To the Editor:
We recently read with great interest the article by Jatwani et al, describing the case of a 65-old woman who experienced psoriatic arthritis (PsA) after 12 weeks of nivolumab (N) therapy for non–small cell lung cancer.1
Here we report, for the first time, a de novo onset of seronegative oligoartritis in a white young man treated with N for classic Hodgkin lymphoma (cHL).
In March 2016, a 29-year-old male patient exhibited yet another relapse of a refractory cHL, which previously failed first-, second-, and third-line standard therapies, as well as the autologous and allogeneic stem cell transplantation.
He thus started an N-monotherapy (3 mg/kg intravenously every 2 weeks), reaching a cHL remission at the minimum follow-up period of 6 months, as determined by the restaging imaging.2 Because of the integrated efficacy and safety profile in this patient, hematologists kept the N-treatment.
In November 2017, after 18 months from the beginning of the N-therapy, the patient referred to our clinic, presenting rapidly progressive arthritis of the right knee and ankles. Increased levels of acute-phase reactants (erythrocyte sedimentation rate, 36 mm/h; C-reactive protein, 78 mg/L) were revealed, whereas no abnormalities in antinuclear antibodies, rheumatoid factor, anti–cyclic citrullinated peptide antibodies, and serum uric acid occurred. Magnetic resonance imaging (MRI) of the right knee showed an active synovitis with fluid distension of the joint capsule and intra-articular effusion (Fig.). MRI of the ankles documented intra-articular effusion and inflammatory changes of the posterior tibial tendons, being representative of acute tenosynovitis. These pathological features were suggestive for an oligoarticular PsA sine psoriasis (PsO). The disease activity score index for PsA (DAPSA) suggested a moderate-high disease severity (DAPSA, 25). According to the hematological consultation, N-therapy was discontinued and methylprednisolone (MP) was introduced (48 mg/d orally), resulting in a disease remission in a month (DAPSA, 4). MP was tapered by 10% of the dose once every 2 weeks, up to a 16 mg/d dose. The restaging scans concurrently documented a stable cHL remission. After 1 week of 16 mg/d MP dose, swollen and pain of knees, ankles, and elbows occurred, registering high disease activity (DAPSA, 27). Methotrexate (MTX) was thus added to therapy (15 mg subcutaneously, once a week). After 4 weeks of MTX, a disease remission was reached (DAPSA, 4) and MP lowering was successfully performed. Owing to a cHL stable remission, hematologists did not reintroduce N-therapy.
FIGURE.
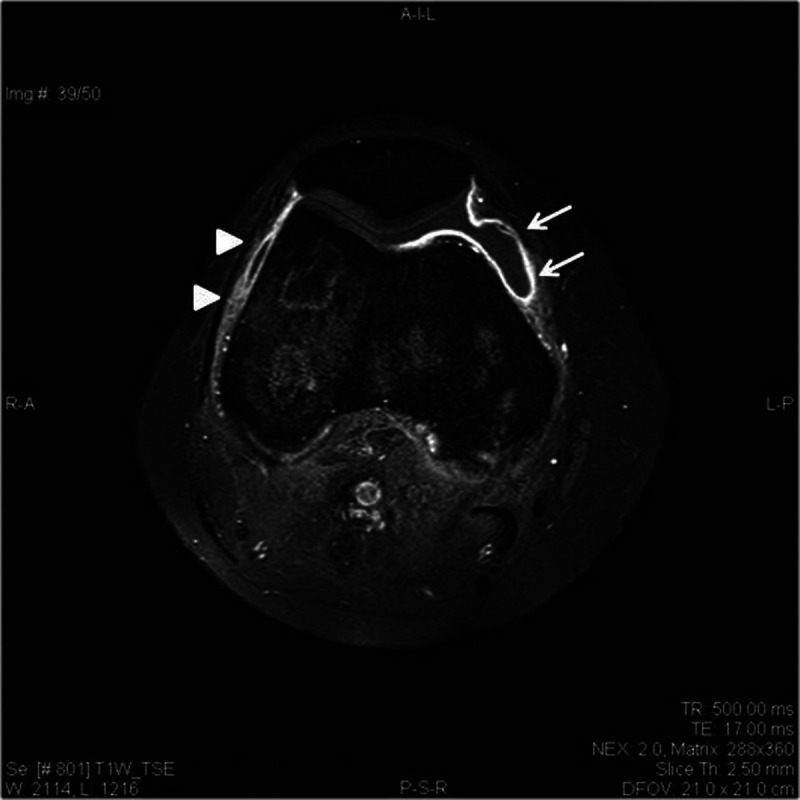
Magnetic resonance imaging (MRI) of right knee at onset of arthritis. MRI (contrast-enhanced T1W fat-suppressed spin-echo image) of the right knee showing extensive effusive synovitis involving the tibiofemoral joint space (arrows) as well as synovial thickening (arrowheads).
Currently, the young patient is still in complete remission of both cHL and oligoarthritis, while being treated with MTX (15 mg/wk) and low-dose MP (4 mg/d).
This is the first report describing the occurrence of a seronegative oligoarthritis sine PsO in a young male undergoing N-treatment for relapsing cHL. Differently from the few related reports describing N-induced PsA, our patient is not 64 years or older, and he is not affected by lung cancer with occasionally clinically overt PsO.1,3,4 Moreover, our case indicates that an N-induced de novo arthritis can occur very late after the beginning of N-treatment5,6 and can persist despite N-discontinuation.4,7 In our patient, we hypothesize that N-induced Th17 upregulation resulted in an imbalance of proinflammatory cytokines, which may contribute to the onset of the arthritis.8 In addition, the onset of oligoarthritis was associated with the cHL remission, suggesting that the induction of inflammatory arthritis may correlate with the antitumor activity.4 However, the arthritis persisted after N-discontinuation, requiring disease-modifying antirheumatic drug therapy: in this context, the persistence of the therapeutic effects after the discontinuation cannot be predicted.
Maria Rosaria Galdiero, MD, PhD
Department of Translational Medical Sciences and Center for Basic and Clinical Immunology Research WAO Center of Excellence University of Naples Federico II Naples, Italy and Institute of Experimental Endocrinology and Oncology “G. Salvatore” National Research Council Naples, Italy mariarosaria.galdiero@unina.it
Emanuela Morelli, MD
Hematology-Oncology and Stem Cell Transplantation Unit Istituto Nazionale Tumori Fondazione “G. Pascale” IRCCS Naples, Italy
Paola Triggianese, MD, PhD
Rheumatology, Allergology and Clinical Immunology Department of “Medicina dei Sistemi” University of Rome Tor Vergata Rome, Italy
Laura Carucci, MD
Alessandra Punziano, MD
Postgraduate Program in Clinical Immunology and Allergy University of Naples Federico II Naples, Italy
Antonio Pinto, MD
Hematology-Oncology and Stem Cell Transplantation Unit Istituto Nazionale Tumori Fondazione “G. Pascale” IRCCSNaples, Italy
Arturo Genovese, MD
Amato de Paulis, MD
Giuseppe Spadaro, MD
Department of Translational Medical Sciences and Center for Basic and Clinical Immunology Research WAO Center of Excellence University of Naples Federico II Naples, Italy
Footnotes
This work has been supported by grants from Regione Campania CISI-Lab Project, CRèME Project, and TIMING Project and by MIUR-PRIN 2017M8YMR8_005 to M.R.G.
The authors have no conflicts of interest to declare.
All the listed authors have made a substantial, direct, and intellectual contribution to the work. They approved it for publication.
Contributor Information
Emanuela Morelli, Email: e.morelli@istitutotumori.na.it.
Paola Triggianese, Email: paola.triggianese@gmail.com.
Laura Carucci, Email: laura.carucci@outlook.it.
Alessandra Punziano, Email: a.punziano@studenti.unina.it.
Antonio Pinto, Email: a.pinto@istitutotumori.na.it.
Arturo Genovese, Email: arturogenovese2@gmail.com.
Amato de Paulis, Email: depaulis@unina.it.
Giuseppe Spadaro, Email: spadaro@unina.it.
REFERENCES
- 1.Jatwani K Kaur H Chugh K, et al. Nivolumab-induced psoriatic arthritis in a patient with advanced small cell lung cancer. J Clin Rheumatol. 2020. [DOI] [PubMed] [Google Scholar]
- 2.Kasamon YL de Claro RA Wang Y, et al. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical Hodgkin lymphoma. Oncologist. 2017;22:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sapalidis K Kosmidis C Michalopoulos N, et al. Psoriatic arthritis due to nivolumab administration a case report and review of the literature. Respir Med Case Rep. 2018;23:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law-Ping-Man S Martin A Briens E, et al. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology (Oxford). 2016;55:2087–2089. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol. 2018;14:569–579. [DOI] [PubMed] [Google Scholar]
- 6.Cappelli LC Gutierrez AK Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komiya K Nakamura T Abe T, et al. Discontinuation due to immune-related adverse events is a possible predictive factor for immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer. 2019;10:1798–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulos J Carven GJ van Boxtel SJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35:169–178. [DOI] [PubMed] [Google Scholar]


