Abstract
Background/Objective
Pegloticase is used for treatment of refractory gout, which has failed maximal medical management, but only 42% of patients respond completely to treatment because of the presumed development of antidrug antibodies, which rapidly clear the pegloticase molecule. Immunomodulatory medications temper antidrug antibody development in other diseases. The aim of this case series was to investigate the utility of adding methotrexate to a pegloticase regimen to increase the response durability in a real-world practice setting.
Methods
In this multicenter, proof-of-concept, observational case series, refractory tophaceous gouty arthropathy patients being started on pegloticase 8 mg every 2 weeks were identified. The patients began oral methotrexate 15 mg/wk and folic acid 1 mg/d, 1 month prior to the initial pegloticase administration, and continued throughout pegloticase treatment. Responders were defined by demonstrating ≥80% of preinfusion serum uric acid (sUA) levels <6.0 mg/dL between months 3 and 6.
Results
Ten sequential patients, aged 35 to 80 years, identified between May 2017 and June 2018, from 3 separate infusion centers were followed for up to 10 months. All patients maintained methotrexate 15 mg/wk without dose adjustments. There were 143 total pegloticase infusions. All 10 patients completed a full course of pegloticase treatment with 100% response and no infusion reactions. No patients stopped pegloticase therapy because of increased sUA, loss of response, or gout flares.
Conclusions
Pretreatment and coadministration of methotrexate with pegloticase resulted in 100% maintenance of pegloticase sUA response with no infusion reactions. These data support the potential use of immunomodulation with methotrexate to improve durability of pegloticase response in the treatment of refractory gout.
Key Words: case series, methotrexate, pegloticase, tophi, uncontrolled gout
Gout affects approximately 4% of the US population. It is the most common form of inflammatory arthritis in men and has been associated with disability and decreased quality of life.1–3 Gout is caused by elevated sUA levels, which leads to precipitation of monosodium urate crystals from the blood into joints and other tissues at sUA concentrations greater than 6.8 mg/dL.4 The frequency of gout is increasing worldwide, with prevalence estimates as high as 7% in older men.5–7 It is estimated that approximately 200,000 persons in the United States experience chronic refractory gout,3 characterized by ongoing symptoms of active disease and failure to maintain sUA less than 6.0 mg/dL despite maximal medical management with xanthine oxidase inhibitors (i.e., allopurinol and febuxostat) and uricosuric agents (i.e., probenecid).8–11 These patients often have significant, disabling urate deposits known as tophi in their skin, soft tissues, and joints. Hyperuricemia has also been associated with metabolic syndrome and hypertension,12,13 suggesting its role in chronic systemic inflammation.
Pegloticase is a recombinant modified mammalian urate oxidase (uricase) produced by a genetically modified strain of Escherichia coli and conjugated to monomethoxypoly(ethylene glycol), which metabolizes relatively insoluble urate to highly soluble allantoin.14 It is used in the treatment of refractory gout that has failed maximal medical management. Pegloticase is dramatically efficacious in lowering sUA, reducing the size of tophi, and resolving clinical signs and symptoms of gout.8,15–18 However, 2 randomized controlled trials (RCTs) have shown a complete responder rate of only 42% when defined as the ability to maintain sUA levels of less than 6.0 mg/dL for ≥80% of the time during months 3 and 6 of treatment.18 Furthermore, administration of pegloticase was associated with infusion reactions in 26% of patients who received pegloticase versus 5% who received placebo. Anaphylaxis, as defined by the National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network criteria, occurred in 5% of patients on pegloticase but 0% on placebo.19
A relationship between the loss of urate-lowering efficacy (indicated by a rapid rise in sUA) and high-titer antibody formation was initially identified in a post hoc analysis of 2 studies.15,18 Subjects with high antipegloticase antibody titers (>1:2560) experienced a significant loss of pegloticase activity that was attributed to more rapid clearance of drug in the presence of these antibodies. Sixty-nine (41%) of 169 patients receiving pegloticase developed high titer antipegloticase antibodies and subsequently lost response to the drug.20 In a separate analysis, only 1 of 52 subjects with high antibody titers maintained a response to pegloticase (sUA <6.0 mg/dL).18 In addition, 60% of subjects with high titers developed infusion reactions.18,19 Antipegloticase antibodies were largely directed to the polyethylene glycol portion of the molecule and increased the pharmacokinetic clearance of pegloticase, resulting in a reduction of sUA lowering activity.20
As is routinely utilized in the treatment of other rheumatologic diseases with biologic medications, coadministration of immunomodulatory medications, such as methotrexate, could potentially temper the development of antidrug antibodies (as defined by maintenance of sUA response) in patients treated with pegloticase for refractory gouty arthropathy.21 The current case series evaluated patients in a real-world practice setting in order to determine the utility of adding methotrexate to a pegloticase regimen to increase the durability of response.
METHODS
This proof-of-concept, observational case series was conducted at 3 separate infusion centers in the United States. The case series methodology was reviewed by the Western Institutional Review Board (Puyallup, WA), and an institutional review board exemption determination was granted. Although not required per institutional review board exemption for study inclusion, patient consent prior to treatment was obtained by the treating physician.
Study Population
All adults with refractory, tophaceous, gouty arthropathy (as determined clinically by their treating physician) who were started on treatment with pegloticase were considered for inclusion. To be included, all patients also had to start oral methotrexate (15 mg/wk) and oral folic acid (1 mg/d) 4 weeks prior to the first pegloticase infusion, as recommended and prescribed by their treating physician. No additional inclusion or exclusion criteria were implemented; however, no patients with previous uricase exposure were considered. Patients were identified sequentially from the start of the study period and followed until completion of pegloticase treatment, which was determined by the treating physician as resolution of tophi or pegloticase failure. Pegloticase failure was determined by the treating physician but recommended as drug intolerability or loss of pegloticase response due to monitoring protocol, which suggests discontinuation of pegloticase if 2 consecutive preinfusion sUA concentrations (obtained within 48 hours prior to each infusion) are greater than 6.0 mg/dL.
Study Medications
All patients received methotrexate, folic acid, and pegloticase in an open-label fashion. Patients began oral methotrexate (15 mg/wk) and oral folic acid (1 mg/d). After 4 weeks, pegloticase 8 mg administered intravenously (IV) every 2 weeks was initiated and taken in combination with methotrexate and folic acid for the remainder of the study. Any infusion premedications, which were consistent with standard practices (i.e., corticosteroids and/or antihistamines), were administered per the individual physician's discretion, as well as management of any gout flares that occurred during the treatment course.
Study Procedures
Demographic information (age/sex), medical comorbidities, year of gout diagnosis, previous gout medication treatments, and baseline sUA information were obtained on patients previously identified by their treating physician as appropriate for pegloticase and willing to take methotrexate. As per standard of care and as part of monitoring protocol, sUA was measured every 2 weeks, within 48 hours prior to each subsequent infusion. Additional laboratory monitoring for methotrexate toxicity was performed at the discretion of the treating physician. Based on the preinfusion sUA levels, discontinuation of pegloticase was considered after application of the monitoring protocol, if 2 or more consecutive levels were greater than 6.0 mg/dL. During treatment, any infusion reactions and gout flares were documented by the infusion staff. All deidentified patient information and sUA data, previously documented by the treating physician in the EMR, were collected on case record forms by the investigators.
Study Endpoints
Completion of pegloticase treatment was determined by the treating physician as resolution of tophi or pegloticase failure. Pegloticase failure was determined by the treating physician but recommended as drug intolerability or loss of pegloticase response due to monitoring protocol as previously described. Patients were responders if able to maintain an sUA of less than 6.0 mg/dL at least 80% of the time between months 3 and 6 of pegloticase treatment. The primary endpoint was a comparison of the response rates in patients coadministered methotrexate versus published response rates from the 2 RCTs (known to be 42%) in those on pegloticase monotherapy.18 The secondary endpoint was to evaluate the number of infusion reactions versus published rates from the 2 RCTs (known to be 26% prior to application of monitoring protocol).18
RESULTS
A total of 10 sequential patients ranging in age from 35 to 80 years were identified, from 3 separate infusion centers (Table), who started methotrexate, folic acid, and pegloticase. All 10 patients were pretreated with 125 mg of IV methylprednisolone and 25 to 50 mg of diphenhydramine (doses varied per provider) 30 minutes prior to the infusion. All 10 patients completed the 4-week methotrexate and folic acid run-in period prior to the first dose of pegloticase without issue. A sum total of 143 pegloticase infusions were performed within the observation period. All 10 patients received at least 10 pegloticase infusions (5 months), 9 patients at least 12 infusions (6 months), 5 patients at least 16 infusions (8 months), 2 patients at least 18 infusions (9 months), and 1 patient received 19 infusions. All 10 patients completed a full course of pegloticase treatment as determined by tophus resolution. Oral methotrexate 15 mg/wk was continued in all patients while on pegloticase therapy, and no methotrexate dose adjustments were made due to intolerance or toxicity concerns.
TABLE.
Patient Baseline Characteristics
| Patient | Sex | Age, y | Comorbidities | Previous Gout Treatment | Year of Gout Diagnosis | Baseline sUA, mg/dL |
|---|---|---|---|---|---|---|
| 1 | Male | 45 | Epilepsy, depression, anxiety, insomnia, sleep apnea | Allopurinol 600 mg every day and febuxostat | 1991 | 8.3 |
| 2 | Male | 50 | None | Allopurinol | Unknown | 8.2 |
| 3 | Male | 41 | Renal insufficiency | Allopurinol and high-dose febuxostat | 1997 | 5.8 |
| 4 | Female | 80 | Psoriatic arthritis, senile osteoporosis, renal failure, hypertension, hyperlipidemia, heart block with pacemaker, valvular heart disease, parathyroid tumor | Febuxostat 80 mg | Unknown | 7.4 |
| 5 | Female | 67 | Hyperlipidemia, neoplasm right foot, palpitations | None | 2017 | 7.8 |
| 6 | Male | 35 | Insomnia | None | >10 years | 12.1 |
| 7 | Male | 50 | Hypertension, type 2 diabetes mellitus | Allopurinol | 1993 | 10.0 |
| 8 | Male | 51 | None | Allopurinol | 2003 | 9.1 |
| 9 | Male | 44 | Type 2 diabetes mellitus, depression, anxiety, osteoarthritis, hypertension, asthma | Allopurinol | 1995 | 9.1 |
| 10 | Male | 55 | Hypertension, hyperlipidemia, nontraumatic subarachnoid hemorrhage, osteoarthritis cervical spine | None | Unknown | 8.4 |
Study Outcomes
Individual patient results are displayed in Figure 1, outlining preinfusion sUA over time. In summary, 100% of patients were responders as defined by ≥80% of sUA levels measured during the observation period, between 3 and 6 months of therapy, being maintained at the goal of less than 6.0 mg/dL. There were 2 patients who experienced sUA levels greater than 6.0 mg/dL during treatment (one following the first infusion and one following the second and third infusions), and both returned to the goal of less than 6.0 mg/dL on all subsequent infusions. None of the 10 patients stopped pegloticase therapy due to increased sUA or loss of response, and no infusion reactions occurred. One patient (patient 1) received 10 infusions because of financial restrictions; however, full resolution of tophi was determined by the treating physician, meeting the study criteria for completion. One patient completed a pretreatment knee x-ray showing tophi below the patellar tendon, as well as serial dual-energy computed tomography scans of the lower extremities, showing reductions in the total volume of tophi from 8.52 to 0.22 cm3.
FIGURE 1.
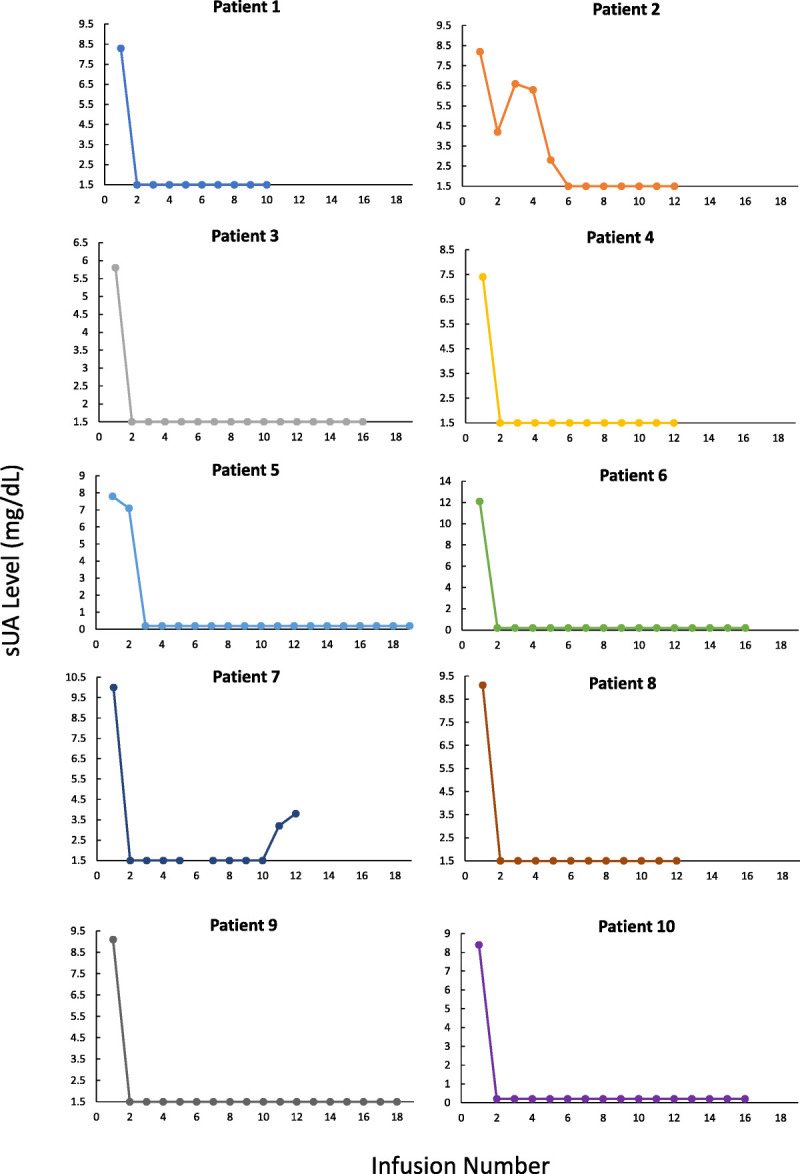
Preinfusion sUA of each patient over time. Note: Y axis minimum set to lower limit of detection for assay (1.5 or 0.2 mg/dL).
Safety
There were no infusion reactions in any of the 143 infusions (Fig. 2), and no other safety concerns were identified. Anaphylaxis was not observed in any patient. Gout flares did occur in 7 of the 10 patients, primarily following the initial infusion, with less severity/prevalence with subsequent infusions. No patients discontinued treatment because of gout flares or methotrexate toxicity.
FIGURE 2.

Percentage of pegloticase (+ methotrexate) responders and percentage of patients with an infusion reaction (IR) as compared with the results of the RCTs of pegloticase every 2 weeks.18
DISCUSSION
Pegloticase was approved by the US Food and Drug Administration for treatment of refractory gouty arthropathy on September 15, 2010. For nearly a decade, the medication has shown efficacy in treating the most severely affected uncontrolled gout patients. Although the treatment response is dramatic in some patients, approximately half of the patients treated with pegloticase will experience a loss of response, manifested by elevated sUA levels.
Immunogenicity, leading to the formation of antidrug antibodies, has been highly correlated with elevated sUA levels in patients receiving pegloticase treatment and is presumed to be the cause of treatment failures. Antidrug antibodies are also associated with infusion reactions in those patients who continued to receive pegloticase.20
As is the case with other biologic medications (used in other disease states), which are known to generate antidrug antibodies,22 the concomitant use of immunomodulatory medications, such as methotrexate, reduces the formation of antidrug antibodies23 and lowers the risk of medication therapeutic failure, adverse effects, and infusion reactions.24 Methotrexate has also been shown to cause a dose-dependent blunting of the response to influenza vaccination.25 Although methotrexate has multiple known toxicity risks, including manifestations such as nausea, vomiting, mucositis, elevated liver function tests, acute renal failure, pneumonitis, and pancytopenia, data have shown that it is well tolerated when weekly low doses were used (starting 10–15 mg) for rheumatologic diseases, with some increased clinical efficacy with patients administered 12.5 to 15 mg/wk versus 5 to 10 mg.26,27 Because of the above data and the familiarity of methotrexate among rheumatologists, the authors selected methotrexate 15 mg orally once weekly for coadministration with pegloticase.
This observational, proof-of-concept case series sought to evaluate the ability of methotrexate, when coadministered with pegloticase to improve the durability of response (response rate) versus pegloticase monotherapy. Patients being prescribed pegloticase and methotrexate were identified from 3 separate infusion centers. All patients were treated with oral methotrexate (15 mg/wk) and oral folic acid (1 mg/d) for 4 weeks prior to beginning pegloticase infusions and continued for the duration of the pegloticase treatment. Using this protocol, 10 of 10 uncontrolled refractory gout patients (100%) were responders to pegloticase and were able to complete full courses of pegloticase treatment, with 100% tophus resolution. Improvement was also demonstrated in 1 patient with serial dual-energy computed tomography scans of the lower extremities.
Patients 2 and 7 had differing courses compared with the group. Patient 2 experienced an initial drop in sUA but then had 2 consecutive sUA levels greater than 6.0 mg/dL. As the patient was clinically improving with a decreasing sUA (albeit >6.0 mg/dL), a clinical decision to continue pegloticase treatment was made by the treating physician, and ultimately, the patient was a responder by study definitions. In hindsight, the second sUA value was likely the aberrancy as the physician changed the place of practice (and laboratories) where this was obtained. Patient 7 experienced a complete clinical response. It was determined by the treating physician that the full course of pegloticase treatment needed was 12 doses. During the last 2 infusions, the sUA remained less than 6.0 mg/dL (meeting the study definition of response); however, the levels did trend upward before the medication was discontinued.
It should be noted that patient 3 had a baseline sUA of 5.8 mg/dL, which technically meets the responder criteria of the study endpoint, but was included in the case series. As previously stated, there were no specific inclusion or exclusion criteria related to baseline sUA for this case series. The patient had the presence of functionally limiting tophi despite maximal medically appropriate treatment (both allopurinol and high-dose febuxostat) and met the treating physician's criteria of refractory gout, which supported treatment with pegloticase.
In this case series, all patients tolerated oral methotrexate (15 mg/wk) and oral folic acid (1 mg/d). No dose adjustments were needed. There were no serious adverse events and no infusion reactions in any patient. Gout flares occurred in 7 (70%) of 10 patients.
There were multiple study limitations inherent in this proof-of-concept case series, including the small sample size, lack of comparator group, and open-label design. However, the objective nature of the outcomes (tophus resolution, decrease in sUA, and prevention of infusion reactions), along with the ability to compare to the 2 previously published RCTs, supports the validity of the results. The lack of consistency across sites pertaining to the definition of refractory gout for inclusion in the case series, medication management preinfusion (specifically use of 125 mg IV methylprednisolone vs. 200 mg IV hydrocortisone from the RCTs), and treatment for gout flares appears at first to be a significant barrier for generalizability to larger populations. However, the dramatic response across this heterogeneous group may actually reflect the strength of the results.
In this proof-of-concept case series of 10 sequential patients, pretreatment and coadministration of oral methotrexate (15 mg/wk) and oral folic acid (1 mg/d) with pegloticase (Botson and Peterson protocol) resulted in a 100% maintenance of pegloticase sUA response with no infusion reactions. The response rate in the case series more than doubled the previously published response rate of 42% from the 2 RCTs and significantly improved on the 26% infusion reaction rate seen in these same RCTs. The addition of methotrexate and the presumed prevention of antidrug antibody formation are surmised to contribute to this improvement. Although additional studies would be needed to corroborate these results, these data support the potential use of immunomodulation with methotrexate in treatment of refractory gout with pegloticase.
KEY POINTS
What is already known about this subject?
Refractory gout represents a significant unmet need in rheumatology.
Pegloticase is highly effective at reducing serum uric acid (sUA) but is also highly immunogenic, resulting in loss of efficacy and increased risk of infusion reactions once neutralizing antidrug antibodies develop.
What does this study add?
Our study demonstrates that immunomodulation with methotrexate improves the durability of pegloticase response with a significant reduction in infusion reactions.
How might this impact clinical practice?
Refractory gout patients are now able to complete a full course of pegloticase treatment, leading to disease resolution, with decreased risk for infusion reactions.
These data support a novel option for the treatment of refractory gouty arthropathy.
ACKNOWLEDGMENTS
The authors thank Michele O'Fallon, MD, for contributing patients to the study.
Footnotes
This study was funded entirely by Alaska Rheumatology Alliance, a 501c(6) organization whose purpose is to promote and improve rheumatologic care in Alaska. J.K.B. and J.P. have received honoraria and are on the speaker's bureau for Horizon Therapeutics. They report personal fees from Horizon Therapeutics, outside the submitted work.
J.K.B. and J.P. actively contributed to all aspects acquisition, analysis, and interpretation of data, as well as writing, revising, and approving the final version of the article.
Patient consent was not required as the study was observational with deidentified data collection only. Ethics approval: Western Institutional Review Board—exemption under 45 CFR 46.101(b)(4). All data relevant to the study are included in the article. Data are available upon reasonable request. Request can be made by email to the corresponding author.
REFERENCES
- 1.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2008;67:1310–1316. [DOI] [PubMed] [Google Scholar]
- 2.Sattui SE, Singh JA, Gaffo AL. Comorbidities in patients with crystal diseases and hyperuricemia. Rheum Dis Clin North Am. 2014;40:251–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. [DOI] [PubMed] [Google Scholar]
- 4.Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443–452. [DOI] [PubMed] [Google Scholar]
- 5.Saag KG, Choi H. Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther. 2006;8(suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikuls TR Farrar JT Bilker WB, et al. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis. 2005;64:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Drug Administration . Arthritis Advisory Committee briefing document. Krystexxa (Pegloticase). 2009. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisDrugsAdvisoryCommittee/UCM165714.pdf. Accessed March 23, 2016.
- 9.Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp. 2013;75:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brook RA Forsythe A Smeeding JE, et al. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin. 2010;26:2813–2821. [DOI] [PubMed] [Google Scholar]
- 11.Khanna P Khanna D Storgard C, et al. A world of hurt: failure to achieve treatment goals in patients with gout requires a paradigm shift. Postgrad Med. 2016;128:34–40. [DOI] [PubMed] [Google Scholar]
- 12.Choi HK Ford ES Li C, et al. Prevalence of the metabolic syndrome in patients with gout: the third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57:109–115. [DOI] [PubMed] [Google Scholar]
- 13.Pan A Teng GG Yuan JM, et al. Bidirectional association between self-reported hypertension and gout: the Singapore Chinese Health Study. PLoS One. 2015;10:e0141749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krystexxa (pegloticase) [package insert]. Lake Forest, IL: Horizon Therapeutics; 2020.
- 15.Sundy JS Becker MA Baraf HS, et al. , Pegloticase Phase 2 Study Investigators. Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout: results of a phase II randomized study. Arthritis Rheum. 2008;58:2882–2891. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. Arthritis Advisory Committee . Krystexxa™ (pegloticase) for intravenous infusion BLA No. 125293 2009. Available at: http://tinyurl.com/j293s5h. Accessed March 23, 2016.
- 17.Baraf HS Matsumoto AK Maroli AN, et al. Resolution of gouty tophi after twelve weeks of pegloticase treatment. Arthritis Rheum. 2008;58:3632–3634. [DOI] [PubMed] [Google Scholar]
- 18.Sundy JS Baraf HS Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711–720. [DOI] [PubMed] [Google Scholar]
- 19.Baraf HS Yood RA Ottery FD, et al. Infusion-related reactions with pegloticase, a recombinant uricase for the treatment of chronic gout refractory to conventional therapy. J Clin Rheumatol. 2014;20:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsky PE Calabrese LH Kavanaugh A, et al. Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res Ther. 2014;16:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershfield MS Ganson NJ Kelly SJ, et al. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther. 2014;16:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strand V Balsa A Al-Saleh J, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017;31:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieckaert CL, Nurmohamed MT, Wolbink GJ. Methotrexate reduces immunogenicity in adalimumab treated rheumatoid arthritis patients in a dose dependent manner. Ann Rheum Dis. 2012;71:1914–1915. [DOI] [PubMed] [Google Scholar]
- 24.O'Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20:1–6. [DOI] [PubMed] [Google Scholar]
- 25.Park JK Lee YJ Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visser K Katchamart W Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E initiative. Ann Rheum Dis. 2009;68:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furst DE Koehnke R Burmeister LF, et al. Increasing methotrexate effect with increasing dose in the treatment of resistant rheumatoid arthritis. J Rheumatol. 1989;16:313–320. [PubMed] [Google Scholar]


