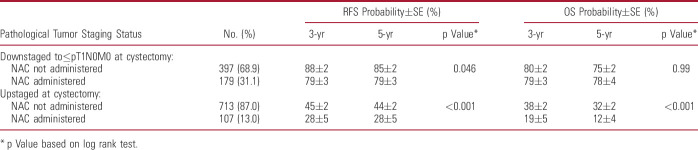
Among patients with ≥cT2 disease, those who were upstaged following NAC had significantly worse RFS and OS (both, p <0.001) compared with those who directly underwent RC (table 3). However, those who were downstaged following NAC had a slightly worse RFS (p=0.046) and no difference in OS (p=0.99) compared with those who directly underwent RC.
Table 3.
Univariable associations of NAC administration with clinical outcomes in patients with ≥cT2 bladder cancer undergoing radical cystectomy, stratified by pathological tumor staging status
Discussion
A prior report of our institutional RC experience highlighted the importance of surgical management for invasive UCB.8 However, it predated important changes in perioperative and surgical management including NAC use, lymph node packeting, ERAS, and robot-assisted RC.5–7,12 This study presents our long-term experience using a meticulously annotated population of 3,347 patients to compare historical and contemporary outcomes following RC in light of evolving changes to UCB management. Through the decades, our patients have trended to being older and with more comorbidities. NAC use has increased, peaking at 36.8% in 2017, with concomitantly decreased AC use that is consistent with national trends;13 29.2% of patients overall (40.8% of patients with ≥cT2 disease) received NAC in the last decade compared with the 22.3% national average, which translated to an improved 23.7% overall pT0N0M0 rate in this subpopulation in the same timeframe.14 Conformity to ERAS resulted in shorter inpatient stay. Despite these advances, there was no change in RFS over time, although OS showed a trend towards improvement. While patients with organ-confined disease have excellent 5-year RFS and OS probabilities after RC (80% and 71%, respectively), corresponding rates in patients with nodal-metastatic disease remain dismal (30% and 25%, respectively). This reflects “real-world” trends and outcomes following RC at a high-volume academic center with a tradition of pioneering UCB management. It underscores the value of aggressive surgical treatment that incorporates advances in operative and perioperative management while emphasizing the need for innovations that improve disease-specific outcomes.
Prior studies have reported an association between improved surgical outcomes and higher number of dissected lymph nodes.15 However, our data indicate that, notwithstanding a consistent template, nodal counts can vary based on sample submission methodology. Our nodal yield increased with concomitant decrease in nodal density without any significant RFS change since specimen submission was changed in 2002 from en bloc to anatomically defined packets.12 Others have emphasized the importance of a thorough lymphadenectomy over nodal counts for achieving optimal oncologic outcomes.16 Our RFS and OS are comparable to the series from Ulm, Germany, where patients underwent super-extended lymphadenectomy since 2001 with a mean of 18 resected nodes.9 We believe that our adherence to a meticulous extended lymphadenectomy template using open and robot-assisted approaches has resulted in high nodal yields and putatively curative resections. The first phase 3 trial to address the extent of node dissection in RC was unable to provide conclusive evidence of oncologic benefit for extended lymphadenectomy over standard dissection, although it was not a noninferiority design and likely underpowered to discern small but clinically relevant differences.17 Results of the SWOG S1011 trial that randomized patients with muscle-invasive disease to standard versus extended lymphadenectomy are pending.
In contrast to national trends, majority of patients at our institution underwent orthotopic ileal neobladder as the modality of urinary diversion.18 This is also oncologically safe and feasible in patients with prostatic stromal invasion and prior pelvic irradiation.19,20 Individual choice of diversion is ultimately a shared decision based on preoperative counseling, patient and disease characteristics, lifestyle, priorities, and other intangible patient preferences.
Our findings have some important differences from the recently reported Memorial Sloan Kettering Cancer Center (MSKCC) experience.21 Detailed pathological staging for the MSKCC population is unavailable, but their overall nodal metastasis rate of 20% suggests a comparable cohort composition as ours. While RFS in our cohort did not improve over time, MSKCC reported a significant improvement in 5-year RFS probabilities from 58% in 1997 to 66% in 2013. Our corresponding probabilities during those decades remained consistent at 64% and 66%, respectively (supplementary table 2, https://www.jurology.com). Indeed, the cumulative 5-year RFS probability across our cohort is 65%, thereby explaining the perceived lack of change. However, it is unclear whether this represents a plateau in overall benefit with the current armamentarium of surgical and medical innovations. The MSKCC report also predated their institutional implementation of ERAS and robot-assisted RC. The authors attributed their RFS improvement to its temporal association with increasing NAC use but did not present any multivariable analyses. Our multivariable analyses employing classical and directed acyclic graphical approaches highlight the independent prognostic roles of age, comorbidities, pathological stage, NAC administration and lymphovascular invasion. Among patients who received NAC, our ypT0N0M0 rate of 21.7% in the last 2 decades was comparable to MSKCC (22%) and other international academic centers (22.7%), and higher than the overall national average (10.6%) during the corresponding time frame.21–23 Our findings indicate that there was discernible benefit for patients receiving NAC who achieved pT0N0M0 or organ-confined disease at RC with no significant outcome differences between these subgroups. In contradistinction, those with extravesical and/or nodal metastatic disease after NAC experienced delay to definitive surgery and worse outcomes than those who directly underwent RC. While these findings should be interpreted cautiously given the study’s retrospective nature, it is clear that current clinical tools for predicting pathological stage and NAC response are relatively imprecise.24 This highlights the need to validate and incorporate novel biomarkers that identify patients who may likely not respond to traditional NAC, and may therefore benefit more from novel agents or expedited surgery.25–27 The need to achieve remission following definitive surgery is crucial as post-cystectomy recurrences are associated with dismal prognosis despite salvage therapy.28
This study has some limitations that warrant consideration. Precise metrics of performance status and comorbidities were not accurately recorded on all patients given the study’s retrospective nature and were therefore excluded from analysis. Subcategories of variant histology were not examined in detail as they comprised a small minority of the population and this was beyond the analytic scope; outcomes of these subgroups have been characterized previously.29,30 This population also predates the increasing use of immune checkpoint inhibitors and other targeted therapies for advanced UCB management. The strength of this study rests on the well-curated clinical information on patients with UCB undergoing RC from a prospectively maintained database. Standards for clinical decision making, indications for RC, surgical intervention and technical fundamentals, and pathological evaluation have remained consistent throughout the study period. Operative principles were meticulously followed by a group of high-volume surgeons, and all relevant clinicopathological annotations were standardized. Granular analysis of the population on a decade-by-decade and annual basis allowed for combined comparison of historical and contemporary data to map the evolution of surgical management of UCB in the context of medical, technical and perioperative advances.
Conclusions
This study presents a homogeneous RC series with long followup where consistency of surgical philosophy and standardized reporting permitted focused comparisons. Taken together, these data suggest that adherence to oncologically sound surgical principles can result in consistent outcomes following RC for UCB. While there has been an improvement in OS, this trend has not been recapitulated with RFS over time. Our real-world evolutionary experience with NAC suggests that a certain subset of UCB patients can derive great benefit. The challenge is to build reliable tools that accurately identify this subgroup, while developing novel therapeutic strategies for those who may not respond to conventional NAC in order to augment the oncologic benefit of radical surgery.
Footnotes
Financial and/or other relationship with BCAN Wright Foundation.
Editor's Note: This article is the second of 5 published in this issue for which category 1 CME credits can be earned. Instructions for obtaining credits are given with the questions on pages 481 and 482.
Contributor Information
Anirban P. Mitra, Email: apmitra@gmail.com.
Jie Cai, Email: jie.cai@med.usc.edu.
Gus Miranda, Email: gmiranda@med.usc.edu.
Sumeet Bhanvadia, Email: sumeet.bhanvadia@med.usc.edu.
David I. Quinn, Email: diquinn@med.usc.edu.
Anne K. Schuckman, Email: anne.schuckman@med.usc.edu.
Hooman Djaladat, Email: djaladat@med.usc.edu.
References
- 1.Abdollah F, Gandaglia G, Thuret R, et al. : Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol 2013; 37: 219. [DOI] [PubMed] [Google Scholar]
- 2.Shah A, Rachet B, Mitry E, et al. : Survival from bladder cancer in England and Wales up to 2001. Br J Cancer, suppl., 2008; 99: S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan TM, Barocas DA, Keegan KA, et al. : Volume outcomes of cystectomy—is it the surgeon or the setting? J Urol 2012; 188: 2139. [DOI] [PubMed] [Google Scholar]
- 4.Scarberry K, Berger NG, Scarberry KB, et al. : Improved surgical outcomes following radical cystectomy at high-volume centers influence overall survival. Urol Oncol 2018; 36: 308.e11. [DOI] [PubMed] [Google Scholar]
- 5.Daneshmand S, Ahmadi H, Schuckman AK, et al. : Enhanced recovery protocol after radical cystectomy for bladder cancer. J Urol 2014; 192: 50. [DOI] [PubMed] [Google Scholar]
- 6.Parekh DJ, Reis IM, Castle EP, et al. : Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018; 391: 2525. [DOI] [PubMed] [Google Scholar]
- 7.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005; 48: 202. [DOI] [PubMed] [Google Scholar]
- 8.Stein JP, Lieskovsky G, Cote R, et al. : Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001; 19: 666. [DOI] [PubMed] [Google Scholar]
- 9.Hautmann RE, de Petriconi RC, Pfeiffer C, et al. : Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 2012; 61: 1039. [DOI] [PubMed] [Google Scholar]
- 10.Shariat SF, Karakiewicz PI, Palapattu GS, et al. : Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol 2006; 176: 2414. [DOI] [PubMed] [Google Scholar]
- 11.Greenland S Pearl J and Robins JM: Causal diagrams for epidemiologic research. Epidemiology 1999; 10: 37. [PubMed] [Google Scholar]
- 12.Zehnder P, Moltzahn F, Mitra AP, et al. : Radical cystectomy with super-extended lymphadenectomy: impact of separate vs en bloc lymph node submission on analysis and outcomes. BJU Int 2016; 117: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macleod LC, Yabes JG, Yu M, et al. : Trends and appropriateness of perioperative chemotherapy for muscle-invasive bladder cancer. Urol Oncol 2019; 37: 462. [DOI] [PubMed] [Google Scholar]
- 14.Duplisea JJ, Mason RJ, Reichard CA, et al. : Trends and disparities in the use of neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma. Can Urol Assoc J 2019; 13: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koppie TM, Vickers AJ, Vora K, et al. : Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer 2006; 107: 2368. [DOI] [PubMed] [Google Scholar]
- 16.Dhar NB, Klein EA, Reuther AM, et al. : Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol 2008; 179: 873. [DOI] [PubMed] [Google Scholar]
- 17.Gschwend JE, Heck MM, Lehmann J, et al. : Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol 2019; 75: 604. [DOI] [PubMed] [Google Scholar]
- 18.Lin-Brande M, Nazemi A, Pearce SM, et al. : Assessing trends in urinary diversion after radical cystectomy for bladder cancer in the United States. Urol Oncol 2019; 37: 180.e1. [DOI] [PubMed] [Google Scholar]
- 19.Djaladat H, Mitra AP, Miranda G, et al. : Radical cystectomy and orthotopic urinary diversion in male patients with pT4a urothelial bladder carcinoma: oncological outcomes. Int J Urol 2013; 20: 1229. [DOI] [PubMed] [Google Scholar]
- 20.Vassantachart A, Daneshmand S, Cai J, et al. : Feasibility and outcomes of orthotopic ileal neobladder reconstruction following pelvic irradiation. Urology 2021; 148: 198. [DOI] [PubMed] [Google Scholar]
- 21.Almassi N, Cha EK, Vertosick EA, et al. : Trends in management and outcomes among patients with urothelial carcinoma undergoing radical cystectomy from 1995 to 2015: the Memorial Sloan Kettering experience. J Urol 2020; 204: 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zargar H, Espiritu PN, Fairey AS, et al. : Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2015; 67: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaid HB, Patel SG, Stimson CJ, et al. : Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 2014; 83: 75. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadi H, Mitra AP, Abdelsayed GA, et al. : Principal component analysis based pre-cystectomy model to predict pathological stage in patients with clinical organ-confined bladder cancer. BJU Int 2013; 111: E167. [DOI] [PubMed] [Google Scholar]
- 25.Mitra AP, Lam LL, Ghadessi M, et al. : Discovery and validation of novel expression signature for postcystectomy recurrence in high-risk bladder cancer. J Natl Cancer Inst 2014; 106: dju290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seiler R, Ashab HAD, Erho N, et al. : Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol 2017; 72: 544. [DOI] [PubMed] [Google Scholar]
- 27.Flaig TW, Tangen CM, Daneshmand S, et al. : A randomized phase II study of coexpression extrapolation (COXEN) with neoadjuvant chemotherapy for bladder cancer (SWOG S1314; NCT02177695). Clin Cancer Res 2021; 27: 2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitra AP, Quinn DI, Dorff TB, et al. : Factors influencing post-recurrence survival in bladder cancer following radical cystectomy. BJU Int 2012; 109: 846. [DOI] [PubMed] [Google Scholar]
- 29.Mitra AP, Bartsch CC, Bartsch G, Jr, et al. : Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol Oncol 2014; 32: 117. [DOI] [PubMed] [Google Scholar]
- 30.Mitra AP, Fairey AS, Skinner EC, et al. : Implications of micropapillary urothelial carcinoma variant on prognosis following radical cystectomy: a multi-institutional investigation. Urol Oncol 2019; 37: 48. [DOI] [PubMed] [Google Scholar]