Abstract
Objective:
To evaluate the audiological and subjective benefit from hearing rehabilitation with an active bone conduction implant in subjects with single-sided sensorineural deafness (SSD).
Study Design:
Prospective, multicenter, single-subject repeated measures.
Setting:
Tertiary referral center, five clinics in Germany and Switzerland.
Patients:
Seventeen subjects aged 18 years and older with severe to profound unilateral sensorineural hearing loss and contralateral normal hearing were followed up for 24 months.
Intervention:
Active bone conduction implant.
Main Outcome Measures:
Speech understanding in noise was assessed in three situations: with signal from front, deaf, or normal hearing side (with noise from front in all set-ups). Subjective benefit was evaluated using the Speech, Spatial, and Qualities of Hearing (SSQ-B) and Bern Benefit in Single-Sided Deafness (BBSS) questionnaire.
Results:
When the signal was coming from the deaf side the mean improvement of the speech reception threshold in noise ranged from 1.5 up to 2.2 dB with the device and was statistically and clinically significant at all tested timepoints. No significant difference between the aided and unaided situation was found when signal and noise were coming from the front. With the signal from the normal hearing side no clinically significant difference, that is, greater than 1 dB between the aided and unaided situation was found. The SSQ-B and BBSS questionnaire showed an overall improvement with no significant difference between time points.
Conclusions:
The study demonstrates long-term efficacy and benefit of the device in adults with SSD. Patients reported substantial and persistent subjective benefit from the active bone conduction implant.
Keywords: Bone conduction, Implant, Single sided deafness
INTRODUCTION
Single-sided Deafness (SSD) is characterized by severe to profound hearing loss in one ear and normal hearing in the other ear. Etiologies of congenital or acquired SSD vary considerably and are often unknown. Possible causes include idiopathic sudden sensorineural hearing loss (SNHL), viral or bacterial inner ear infections, acoustic neuromas, physical trauma to the ear or head, and unilateral Menière's disease.
Although some SSD patients report few hearing problems in their daily lives, many suffer in various listening situations and seek treatment. Individuals with SSD report good comprehension in quiet environments. However, SSD patients often have poor sound localization abilities (1,2) as well as difficulties with speech recognition in noise and other challenging listening situations due to the loss of binaural processing (3).
Patients with SSD have different hearing rehabilitation options ranging from cochlear implants, contralateral routing of signals (CROS) hearing aids to bone conduction (BC) devices. In patients with a functioning cochlear nerve, cochlear implants may restore binaural hearing but require surgical access to the inner ear and long-term rehabilitation. In conventional CROS devices, sound is recorded through a microphone on the deaf side and transmitted to a hearing aid worn on the better ear to overcome the acoustic head shadow effect. CROS devices require no surgery and are thus the least invasive option, although the patient will have to wear a device at each ear (4,5).
BC devices employ the same principle as CROS devices by transmitting sound from the ear with SSD to the better ear, using BC. Device options include BC sound processors held in place via Softband (6), an adhesive adapter (7,8), an implanted magnet (9), a percutaneous abutment (i.e., bone-anchored hearing aids, BAHA) and an active transcutaneous bone conduction implant system (tBCI). Up-to-date, the largest number of studies on BC solutions in SSD have been published on BAHA, with significant improvements in speech perception and quality of life noted (10–15). Fewer studies have been published on the tBCI in SSD patients, but initial results (16–19), including a temporal bone study comparing the tBCI to the BAHA (20), have shown similar outcomes for both devices. The tBCI implantation is more complex and requires more space in the temporal bone than BAHA, but the advantage is that the skin is intact after postoperative healing. Some SSD users require support only in certain hearing situations; thus, the tBCI is completely invisible when not in use. For all BC devices a trial period with a non-implantable BC device and intensive counseling is essential to assess preoperatively if the SSD candidate would benefit from such a treatment option. A systematic review concluded that after a trial period 32 to 70% of SSD patients would decline BAHA implantation (21). Others reported that the subjectively perceived benefit was less than expected or decreased with time (22,23), suggesting that SSD patients are a challenging cohort.
The tBCI is intended to treat patients with either conductive or mixed hearing loss (C/MHL) or SSD. Previous studies have shown long-term benefit from the tBCI in patients with C/MHL (24). The number of tBCI users with SSD is still limited. The aim of this prospective study was to evaluate the long-term audiological and subjective benefit in 17 SSD patients implanted with the tBCI followed-up for up to 24 months.
MATERIALS AND METHODS
Device Description
The BONEBRIDGE system (MED-EL, Innsbruck, Austria) tBCI is composed of an external audio processor (AP) and an active BC implant under the intact skin (25). Surgical implantation is performed under general or local anesthesia as described previously (25–27). If the AP is removed, the active tBCI is MRI-conditional up to 1.5 T, allowing postoperative control of pathologies only detectable in MRI (e.g., vestibular schwannoma). An adapted imaging protocol was recently introduced that enables diagnostically useful MRIs in tBCI patients by reducing the artefact around the implant site (28).
Study Design
We used a prospective, single-subject repeated-measures design in which each subject serves as his/her own control. The study was approved by the local institutional review boards: cantonal ethics commission (EC) Zurich ECNo. 2012_0240, cantonal EC Bern ECNo. 148/12, EC Northwest- and central Switzerland ECNo. 12062, EC LMU Munich ECNo. 276-12, EC University Lübeck ECNo. 13-154. All subjects signed the informed consent form upon study inclusion.
Subjects
Seventeen subjects from five centers in Germany and Switzerland were enrolled. The inclusion criteria were: 1) unilateral severe-to-profound SNHL in one ear and 2) contralateral normal hearing. All patients were given the choice between CROS hearing aids, percutaneous BCI, transcutaneous passive BCI, transcutaneous active BCI, or cochlear implant (CI). However, at the time of the study, the CI was not reimbursed in one of the countries. Preoperatively, all patients underwent a two-week trial of a commercially available BCI on a headband. Mean duration of hearing loss was 12 years (range 3–44 years). All patients reported difficulties communicating in their professional and social lives. All surgeries were performed under general anesthesia. One of these 17 subjects (ID6) suffered from moderate-to-severe unilateral SNHL (AC PTA4 = 57.5 dB HL), and was excluded from the analysis. Patient demographics are found in Table 1. Subjects were followed for 24 months except for subject ID7 who decided not to participate further after baseline testing and subject ID16 who moved away after the 12-month evaluation. Three subjects were lost to follow-up: subjects ID12 and ID15 participated until the 18-month evaluation; subject ID9 until the 12-month evaluation. Adverse events were reported according to ISO 14155:2011.
TABLE 1.
Baseline demographics and etiology
| Subject ID | Gender | Age in Years | Implanted Side | Contralateral AC PTA4 (dB HL) | Etiology |
| 1 | M | 46 | R | 8.75 | Otitis Media |
| 2 | F | 56 | R | 18.75 | Sudden Hearing Loss |
| 3 | M | 56 | L | 15 | Sudden Hearing Loss |
| 4 | F | 62 | R | 20 | Barotrauma |
| 5 | F | 18 | L | 18.75 | Unknown |
| 6 | F | 48 | R | 13.75 | Menière’ Disease |
| 7 | F | 22 | R | 3.75 | Meningitis |
| 8 | F | 48 | L | 25 | Unknown |
| 9 | M | 32 | L | 5 | Congenital |
| 10 | M | 35 | R | 1.25 | Congenital |
| 11 | M | 28 | R | 12.5 | Congenital |
| 12 | M | 46 | R | 12.5 | Congenital |
| 13 | M | 23 | R | 0 | Mumps |
| 14 | F | 45 | L | 5 | Unknown |
| 15 | F | 33 | L | N/A | Hypoxia during birth |
| 16 | F | 24 | R | 9 | Acoustic shock |
| 17 | M | 58 | R | 11.5 | Cholesteatoma |
Air conduction (AC) PTA4 (four frequency pure tone average) was calculated across 0.5, 1, 2, and 3 kHz.
F indicates female; L, left; M, male; N/A not available; R, right.
Audiometric Testing
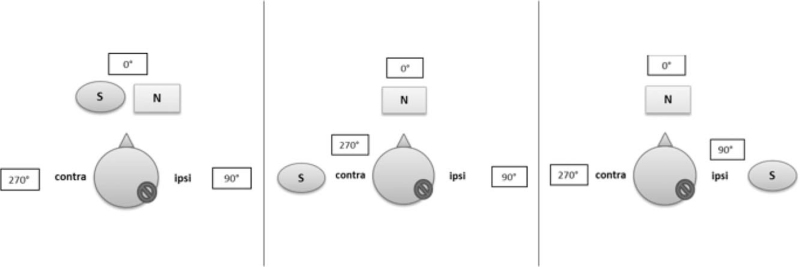
Speech understanding in noise was tested using the OLSA (German Oldenburg Sentence Test, (29)) at a fixed noise level of 65 dB SPL at baseline (within 3 months after surgery), the 6-month, 12-month, 18-month, and 24-month evaluations. The signal level was adapted until the speech reception threshold (SRT) for 50% correct recognition was determined and the signal-to-noise ratio (SNR) calculated. The tBCI-aided and unaided conditions were tested with noise always from the front (0° azimuth) and speech presented frontally (Scenario 1, S0° azimuth), at the contralateral side (Scenario 2, 270° azimuth) or at the implanted ear (Scenario 3, 90° azimuth) (see Fig. 1).
FIG. 1.
Test scenarios for speech understanding in noise. Test scenario 1 (left). S0° N0°: Both, Signal and noise presented frontally (0° azimuth). Test scenario 2 (middle). S270° N0°: Signal presented contralateral to the SSD side (270° azimuth) and noise presented frontally (0° azimuth). Test scenario 3 (right). S90° N0°: Signal presented at the implanted side (90° azimuth) and noise presented frontally (0° azimuth). Contra indicates contralateral: normal hearing side/ear; ipsi, ipsilateral: SSD affected/implanted side; S, Signal; N, Noise.
Subjective Benefit
Subjective benefit was determined at the 6, 12, and 24-month evaluations using two questionnaires. The “Speech, Spatial, and Qualities of Hearing” (SSQ) questionnaire measures self-reported auditory disability across a variety of domains that reflect the patient's everyday hearing perception (30). This study used the benefit version of the questionnaire (SSQ-B). The “Bern Benefit in Single-Sided Deafness” (BBSS) (31) comprises 10 questions to assess SSD patients’ benefit with a CROS or BC device. Answers on both questionnaires range from −5 to +5, where −5 indicates “much worse” with the device than without and +5 “much better.” The midpoint of the scale (0) indicates that the ability or experience is “unchanged.”
Statistical Analysis
Data were tested for normal distribution with the Kolmogorov–Smirnov and Shapiro–Wilk tests. Normally distributed datasets were analyzed with the paired-sample t test; the non-normally distributed datasets with the Wilcoxon signed-rank test. A p-value of ≤0.05 was considered statistically significant. For pairwise comparisons the p-value was adjusted with the Bonferroni correction method.
Pairwise comparisons between the single test intervals were made with the paired-sample t test for the SSQ-B questionnaire and with the Wilcoxon signed-rank test for the BBSS questionnaire. The SSQ-B subscale total scores were calculated as the average of all items within a subscale. The Total BBSS score is the average of all 10 items. Missing values were treated as missing values. Only subjects with missing values on ≤3 questions were included in the calculation of the total score.
RESULTS
Speech Understanding in Noise
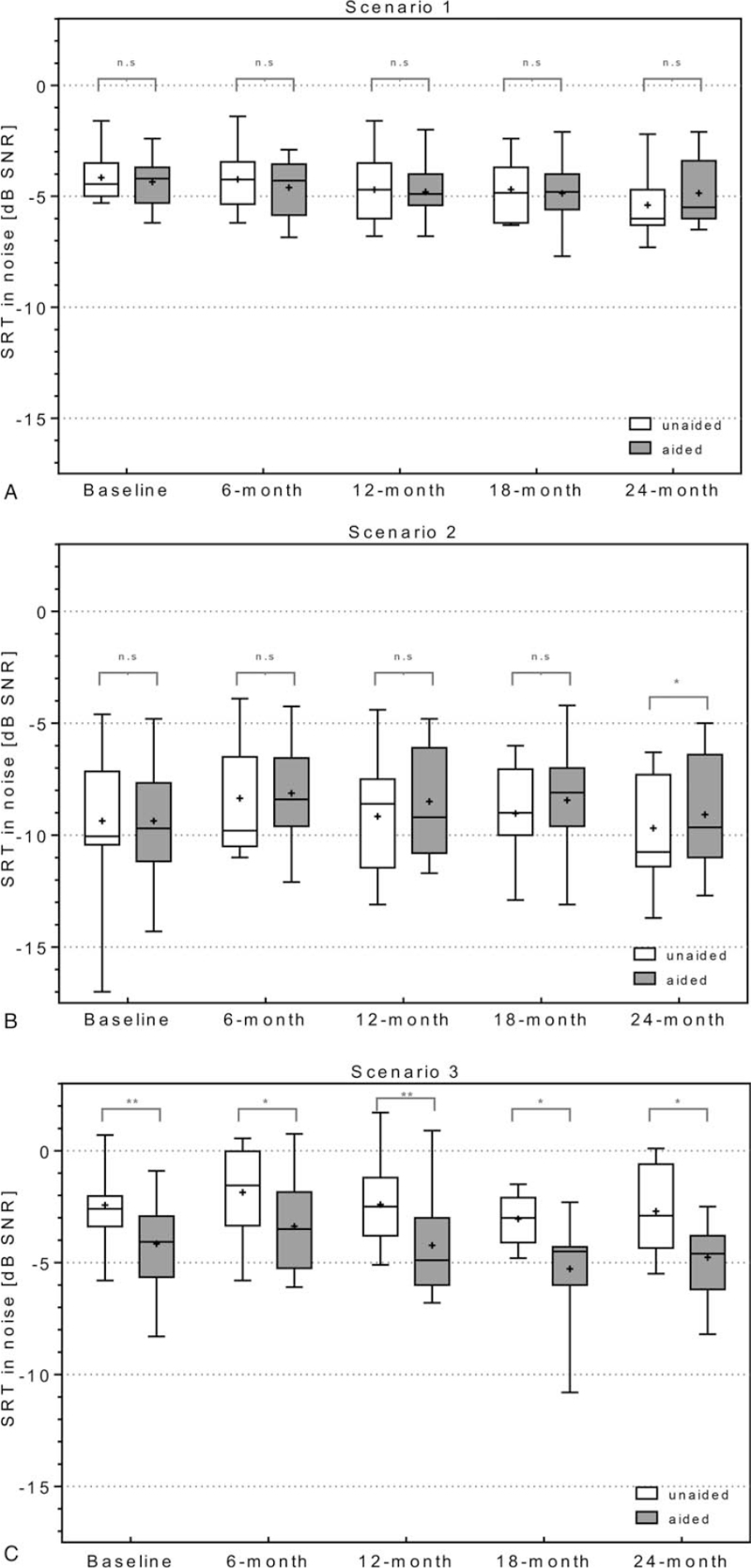
In scenario 1 with signal and noise from front (S0° N0°), no statistically significant difference was found between the aided and the unaided test conditions at any time points (Fig. 2A). In scenario 2, with signal to the normal-hearing ear and noise from front (S270° N0°) (Fig. 2B), no statistically significant difference was found at baseline, the 6-month, 12-month, and 18-month evaluations (p = 0.393 at baseline; p = 0.807 at 6-month; p = 0.109 at 12-month; p = 0.139 18-month). However, at the 24-month evaluation, a statistically significant increase in the SRT in noise of 0.6 dB was found when comparing the aided to unaided conditions (p = 0.008). In Scenario 3 (S90° N0°) with speech at the implanted side and noise from the front, tBCI-aided SRT in noise improved in all subjects compared to the unaided situation, with a mean improvement of 1.7 dB at baseline, 1.5 dB at 6-month, 1.8 dB at 12-month, 2.2 dB at 18-month, and 2.1 dB at 24-month (Fig. 2C, p = 0.001 baseline; p = 0.011 6-month evaluation; p = 0.001 12-month evaluation; p = 0.003 18-month evaluation; p = 0.004 24-month evaluation). Detailed descriptive statistics are provided in Supplement 1.
FIG. 2.
Speech understanding in noise. OLSA at a fixed noise level of 65 dB SPL from front and speech adapted level from front (A, Scenario 1, S0°N0°), from the normal hearing side (B, Scenario 2, S270° N0°) and from the SSD side (C, Scenario 3 S90° N0°). Box Plots: median = horizontal lines; + = mean; whiskers = min.–max. values; n.s. = nonsignificance; ∗ = significance (p ≤ 0.01); ∗∗ = significance (p ≤ 0.001).
Subjective Benefit
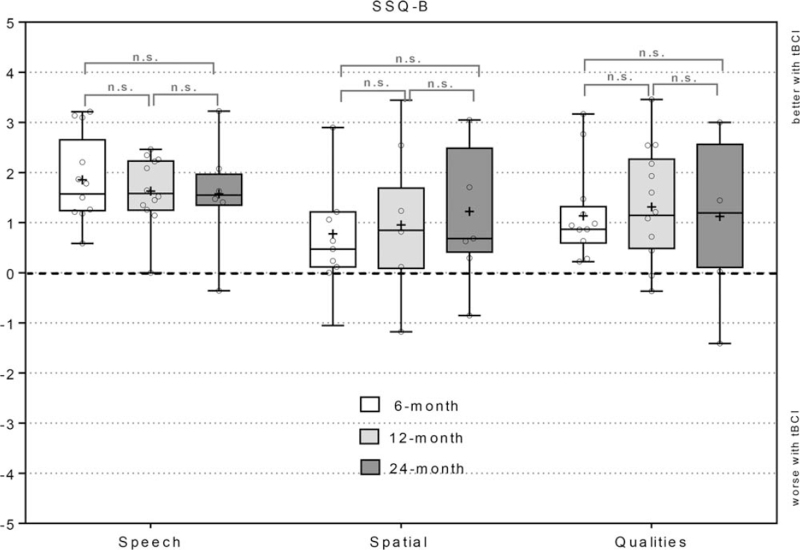
All subscales of the SSQ-B questionnaire showed overall improvement. The speech hearing subscale scores were 1.85 ± 0.83 at the 6-month, 1.63 ± 0.65 at the 12-month and 1.57 ± 0.93 at the 24-month evaluations. The spatial hearing subscale scores were 0.78 ± 1.14, 0.96 ± 1.32, and 1.22 ± 1.30, while the quality scale scores were 1.13 ± 0.88, 1.22 ± 1.09, and 1.12 ± 1.47 at these timepoints (Fig. 3). No statistical significance was found between the different timepoints. The speech section of the SSQ-B yielded the most positive scores, while results were closer to 0 for the spatial section. Detailed results for the SSQ-B individual subscales can be found in Supplement 2 (speech), Supplement 3 (spatial) and Supplement 4 (qualities). Out of 16 subjects who completed the questionnaire, 13 scored positively, one scored (ID12) 0 (i.e., no difference) and two subjects (ID5, ID6) scored negatively on the speech domain at their latest evaluation. On the spatial domain, 10 scored positively, 2 scored 0 (ID5, ID12), and 4 subjects (ID4, ID6, ID11, and ID15) scored negatively at their latest evaluation. On the qualities domain, 12 scored positively and four (ID4, ID6, ID11, and ID12) negatively.
FIG. 3.
Subjective benefit based on the SSQ-B questionnaire. The score for the subdomains Speech, Spatial and Qualities was calculated as the average of all items within a subdomain. Positive scores indicate a better hearing perception with the device, negative scores indicate a worse hearing perception with the device. Only subjects with missing values on ≤3 questions were included in the calculation of the total score. Box Plots: median = horizontal lines; + = mean; whiskers = min.-max. values; circles = individual scores; n.s. = no significance.
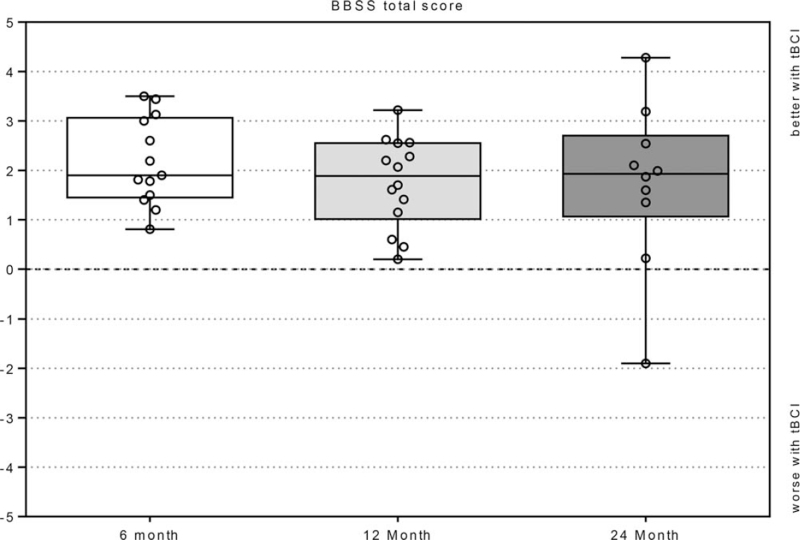
BBSS questionnaire results showed an overall improvement with the tBCI at all timepoints (Fig. 4). The patients reported an average device-aided benefit of 2.2 ± 0.9 at the 6-month, 1.7 ± 0.9 at the 12-month and 1.7 ± 1.7 at the 24-month evaluations compared to unaided. Subject ID5's average benefit was negative at the 24-month evaluation but was 3 at baseline. The subjects rated their hearing with the tBCI slightly more positively at the 6-month evaluation compared to the 12- and 24-month timepoints, though this was not statistically significant (Wilcoxon signed-rank test: p = 0.158 and p = 0.173). The average benefit was positive for all items on the BBSS. Across all timepoints the highest benefit was reported for overall hearing (question 10), participation in a group conversation (question 8), holding a conversation while driving a car (question 6) and conversation in a quiet environment (question 1). Questions concerning tBCI-aided hearing in noise and background sound (question 5) showed the lowest benefit, as well as hearing indoors, such as churches or entrance halls (question 7). Average daily wearing time was 10.1/11.3/8.4 hours at the 6-, 12-, and 24-month assessments. BBSS questionnaire individual question results are found in Supplement 5.
FIG. 4.
BBSS Total Score. The BBSS total score is the average of all 10 items. Box Plots: median = horizontal lines; + = mean; whiskers = min.–max. values; circles = individual scores; n.s. = no significance.
Adverse Events
Out of 10 adverse events reported during the study, 4 were deemed procedure- or device-related. One patient experienced retroauricular wound healing problems that were locally treated with medication. One patient had local swelling and pain after implantation that resolved without treatment. Another patient experienced occasional local swelling without pain at the implant site in the evening that resolved in the morning. One patient reports headache once per month. Two nonrelated serious adverse events required hospitalization due to an inguinal hernia and acute cholecystitis.
DISCUSSION
This study assessed hearing performance in challenging real-life listening situations and subjective benefit in SSD adult subjects using a tBCI. They were followed for up to 24 months after surgery. Subjective benefit was demonstrated in two questionnaires reporting on everyday listening situations with and without the tBCI.
Speech Understanding in Noise
Three different scenarios were employed to test speech intelligibility in noise in real-world listening situations. For test scenario 1 (S0°N0°), in which both the signal and noise originate from the front of the user, no significant improvement in speech intelligibility was observed in the aided compared to the unaided condition. In this test scenario, there is no spatial separation between signal and noise and both sound and speech are processed by the normal hearing ear without obstruction/attenuation by the head. Similarly, no improvement was found for the percutaneous BAHA device in this test condition (32,33). As expected, the user appears to gain no benefit from the tBCI in this listening situation, but no significant deterioration in speech intelligibility was observed at any timepoint investigated.
In test scenario 2, with speech presented to the normal hearing side, SSD patients typically have fewer difficulties understanding speech in noise. However, this is also the most unfavorable test situation for the device under investigation, as it mainly amplifies noise. Previous publications have shown that if speech is presented to the normal hearing side, decreased speech understanding is observed in the aided condition with conventional and BC-CROS devices (34). In our study, no statistically or clinically significant difference was found between the aided and unaided conditions at baseline, the 6-month, 12-month, or 18-month evaluations. At the 24-month evaluation, we found a statistically significant difference in test scenario 2. The mean speech reception threshold in noise was 0.6 dB better in the unaided compared to the aided situation. However, this difference was within the 1-dB test–retest variability of the speech in noise test, thus it was considered not clinically significant (35). In the most recent individual data, three subjects experienced a deterioration of more than 1 dB in this test set-up: ID4 (1.3 dB), ID10 (1.1 dB), and ID11 (1.75 dB). ID1, ID13, and ID14 experienced difficulties with the device at baseline in this scenario, but not at the later evaluations. In a previous study with speech presented from the front and noise at the SSD side, no significant difference was found between the unaided and tBCI aided condition (36).
The most complicated real-world hearing situation for unaided SSD individuals occurs in scenario 3 (S90°N0°), when the signal is presented to the deaf ear and noise to the front. In this scenario, the head shadow is present, as the head obstructs the signal which is therefore attenuated at the normal hearing ear and may not be separated from the noise. Device benefit would be most visible in this condition. Indeed, the greatest decrease in SRT in noise and thus the greatest speech understanding improvement was observed in test scenario 3. The primary endpoint for all subjects was reached and a clinically as well as statistically significant improvement was observed between the tBCI-aided and unaided conditions at all timepoints. At the latest individual-subject evaluation, 13 of 17 subjects showed clinically significant improvement of at least 1 dB in this scenario. Subjects ID6 (0.4 dB decrease), ID13 (0.4 dB decrease), and ID15 (0.8 dB decrease) did not reach this at the last evaluation although they showed an improvement of 2.2, 2.1, and 5 dB at baseline. Subject ID16 showed an improvement of 1, 1.2, and 0.9 dB at the 12-, 18-, and 24-month evaluations. The results for test scenario 3 are in accordance with results reported by Laske (16) in a prospective cohort study on adults implanted with the tBCI and by Linstrom (1) and Bosman (2003) (37) on SSD subjects using a BAHA. The large decrease in SNR for test scenario 3 shows that the tBCI can reduce the head shadow effect in this difficult listening situation for SSD patients.
Although our study involved patients with sensorineural SSD (both congenital and acquired), a recent study explored the possibility that the tBCI might also benefit patients with unilateral conductive hearing loss (UCHL) as well. Vyskocil et al. (38) tested sound source localization in five congenital UCHL patients with tBCI, with all patients showing improvement over the unaided condition; however, more examinations with a larger number of patients and/or longer follow-up are necessary to draw any conclusions. Similar studies in congenital UCHL patients using a BAHA (39,40) showed very little sound localization benefit with the device. Fan et al. investigated sound localization abilities in 32 atresia patients implanted with the tBCI in this study, with and without an additional adhesive bone conduction device on the contralateral ear, interestingly finding a statistically significant improvement in the bilateral condition (41).
Subjective Benefit
In addition to the significant improvement in speech understanding in noise in scenario 3, an increase in subjective benefit was documented on the SSQ-B and BBSS questionnaires at the 6-month, 12-month, and 24-month evaluations. The subjective benefit from the tBCI was most prominent in the “Speech” subscale of the SSQ-B, compared to the “Spatial” and “Quality” subscales. The “Speech” subscale contains six questions that are mostly related to hearing situations that are challenging for SSD patients, for example, background noise, conversations taking place without eye contact, environments with an echo, interference of voices with the same/different pitch and following conversations with many people talking (see questions 5 to 9 and 11 in supplement 2). The tBCI users benefited the most from their device in these situations.
Although it is not expected that treatment with the tBCI restores binaural hearing, patients in our study reported having better lateralization after receiving their devices. They particularly reported an improved ability to locate a speaker to the right or the left (see question 3 in supplement 3). This question scored highest on average on the SSQ-B “spatial” subscale.
Since speech understanding can be exhausting over the course of the day for the hearing impaired, it is important to point out that the subjects also experienced a benefit with the tBCI in the “Qualities” subscale of the SSQ-B for hearing situations associated to concentration and attention (see questions 14–17 in Supplement 4). However, the subjects did not experience a benefit regarding the naturalness of other voices, everyday sounds or their own voice (see questions 10–12 in Supplement 4).
The total BBSS score of 2.2 at 6, 1.7 at 12 and 1.7 at 24 months postimplantation demonstrate that the subjects in this study benefited from the device. The decrease in the subjectively perceived benefit over time in the BBSS score was not significant. Such a decrease over time has been previously noted in SSD subjects receiving another BC device (15). BBSS outcomes for conversations in groups or cars and overall hearing (see Supplement 5) further indicate that the SSD subjects treated with the tBCI benefit in situations where the head shadow effect plays a role.
To assess whether there are different outcomes after tBCI treatment in subjects suffering from congenital SSD (ID9, ID10, ID11, and ID12), a comparison to cases with acquired SSD was done. Due to the limited number of subjects no statistical analysis was performed. Another limitation of this comparison is that all congenital SSD patients were enrolled in the same study center. In scenario 2, two (ID10 and ID11) of the four congenital SSD cases experienced a clinically significant worsening of speech understanding in noise with the tBCI switched on, whereas this was only the case for one of the remaining 14 subjects. In scenario 3 all of them experienced a clinically significant improvement of 1 to 2.4 dB for speech understanding in noise. Three of the four subjects also reported a subjective benefit from the device in the BBSS and the speech domain of the SSQ-B, but not subject ID12. None of the congenital SSD patients reported an improvement greater than 1 in the spatial hearing or hearing quality domain.
Safety
Safety of the device was confirmed by a lack of revision surgeries, serious device-related adverse events or serious device deficiencies up to 2 years postoperatively. Also, no explantation was reported. Changes in bone and air conduction thresholds are not associated with tBCI implantation (18,42). Clinical experience with the tBCI in subjects with conductive and mixed hearing losses has confirmed that the implantation procedure involves risks comparable to other implantable BC hearing implants, but avoids the most frequent postoperative complications observed with BAHA, namely those associated with the percutaneous abutment (19,43–48). Up-to-date, all reported complications with the tBCI have been comparable to those of other BC hearing implants.
Although this is the first study following up SSD patients implanted with the tBCI for up to 24 months, some limitations should be noted: Only 17 patients were enrolled with only 11 completing the 24-month assessment. The effect of treatment on spatial hearing was not assessed with an objective test, only within a questionnaire. Evaluation of the subjective measurement instruments would have benefitted from an untreated control group.
CONCLUSION
This study demonstrates the long-term benefit from the tBCI in adults with SSD. The device alleviates the head shadow effect in difficult listening situations in noise when speech is lateralized to the hearing-impaired ear. Complications were considerably lower than those found with other, especially percutaneous, bone-anchored hearing aids. The patients reported a substantial persistent subjective benefit for up to 2 years of device usage.
Supplementary Material
Acknowledgments
We appreciate the time and commitment given by the participants during this study. The authors thank Dr. Gabriella Bock and Dr. Carmen Giefing-Kröll for their writing support during manuscript preparation.
Footnotes
The study was sponsored by MED-EL (Innsbruck, Austria). JMH works as consultant for MED-EL (Innsbruck, Austria). JHM, WW, MC, HF and CR received reimbursements for travel expenses from MED-EL.
The authors disclose no conflicts of interest.
Supplemental digital content is available for this article.
REFERENCES
- 1. Linstrom CJ, Silverman CA, Yu GP. Efficacy of the bone-anchored hearing aid for single-sided deafness. Laryngoscope 2009; 119:713–720. [DOI] [PubMed] [Google Scholar]
- 2. Welsh LW, Welsh JJ, Rosen LF, et al. Functional impairments due to unilateral deafness. Ann Otol Rhinol Laryngol 2004; 113:987–993. [DOI] [PubMed] [Google Scholar]
- 3. Algazi VR, Duda RO, Duralswami R, et al. Approximating the head-related transfer function using simple geometric models of the head and torso. J Acoust Soc Am 2002; 112 (5 Pt 1):2053–2064. [DOI] [PubMed] [Google Scholar]
- 4. Ryu NG, Moon IJ, Byun H, et al. Clinical effectiveness of wireless CROS (contralateral routing of offside signals) hearing aids. Eur Arch Otorhinolaryngol 2015; 272:2213–2219. [DOI] [PubMed] [Google Scholar]
- 5. Harford E, Dodds E. The clinical application of CROS. A hearing aid for unilateral deafness. Arch Otolaryngol 1966; 83:455–464. [DOI] [PubMed] [Google Scholar]
- 6. Choi JE, Ma SM, Park H, et al. A comparison between wireless CROS/BiCROS and soft-band BAHA for patients with unilateral hearing loss. PLoS One 2019; 14:e0212503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mertens G, Gilles A, Bouzegta R, et al. A prospective randomized crossover study in single sided deafness on the new non-invasive adhesive bone conduction hearing system. Otol Neurotol 2018; 39:940–949. [DOI] [PubMed] [Google Scholar]
- 8. Gawliczek T, Munzinger F, Anschuetz L, et al. Unilateral and bilateral audiological benefit with an adhesively attached, noninvasive bone conduction hearing system. Otol Neurotol 2018; 39:1025–1030. [DOI] [PubMed] [Google Scholar]
- 9. Hougaard DD, Boldsen SK, Jensen AM, et al. A multicenter study on objective and subjective benefits with a transcutaneous bone-anchored hearing aid device: first Nordic results. Eur Arch Otorhinolaryngol 2017; 274:3011–3019. [DOI] [PubMed] [Google Scholar]
- 10. Pfiffner F, Kompis M, Stieger C. Bone-anchored Hearing Aids: correlation between pure-tone thresholds and outcome in three user groups. Otol Neurotol 2009; 30:884–890. [DOI] [PubMed] [Google Scholar]
- 11. Yuen HW, Bodmer D, Smilsky K, et al. Management of single-sided deafness with the bone-anchored hearing aid. Otolaryngol Head Neck Surg 2009; 141:16–23. [DOI] [PubMed] [Google Scholar]
- 12. Pfiffner F, Kompis M, Flynn M, et al. Benefits of low-frequency attenuation of Baha(R) in single-sided sensorineural deafness. Ear Hear 2011; 32:40–45. [DOI] [PubMed] [Google Scholar]
- 13. Wesarg T, Aschendorff A, Laszig R, et al. Comparison of speech discrimination in noise and directional hearing with 2 different sound processors of a bone-anchored hearing system in adults with unilateral severe or profound sensorineural hearing loss. Otol Neurotol 2013; 34:1064–1070. [DOI] [PubMed] [Google Scholar]
- 14. Wazen JJ, Van Ess MJ, Alameda J, et al. The Baha system in patients with single-sided deafness and contralateral hearing loss. Otolaryngol Head Neck Surg 2010; 142:554–559. [DOI] [PubMed] [Google Scholar]
- 15. Kompis M, Wimmer W, Caversaccio M. Long term benefit of bone anchored hearing systems in single sided deafness. Acta Otolaryngol 2017; 137:398–402. [DOI] [PubMed] [Google Scholar]
- 16. Laske RD, Roosli C, Pfiffner F, et al. Functional results and subjective benefit of a transcutaneous bone conduction device in patients with single-sided deafness. Otol Neurotol 2015; 36:1151–1156. [DOI] [PubMed] [Google Scholar]
- 17. Salcher R, Zimmermann D, Giere T, et al. Audiological results in SSD with an active transcutaneous bone conduction implant at a retrosigmoidal position. Otol Neurotol 2017; 38:642–647. [DOI] [PubMed] [Google Scholar]
- 18. Schmerber S, Deguine O, Marx M, et al. Safety and effectiveness of the Bonebridge transcutaneous active direct-drive bone-conduction hearing implant at 1-year device use. Eur Arch Otorhinolaryngol 2016. [DOI] [PubMed] [Google Scholar]
- 19. Wimmer W, von Werdt M, Mantokoudis G, et al. Outcome prediction for Bonebridge candidates based on audiological indication criteria. Auris Nasus Larynx 2019; 46:681–686. [DOI] [PubMed] [Google Scholar]
- 20. Huber AM, Sim JH, Xie YZ, et al. The Bonebridge: preclinical evaluation of a new transcutaneously-activated bone anchored hearing device. Hear Res 2013; 301:93–99. [DOI] [PubMed] [Google Scholar]
- 21. Wendrich AW, Kroese TE, Peters JPM, et al. Systematic review on the trial period for bone conduction devices in single-sided deafness: rates and reasons for rejection. Otol Neurotol 2017; 38:632–641. [DOI] [PubMed] [Google Scholar]
- 22. Desmet J, Wouters K, De Bodt M, et al. Long-term subjective benefit with a bone conduction implant sound processor in 44 patients with single-sided deafness. Otol Neurotol 2014; 35:1017–1025. [DOI] [PubMed] [Google Scholar]
- 23. Saroul N, Akkari M, Pavier Y, et al. Long-term benefit and sound localization in patients with single-sided deafness rehabilitated with an osseointegrated bone-conduction device. Otol Neurotol 2013; 34:111–114. [DOI] [PubMed] [Google Scholar]
- 24. Rader T, Stover T, Lenarz T, et al. Retrospective analysis of hearing-impaired adult patients treated with an active transcutaneous bone conduction implant. Otol Neurotol 2018; 39:874–881. [DOI] [PubMed] [Google Scholar]
- 25. Sprinzl G, Lenarz T, Ernst A, et al. First European multicenter results with a new transcutaneous bone conduction hearing implant system: short-term safety and efficacy. Otol Neurotol 2013; 34:1076–1083. [DOI] [PubMed] [Google Scholar]
- 26. Manrique M, Sanhueza I, Manrique R, et al. A new bone conduction implant: surgical technique and results. Otol Neurotol 2014; 35:216–220. [DOI] [PubMed] [Google Scholar]
- 27. Wimmer W, Gerber N, Guignard J, et al. Topographic bone thickness maps for Bonebridge implantations. Eur Arch Otorhinolaryngol 2015; 272:1651–1658. [DOI] [PubMed] [Google Scholar]
- 28. Wimmer W, Hakim A, Kiefer C, et al. MRI metal artifact reduction sequence for auditory implants: first results with a transcutaneous bone conduction implant. Audiol Neurootol 2019; 24:56–64. [DOI] [PubMed] [Google Scholar]
- 29. Wagener KB, Kollmeier B. Development and evaluation of a German sentence test Part II: optimization of the Oldenburg sentence test. Z Audiol 1999; 38:44–56. [Google Scholar]
- 30. Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol 2004; 43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karger Publishers, Kompis M, Pfiffner F, Krebs M, et al. Factors influencing the decision for Baha in unilateral deafness: the Bern benefit in single-sided deafness questionnaire. Implantable Bone Conduction Hearing Aids 2011; 103–111. [DOI] [PubMed] [Google Scholar]
- 32. Boucek J, Vokral J, Cerny L, et al. Baha implant as a hearing solution for single-sided deafness after retrosigmoid approach for the vestibular schwannoma: audiological results. Eur Arch Otorhinolaryngol 2017; 274:133–141. [DOI] [PubMed] [Google Scholar]
- 33. Martin TP, Lowther R, Cooper H, et al. The bone-anchored hearing aid in the rehabilitation of single-sided deafness: experience with 58 patients. Clin Otolaryngol 2010; 35:284–290. [DOI] [PubMed] [Google Scholar]
- 34. Snapp HA, Hoffer ME, Liu X, et al. Effectiveness in rehabilitation of current wireless CROS technology in experienced bone-anchored implant users. Otol Neurotol 2017; 38:1397–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bis Bibliotheks- und Informations system der Universität Oldenburg, Wagener KC. Factors Influencing Sentence Intelligibility in Noise. 2004; ISBN: 3-8142-0897-8. [Google Scholar]
- 36. Schmerber S, Deguine O, Marx M, et al. Safety and effectiveness of the Bonebridge transcutaneous active direct-drive bone-conduction hearing implant at 1-year device use. Eur Arch Otorhinolaryngol 2017; 274:1835–1851. [DOI] [PubMed] [Google Scholar]
- 37. Bosman AJ, Hol MK, Snik AF, et al. Bone-anchored hearing aids in unilateral inner ear deafness. Acta Otolaryngol 2003; 123:258–260. [DOI] [PubMed] [Google Scholar]
- 38. Vyskocil E, Liepins R, Kaider A, et al. Sound localization in patients with congenital unilateral conductive hearing loss with a transcutaneous bone conduction implant. Otol Neurotol 2017; 38:318–324. [DOI] [PubMed] [Google Scholar]
- 39. Kunst SJ, Leijendeckers JM, Mylanus EA, et al. Bone-anchored hearing aid system application for unilateral congenital conductive hearing impairment: audiometric results. Otol Neurotol 2008; 29:2–7. [DOI] [PubMed] [Google Scholar]
- 40. Priwin C, Jonsson R, Magnusson L, et al. Audiological evaluation and self-assessed hearing problems in subjects with single-sided congenital external ear malformations and associated conductive hearing loss. Int J Audiol 2007; 46:162–171. [DOI] [PubMed] [Google Scholar]
- 41. Fan X, Ping L, Yang T, et al. Comparative effects of unilateral and bilateral bone conduction hearing devices on functional hearing and sound localization abilities in patients with bilateral microtia-atresia. Acta Otolaryngol 2020; 140:575–582. [DOI] [PubMed] [Google Scholar]
- 42. Rahne T, Seiwerth I, Gotze G, et al. Functional results after Bonebridge implantation in adults and children with conductive and mixed hearing loss. Eur Arch Otorhinolaryngol 2015; 272:3263–3269. [DOI] [PubMed] [Google Scholar]
- 43. Schwab B, Wimmer W, Severens JL, et al. Adverse events associated with bone-conduction and middle-ear implants: a systematic review. Eur Arch Oto-Rhino-Laryngol 2020; 277:423–438. [DOI] [PubMed] [Google Scholar]
- 44. Sprinzl GM, Wolf-Magele A. The Bonebridge Bone Conduction Hearing Implant: indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol 2016; 41:131–143. [DOI] [PubMed] [Google Scholar]
- 45. Baumgartner WD, Hamzavi JS, Boheim K, et al. A new transcutaneous bone conduction hearing implant: short-term safety and efficacy in children. Otol Neurotol 2016; 37:713–720. [DOI] [PubMed] [Google Scholar]
- 46. Kulasegarah J, Burgess H, Neeff M, et al. Comparing audiological outcomes between the Bonebridge and bone conduction hearing aid on a hard test band: our experience in children with atresia and microtia. Int J Pediatr Otorhinolaryngol 2018; 107:176–182. [DOI] [PubMed] [Google Scholar]
- 47. Hobson JC, Roper AJ, Andrew R, et al. Complications of bone-anchored hearing aid implantation. J Laryngol Otol 2010; 124:132–136. [DOI] [PubMed] [Google Scholar]
- 48. Eberhard KE, Olsen SO, Miyazaki H, et al. Objective and subjective outcome of a new transcutaneous bone conduction hearing device: half-year follow-up of the first 12 nordic implantations. Otol Neurotol 2016; 37:267–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.