Abstract
Objective:
To determine prevalence and health-related quality of life (HRQOL) of moderate-to-severe vasomotor symptoms (VMS) in postmenopausal women in Europe, the US, and Japan, and among subgroups of women not taking hormone therapy (HT).
Methods:
Screening surveys were sent to a random sample of women aged 40 to 65 years; full questionnaires followed to those who completed them and met inclusion criteria. Women with successfully treated VMS, breast cancer, or on HT for medical conditions were excluded. The Menopause-Specific QOL (MENQOL) and Work Productivity and Activity Impairment (WPAI) questionnaires were included in the questionnaire.
Results:
Of 25,161 women completing the screening survey, 11,771 were postmenopausal and 3,460 met inclusion criteria and completed the full questionnaire. Prevalence of moderate-to-severe VMS was 40%, 34%, and 16% in Europe, the US, and Japan, respectively. A large proportion were HT averse, albeit eligible (Europe 56%, US 54%, Japan 79%). In total, 12%, 9%, and 8% in Europe, the US, and Japan, respectively, were HT-contraindicated. A high proportion were HT-cautious (Europe 70%, US 69%, Japan 52%). Most common menopausal symptoms reported in the MENQOL were feeling tired or worn out (Europe/US 74%, Japan 75%), aching in muscles and joints (Europe 69%, US 68%, Japan 61%), difficulty sleeping (Europe 69%, US 66%, Japan 60%), and hot flashes (Europe 67%, US 68%, Japan 62%). Overall, the most bothersome symptom was weight gain. As measured by the WPAI, hot flashes and night sweats had a greater impact on daily activities than on working activities.
Conclusions:
A high proportion of women experienced moderate-to-severe VMS, with associated symptoms impacting QOL.
Keywords: Hormone therapy, Menopause, Quality of life, Vasomotor symptoms
Vasomotor symptoms (VMS), characterized by hot flashes (also called hot flushes) and/or night sweats, are the main symptoms of menopause, and are experienced by up to 80% of women during the menopausal transition.1,2 The majority of women rate their VMS as moderate-to-severe,2 meaning that they experience a sensation of heat with sweating.3 VMS can persist on average for 7 to 10 years;4,5 although this seems dependent on the timing of onset.5
Prevalence of menopause-related VMS seems to vary widely depending on geographic region, selection of criteria, and method of symptom identification. A systematic review of published data on menopausal symptoms reported prevalence rates ranging from 22% to 63% in Asia to 36% to 50% in North America, and 74% in Europe;6 however, cross-cultural studies have mostly been restricted to one country or continent and only included small numbers of participants.
The impact of VMS on individuals’ quality of life (QOL) can be significant. Women with moderate-to-severe VMS may experience sleep problems, fatigue, anxiety, and depression, that may impact the ability to work and carry out day-to-day activities.7-13 VMS are the primary driver for seeking medical attention for menopause-related symptoms.14 However, many women with VMS do not seek advice from healthcare professionals; instead they try complementary therapies and over-the-counter medications.15,16 These include cognitive behavioral therapy and herbal remedies such as black cohosh, ginseng, gingko biloba, St. John's wort, and dong quai. As a result, it is difficult to estimate the burden of VMS in terms of both prevalence and impact on QOL through available data.
Given that moderate-to-severe VMS can have a significant impact on QOL over a number of years, it is important to better understand its impact and the number of women affected. Thus, we conducted a survey of postmenopausal women aged 40 to 65 years, with the primary objective of determining the prevalence of moderate-to-severe VMS and the impact on health-related QOL (HRQOL) in women in Europe, the US, and Japan, and among subgroups of women not taking hormone therapy (HT). The secondary objective was to understand the proportion of women with moderate-to-severe VMS who seek medical advice and treatment.
METHODS
Study design
This was a cross-sectional online survey of postmenopausal women currently experiencing moderate-to-severe VMS, or who reported having symptoms in the prior 12 months. The survey was conducted in five countries in Europe (France, Germany, Italy, Spain, UK), the US, and Japan between December 2019 and February 2020. An online screening survey (available on computer, tablet, or smartphone) was sent by global consumer panels (belonging to Lightspeed in Europe and the US and Toluna in Japan) to a random sample of women aged 40 to 65 years. The full questionnaire was sent to those who completed the screening survey and met inclusion criteria.
Study population
Women in consumer panels who were 40 to 65 years of age were eligible to receive the screening survey. The full questionnaire was sent to those who completed the screening survey and met the following criteria: 40 to 65 years old, postmenopausal (without periods for ≥12 mo), ≥1 VMS symptom in the last 12 months (had chosen at least hot flashes or night sweats from the list of menopause symptoms in the screening survey), experiencing moderate-to-severe VMS symptoms in the last 12 months, never diagnosed with breast cancer or treated with antiestrogens, aromatase inhibitors, or gonadotropin-releasing treatments in the last 12 months. Ethics committee exemption was sought and granted for the market research involved in the study. Women received a small financial incentive to participate in the study.
Women not meeting these inclusion criteria based on their responses to the screening survey (eg, those who experienced no or only mild VMS symptoms) were not sent the full questionnaire. Women successfully treated for VMS with no residual VMS were, therefore, by default, not eligible.
VMS severity was defined based on the 2003 US Food and Drug Administration Guidance for Industry3 (the same definition used in Europe17), where mild is defined as “a sensation of heat without sweating,” moderate is “a sensation of heat with sweating, able to continue activity,” and severe is “a sensation of heat with sweating, causing cessation of activity.”
Questionnaires and assessments
The screening survey included six screening questions on age, current menstrual status, prior diagnosis of breast cancer, treatments received in the previous 12 months (including HT), menopause symptoms, and whether participants had experienced mild/moderate/severe VMS. The full questionnaire included 37 questions on medical conditions, frequency and duration of VMS, perceptions of menopause, whether they had seen an healthcare professional for advice on menopause symptoms, treatment for VMS symptoms, perceptions of HT use, out-of-pocket costs, and other approaches to coping with menopause.
The Menopause-Specific QOL (MENQOL) questionnaire18 was included (as Question 4) to assess QOL and impact of menopause symptoms. Participants selected whether they had experienced any of the 30 common menopause symptoms in the past week. If they had experienced a symptom, they indicated how much the symptom bothered them on a scale of 1 (not at all bothered) to 6 (extremely bothered). In addition, the Work Productivity and Activity Impairment (WPAI) questionnaire19 was included (Questions 7-12) to assess the level of impact of hot flashes or night sweats on daily activities or working activities. Participants indicated the impact of each symptom on a scale of 0 (had no effect) to 10 (completely prevented me from working or daily activities).
Calculation for prevalence of VMS
To calculate the prevalence of postmenopausal women with moderate-to-severe VMS, data were taken from public sources,20 as well as detailed statistics from the study (Supplementary Fig. 1). The denominator for prevalence was defined as the number of women aged 40 to 65 years completing the screening survey who classified themselves as postmenopausal (‘after menopause, without periods for at least 12 months’). The numerator was defined as the number of women who met the criteria for age 40 to 65 years, menopausal stage (postmenopausal) and VMS severity (moderate-to-severe), and who completed the main survey.
Prevalence was determined overall and in the following subpopulations (these were not mutually exclusive):
HT-willing: women currently prescribed HT or bioidentical HT or who are willing to take HT
HT-averse: women who are NOT currently treated with hormonal prescription therapies or prescribed bioidentical hormones and who are NOT willing to take HT
HT-contraindicated: women who have been assessed by a physician and HT was deemed not appropriate due to certain conditions/circumstances: bleeding from the genital tract without a determined cause, acute liver failure/active liver disease, deep vein thrombosis, uterine cancer, ovarian cancer, heart attack/stroke/angina/myocardial infarction (adapted from NAMS Position Statement, 201721)
HT-stoppers: women who previously received hormonal prescription therapies or prescribed bioidentical “natural” hormones, but are NOT currently on treatment
HT-caution: women with underlying medical conditions (eg, smoking, first degree relative with breast cancer, high cholesterol or triglycerides, migraine, diabetes) that warrant a cardiovascular or breast cancer risk assessment before prescribing HT (adapted from22).
Statistical analysis
The analysis was carried out with the Statistical Package for the Social Sciences (SPSS) and the output exported (Word/Excel). Continuous variables were summarized descriptively including the number of respondents (n), mean, standard deviation, median, minimum, and maximum. Categorical data was summarized by frequencies and percentages. Percentages by categories were based on the number of respondents with no missing data, such that the percentages for the nonmissing categories totalled 100%. Significant differences between subgroups were analyzed using the T test and/or ANOVA, plus the Z test. Data showed a normal distribution for key subgroups, resulting in the decision to employ the T test only for comparison of two subgroup means, ANOVA for comparison of >2 subgroup means, and the Z test to compare population means to that of a sample. Since normal distribution of data was confirmed for usage of T and Z tests, statistical comparison of the regions/countries was allowed, regardless of the sample size.
RESULTS
Characteristics of study participants
In total, 69,000 women aged 40 to 65 years were sent the screening survey (Supplementary Fig. 2). Of 25,161 women who completed the screening survey, 11,771 were postmenopausal and received the full questionnaire. A total of 3,460 women completed the full questionnaire (n = 2,035 in Europe [France, n = 406; Germany, n = 405; Italy, n = 413; Spain, n = 406; UK, n = 405], n = 675 in US, n = 750 in Japan). The denominator was therefore 11,771 and the numerator was 3,460 for the prevalence calculation.
Table 1 displays baseline characteristics. Mean age of participants was 56 years in Europe, 57 years in the US, and 55 years in Japan; most women were aged 51 to 60 years. In Europe and Japan, most women were employed (working for pay); this proportion was slightly lower in the US. The most common concurrent medical conditions reported from those in the questionnaire were high cholesterol or triglycerides, hypertension in Europe and the US, and myoma of the uterus and migraine in Japan. In Europe, 34% were smokers; this percentage was lower in the US (22%) and Japan (20%).
TABLE 1.
Baseline participant characteristics
| Characteristic | Europe N = 2,035 | US N = 675 | Japan N = 750 |
| Age, mean, y | 56 | 57 | 55 |
| Age group, % | |||
| 40-50 | 11 | 10 | 11 |
| 51-60 | 67 | 59 | 79 |
| 61-65 | 22 | 31 | 10 |
| Marital status, % | |||
| Never married | 11 | 9 | 17 |
| Not married, living with partner | 11 | 6 | 2 |
| Married | 58 | 57 | 63 |
| Divorced/separated | 17 | 22 | 16 |
| Widowed | 3 | 7 | 2 |
| Other | 0 | 0 | 0 |
| Employed, % | 57 | 48 | 54 |
| Education, % | |||
| University/Doctorate | 31 | 55 | 45 |
| Middle/high school | 67 | 45 | 55 |
| Elementary | 2 | – | – |
| Severity of VMS symptoms, % | |||
| Mild | 9 | 14 | 5 |
| Moderate | 72 | 84 | 15 |
| Severe | 32 | 24 | 87 |
| Hot flashes and/or night sweats experienced in past 12 mo, % | |||
| Hot flashes only | 24 | 24 | 48 |
| Only night sweats | 14 | 17 | 22 |
| Both hot flashes and night sweats | 62 | 59 | 29 |
| Smoker, % | 34 | 22 | 20 |
| Top six medical conditions, % | |||
| High cholesterol or triglycerides | 24 | 37 | 14 |
| Hypertension | 24 | 34 | 14 |
| Migraine | 21 | 18 | 19 |
| Myoma of the uterus | 14 | 11 | 23 |
| Diabetes | 8 | 12 | 4 |
| Endometriosis | 5 | 9 | 10 |
| None of the above | 36 | 33 | 41 |
VMS, vasomotor symptoms.
In Europe and the US, the majority of women experienced moderate VMS (“I have had a sensation of heat with sweating, but was able to continue activities”); however, in Japan, 87% experienced severe VMS (“I have had a sensation of heat with sweating, causing me to stop activities”). In the previous 12 months, 62% in Europe, 59% in the US, and 29% in Japan had experienced both hot flashes and night sweats.
Prevalence of moderate-to-severe VMS
Prevalence of moderate-to-severe VMS among postmenopausal women aged 40 to 65 years was calculated in those who completed the screening survey and full survey. European countries reported the highest moderate-to-severe VMS (40%), followed by the US (34%) and Japan (16%) (Table 2). Prevalence in Europe ranged from 31% in France to 52% in Italy.
TABLE 2.
Prevalence of moderate-to-severe VMS amongst women aged 40-65 yearsa
| Europeb N = 2,035 | US N = 676 | Japan N = 760 | |
| Estimated prevalence of moderate-to-severe VMS in country/region, % | 40c | 34 | 16c |
| Prevalence by HT group, %d | |||
| HT-willinge | 24 | 25 | 11c |
| HT-aversef | 56 | 54 | 79c |
| HT-contraindicatedg | 12 | 9 | 8 |
| HT-stoppersh | 9 | 11 | 5c |
| HT-cautioni | 70 | 69 | 52c |
HT, hormone therapy; VMS, vasomotor symptoms.
Percentages estimated based on respondents’ self-assessment and perceptions.
Europe includes France, Germany, Italy, Spain, and UK. Prevalence by individual European country is shown in Supplementary Table 1.
Difference statistically significant vs both other regions at 95% CI level.
Groups are not mutually exclusive. Self-reported comorbidities do not indicate severity levels, associated treatments, etc.
HT-willing: women currently prescribed HT or bioidentical HT or who are willing to take HT.
HT-averse: women who are NOT currently treated with hormonal prescription therapies or prescribed bioidentical hormones and who are NOT willing to take HT.
HT-contraindicated: women who have been assessed by a physician and HT was deemed not appropriate due to certain conditions/circumstances: bleeding from the genital tract without a determined cause, acute liver failure/active liver disease, deep vein thrombosis, uterine cancer, ovarian cancer, heart attack/stroke/ angina/myocardial infarction (adapted from The North American Menopause Society Position Statement, 201721).
HT-stoppers: women who previously received hormonal prescription therapies or prescribed bioidentical “natural” hormones, but are NOT currently on treatment.
HT-caution: women with underlying medical conditions (eg, smoking, 1st degree relative with breast cancer, high cholesterol or triglycerides, migraine, diabetes) that warrant a cardiovascular or breast cancer risk assessment before prescribing HT (adapted from Reference 22).
Considering the subpopulations, a large proportion of women with moderate-to-severe VMS were HT-averse, albeit eligible: 56% in Europe, 54% in the US, 79% in Japan. A total of 12% of women in Europe, 9% in the US, and 8% in Japan were defined as HT-contraindicated. Additionally, a high proportion were in the HT-caution subgroup (70% in Europe, 69% in the US, 52% in Japan), indicating that risk-benefit assessment for cardiovascular or breast cancer would be needed before decisions could be made on initiating HT. Findings for Europe were generally consistent across each country (Supplementary Table 1). The top three comorbidities associated with HT-caution were high cholesterol/triglycerides (34%, 53%, and 36% in Europe, the US, and Japan, respectively), migraine (30%, 27%, and 26%, respectively), and diabetes (11%, 18%, and 8%, respectively). Of participants in the HT-caution subgroup, 48% in Europe, 32% in the US, and 37% in Japan were smokers, and 23%, 26%, and 20%, respectively, had relatives with breast cancer.
Perception of bother associated with VMS
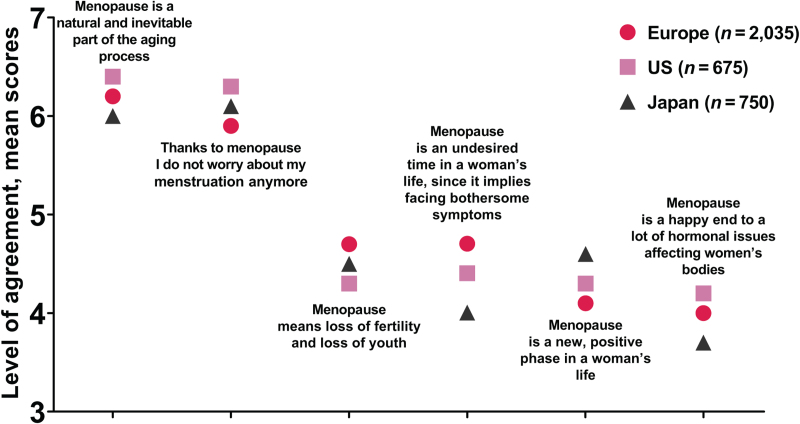
There was an overall acceptance among respondents of menopause as a natural and inevitable part of the aging process, associated with the end of worrying about menstruation; this was significantly more the case in the US (Fig. 1). In Europe, menopause was significantly more associated with loss of fertility and the onset of bothersome symptoms versus the other regions. There was a lower agreement overall that “menopause is a happy end to a lot of hormonal issues affecting women's bodies” (significantly less so in Japan).
FIG. 1.
Participants’ opinions about the menopause. Data taken from Q6. Below you will find a list of statements related to possible opinions about the impact of the menopause. Please indicate your personal level of agreement or disagreement using a 7-point scale where 1 means “I strongly disagree” and 7 means “I strongly agree.”
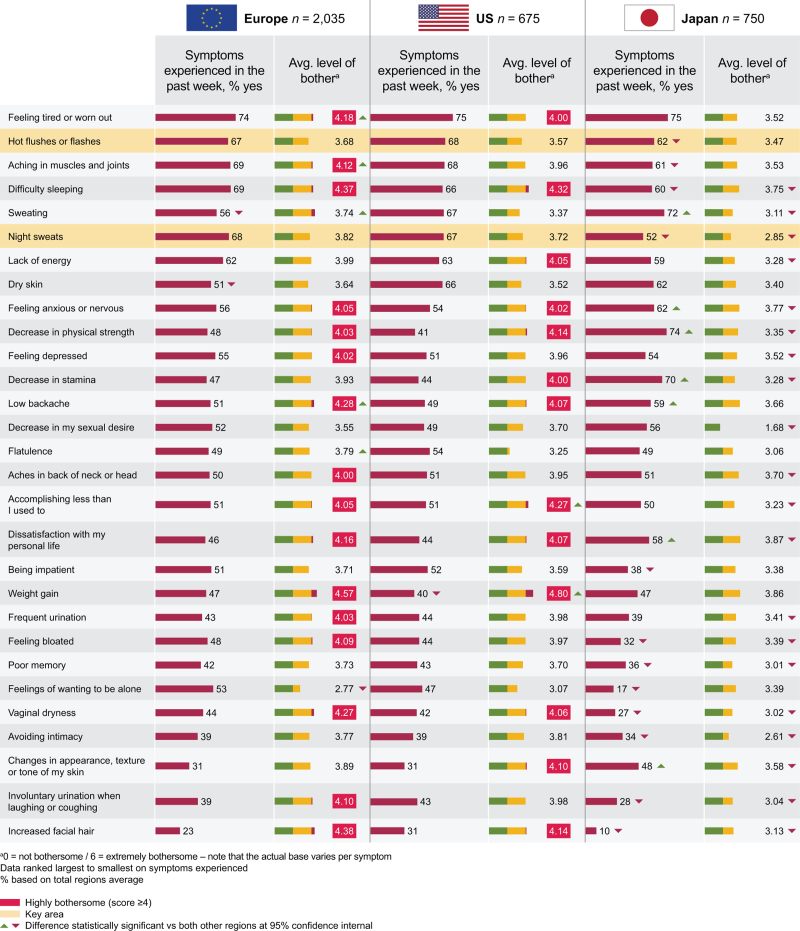
The most commonly reported menopausal symptom in the past week was feeling tired or worn out (74% in Europe, 75% in both the US and Japan) (Fig. 2). In Japan, decrease in physical strength was reported by 74%, sweating in 72%, and decrease in stamina in 70%. Other symptoms reported by a high proportion of women were aching in muscles and joints (69% in Europe, 68% in the US, 61% in Japan), difficulty sleeping (69% in Europe, 66% in the US, 60% in Japan), hot flashes (67% in Europe, 68% in the US, 62% in Japan), and night sweats (68% in Europe, 67% in the US, 52% in Japan).
FIG. 2.
Menopause symptoms experienced in the past week by participants, and associated level of bother for each symptom using the MENQOL.
Overall, the most bothersome symptom of menopause was weight gain (Fig. 2), considered highly bothersome in Europe (a level of bother of 4.57) and the US (4.80), and moderately bothersome in Japan (3.86), where 0 = not bothersome and 6 = extremely bothersome. Dissatisfaction with personal life was the most bothersome symptom in Japan (3.87). Difficulty sleeping was also considered to be highly bothersome (4.37 in Europe, 4.32 in the US, 3.75 in Japan). Notably, women in Japan generally had lower average level of bother across all symptoms (<4) versus women in Europe and the US.
Women in Europe experienced an average of 4.6 times/d of hot flashes (mild, moderate, or severe) and/or night sweats; this was lower in the US (3.8 times/d), and Japan (3.0 times/d). A similar number of moderate or severe VMS were experienced by women in Europe (2.9/d) and the US (2.6/d); women in Japan reported a lower rate (1.4/d). Women in the US reported a longer average duration of each episode (34 min/episode) than women in Europe or Japan (23 min/episode reported by both).
Menopause impact on work productivity and daily activities
According to WPAI findings, hot flashes and night sweats had a greater impact on daily activities (eg, work around the house, shopping, childcare, exercising, studying, etc.) than on working activities. Impact scores were highest in Japan: 1.72 for working activities versus 1.41 in Europe and 1.14 in the US, and 2.41 in Japan for daily activities versus 2.19 in Europe and 1.99 in the US. However, generally the impact of VMS on work productivity or ability to do daily activities was low. This trend was amplified when data were filtered on women with sleep disturbances, with impact scores highest in Japan: 2.36 on working activities versus 1.62 in Europe and 1.2 in the US, and 3.16 on daily activities in Japan versus 2.50 in Europe and 2.25 in the US.
Managing menopause-related symptoms: hormone therapy usage and attitudes
Overall, 83% of participants in Europe, 81% in the US, and 91% in Japan had never received HT.
Of those who had not previously received HT, a high proportion were HT-averse (Table 3); this was highest in Japan (87%). The predominant reason for not receiving HT treatment was the belief that menopause will pass by itself (52% in Europe, 53% in the US, 66% in Japan). Other reasons included worries about HT side effects (28% in Europe, 34% in the US, 11% in Japan), worries about long-term risks associated with HT (25% in Europe, 30% in the US, 8% in Japan), lack of information about HT (8% in Europe, 6% in the US, 15% in Japan), and risk factors in the family (10% in Europe, 11% in the US, 2% in Japan).
TABLE 3.
Reasons participants had never received hormone therapya
| % | Europe (N=2,035) | US (N = 675) | Japan (N = 750) |
| Never taken HT | 83 | 81 | 91 |
| HT-averse | 70 | 68 | 87 |
| Willing to receive HT in the future | 30 | 32 | 13 |
| Reasons for never having taken HT | |||
| No need to treat menopause with drugs since it will pass by itself | 52 | 53 | 66 |
| Worried about side effects of HT | 28 | 34 | 11 |
| Worried about long-term risks associated with HT | 25 | 30 | 8 |
| Risk factors in the family | 10 | 11 | 2 |
| Lack of information about HT | 8 | 6 | 15 |
| HT was discussed with HCP but was not advised due to an underlying condition(s) that places me at higher risk if HT is taken | 6 | 3 | 2 |
| Discussed with HCP but was not advised due to underlying conditions | 5 | 3 | 1 |
Europe includes France, Germany, Italy, Spain, and UK.
HCP, healthcare professional; HT, hormone therapy.
Data from Q25: “You have never received hormonal treatment for your menopause related symptoms. Is this due to…” and Q26: “Would you consider taking hormones (Hormone Replacement Therapy or HT; eg, estrogen therapy, progestin therapy) for your menopause related hot flashes/night sweats if this was advised/ prescribed to you by an HCP?”. Percentages estimated based on respondents’ self-assessment and perceptions.
Managing menopause-related symptoms: lifestyle changes and over-the-counter medicines
The majority of women in the study adopted lifestyle changes: 75% in Europe, 76% in the US, 53% in Japan). These included keeping cool, rest and relaxation, wearing loose clothing, and increased level of exercise. In those women who made lifestyle changes, over half reported some improvement in hot flashes/night sweats. However, between 38% (Europe) and 46% (Japan) reported no change in symptoms. Over-the-counter products for coping with menopause-related symptoms were taken by 45%, 43%, and 27% of participants in Europe, the US, and Japan, respectively. Of these, 45% in Europe, 52% in the US, and 49% in Japan reported no change in symptoms; 35% in Europe, 30% in the US, and 34% in Japan reported that symptoms had slightly improved.
DISCUSSION
The results from this global survey demonstrate that a high proportion of postmenopausal women aged 40 to 65 years experience moderate-to-severe VMS. This prevalence seems to be higher in Europe and the US (40% and 34%, respectively) than Japan (16%). In comparison, a prevalence of 65% of VMS was demonstrated in postmenopausal women (n = 4,402) in the US Menopause Epidemiology Study.23
A number of previous studies have reported similarly low incidence of VMS in Japanese women. For example, Lock et al reported around 10% of women with hot flashes.24-26 Similar to our results, in the US SWAN study that included women of Japanese, African American, Chinese, and Hispanic origin in addition to White women, Japanese women reported the fewest VMS.1,4,9,27 However, there were variations in incidences reported by Japanese women living in the US and those living in Japan.28,29 It is notable that there is no accepted definition in Japan for hot flashes and their severity, and reporting of hot flashes by Japanese women may be dependent on the terminology used.30 In the current study, more Japanese women reported severe rather than moderate VMS. However, it is possible that this is due to translation and interpretation issues.
When considering the prevalence of moderate-to-severe VMS by HT subgroup, the top three comorbidities associated with HT-caution were high cholesterol/triglycerides, migraine, and diabetes. The HT-caution subgroup also contained a larger percentage of smokers and relatives with breast cancer versus overall respondents. Only a small proportion of women across each region had absolute contraindications for HT. Over half of women were HT-eligible but averse to taking HT, with Japan having the highest proportion who were HT-averse. A high proportion of women also had comorbidities that usually require a benefit-risk assessment before initiating HT. Indeed, many barriers to HT are present and include fears of increasing breast cancer risk and vascular events, especially in women with a family history representing a point of concern.31
Across all regions, there was an overall acceptance of menopause as a natural and inevitable part of aging. Despite this, menopause is associated with a significant burden. Women reported a wide range of psychological and physical symptoms of VMS, most frequently feeling tired and worn out, which was consistently the highest reported symptom across all regions. The most bothersome VMS symptom reported in the US and Europe was weight gain and—in Japan—dissatisfaction with personal life. Difficulty sleeping was also considered highly bothersome in all regions. Results from the SWAN study have also shown strong associations between VMS and perceived sleep disturbance.32
The average number of VMS (hot flashes and/or night sweats) experienced by women in this survey (4.6, 3.8, and 3.0 times/d in Europe, the US, and Japan, respectively) is relatively low compared with regulatory interventional studies where the criteria are usually a minimum of 7 moderate-to-severe VMS/24 hours. This difference in frequency must be considered when interpreting the results. Overall, night sweats and hot flashes were reported as moderately bothersome. However, women who suffered from both hot flashes and night sweats reported that combination of both symptoms was more bothersome than either of these symptoms alone. Women in Japan reported a lower level of bother and fewer moderate-to-severe VMS versus those in Europe or the US. This finding is consistent with other studies, such as SWAN, where Japanese women were least likely to describe VMS as bothersome.9 Across the regions, night sweats and hot flashes had a greater impact on daily activities than on working activities. However, the perceived impact of these symptoms was low. Other studies have previously shown an increase in work days lost in women with untreated VMS.33
The majority of respondents across all regions had never received HT and were HT-averse. Given that women with controlled VMS were excluded from completing the full questionnaire, this finding is not surprising, as women successfully taking HT with no residual VMS would be ineligible for the full questionnaire, and thus respondents may be more likely to be those who were HT-averse. The main reason for not taking HT was belief that menopausal symptoms would pass without pharmacological intervention (reported by ≥52% of participants in any country/region). In comparison, worries about side effects and concern about long-term risks and risk factors in the family were cited by fewer participants. However, many women made lifestyle changes or tried over-the-counter medications to manage symptoms. Usage of HT has significantly reduced in the US and in most European countries since publication of the Women's Health Initiative study results raised questions about the long-term safety of this treatment.34-36 HT is, however, recognized by international clinical practice guidelines as the most effective treatment for VMS in symptomatic women <60 years of age/within 10 years of menopause.21,37-39
The current study is the most robust analysis of VMS to date. Key strengths are the global scope of this study, including a large number of respondents from across Europe, the US, and Japan, and the breadth and variety of outcomes assessed, including the validated MENQOL and WPAI questionnaires. However, whilst condition-specific PRO tools like MENQOL were utilized in this study, a study of this type may not allow us to fully distinguish between symptoms related to the menopause and symptoms merely related to aging. With respect to interpreting the results of this study, it is important to consider the cross-sectional design and the limitation of cross-sectional data points. Also, despite consumer panels being used to generate the sample for the screening survey, they still have biases—such as willingness to be proactive in research, access to the internet, and a higher education level (shown in Table 1). Further, there is a difference in the average frequency of VMS in this study population versus that in regulatory (compliant) interventional studies. The data were collected through a computer-aided web interview; therefore, the responder recall period should be considered when interpreting the findings. In addition, a clinician-confirmed diagnosis was not required, but confirmation of disease severity was supported by requesting information on number and severity of hot flashes and night sweats. Also, patients completely controlled with treatment such as HT, therefore having no moderate-to-severe VMS, did not qualify to receive the full questionnaire. Therefore, women completing the full questionnaire could be a relatively unique group with unmet need. Although this group is likely to represent the real-world situation, this should be taken into consideration when interpreting the results.
CONCLUSIONS
A high proportion of women experience moderate-to-severe VMS, especially in Europe and the US. Associated symptoms such as feeling tired and worn out and problems with sleeping impact women's QOL and can have a significant burden. However, since not all women who experience moderate-to-severe VMS seek healthcare advice, this burden is likely to be underestimated when assessing only women diagnosed with VMS in a healthcare setting.
For women who are currently not able to or are unwilling to receive HT treatment for VMS, the availability of new treatment options may help improve the management of VMS.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank the study investigators, and all participants and their legal representatives who took part in the study. This study was funded by Astellas Pharma Inc. Medical writing support was provided by Sue Cooper and Catherine Elliott of Elevate Scientific Solutions and funded by Astellas Pharma.
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Footnotes
Funding/support: This study was funded by Astellas Pharma Inc.
Financial disclosure/conflicts of interest: R.E.N. is a lecturer and consultant for Bayer, Exceltis, and Theramex; a researcher for Novo Nordisk; a researcher and consultant for Fidia; a lecturer for MSD and Pfizer; a consultant for Astellas and Palatin; and a researcher and lecturer for Shionogi. R.K. is a consultant and researcher for Astellas, Allergan, Estetra, Therapeutics MD, and a researcher for Bayer and Mithra. C.R. and E.G. have no disclosures to report. B.S. is a former employee of Astellas. E.S. and N.S. are employees of Astellas.
Previous publications of the data: abstract presented at NAMS 2020.
Supplemental digital content is available for this article.
REFERENCES
- 1.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health 2006; 96:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause 2014; 21:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Drug Administration. Guidance for industry: estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms — recommendations for clinical evaluation. Available at: https://www.fda.gov/media/71359/download. Accessed April 22, 2021. [Google Scholar]
- 4.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015; 175:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol 2011; 117:1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 2010; 13:419–428. [DOI] [PubMed] [Google Scholar]
- 7.Whiteley J, Wagner JS, Bushmakin A, Kopenhafer L, Dibonaventura M, Racketa J. Impact of the severity of vasomotor symptoms on health status, resource use, and productivity. Menopause 2013; 20:518–524. [DOI] [PubMed] [Google Scholar]
- 8.Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas 2009; 62:153–159. [DOI] [PubMed] [Google Scholar]
- 9.Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: who is most bothered by vasomotor symptoms? Menopause 2008; 15:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the Study of Women's Health across the Nation. Obstet Gynecol Clin North Am 2011; 38:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worsley R, Bell RJ, Gartoulla P, Robinson PJ, Davis SR. Moderate-severe vasomotor symptoms are associated with moderate-severe depressive symptoms. J Womens Health (Larchmt) 2017; 26:712–718. [DOI] [PubMed] [Google Scholar]
- 12.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burleson MH, Todd M, Trevathan WR. Daily vasomotor symptoms, sleep problems, and mood: using daily data to evaluate the domino hypothesis in middle-aged women. Menopause 2010; 17:87–95. [DOI] [PubMed] [Google Scholar]
- 14.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas 2007; 58:348–358. [DOI] [PubMed] [Google Scholar]
- 15.Gentry-Maharaj A, Karpinskyj C, Glazer C, et al. Use and perceived efficacy of complementary and alternative medicines after discontinuation of hormone therapy: a nested United Kingdom Collaborative Trial of Ovarian Cancer Screening cohort study. Menopause 2015; 22:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posadzki P, Lee MS, Moon TW, Choi TY, Park TY, Ernst E. Prevalence of complementary and alternative medicine (CAM) use by menopausal women: a systematic review of surveys. Maturitas 2013; 75:34–43. [DOI] [PubMed] [Google Scholar]
- 17. European Medicines Agency. Guideline on clinical investigation of medicinal products for hormone replacement therapy of oestrogen deficiency symptoms in postmenopausal women. Available at: https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-hormone-replacement-therapy-oestrogen-deficiency-symptoms. Accessed April 5, 2021. [Google Scholar]
- 18.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas 1996; 24:161–175. [DOI] [PubMed] [Google Scholar]
- 19.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4:353–365. [DOI] [PubMed] [Google Scholar]
- 20. Population pyramids of the world from 1950 to 2100: France, Germany, Italy, Spain, UK, Japan and US. Available at: https://www.populationpyramid.net/world/2017/. Accessed April 22, 2021. [Google Scholar]
- 21.The NAMS Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017; 24:728–753. [DOI] [PubMed] [Google Scholar]
- 22.Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/non-hormonal therapy decision making: a clinical decision-support tool from The North American Menopause Society. Menopause 2015; 22:247–253. [DOI] [PubMed] [Google Scholar]
- 23.Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric 2008; 11:32–43. [DOI] [PubMed] [Google Scholar]
- 24.Lock M. Ambiguities of aging: Japanese experience and perceptions of menopause. Cult Med Psychiatry 1986; 10:23–46. [DOI] [PubMed] [Google Scholar]
- 25.Lock M. Menopause in cultural context. Exp Gerontol 1994; 29:307–317. [DOI] [PubMed] [Google Scholar]
- 26.Lock M, Kaufert P, Gilbert P. Cultural construction of the menopausal syndrome: the Japanese case. Maturitas 1988; 10:317–332. [DOI] [PubMed] [Google Scholar]
- 27.Reed SD, Lampe JW, Qu C, et al. Self-reported menopausal symptoms in a racially diverse population and soy food consumption. Maturitas 2013; 75:152–158. [DOI] [PubMed] [Google Scholar]
- 28.Anderson D, Yoshizawa T, Gollschewski S, Atogami F, Courtney M. Menopause in Australia and Japan: effects of country of residence on menopausal status and menopausal symptoms. Climacteric 2004; 7:165–174. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda T, Makita K, Ishitani K, Takamatsu K, Horiguchi F, Nozawa S. Status of climacteric symptoms among middle-aged to elderly Japanese women: comparison of general healthy women with women presenting at a menopausal clinic. J Obstet Gynaecol Res 2005; 31:164–171. [DOI] [PubMed] [Google Scholar]
- 30.Melby MK. Vasomotor symptom prevalence and language of menopause in Japan. Menopause 2005; 12:250–257. [PubMed] [Google Scholar]
- 31.Parish SJ, Nappi RE, Kingsberg S. Perspectives on counseling patients about menopausal hormone therapy: strategies in a complex data environment. Menopause 2018; 25:937–949. [DOI] [PubMed] [Google Scholar]
- 32.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 2008; 31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 33.Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause 2015; 22:260–266. [DOI] [PubMed] [Google Scholar]
- 34.Ameye L, Antoine C, Paesmans M, de Azambuja E, Rozenberg S. Menopausal hormone therapy use in 17 European countries during the last decade. Maturitas 2014; 79:287–291. [DOI] [PubMed] [Google Scholar]
- 35.Crawford SL, Crandall CJ, Derby CA, et al. Menopausal hormone therapy trends before versus after 2002: impact of the Women's Health Initiative Study Results. Menopause 2018; 26:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: results from the National Health and Nutrition Examination Survey, 1999-2010. Obstet Gynecol 2012; 120:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2015; 100:3975–4011. [DOI] [PubMed] [Google Scholar]
- 38.ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol 2014; 123:202–216. [DOI] [PubMed] [Google Scholar]
- 39.de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Maturitas 2016; 91:153–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.