Abstract
Previously, Rhodotorula glutinis was reported to produce a large amount of exocellular mannan, having a repeating unit of →3)-d-Manp-(1→4)-d-Manp-(1→. Recently, we found that antigenic polysaccharides of Leptospira biflexa serovar patoc strain Patoc I have the same repeating unit and cross-react with antisera raised against extended strains of other leptospires (K. Matsuo, E. Isogai, and Y. Araki, Carbohydr. Res., in press). This structural identity and the difficulty of producing and isolating antigens led us to confirm the usefulness of Rhodotorula mannan as an immunoreactive antigen in a serological diagnosis of leptospirosis. In the present investigation, we confirmed the structural identity of an exocellular mannan isolated from R. glutinis AHU 3479 and tried to use it as an immunoreactive antigen in a serological diagnosis of leptospirosis. From its chemical analysis and 1H- and 13C-labeled nuclear magnetic resonance spectrometry, the Rhodotorula mannan was confirmed to consist of the same disaccharide units. Furthermore, such a preparation was shown to immunoreact to various sera from patients suffering with leptospirosis as well as to most rabbit antiserum preparations obtained from immunization with various strains of pathogenic leptospires. Therefore, the Rhodotorula mannan preparation is useful as an immunoreactive antigen in the serological diagnosis for leptospirosis.
Leptospires are known to be causative bacteria of an acute and febrile illness, leptospirosis. Several serological methods have been developed for detecting anti-Leptospira antibodies in serum samples from various patients suffering from leptospirosis (1, 4, 14, 15, 17); however, such methods seem laborious as well as expensive. Thus, the development of more conventional methods has been expected for a long time.
It has been reported that nonpathogenic Leptospira biflexa serovar patoc strain Patoc I contains any genus-specific antigen (9, 10). In a previous paper (8), we reported purification of such genus-specific antigens and showed them to have a common backbone structure, →3)-β-d-Manp-(1→4)-β-d-Manp-(1→, and to cross-react with most antisera obtained from rabbits immunized with various strains of pathogenic leptospires. The cross-reactivity strongly suggests the usefulness of this genus-specific antigen as an immunoreactive antigen in the diagnosis of leptospirosis. However, there are several problems in such an application, particularly because of its poor yield. In the course of its structural determination, we noticed that an exocellular mannan isolated from Rhodotorula glutinis (5) and the antigenic polysaccharides of L. biflexa Patoc I (designated patoc-APs) had the same repeating units. According to this previous report (5), a high yield of mannan with good purity can be isolated from R. glutinis. Thus, we tried to isolate a similar exocellular mannan from R. glutinis AHU 3479 (designated Rhodotorula mannan) and to confirm its identity by analyzing its structure and immunoreactivity. Several serum samples obtained from leptospirosis patients were shown to immunoreact with Rhodotorula mannan, suggesting the usefulness of Rhodotorula mannan in the detection of anti-Leptospira antibodies.
MATERIALS AND METHODS
Cultivation of R. glutinis AHU 3479, isolation of exocellular mannan, and its structural determination.
R. glutinis AHU 3479 was grown in a yeast nitrogen base (Difco, Detroit, Mich.) medium containing 5% glucose (5) at 27°C for 4 days with vigorous shaking. After removal of cells by centrifugation, the supernatant was filtered through a glass filter. Exocellular polysaccharides were recovered from the filtrate by ethanol precipitation. The precipitate was dissolved in water, and a mannan-rich fraction was differentially precipitated as a copper-mannan complex by stepwise addition of Fehling's solution (3). The complex was suspended in water and decomposed by addition of 4 M HCl solution to give a final concentration of 0.4 M HCl. After complete dissolution, the mannan fraction was recovered by ethanol precipitation, and the precipitate was used as a Rhodotorula mannan preparation (typical yield, 38 mg from a 100-ml culture). Its structural characterization was performed by methylation analysis and Smith degradation, as reported previously (8). 1H- and 13C-labeled NMR measurements were performed with a JEOL ALPHA-600 spectrometer at the high-resolution nuclear magnetic resonance (NMR) laboratory (Hokkaido University). Gas chromatography-mass spectrometry (GC-MS) was carried out with a JEOL JMS-AX500 at the GC-MS & NMR laboratory (Faculty of Agriculture, Hokkaido University). The absolute configuration of mannose was determined by using d-hexokinase (11). Hexose was determined by the phenol-H2SO4 method (2); hexosamine, by the method of Tsuji et al. (16) after N-deacylation of samples by acid hydrolysis in 2 M HCl at 100°C for 2 h; protein, by DC protein assay (Bio-Rad, Richmond, Calif.).
Sera.
Ten serum samples from leptospirosis patients in Japan were obtained from the National Institute of Infectious Disease (Tokyo, Japan). Serological analysis indicated that these patients were infected with a strain belonging to serogroup Icterohaemorrhagiae of L. interrogans. Five similar serum samples from leptospirosis patients in the Philippines were provided by Y. Yanagihara (University of Philippines, Manila). Serological analysis indicated that these patients were infected by strains belonging to serogroup Pyrogenes. Another 30 serum samples obtained in Japan (15 paired sera from serologically or genetically diagnosed leptospirosis patients) were provided by K. Akiyama (Miyagi Prefectural Institute of Public Health and Environment, Sendai, Japan). Antisera samples from patients with Lyme disease and from patients with syphilis were obtained from the collection of the Health Sciences University of Hokkaido (Ishikari-Tobetsu, Japan) and Hitachi Kasei (Tokyo, Japan), respectively. Specimens of rabbit antisera elicited against whole cells of leptospires were the same as those reported previously (7). Sera were appropriately diluted with phosphate-buffered saline (PBS [pH 7.4]) containing 0.05% Tween 20 and were used in enzyme-linked immunosorbent assays (ELISAs).
ELISA.
ELISA was performed by the same protocol as reported previously (8), except that Rhodotorula mannan (0.2 μg/50 μl) was used as the antigen and that a poly-l-lysine coating step was omitted. Peroxidase-conjugated goat anti-human immunoglobulin G (IgG) and IgM preparations were purchased from Chemicon International, Inc., Temecula, Calif.); peroxidase-conjugated goat anti-rabbit IgG (H+L) was from American Qualex (San Clemente, Calif.).
RESULTS
Structural characterization of Rhodotorula mannan.
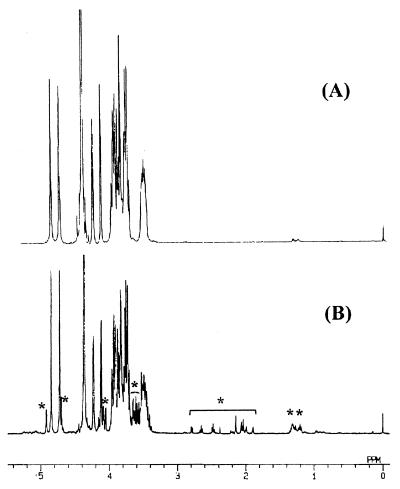
A Rhodotorula mannan was isolated from the culture filtrate of R. glutinis AHU 3479 and purified as its copper complex. From analytical data, this mannan was shown to contain mannose alone and to be free from proteins and hexosamines. All signals exhibited in 1H- (Fig. 1A) and 13C- (Fig. 2A) labeled NMR spectra were derived from two kinds of mannose residues substituted at different positions, consistent with the absence of any contaminated material. An enzymatic analysis using d-hexokinase indicated that all mannose components were in a d configuration. Its methylation products gave equimolar amounts of 1,3,5-tri-O-acetyl-2,4,6-tri-O-methylmannitol and 1,4,5-tri-O-acetyl-2,3,6-tri-O-methylmannitol on the basis of their retention times as measured by gas-liquid chromatography (GLC) and their fragmentation patterns in GC-MS. Thus, Rhodotorula mannan consists of 3-O- and 4-O-substituted d-mannopyranose residues in an equimolar ratio. Smith degradation gave 2-O-d-mannopyranosyl-d-erythritol alone as the product, suggesting the presence of a repeating disaccharide unit, →3)-d-Manp-(1→4)-d-Manp-(1→. 1H- and 13C-labeled NMR spectra of Rhodotorula mannan (Fig. 1A and 2A) gave much simpler signals than those of patoc-APs. The latter polysaccharides contained additional sugars as their minor components (8); therefore, they exhibited a large number of minor signals arising from the additional sugar residues (shown by asterisks in Fig. 1B and 2B). However, all of the major signals were fully consistent with the corresponding signals observed in the NMR spectra of Rhodotorula mannan, strongly suggesting that both polysaccharides have the same repeating unit. All values of chemical shifts and coupling constants found in the 1H- and 13C-labeled NMR spectra of Rhodotorula mannan were in agreement with those of patoc-APs in our previous report (8). Particularly the chemical shifts, as well as the coupling constants, for two each of the anomeric protons (4.72 ppm, JH1, H2 = 0.6 Hz; 4.85 ppm, JH1, H2 = 0.5 Hz) and carbons (101.7 ppm, JH1, C1 = 161 Hz; 98.6 ppm, JH1, C1 = 160 Hz) agreed with those for β-mannoside, indicating β-glycosidic forms of all mannose residues.
FIG. 1.
1H-NMR spectra of Rhodotorula mannan (A) and AP-2 of L. biflexa patoc Patoc I (B). The spectra were recorded in D2O at 65°C. A large signal at 4.36 ppm was derived from HOD. Asterisks in panel B show signals arising from minor sugar residues.
FIG. 2.
13C-NMR spectra of Rhodotorula mannan (A) and AP-2 of L. biflexa patoc Patoc I (B). The spectra were recorded at 65°C. Asterisks in panel B show signals arising from minor sugar residues.
Immunoreaction between Rhodotorula mannan and rabbit antisera against different leptospires.
Previously, we reported that three patoc-APs were extensively immunoreactive with most rabbit antisera elicited against whole cells of various strains of leptospires (24 of 28 strains) and that the immunoreaction was specifically inhibited by β-1,4-mannobiose (8). Because Rhodotorula mannan contained the same epitope, it was predicted to show a similar immunoreactivity to the above rabbit antisera. As shown in Table 1, most rabbit antisera could be immunoreacted with Rhodotorula mannan at 4 μg/ml, which concentration was 10-fold lower than that for patoc-APs (40 μg/ml). Probably the apparent high reactivity of Rhodotorula mannan reflects its large molecular size and strong adhesive property to polystyrene plates. Owing to large difference in the antigen concentrations used, the antigenicities of Rhodotorula mannan and patoc-APs could not be directly compared, but Rhodotorula mannan is presumed to serve as a more effective antigen in the detection of anti-Leptospira antibodies by ELISA. The immunoreactions between Rhodotorula mannan and rabbit antisera were also specifically inhibited by β-1,4-mannobiose (data not shown). These results indicate that Rhodotorula mannan has an immunoreactivity similar to or identical with patoc-APs and may be useful as an immunoreactive antigen in the ELISA of leptospirosis diagnosis.
TABLE 1.
Cross-reactivity of Rhodotorula mannan with various rabbit antisera against Leptospira strains
| Strain of leptospires used in immunization | Serogroup | Extent of cross-reactivity (avg absorbance)a |
|---|---|---|
| L. biflexa patoc Patoc I | Semaranga | S (1.093) |
| L. borgpetersenii jules Jules | Hebdomadis | M (0.406) |
| L. borgpetersenii nona Nona | Hebdomadis | S (0.990) |
| L. borgpetersenii worsfoldi Worsford | Hebdomadis | M (0.390) |
| L. interrogans autumnalis Akiyami A | Autumnalis | S (0.983) |
| L. interrogans canicola Galtoni | Canicola | W (0.247) |
| L. interrogans canicola Malaya | Canicola | M (0.500) |
| L. interrogans canicola Moulton | Canicola | S (0.816) |
| L. interrogans copenhageni Shibaura | Icterohaemorrhagiae | W (0.186) |
| L. interrogans hebdomadis Hebdomadis | Hebdomadis | M (0.712) |
| L. interrogans icterohaemorrhagiae Okinawa | Icterohaemorrhagiae | W (0.184) |
| L. interrogans icterohaemorrhagiae CF-1 | Icterohaemorrhagiae | S (0.842) |
| L. interrogans icterohaemorrhagiae RGA | Icterohaemorrhagiae | W (0.273) |
| L. interrogans kremastos Kyoto | Hebdomadis | W (0.385) |
| L. interrogans naam Naam | Icterohaemorrhagiae | S (0.953) |
| L. interrogans smithi Smith | Icterohaemorrhagiae | M (0.536) |
| L. kirschneri kabura Kabura | Hebdomadis | S (1.129) |
| L. kirschneri kambale Kambale | Hebdomadis | S (1.105) |
| L. kirschneri ndahmbukuje Ndahmbukuje | Icterohaemorrhagiae | M (0.581) |
| L. meyeri perameles Perameles | Mini | S (0.894) |
| L. santarosai beye Beye | Mini | M (0.691) |
| L. santarosai borincana Borincana | Hebdomadis | S (1.048) |
| L. santarosai maru Maru | Hebdomadis | S (0.835) |
| L. weilii samin Sarmin | Sarmin | W (0.196) |
| L. interrogans australis Ballico | Australis | — (0.041) |
| L. interrogans canicola HondUtrecht IV | Canicola | — (0.037) |
| L. interrogans pomona Pomona | Pomona | — (0.055) |
| L. interrogans wolffi 3705 | Sejroe | — (0.031) |
Triplicate assays were carried out by using each 1,000-fold-dilution serum, and the average data are shown in parentheses. Positive cross-reactivity is shown depending on its extent: strong (S), medium (M), or weak (W). —, no reaction.
Trial use of Rhodotorula mannan in the detection of human anti-Leptospira antibodies.
Before Rhodotorula mannan can be recommended in the diagnosis of leptospirosis, its immunoreactivity must be confirmed to be specific. Thus, we tested serum samples obtained from patients infected by L. interrogans (40 samples from 25 Japanese patients, samples J-1 to J-10 [Table 2] and JM-1 to JM-15 [Table 3], and 5 samples from Filipino patients, samples P-1 to P-5 [Table 2]), Borrelia (Lyme disease; samples L1 to L-10 [Table 2]), and Treponema (syphilis; samples S1 to S10 [Table 2]) by ELISA. Sera from 10 healthy humans were used as negative controls. Because the appearance periods of IgM and IgG are known to be different and the number of elapsed days after bacterial infection can not be exactly determined, we tried to detect IgG or IgM class antibodies specific to leptospires in serum samples by using peroxidase-conjugated goat anti-human IgG or IgM. As shown in Tables 2 and 3, except for a few serum samples (e.g., J-10 in Table 2, as well as JM-9f and JM-9s in Table 3), almost all of the antisera collected from leptospirosis patients (42 of 45 serum samples) gave distinct immunoreactions with the Rhodotorula mannan antigen although these positive sera contained a different set of IgG and/or IgM. Twelve serum samples (JM-10 to JM-15), which were negative against the respective cells belonging to serovars copenhageni, autumnalis, hebdomadis, and australis in a standard microscopic agglutination test (MAT) (4), were also immunoreactive with the same Rhodotorula mannan antigen (Table 3). This result suggests that the ELISA using Rhodotorula mannan is more sensitive than MAT in the detection of anti-Leptospira antibodies. On the other hand, no sera from healthy humans, Lyme disease patients, and syphilis patients gave any immunoreaction to the Rhodotorula mannan antigen (Table 2). From these results, we concluded that the Rhodotorula mannan antigen can specifically cross-react to IgG and/or IgM specific to leptospires, strongly supporting our prediction that Rhodotorula mannan may be a useful antigen in the serological diagnosis of leptospirosis.
TABLE 2.
Cross-reactivity in ELISA between Rhodotorula mannan and sera collected from several spirochetosis patientsa
| Sampleb | Genus of Spirocheta | Serogroup | Detection (avg absorbance)
|
|
|---|---|---|---|---|
| IgM | IgG | |||
| J-1 | Leptospira | Icterohaemorrhagiae | + (0.061)c | + (0.500)c |
| J-2 | Leptospira | Icterohaemorrhagiae | + (0.051)c | + (0.453)c |
| J-3 | Leptospira | Icterohaemorrhagiae | + (0.063)e | + (0.079)c |
| J-4 | Leptospira | Icterohaemorrhagiae | − (0.025) | + (0.131)c |
| J-5 | Leptospira | Icterohaemorrhagiae | − (0.030) | + (0.103)d |
| J-6 | Leptospira | Icterohaemorrhagiae | + (0.063)e | + (0.077)e |
| J-7 | Leptospira | Icterohaemorrhagiae | − (0.047) | + (0.082)e |
| J-8 | Leptospira | Icterohaemorrhagiae | − (0.049) | + (0.102)e |
| J-9 | Leptospira | Icterohaemorrhagiae | − (0.048) | + (0.103) |
| J-10 | Leptospira | Icterohaemorrhagiae | − (0.049) | − (0.049) |
| P-1 | Leptospira | Pyrogenes | + (0.568) | + (0.333) |
| P-2 | Leptospira | Pyrogenes | − (0.039) | + (0.053) |
| P-3 | Leptospira | Pyrogenes | + (0.253) | + (0.050) |
| P-4 | Leptospira | Pyrogenes | + (0.217) | + (0.310) |
| P-5 | Leptospira | Pyrogenes | + (0.271) | + (0.168) |
| L-1 through L-10 | Borrelia | — | − (0.020) | − (0.020) |
| S-1 through S-10 | Treponema | — | − (0.023) | − (0.024) |
Unless otherwise indicated, triplicate assays were done by using each 500-fold-dilution serum, and the average data are shown in parentheses. +, positive samples giving an average absorbance higher than twice the control value (0.025); −, negative samples.
Sera J-1 to J-10 and sera P-1 to P-5 were collected from Japanese and Filipino patients, respectively.
Assayed with 5,000-fold-dilution sera.
Assayed with 2,000-fold-dilution sera.
Assayed with 1,000-fold-dilution sera.
TABLE 3.
Cross-reactivity in ELISA between Rhodotorula mannan and sera of leptospirosisa
| Sample | Blood-collecting days after onset of disease | Serogroup | Detection (avg absorbance)
|
|
|---|---|---|---|---|
| IgM | IgG | |||
| JM-1f | 25 | Autumnalis | + (0.461) | + (0.420)b |
| s | 49 | − (0.103) | + (0.419) | |
| JM-2f | 10 | Autumnalis | − (0.048) | + (0.401) |
| s | 114 | − (0.096) | + (0.552)c | |
| JM-3f | 5 | Icterohaemorrhagiae | − (0.052) | + (0.240) |
| s | 20 | + (0.220) | + (0.461) | |
| JM-4f | 6 | Icterohaemorrhagiae | − (0.100) | + (0.185) |
| s | 24 | + (0.148) | + (0.444) | |
| JM-5f | 10 | Icterohaemorrhagiae | + (0.407)c | + (0.426)b |
| s | 17 | + (0.495)d | + (0.494)c | |
| JM-6f | 3 | Icterohaemorrhagiae | + (0.074) | + (0.167) |
| s | 17 | − (0.096) | + (0.162) | |
| JM-7f | 8 | Icterohaemorrhagiae | + (0.351)d | + (1.178)d |
| s | 36 | + (0.387)c | + (0.920)d | |
| JM-8f | 11 | Icterohaemorrhagiae | + (0.358)d | + (0.989)d |
| s | 24 | − (0.080) | + (0.442) | |
| JM-9f | 21 | Icterohaemorrhagiae | − (0.019) | − (0.057) |
| s | 65 | − (0.031) | − (0.070) | |
| JM-10f | 43 | Unknowne | + (0.250) | + (0.353)b |
| s | 149 | + (0.193) | + (0.391)b | |
| JM-11f | 7 | Unknowne | + (0.171) | + (0.174) |
| s | 17 | + (0.199) | + (0.172) | |
| JM-12f | 9 | Unknowne | + (0.262) | + (0.220) |
| s | 36 | + (0.384) | + (0.307) | |
| JM-13f | 30 | Unknowne | + (0.266) | + (0.390) |
| s | 37 | + (0.244) | + (0.392) | |
| JM-14f | 3 | Unknowne | − (0.101) | + (0.126) |
| s | 15 | + (0.129) | + (0.149) | |
| JM-15f | 30 | Unknowne | + (0.311) | + (0.337)b |
| s | 74 | + (0.147) | + (0.338) | |
Unless otherwise indicated, triplicate assays were done by using each 500-fold-dilution serum, and the average data are shown in parentheses. +, positive samples giving an average absorbance higher than twice the control value (0.045).
b–d Assayed with 1,000-, 2,000-, or 5,000-fold-dilution sera, respectively.
Serum which did not agglutinate the respective cells of serovars copenhageni, autumnalis, hebdomadis, and australis in MAT procedure.
DISCUSSION
Different serological methods have been used for the diagnosis of leptospirosis (1, 4, 14, 15, 17), but there are some difficulties with such methods. For example, in the MAT procedure, many different living cells may be required as the antigens for detection of anti-Leptospira antibodies in serum samples; for the detection of anti-Leptospira antibodies in serum samples by ELISA, considerably more antigens of many leptospires may be needed to obtain a reliable diagnosis. Recently, on the basis of a finding that heat-stable antigens from nonpathogenic L. biflexa are cross-reactable with a variety of serum samples from leptospirosis patients, a dipstick assay has been developed by using such antigens bound to nitrocellulose membranes (6, 12). In a previous study (8), we reported the structural characteristics of antigenic polysaccharides of L. biflexa patoc Patoc I (patoc-APs) and their cross-reactivity with rabbit antisera elicited against many other strains of leptospires. Owing to a low yield of patoc-APs, any application of patoc-APs to the clinical diagnosis of leptospirosis seems difficult. Fortunately, an exocellular mannan produced by R. glutinis is reported to have the same disaccharide unit (5). Thus, we tried to confirm the usefulness of the above application. From the structural characterization of Rhodotorula mannan produced by arbitrarily selected R. glutinis AHU 3479, this exocellular polysaccharide, which is available in high purity and large amounts, was confirmed to have the same repeating disaccharide and to show the same immunoreactivity as that of patoc-APs. In ELISA, rabbit anti-Leptospira antibodies were immunoreactable in much lower concentrations of Rhodotorula mannan (4 μg/ml) than patoc-APs (40 μg/ml). The key difference may be the large molecular size of the former antigen and its better adsorption property on uncoated polystyrene plates as well as on poly-l-lysine-coated ones. A similar ELISA using Rhodotorula mannan as the antigen was also useful to detect human anti-Leptospira antibodies. The IgG and/or IgM class of antibodies specific to leptospires was detectable in almost all serum samples (42/45) from leptospirosis patients, but there was a large fluctuation in their titers. Such fluctuation is consistent with the previous finding that the titers of IgM and IgG are changed during the infection processes by leptospires (1, 14). Therefore, in the serological diagnosis, both Ig species specific to leptospires must be measured. We confirmed the antigenic specificity of Rhodotorula mannan; namely, we found that tested sera from healthy humans and other spirochetosis patients can not immunoreact with this ELISA antigen (Table 2). Thus, we conclude that the Rhodotorula mannan antigen specifically cross-reacts with anti-Leptospira antibodies. Furthermore, such Rhodotorula mannan is a useful ELISA antigen in the detection of anti-Leptospira antibodies in serum samples of leptospirosis patients. More recently, a convenient latex agglutination assay also has been developed by using heat-stable, broadly reactive antigens of L. interrogans hardjo Lely 607 (13). Rhodotorula mannan is also applicable for the latex agglutination assay and dipstick assay (6, 12). A dipstick or latex beads conjugated with Rhodotorula mannan may be a sensitive method. Moreover, a vaccine containing Rhodotorula mannan may provide potent protection against many leptospires in vaccinated mammals.
REFERENCES
- 1.Adler B, Murphy A M, Locarnini S A, Faine S. Detection of specific anti-leptospiral immunoglobulins M and G in human serum by solid-phase enzyme-linked immunosorbent assay. J Clin Microbiol. 1980;11:452–457. doi: 10.1128/jcm.11.5.452-457.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 3.Edwards T E. Yeast polysaccharide: isolation of glycogen, glucan, and mannan polysaccharides from Baker's yeast. In: Whistler R L, editor. Methods in carbohydrate chemistry, vol. V: general polysaccharides. New York, N.Y: Academic Press, Inc.; 1965. pp. 176–179. [Google Scholar]
- 4.Faine S, Adler B, Bolin C, Perolat P. Leptospira and leptospirosis. 2nd ed. Melbourne, Australia: MediSci; 1999. [Google Scholar]
- 5.Gorin P A, Horitsu K, Spencer J F T. An exocellular mannan, alternately linked 1,3-β and 1,4-β from Rhodotorula glutinis. Can J Chem. 1965;43:950–954. [Google Scholar]
- 6.Gussenhoven G C, van der Hoorn M A W G, Goris M G A, Terpstra W J, Hartskeerl R A, Mol B W, van Ingen C W, Smits H L. LEPTO dipstick, a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human sera. J Clin Microbiol. 1997;35:92–97. doi: 10.1128/jcm.35.1.92-97.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isogai E, Isogai H, Kurebayashi Y, Ito N. Biological activities of leptospiral lipopolysaccharide. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1986;261:53–64. doi: 10.1016/s0176-6724(86)80062-1. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo K, Isogai E, Araki Y. Occurrence of [→3)-β-d-Manp-(1→4)-β-d-Manp-(1→]n units in the antigenic polysaccharides from Leptospira biflexa serovar patoc strain Patoc I. 2000. Carbohydr. Res., in press. [DOI] [PubMed] [Google Scholar]
- 9.Palit A, Gulasekharam J. Genus-specific leptospiral antigen and its possible use in laboratory diagnosis. J Clin Pathol. 1973;26:7–16. doi: 10.1136/jcp.26.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palit A, Hamilton R C, Gulasekharam J. Further studies on leptospiral genus-specific antigen: its ultrastructure and immunochemistry. J Gen Microbiol. 1974;82:223–236. doi: 10.1099/00221287-82-2-223. [DOI] [PubMed] [Google Scholar]
- 11.Schachter H. Enzymic microassay for d-mannose, d-glucose, d-galactose, l-fucose, and d-glucosamine. Methods Enzymol. 1975;41:3–10. doi: 10.1016/s0076-6879(75)41003-5. [DOI] [PubMed] [Google Scholar]
- 12.Smits H L, Ananyina Y V, Chereshsky A, Dancel L, Lai-a-fat R F M, Chee H D, Levett P N, Masuzawa T, Yanagihara Y, Muthusethupathi M A, Sanders E J, Sasaki D M, Domen H, Yersin C, Aye T, Bragg S L, Gussenhoven G C, Goris M G A, Terpstra W J, Hartskeerl R A. International multicenter evaluation of the clinical utility of a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human sera specimens. J Clin Microbiol. 1999;37:2904–2909. doi: 10.1128/jcm.37.9.2904-2909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smits H L, van der Hoorn M A W G, Goris M G A, Gussenhoven G C, Yersin C, Sasaki D M, Terpstra W J, Hartskeerl R A. Simple latex agglutination assay for rapid serodiagnosis of human leptospirosis. J Clin Microbiol. 2000;38:1272–1275. doi: 10.1128/jcm.38.3.1272-1275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terpstra W J, Ligthart G S, Schoone G J. ELISA for the detection of specific IgM and IgG in human leptospirosis. J Gen Microbiol. 1985;131:377–385. doi: 10.1099/00221287-131-2-377. [DOI] [PubMed] [Google Scholar]
- 15.Torten M, Shenberg E, van der Hoeden J. The use of immunofluorescence in the diagnosis of human leptospirosis by a genus-specific antigen. J Infect Dis. 1966;116:537–543. doi: 10.1093/infdis/116.5.537. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji A, Kinoshita T, Hoshino M. Microdetermination of hexosamines. Chem Pharm Bull. 1969;17:217–218. doi: 10.1248/cpb.17.217. [DOI] [PubMed] [Google Scholar]
- 17.Winslow W E, Merry D J, Pirc M L, Devine P L. Evaluation of commercial enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody in diagnosis of human leptospiral infection. J Clin Microbiol. 1997;35:1938–1942. doi: 10.1128/jcm.35.8.1938-1942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]