A respondent-driven sampling survey among men who have sex with men and transwomen, and genderqueer in Zimbabwe found that active syphilis overall and among participants living with HIV was >6 times and ~4 times higher, respectively, than estimates among the general adult male population in the country.
Abstract
Background
Syphilis increases human immunodeficiency virus (HIV) acquisition risk and impacts the immunologic and virologic response among people living with HIV (PLHIV). We assessed the prevalence of active or current syphilis and HIV/syphilis and their correlates among men who have sex with men (MSM), transwomen, and genderqueer (TGW/GQ) individuals in Zimbabwe.
Methods
Among a respondent-driven sample of MSM and TGW/GQ who were tested for HIV and syphilis in Harare and Bulawayo, Zimbabwe in 2019 (n = 1511), multiple logistic regression was used to assess correlates of active syphilis. Unadjusted logistic regression was used among PLHIV (n = 340) due to small sample size. All analyses were unweighted as data did not reach convergence for HIV.
Results
Prevalence of active syphilis overall and among PLHIV was 5.5% and 10.1%, respectively, in Harare, and 5.6% and 11.0%, respectively, in Bulawayo. Participants were more likely to have active syphilis if they were PLHIV (adjusted odds ratio [aOR], 2.2; 95% confidence interval [CI], 1.4–3.6), aged 25–34 years (aOR, 2.2 years; 95% CI, 1.3–3.8 years; reference, 18–24 years), or self-report sexually transmitted infection symptoms (aOR, 1.8; 95% CI, 1.1–3.0). Compared with Bulawayo TGW/GQ, MSM in Harare (aOR, 0.2; 95% CI, 0.1–0.5) and Bulawayo (aOR, 0.2; 95% CI, 0.1–0.4), and TGW/GQ in Harare (aOR, 0.2; 95% CI, 0.1–0.6) were less likely to have active syphilis. Among PLHIV, coinfection was 13.0% among TGW/GQ and 9.7% among MSM. Odds of coinfection were higher for those aged 25 to 34 years (OR, 3.7 years; 95% CI, 1.2–11.1 years) and lower among Harare MSM (OR, 0.2; 95% CI, 0.1–0.7), Bulawayo MSM (OR, 0.1; 95% CI, 0.0–0.4), and Harare TGW/GQ (OR, 0.1; 95% CI, 0.0–0.4) compared with Bulawayo TGW/GQ.
Conclusions
Findings highlight a high burden of syphilis among MSM and TGW/GQ and underscore the importance of HIV/syphilis detection and improved service delivery for these groups.
Active syphilis increases human immunodeficiency virus (HIV) acquisition risk for HIV-negative persons1 and impacts the immunologic and virologic response among people living with HIV (PLHIV). Among HIV-negative individuals, ulcerative sexually transmitted infections (STIs), such as syphilis, facilitate sexual acquisition of HIV through disruptions to the epithelium and mucosa.1 Genital sores caused by syphilis may also allow for more efficient transmission of HIV from a coinfected partner.2 Syphilis has been associated with increases in HIV viral load,2 which escalates transmission risk and can result in adverse health outcomes for coinfected individuals living with HIV including more rapid progression of disease and increases in HIV-related morbidity and mortality. Other adverse health outcomes of syphilis include neurologic and cardiac disease, fetal loss or stillbirth, neonatal death, and congenital syphilis, among others.3,4
Given similar sexual risk profiles of those infected with HIV and/or syphilis, rates of coinfection among PLHIV are high. In Zimbabwe, the prevalence of active syphilis among the general population is less than 1% though is greater among PLHIV compared with HIV-negative persons5; female and male PLHIV in the general population are nearly 4 and 6 times more likely to have active syphilis, respectively, than HIV-negative individuals.5
Although data on the burden of active syphilis and HIV/syphilis coinfection among the general population in Zimbabwe are available, estimates for men who have sex with men (MSM) and transwomen (TGW), key populations with heightened HIV risk globally6,7 are not, largely because of the political and legal marginalization of these populations. In Zimbabwe, sexual and gender minorities have few legal and social protections; there is no legal recognition of trans or nonbinary/genderqueer (GQ) persons and same-sex sexual relationships remain prohibited by law.8
Globally, gay, bisexual, and other MSM and transgender people combined represent a quarter of all new HIV infections and have 26 and 13 times greater risk of acquiring HIV, respectively, compared with the rest of the population.9 Considering the disproportionate burden of HIV among MSM and TGW/GQ globally and the relationship between HIV and syphilis, epidemiologic data on prevalence and correlates of syphilis and HIV/syphilis coinfection among these groups in Zimbabwe are necessary to inform HIV and sexual and reproductive health and rights (SRHR) services and their improved integration for key populations. We assessed the prevalence and correlates of active syphilis among a sample of MSM and TGW/GQ overall and among MSM and TGW/GQ living with HIV in Zimbabwe.
MATERIALS AND METHODS
Study Setting
Harare and Bulawayo, Zimbabwe’s 2 largest cities, were selected as survey sites to ensure the survey had northern and southern geographic representation. The HIV prevalence among the general population overall (Harare, 13.0%; Bulawayo, 16.5%) and among men (Harare, 10.0%; Bulawayo, 13.8%) differ by city.10 Whereas both cities had established organizations serving MSM and TGW/GQ communities at the time of data collection, anecdotal accounts from MSM and TGW/GQ suggested higher tolerance of these groups in Bulawayo because of its proximity to the South African border.
Data Collection
From March to July 2019, MSM and TGW/GQ (n = 1538) in Harare and Bulawayo, Zimbabwe were recruited into cross-sectional surveys using respondent-driven sampling (RDS),11 a chain referral recruitment approach used to reach populations for whom no sampling frame exists. Before the survey, formative research confirmed there were strong network ties among MSM and TGW/GQ populations in both cities, a requirement for RDS success. First, purposively selected, and eligible seeds were recruited to maximize diversity across key variables including age, sexual orientation, education, marital status, socioeconomic status, key population, area of residence, awareness of HIV status, and engagement with key populations organizations. Seeds were then asked to distribute up to 3 coupons to recruit their peers into the survey, who were then asked to recruit 3 of their peers via coupon distribution, and so on. Fourteen seeds (Harare, 8; Bulawayo, 6) were recruited to initiate recruitment chains and an additional 5 seeds (Harare, 3; Bulawayo, 2) were added during data collection to recruit under-represented subpopulations. Eligibility criteria for the survey included: assigned male at birth, 18 years or older; engaged in anal or oral sex with a man in the past 12 months; and ability to speak English, Shona, or Ndebele. Recruiter-recruit relationships and coupon eligibility were tracked using an electronic coupon manager software. Initially, each participant was given 3 coupons to recruit peers. This number reduced as recruitment needs slowed and stopped when sample size was reached.
Once eligible, participants provided written informed consent for survey participation and biomarker testing separately. Interviewer-administered tablet-based surveys were conducted in English, Shona, or Ndebele. Survey modules included information on demographics, health services utilization, STI symptoms and diagnosis, mental health and substance use, stigma and discrimination, and HIV knowledge and attitudes. After questionnaire completion, consenting participants were tested for HIV, using a 3-test algorithm adapted from the national HIV testing algorithm (Alere HIV Combo, Chembio HIV 1/2 STAT-PAK, INSTI HIV-1/HIV-2 antibody test), and syphilis using Chembio Dual-Path Platform Syphilis Screen and Confirm Assay. Samples from PLHIV were also tested for CD4+ cell count and viral load. Participants who tested HIV-positive and did not report being in care and those who tested positive for syphilis were offered an escorted referral that day to a designated key population-friendly health facility. If a participant declined the escorted referral, they were provided with a referral card to a designated facility to which they could visit on their own. Participants not willing to receive care at designated facilities were asked to share information about their preferred health facility, as well as the date when they intended to visit the facility. All referred participants were followed up a maximum of 3 times to provide additional counseling to motivate care seeking and confirm receipt of care. All participants were asked to return for a second visit, which occurred approximately 2 weeks after participation in the survey. During the second visit, participants were asked to complete a brief questionnaire on peer recruitment and were provided their viral load results (if HIV-positive). All participants received $5 for participation in the survey and an additional $5 was provided to participants for each successful recruit (maximum of 3 recruits) during their second visit.
Sample size was calculated to achieve primary survey objectives: (1) to estimate HIV prevalence and HIV viral load suppression with acceptable precision (at 95% confidence level) among MSM and TGW/GQ in Harare and Bulawayo; and (b) to detect a change in HIV prevalence between the current survey and future surveys conducted among these groups should the Ministry of Health and Child Care integrate MSM and TGW/GQ into the routine surveillance system. Target sample size overall was 1538 participants (Harare, 718; Bulawayo, 820), inclusive of MSM and TGW/GQ. Additional information on the survey methods and results of the primary survey objectives have been published elsewhere.12
Ethical and administrative approvals were received from the Columbia University Institutional Review Board and the Medical Research Council of Zimbabwe. The protocol was reviewed in accordance with the US Centres for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens. This article adheres to STROBE-RDS guidelines.
Measures
Per manufacturer’s instructions, participants with samples reactive for treponemal and nontreponemal antibodies were considered to have active syphilis. Participants with samples reactive only for treponemal antibodies were considered to have a previous infection that was resolved or treated and were categorized as not having active syphilis along with those who were nonreactive to both antibodies (no previous or active syphilis) and reactive for nontreponemal antibodies only (biological false positive). Participants who self-reported 1 or more of the following symptoms in the past 12 months were categorized as having experienced an STI in the past 12 months: (1) abnormal discharge from penis, (2) ulcer/sore on or near penis, (3) pain on urination, (4) ulcer/sore on or near anus, (5) abnormal discharge from anus, or (6) anal warts. Alcohol dependence was defined using an Alcohol Use Disorders Identification Test score of 15 or greater. Viral load suppression was defined as less than 1000 copies/mL. CD4+ cell count thresholds for advanced HIV disease (200 ≤ CD4+ count <350) and acquired immunodeficiency syndrome (AIDS) (<200 CD4+ count) according to the World Health Organization stages of HIV infection were used. Early sexual debut was defined as sexual debut with a male sex partner before the age of 15 years and recent transactional sex was defined as exchanging sex for money, goods, or services with 1 or more male or TGW sex partners in the past 6 months. Gender identity was assessed using a 2-step question; participants who reported their current gender and sex assigned at birth to be male were categorized as MSM. Participants who reported their current gender to be female/transfemale/transwoman and sex assigned at birth to be male were categorized as TGW and those who reported their current gender to be GQ and sex assigned at birth to be male were categorized as GQ however due to small sample sizes and based on feedback from Zimbabwe key population organizations, TGW and GQ were combined for the purposes of analysis.
Data Analysis
All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC) and were restricted to those who received biomarker testing. Univariate analyses were used to calculate sample prevalence estimates. χ2 Tests with continuity adjustment and Fisher’s exact tests were used to assess associations between sociodemographic, behavioral, and health outcome variables and active syphilis among biomarker consenting participants and among PLHIV. A multiple logistic regression model was built to assess correlates of active syphilis among biomarker consenting participants. Variables that met a threshold of P < 0.1 in bivariate logistic regression were included in multiple logistic regression; however, only significant (P < 0.05) variables in adjusted analyses were maintained in the final model. Survey data from both cities were combined in regression analyses, and an interaction term between city and key population group (MSM and TGW/GQ) were included in the model based on evidence of interaction. No multivariable model was created for PLHIV due to small sample size in strata. Complete case analyses were used given minimal missing data. All analyses were unweighted and did not account for survey design as the sample did not reach convergence for HIV, the primary study objective. Harare and Bulawayo populations were treated as convenience samples due to inability to apply RDS weights to adjust for nonrandom recruitment patterns. Therefore, results of this analysis do not represent population estimates and may not be generalizable to all MSM and TGW/GQ in Harare and Bulawayo.
RESULTS
A total of 19 seeds participated in the survey ([Harare: 11]; [Bulawayo: 8]). In Harare, the mean number of recruits per seed was 64 and the longest recruitment wave was 17. In Bulawayo, the mean number of recruits per seed was 102 and the longest recruitment wave was 14. Overall, 1927 coupons were distributed in Harare (coupon return rate, 42.8%) and 1913 coupons were distributed in Bulawayo (coupon return rate: 52.3%). A flowchart of the number of individuals screened for eligibility, number eligible, and number consented for survey and biomarker testing by city and key population are described in Figure 1 and demographic information for biomarker consenting participants provided in Table 1. Participants identified primarily as gay/homosexual ([59.2%]; [894/1511]) and the majority were between the ages of 18 and 24 years ([46.9%]; [709/1511]).
Figure 1.
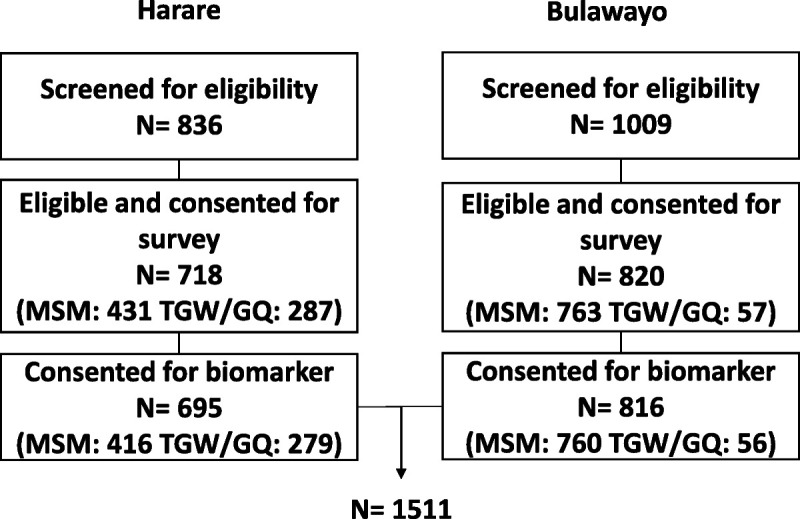
Flowchart of the number of individuals screened for eligibility, number of individuals eligible and consented for survey, and number of participants consented for biomarker testing by city and key population in Zimbabwe, 2019.
TABLE 1.
Demographic and Behavioral Characteristics and ORs of Active Syphilis Among MSM and TGW/GQ Participants Consenting to Biomarker Testing, Zimbabwe, 2019
| Characteristics | Active Syphilis (n = 84) | No Active Syphilis (n = 1427) | Total (N = 1511) | Active Syphilis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Row % | n | Row % | n | Col % | OR | 95% CI | AOR* | 95% CI | |
| Key populationxcity | ||||||||||
| MSM Harare | 21 | 5.1 | 395 | 95.0 | 416 | 27.5 | 0.2 | 0.1–0.5 | 0.2 | 0.1–0.5 |
| TGW/GQ Harare | 17 | 6.1 | 262 | 93.9 | 279 | 18.5 | 0.3 | 0.1–0.6 | 0.2 | 0.1–0.6 |
| MSM Bulawayo | 35 | 4.6 | 725 | 95.4 | 760 | 50.3 | 0.2 | 0.1–0.4 | 0.2 | 0.1–0.4 |
| TGW/GQ Bulawayo | 11 | 19.6 | 45 | 80.4 | 56 | 3.7 | 1 | — | 1 | — |
| HIV status | ||||||||||
| HIV-positive | 36 | 10.6 | 304 | 89.4 | 340 | 22.5 | 2.8 | 1.8–4.4 | 2.5 | 1.5–4.0 |
| HIV-negative | 48 | 4.1 | 1123 | 95.9 | 1171 | 77.5 | 1 | — | 1 | — |
| Age, y | ||||||||||
| 18–24 | 26 | 3.7 | 683 | 96.3 | 709 | 46.9 | 1 | — | 1 | — |
| 25–34 | 43 | 8.0 | 492 | 92.0 | 535 | 35.4 | 2.3 | 1.4–3.8 | 2.1 | 1.2–3.5 |
| 35–44 | 8 | 4.4 | 176 | 95.7 | 184 | 12.2 | 1.2 | 0.5–2.7 | 1.1 | 0.5–2.5 |
| 45+ | 7 | 8.4 | 76 | 91.6 | 83 | 5.5 | 2.4 | 1.0–5.8 | 2.3 | 0.9–5.9 |
| Sexual orientation† | ||||||||||
| Gay/homosexual | 60 | 6.7 | 834 | 93.3 | 894 | 59.3 | 1.8 | 1.1–2.9 | ||
| Bisexual | 24 | 3.9 | 587 | 96.1 | 611 | 40.5 | 1 | — | ||
| Straight/heterosexual | 0 | 0 | 2 | 100.0 | 2 | 0.1 | — | — | ||
| Highest education attended | ||||||||||
| Primary or less | 7 | 8.5 | 75 | 91.5 | 82 | 5.4 | 1 | — | ||
| Secondary | 58 | 5.4 | 1014 | 94.6 | 1072 | 70.9 | 0.6 | 0.3–1.4 | ||
| Tertiary or vocational | 19 | 5.3 | 338 | 94.7 | 357 | 23.6 | 0.6 | 0.2–1.5 | ||
| Self-report STI symptoms past 12 months | ||||||||||
| Yes | 26 | 9.0 | 263 | 91.0 | 289 | 19.1 | 2.0 | 1.2–3.2 | 1.8 | 1.1–2.9 |
| No | 58 | 4.8 | 1164 | 95.3 | 1222 | 80.9 | 1 | — | 1 | — |
| Alcohol dependence (AUDIT score of ≥15) | ||||||||||
| Yes | 27 | 8.0 | 312 | 92.0 | 339 | 22.4 | 1.7 | 1.1–2.7 | ||
| No | 57 | 4.9 | 1115 | 95.1 | 1172 | 77.6 | 1 | — | ||
| Early sexual debut with male sex partner‡ | ||||||||||
| Early sexual debut (<15 y) | 7 | 7.4 | 88 | 92.6 | 95 | 6.4 | 1.4 | 0.6–3.0 | ||
| No early sexual debut (≥15 y) | 77 | 5.5 | 1318 | 94.5 | 1395 | 93.6 | 1 | — | ||
| Recent (≤6 months) transactional sex§ | ||||||||||
| Yes | 15 | 12.3 | 107 | 87.7 | 122 | 8.2 | 2.6 | 1.5–4.8 | ||
| No | 69 | 5.1 | 1298 | 95.0 | 1367 | 91.8 | 1 | — | ||
| No. recent (≤6 months) male sex partners‡ | ||||||||||
| 0–1 | 31 | 4.2 | 713 | 95.8 | 744 | 49.9 | 1 | — | ||
| 2 | 16 | 5.2 | 292 | 94.8 | 308 | 20.7 | 1.3 | 0.7–2.3 | ||
| 3+ | 37 | 8.5 | 401 | 91.6 | 438 | 29.4 | 2.1 | 1.3–3.5 | ||
*Adjusted for key populationxcity, HIV status, age, and self-reported STI symptoms past 12 months using multiple logistic regression. Bolded results are significant (P < 0.05).
†n = 4 other/do not know/refuse to answer.
‡n = 21 missing.
§n = 22 missing.
AUDIT, Alcohol Use Disorders Identification Test.
HIV prevalence was 22.5% overall ([340/1511]; [Harare, 21.4%]; [Bulawayo, 23.4%]), 21.1% among MSM ([248/1176]; Harare, 17.1%]; [Bulawayo, 23.3%]), and 27.5% among TGW/GQ ([92/335]; [Harare, 28.0%]; [Bulawayo, 25.0%]). Overall, 9.1% of participants had ever had syphilis, including past and current infections ([137/1511]; [Harare, 8.1%]; [Bulawayo, 9.9%]) and 0.5% had a biological false positive ([8/1511]; [Harare, 0.6%]; [Bulawayo, 0.4%]). The prevalence of HIV/syphilis coinfection among all participants was 2.4% ([36/1511]; [Harare, 2.2%]; [Bulawayo, 2.6%]). In Harare, 5.5% of participants had active syphilis (38/695) and 10.1% of PLHIV were coinfected (15/149). MSM and TGW/GQ in Harare had a similar prevalence of active syphilis (5.1% v. 6.1%, P > 0.5) and HIV/syphilis coinfection among PLHIV (12.7% v. 7.7%, P > 0.5). In Bulawayo, 5.6% of participants had active syphilis (46/816), and 11.0% of PLHIV were coinfected (21/191). Less than 5% of MSM ([4.6%]; [35/760]) had active syphilis compared with 19.6% among TGW/GQ (11/56) in Bulawayo (P = 0.0001) and HIV/syphilis coinfection among PLHIV was 8.5% (15/177) and 42.9% ([6/14]; [P = 0.0013]), respectively. Among all participants, 19.1% (289/1511) self-reported experiencing 1 or more STI symptoms in the past 12 months. Less than a third of participants with active syphilis ([31.0%]; [26/84]) and HIV/syphilis coinfection ([27.8%]; [10/36]) self-reported STI symptoms. Of those with active syphilis reporting STI symptoms, 57.7% reported 1 symptom (15/26), 26.9% reported 2 symptoms (7/26), and 15.4% reported 3 symptoms (4/26).
Participants were more likely to have active syphilis if they were HIV-positive (adjusted odds ratio [aOR], 2.2; 95% confidence interval [CI], 1.4–3.6), (Table 1), 25–34 years compared with 18 to 24 years (aOR, 2.2; 95% CI, 1.3–3.8), or had self-reported STI symptoms in the past 12 months (aOR, 1.8; 95% CI, 1.1–3.0). Compared with TGW/GQ in Bulawayo, MSM in Harare (aOR, 0.2; 95% CI, 0.1–0.5), MSM in Bulawayo (aOR, 0.2; 95% CI, 0.1–0.4), and TGW/GQ in Harare (aOR, 0.2; 95% CI, 0.1–0.6) were less likely to have active syphilis.
Among PLHIV, less than half were self-reported aware of their HIV status ([45.0]; [153/340]) and 61.5% were virally suppressed (209/340) (Table 2). Overall, 23.5% of PLHIV had advanced HIV disease (80/340) and 11.8% had AIDS (40/340). Among PLHIV, coinfection was 13.0% among TGW/GQ (12/92) and 9.7% among MSM (24/248). In logistic regression, the crude odds of coinfection among PLHIV were higher for those aged 25 to 34 years (odds ratio [OR], 3.7; 95% CI, 1.2–11.1) than those 18 to 24 years and lower among MSM in Harare (OR, 0.2; 95% CI, 0.1–0.7), MSM in Bulawayo (OR, 0.1; 95% CI, 0.0–0.4), and TGW/GQ in Harare (OR, 0.1; 95% CI, 0.0–0.4) compared with TGW/GQ in Bulawayo. There were no significant differences in syphilis coinfection among PLHIV by sexual orientation, education, viral load suppression, advanced HIV disease or AIDS, AIDS alone, awareness of HIV status, self-reported STI symptoms, early sexual debut, recent transactional sex, number of recent male sex partners, or alcohol dependence.
TABLE 2.
Demographic and Behavioral Characteristics and ORs of HIV/Syphilis coinfection Among MSM and TGW/GQ PLHIV, Zimbabwe, 2019
| Characteristic | HIV/Syphilis Coinfection (n = 36) | HIV Infection (No Syphilis Coinfection) (n = 304) | Total (N = 340) | HIV/Syphilis Coinfection | ||||
|---|---|---|---|---|---|---|---|---|
| n | Row % | n | Row % | n | Col % | OR | 95% CI | |
| Key populationxcity | ||||||||
| MSM Harare | 9 | 12.7 | 62 | 87.3 | 71 | 20.9 | 0.2 | 0.1–0.7 |
| TGW/GQ Harare | 6 | 7.7 | 72 | 92.3 | 78 | 22.9 | 0.1 | 0.0–0.4 |
| MSM Bulawayo | 15 | 8.5 | 162 | 91.5 | 177 | 52.1 | 0.1 | 0.0–0.4 |
| TGW/GQ Bulawayo | 6 | 42.9 | 8 | 57.1 | 14 | 4.1 | 1 | — |
| Age, y | ||||||||
| 18–24 | 4 | 4.9 | 77 | 95.1 | 81 | 23.8 | 1 | — |
| 25–34 | 24 | 16.1 | 125 | 83.9 | 149 | 43.8 | 3.7 | 1.2–11.1 |
| 35–44 | 3 | 4.1 | 71 | 96.0 | 74 | 21.8 | 0.8 | 0.2–3.8 |
| 45+ | 5 | 13.9 | 31 | 86.1 | 36 | 10.6 | 3.1 | 0.8–12.3 |
| Sexual orientation* | ||||||||
| Gay/homosexual | 24 | 10.0 | 217 | 90.0 | 241 | 71.5 | 0.8 | 0.4–1.6 |
| Bisexual | 12 | 12.8 | 82 | 87.2 | 94 | 27.9 | 1 | — |
| Straight/heterosexual | 0 | 0 | 2 | 100.0 | 2 | 0.6 | — | — |
| Highest education attended | ||||||||
| Primary or less | 3 | 12.0 | 22 | 88.0 | 25 | 7.4 | 1 | — |
| Secondary | 24 | 9.8 | 221 | 90.2 | 245 | 72.1 | 0.8 | 0.2–2.9 |
| Tertiary or vocational | 9 | 12.9 | 61 | 87.1 | 70 | 20.6 | 1.1 | 0.3–4.4 |
| Self-report STI symptoms past 12 m | ||||||||
| Yes | 10 | 13.0 | 67 | 87.0 | 77 | 22.7 | 1.4 | 0.6–3.0 |
| No | 26 | 9.9 | 237 | 90.1 | 263 | 77.4 | 1 | — |
| Alcohol dependence (AUDIT score of ≥15) | ||||||||
| Yes | 9 | 9.8 | 83 | 90.2 | 92 | 27.1 | 0.9 | 0.4–2.0 |
| No | 27 | 10.9 | 221 | 89.1 | 248 | 72.9 | 1 | — |
| Early sexual debut with male sex partner† | ||||||||
| Early sexual debut (<15 y) | 5 | 15.2 | 28 | 84.9 | 33 | 9.8 | 1.6 | 0.6–4.4 |
| No early sexual debut (≥15 y) | 31 | 10.2 | 274 | 89.8 | 305 | 90.2 | 1 | — |
| Recent (≤6 mo) transactional sex‡ | ||||||||
| Yes | 6 | 14.6 | 35 | 85.4 | 41 | 12.2 | 1.5 | 0.6–3.9 |
| No | 30 | 10.1 | 266 | 89.9 | 296 | 87.8 | 1 | — |
| No. recent (≤6 mo) male sex partners† | ||||||||
| 0–1 | 13 | 8.7 | 137 | 91.3 | 150 | 44.4 | 1 | — |
| 2 | 7 | 10.1 | 62 | 89.9 | 69 | 20.4 | 1.2 | 0.5–3.1 |
| 3+ | 16 | 13.5 | 103 | 86.6 | 119 | 35.2 | 1.6 | 0.8–3.6 |
| Viral load suppression (<1000 copies/mL) | ||||||||
| Virally suppressed | 21 | 10.1 | 188 | 90.0 | 209 | 61.5 | 1 | — |
| Not virally suppressed | 15 | 11.5 | 116 | 88.6 | 131 | 38.5 | 1.2 | 0.6–2.3 |
| CD4+ cell count | ||||||||
| <350 | 14 | 11.7 | 106 | 88.3 | 120 | 35.3 | 1.2 | 0.6–2.4 |
| ≥350 | 22 | 10.0 | 198 | 90.0 | 220 | 64.7 | 1 | — |
| Known HIV-positive | ||||||||
| Yes | 15 | 9.8 | 138 | 90.2 | 153 | 45.0 | 1 | — |
| No | 21 | 11.2 | 166 | 88.8 | 187 | 55.0 | 1.2 | 0.6–2.3 |
*n = 3 other/do not know/refuse to answer.
†n = 2 missing.
‡n = 3 missing.
Bolded results are significant (P < 0.05).
DISCUSSION
In this survey, active syphilis was more than 6 times higher overall than estimates for adult males in the general population of Harare and Bulawayo, and HIV/syphilis coinfection was nearly 4-fold that among male PLHIV in Zimbabwe.8 Whereas active syphilis estimates among MSM and TGW/GQ in Southern Africa are limited, findings in this survey are substantially higher than available estimates from RDS surveys with MSM in West and Central Africa (0% in Nigeria,13 <1% in Cameroon,14 <2% in Burkina Faso15), where HIV prevalence among the general population is 9 times lower than that of Zimbabwe's and coverage of male circumcision, which reduces risk of HIV and syphilis, is considerably higher.16
Zimbabwe, like most countries in sub-Saharan Africa, has largely employed syndromic management—provision of STI treatment to patients presenting with symptoms where etiological diagnosis is unavailable—as a means of STI control and prevention. In this sample, less than a third of participants with syphilis and HIV/syphilis coinfection reported STI symptoms in the past 12 months; the prevalence of asymptomatic infection among these groups suggests that syndromic management alone is insufficient. To adequately address the SRHR needs of MSM and TGW/GQ and prevent and control STIs, accessibility to diagnostics for syphilis and other STIs and routine STI testing is necessary.
High prevalence of coinfection among PLHIV provides evidence of the need for improved HIV/syphilis service integration, in line with country priorities.17 For key populations, HIV and SRHR services are largely provided by nongovernmental organizations, and minimally integrated, though efforts to train staff to provide comprehensive, integrated care within public facilities have increased and integrated services are available at some facilities.17 Human immunodeficiency virus services can serve as an entry point for syphilis testing and improve detection of syphilis cases. Equally, HIV testing among individuals with active syphilis and their partners can support identification of new HIV cases. Improved case finding for HIV and syphilis may facilitate more timely access to treatment and support viral load suppression for key populations living with HIV. Moreover, rapid point-of-care tests that detect both HIV and syphilis, while not widely available in Zimbabwe, offer opportunities for case detection and integration of services; in settings where resources may be limited, MSM and TGW/GQ can be prioritized in the rollout of dual HIV/syphilis assays given disparities in the burden of HIV/syphilis and their coinfection between these groups and the general population.
Findings from this survey also indicate differences in disease burden within key populations. Although sample size was small, nearly a quarter of TGW/GQ in Bulawayo had active syphilis and almost half of TGW/GQ in Bulawayo living with HIV were coinfected with syphilis; this group had significantly higher odds of active syphilis compared with other key population groups in both cities, underscoring an urgent need for targeted syphilis prevention interventions for TGW/GQ in Bulawayo.
In this survey, HIV/syphilis coinfection among PLHIV was not associated with unsuppressed viral load, advanced HIV disease or AIDS, or AIDS alone according to CD4+ cell count. Emerging evidence suggests ART may mitigate the effect of syphilis on increased viral load replication,18,19 indicating syphilis may have a more significant effect on viral load for PLHIV who are already viremic. Temporal relationships, such as the interaction between syphilis and HIV biomarkers and the nuance of ART's role in this relationship, cannot be assessed in this survey given the cross-sectional study design. While participants were asked about history of STI symptoms in the past 12 months as part of the survey, symptoms asked were not specific for syphilis, limiting our ability to characterize syphilis stages. All self-reported results are subject to social desirability bias; given prevalence of viral load suppression among PLHIV in this sample, we suspect some PLHIV who self-reported to be newly diagnosed through the survey were aware of their HIV-positive status. As earlier described, findings presented do not represent population estimates and may not be generalizable to all MSM and TGW/GQ in survey cities.
Despite these limitations, this survey is the first to describe the prevalence of active syphilis and HIV/syphilis coinfection among MSM and TGW/GQ, populations vulnerable to HIV and syphilis, in Zimbabwe, and highlight disparities in disease burden among and between key populations. Findings from this survey provide evidence of the need for improved integration of HIV/SRHR services to support syphilis and HIV case identification among these groups in Zimbabwe.
Footnotes
Acknowledgments: The authors thank the MSM and TGW/GQ who participated in this survey and provided meaningful information to inform HIV programs for key populations in Zimbabwe. The authors also acknowledge the Zimbabwe Ministry of Health and Child Care, Gay and Lesbians of Zimbabwe (GALZ), and survey staff for supporting the implementation of the survey.
Conflict of Interest: None declared.
Sources of Funding: This project has been supported by the President’s Emergency Plan for AIDS Relief through the CDC under the terms of cooperative agreement U2GGH001939. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the funding agencies.
Contributor Information
Innocent Chingombe, Email: ic2421@cumc.columbia.edu.
Yingfeng Wu, Email: yw2322@cumc.columbia.edu.
Munyaradzi Mapingure, Email: mpm2189@cumc.columbia.edu.
Owen Mugurungi, Email: mugurungi@gmail.com.
Chesterfield Samba, Email: director@galz.co.
John H. Rogers, Email: yet6@cdc.gov.
Avi J. Hakim, Email: hxv8@cdc.gov.
Perpetua Gozhora, Email: perpetua.gozhora@gmail.com.
Sophia S. Miller, Email: sm4594@cumc.columbia.edu.
Godfrey Musuka, Email: gm2660@cumc.columbia.edu.
Tiffany G. Harris, Email: th2604@cumc.columbia.edu.
REFERENCES
- 1.Rottingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: How much really is known? Sex Transm Dis 2001; 28:579–597. [DOI] [PubMed] [Google Scholar]
- 2.Karp G Schlaeffer F Jotkowitz A, et al. Syphilis and HIV co-infection. Eur J Intern Med 2009; 20:9–13. [DOI] [PubMed] [Google Scholar]
- 3.Gomez GB Kamb ML Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: A systematic review and meta-analysis. Bull World Health Organ 2013; 91:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hook EW, 3rd, Marra CM. Acquired syphilis in adults. N Engl J Med 1992; 326:1060–1069. [DOI] [PubMed] [Google Scholar]
- 5.Ruangtragool L Rogers JH Silver R, et al. Factors associated with active syphilis infection among men and women aged 15 years and older in the Zimbabwe Population-based HIV Impact Assessment (2015–2016) (unpublished). In: 2021. [DOI] [PMC free article] [PubMed]
- 6.Beyrer C Baral SD van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poteat T Scheim A Xavier J, et al. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr 2016; 72 Suppl 3(Suppl 3):S210–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Country Policy and Information Note Zimbabwe : Sexual orientation and gender identity and expression. 2019. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/775001/CPIN-_ZIM_-_SOGIE._V4.0e__Jan_2019_.pdf. Accessed January 28, 2021.
- 9.UNAIDS . New HIV infections increasingly among key populations. 2020. Available at: https://www.unaids.org/en/resources/presscentre/featurestories/2020/september/20200928_new-hiv-infections-increasingly-among-key-populations. Accessed January 28, 2021.
- 10.Zimbabwe Population-based HIV Impact Assessment (ZIMPHIA) 2015–2016: Final Report . Harare: Ministry of Health and Child Care, 2019. [Google Scholar]
- 11.Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl 1997; 44:174–199. [Google Scholar]
- 12.HIV and STI Biobehavioral Survey among Men Who Have Sex with Men, Transgender Women, and Genderqueer Individuals in Zimbabwe - Final Report . New York: ICAP at Columbia University, 2020. [Google Scholar]
- 13.Merrigan M Azeez A Afolabi B, et al. HIV prevalence and risk behaviours among men having sex with men in Nigeria. Sex Transm Infect 2011; 87:65–70. [DOI] [PubMed] [Google Scholar]
- 14.Park JN Papworth E Kassegne S, et al. HIV prevalence and factors associated with HIV infection among men who have sex with men in Cameroon. J Int AIDS Soc 2013; 16 Suppl 3(4 Suppl 3):18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouedraogo HG Zida S Compaore TR, et al. Seroepidemiology of syphilis among men who have sex with men in Burkina Faso, West Africa. Eur J Clin Microbiol Infect Dis 2019; 38:1803–1809. [DOI] [PubMed] [Google Scholar]
- 16.Cork MA Wilson KF Perkins S, et al. Mapping male circumcision for HIV prevention efforts in sub-Saharan Africa. BMC Med 2020; 18:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Extended Zimbabwe National HIV and AIDS Strategic Plan 2015–2020. Harare: Ministry of Health and Child Care, 2017. [Google Scholar]
- 18.Champredon D Bellan SE Delva W, et al. The effect of sexually transmitted co-infections on HIV viral load amongst individuals on antiretroviral therapy: A systematic review and meta-analysis. BMC Infect Dis 2015; 15:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grewal R Allen VG Bayoumi AM, et al. Brief report: Syphilis coinfection is not associated with an increased risk of virologic failure among HIV-positive men who have sex with men on antiretroviral therapy. J Acquir Immune Defic Syndr 2019; 80:585–589. [DOI] [PubMed] [Google Scholar]


