Objective:
To assess the effects of a 3-week community-based exercise program on 30-day postoperative complications in high-risk patients scheduled for elective colorectal resection for (pre)malignancy.
Summary Background Data:
Patients with a low preoperative aerobic fitness undergoing colorectal surgery have an increased risk of postoperative complications. It remains, however, to be demonstrated whether prehabilitation in these patients reduces postoperative complications.
Methods:
This 2-center, prospective, single-blinded randomized clinical trial was carried out in 2 large teaching hospitals in the Netherlands. Patients (≥60 years) with colorectal (pre)malignancy scheduled for elective colorectal resection and with a score ≤7 metabolic equivalents on the veterans-specific activity questionnaire were randomly assigned to the prehabilitation group or the usual care group by using block-stratified randomization. An oxygen uptake at the ventilatory anaerobic threshold <11 mL/kg/min at the baseline cardiopulmonary exercise test was the final inclusion criterion. Inclusion was based on a power analysis. Patients in the prehabilitation group participated in a personalized 3-week (3 sessions per week, nine sessions in total) supervised exercise program given in community physical therapy practices before colorectal resection. Patients in the reference group received usual care. The primary outcome was the number of patients with one or more complications within 30 days of surgery, graded according to the Clavien-Dindo classification. Data were analyzed on an intention-to-treat basis.
Results:
Between February 2014 and December 2018, 57 patients [30 males and 27 females; mean age 73.6 years (standard deviation 6.1), range 61–88 years] were randomized to either prehabilitation (n = 28) or usual care (n = 29). The rate of postoperative complications was lower in the prehabilitation group (n = 12, 42.9%) than in the usual care group (n = 21, 72.4%, relative risk 0.59, 95% confidence interval 0.37–0.96, P = 0.024).
Conclusions:
Exercise prehabilitation reduced postoperative complications in high-risk patients scheduled to undergo elective colon resection for (pre)malignancy. Prehabilitation should be considered as usual care in high-risk patients scheduled for elective colon, and probably also rectal, surgery.
Keywords: aerobic fitness, colorectal surgery, exercise training, morbidity, physical fitness, physical therapy, postoperative complications, prehabilitation, ventilatory anaerobic threshold
Colorectal cancer is the third most common cancer worldwide, with an estimated 1.8 million new cases diagnosed and 881,000 deaths associated with the disease in 2018.1 Surgical resection of the tumor remains the cornerstone of curative treatment.2 However, approximately one third of patients who undergo colorectal resection experience postoperative complications,3,4 which can delay recovery, prolong hospitalization, cause unplanned hospital readmission and chronic illness, and severely impair short- and long-term physical functioning and health-related quality of life.5 Reducing complications would considerably reduce the patient burden and costs.3 More than 65% of patients with colorectal cancer are older than 65 years.6 In the elderly, the physiological function and reserves of multiple organ systems gradually decline, which affects their tolerance to surgery,7,8 with patients with a low preoperative aerobic fitness [oxygen uptake (VO2) at the ventilatory anaerobic threshold (VAT) <11 mL/kg/min] being particularly at increased risk of postoperative complications.9,10 These high-risk, less physically fit patients might therefore benefit from preoperative exercise training (prehabilitation) to optimize their aerobic fitness to reduce their risk of morbidity and to facilitate a prompt recovery of physical functioning.11,12
To date, studies have provided inconclusive evidence that prehabilitation reduces postoperative complications, as most studies tended to be underpowered, heterogeneous, and biased towards patients with a low risk of postoperative complications.5,12–15 A recent systematic review of Thomas and colleagues12 found that prehabilitation before major intra-abdominal cancer surgery16,17 improved postoperative outcomes. They concluded that future prehabilitation research should focus more on the adequate selection of high-risk surgical patients and provide personalized, and probably multimodal, (partly)supervised prehabilitation at home or in a community-based setting with the objective monitoring of a patient's progress.12 Here, we describe a randomized clinical trial to study the effects of a 3-week community-based exercise program on 30-day postoperative complications in high-risk patients (VO2 at the VAT <11 mL/kg/min) scheduled for elective colorectal resection for (pre)malignancy.
METHODS
Study Design and Participants
This prospective, single-blinded, randomized clinical trial was carried out at 2 large Dutch teaching hospitals (Medisch Spectrum Twente in Enschede and Ziekenhuisgroep Twente in Almelo). Trial methodology and experimental intervention were designed to conform state-of-the-art recommendations (using the Cochrane risk of bias tool and CONTENT scale, respectively), as well as by using a clinical decision rule to select the right (high-risk) patients for prehabilitation18; this way, methodological risk of bias is minimized19 and therapeutic validity is ensured.20 The trial started in February 2014 and inclusion was completed in December 2018. Patients with colorectal cancer or premalignant colorectal lesions (polyps with grade I-III dysplasia that could not be removed endoscopically) scheduled for elective colorectal resection were recruited. Eligible patients were ≥60 years, had a life expectancy >6 months as estimated by the surgeon, had a metabolic equivalent of task (MET) score ≤7 on the veterans-specific activity questionnaire (VSAQ), were willing to perform community-based prehabilitation at a physical therapy practice in the catchment area of both hospitals, and were able to perform a progressive cardiopulmonary exercise test (CPET). The VSAQ was used to preselect those patients with a potentially low preoperative aerobic fitness.21 We obtained written informed consent from all patients who met the inclusion criteria and were willing to participate. For definite inclusion, patients also had to have a low preoperative aerobic fitness (high risk for postoperative complications) at the baseline CPET, defined as a VO2 at the VAT <11 mL/kg/min. Patients in the prehabilitation group and in the usual care group were all treated within an enhanced recovery pathway according to the enhanced recovery after surgery protocol.22 The study was approved by the local medical ethics committee Twente in Enschede and by the institutional review boards of Ziekenhuisgroep Twente, the Netherlands (registration number P13–18), and was registered in the Netherlands Trial Registry (NTR4032). The trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline23 and was conducted according with the ethical standards of the Helsinki Declaration of 1975. A complete overview of the study protocol has been published.24
Randomization
All patients were identified at multidisciplinary oncology meetings and were enrolled at the outpatient clinic by the surgeon via the following procedure: patients with a VSAQ score ≤7 METs were invited to participate in the study and, after providing their informed consent, they were randomly assigned to the prehabilitation group or the usual care group by block-stratified randomization.25 After randomization, all participants performed a CPET to verify study eligibility (VO2 at the VAT <11 mL/kg/min). A research nurse who did not help recruit patients or perform data analyses performed the randomization by using sealed opaque envelopes, based on computer-generated randomization lists. Randomization was stratified by disease and treatment type: (1) patients with colon cancer or premalignancy, (2) patients with rectal cancer who would receive 1 week of neoadjuvant radiotherapy, and (3) patients with rectal cancer who would receive 5 weeks of neoadjuvant chemoradiotherapy. Participants were unaware of the study hypothesis: they knew they were allocated to one of 2 groups to assess the effects of physical fitness on postoperative outcome, but they did not know that 1 program was developed as an intervention and the other as a control. Subsequently, patients in the prehabilitation group were informed about all aspects of the study. No advice about preoperative exercise training was offered to patients in the usual care group to avoid the risk that these patients initiated preoperative exercise interventions themselves, and they were planned for surgery at the earliest convenience (with explicit permission from the medical ethics committee). Participants and care providers were not masked to randomization, because of the type of intervention. Data collection and analysis were performed blinded to the group allocation of the patients.
Intervention
Ideally, the baseline assessment of the patients was performed within a week of their first visit at the outpatient clinic. Patients in the prehabilitation group were reassessed after the preoperative exercise program a few days before the surgical procedure. Patients in the usual care group underwent surgery as soon as possible (normally within 2–3 weeks). Usual care consisted of nutritional counseling and advice on smoking cessation.
Patients in the prehabilitation group participated in a personalized 3-week (3 sessions per week, 9 sessions in total) supervised exercise program before colorectal resection. These exercises were executed and/or supervised by a group of trained physical therapists, under the guidance of HK and PW, in community physical therapy practices in the catchment area of both hospitals. Patients with colon (pre)malignancy participated in the prehabilitation program during the period between the decision to undergo surgery and the actual procedure, whereas patients with rectal cancer receiving neoadjuvant therapy completed the prehabilitation program before radiotherapy (in case of neoadjuvant radiotherapy, 5 × 5 Gy) or in the 12-week period after completing neoadjuvant chemoradiotherapy (in week 10–12). Each 60-minute training session consisted of moderate-to-high intensity interval training on a cycle ergometer (TechnoGym, Bike Med, Gambettola, Italy) to improve aerobic fitness (40 minutes), and resistance training to improve peripheral muscle strength (20 minutes). Detailed information about the intervention can be found in the Supplemental Digital Content 1.
Outcomes
The primary endpoint was the number of patients with one or more complications within 30 days of surgery. Complications were divided into surgical and nonsurgical complications and graded according to the Clavien-Dindo classification.26,27 Intermediate outcome measures were changes in preoperative aerobic fitness (the VO2 at the VAT) in the prehabilitation group, length of hospital stay, and unplanned readmissions within 30 and 90 days after surgery.
At baseline, participants in both groups underwent a progressive CPET (Oxycon Pro, Jaeger, Hoechberg, Germany in both hospitals) on a calibrated electronically braked cycle ergometer (Ergoline, Ergoselect 100, Bitz, Germany at Medisch Spectrum Twente and Lode Excalibur Sport, Lode BV, Groningen, the Netherlands at Ziekenhuisgroep Twente). Throughout the CPET, breath-by-breath measurement of VO2, carbon dioxide production, respiratory flow, and volume parameters, and 12-lead electrocardiography was performed to verify study eligibility, to assess baseline aerobic fitness (eg, the VO2 at the VAT), to check for potential contraindications, and to personalize the interval training program.24 Additional information concerning the interpretation of the CPET data can be found in Supplemental Digital Content 2.
Muscle strength assessment included handgrip strength and quadriceps strength.24 In addition to clinical history and physical examination, the following descriptive tests were also included (see for detailed information our study protocol)24: timed up-and-go test, short nutritional assessment questionnaire, and the Groningen frailty indicator. Only the prehabilitation group performed the second CPET after the 3-week exercise program.
Sample Size Calculation
On the basis of morbidity rates in colorectal patients reported in the Dutch ColoRectal Audit (30%)2 and the literature (21% in nonfrail patients, 40% in prefrail patients, and 58% in frail patients),28 we hypothesized that the complication rate in high-risk patients (VO2 at the VAT <11 mL/kg/min) undergoing colorectal surgery without prehabilitation would be around 50%, and after prehabilitation about 20%. To detect a statistically significant difference between groups, we calculated that 43 patients in each group would be required (α of 0.0492, due to 1 interim analysis, ß of 80%, taking a 10% drop out rate into account). Our interim analysis (date July 11, 2017), with a stopping rule according to O’Brien-Fleming that aimed to test if the study should be stopped due to superiority or futility,29 showed a significantly lower incidence of complications (33% vs 73%; P = 0.0096) in patients in the prehabilitation group (n = 21) than in patients in the usual care group (n = 22). These data were used to recalculate our sample size, with explicit permission from the Medical Ethics Committee. Now, 27 patients in each group were required to detect statistically significant differences between groups (α of 0.0492, ß of 80%, taking a 10% dropout rate into account). See Supplemental Digital Content 3 for more information.
Statistical Analysis
Data (coded on study code) were analyzed with the Statistical Package for the Social Sciences for Windows (version 23.0; IBM, SPSS Inc., Chicago, IL). For continuous variables, independent samples t-tests or Mann-Whitney U tests, as appropriate, were performed to analyze differences between the 2 groups. For categorical variables, this was done with chi-squared tests or Fisher exact tests, as appropriate. Variables were tested for their association with postoperative complications (P < 0.05), using the t-test, Mann Whitney U test, Fisher exact test, or Chi2 test, as appropriate. A repeated measurements analysis (mixed models) was performed to assess changes over time in continuous variables. A multivariable logistic regression analysis was performed to identify independent predictors of a postoperative complication, using a forward stepwise procedure (P in 0.05, P out 0.10). If there was multicollinearity between variables, the variable that produced the best model fit (based on the –2 log-likelihood) was included in the model. A new logistic regression model was made (method: enter) incorporating the selected significant variables, to utilize the maximum number of observations. Receiver operator curve (ROC) analysis was used to assess the independent ability of predictive variables to discriminate between patients with and without a postoperative complication. All tests were performed on both the intention-to-treat population and the perprotocol population. Because of the interim analysis, the final P-value considered to be statistically significant was reduced to <0.0492 (2-sided).
RESULTS
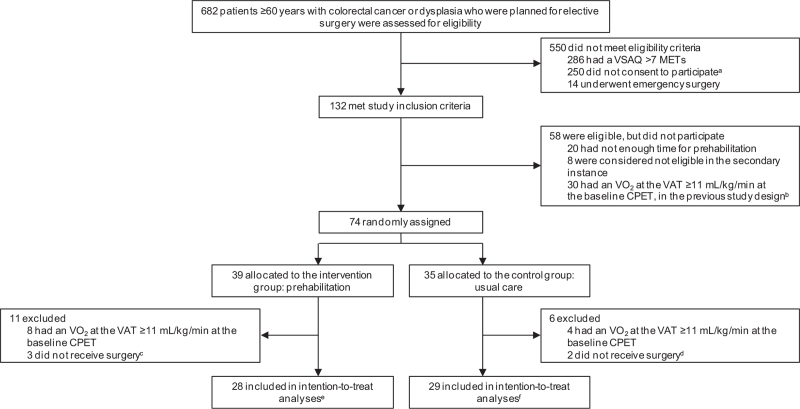
Between February 1, 2014 and the final inclusion on December 31, 2018, 682 potential participants were assessed for study participation. Of these, 132 met the inclusion criteria, of which 74 (56%) gave their consent to participate (Fig. 1). Of these 74 patients, 17 were excluded from the analysis (n = 12 had a VO2 at the VAT >11 mL/kg/min and n = 5 did not undergo surgery). Of the remaining 57 patients {30 males, 27 females; mean age 73.6 years [standard deviation (SD) 6.1], range 61–88 years}, 28 were randomized to the prehabilitation group and 29 to the usual care group. Of the 57 patients, 3 had rectal cancer, of which 2 received neoadjuvant chemoradiation.
FIGURE 1.
Trial profile. CPET = cardiopulmonary exercise test. MET = metabolic equivalent of task. VAT = ventilatory anaerobic threshold. aReasons why patients did not consent to participate: 61 did not feel like it, 19 said they could not cycle, 14 had transportation difficulties, 8 patients believed they were already physically fit for surgery, 7 patients wanted their tumor removed as soon as possible, 5 could not find the time, 90 unknown reasons and/or were not asked to participate, and 46 other reasons. bIn the previous version of the study design, a CPET was performed before randomization took place. cWait-and-see policy in 1 patient with rectal cancer with complete remission after neoadjuvant therapy and 2 patients withdrew from surgery. dWait-and-see policy in 2 patients with rectal cancer with complete remission after neoadjuvant therapy. eOne out of these 28 patients withdrew, because he already knew his date of surgery and was not willing to postpone this date, but was included in the analyses according to the intention-to-treat principle. fTwo out of these 29 patients withdrew, 1 patient because she was overwhelmed by all appointments, and 1 patient randomized to the usual care group wanted to start training herself, but were included in the analyses according to the intention-to-treat principle.
Baseline characteristics of study participants are presented in Table 1 and were largely similar between both groups, except for time between inclusion and surgery (P < 0.001).
TABLE 1.
Baseline characteristics.
| Parameter | Prehabilitation group (n = 28) | Usual care group (n = 29) |
| Age (yrs) | 74 (7) | 73 (6) |
| Sex ratio (M: F) | 16 (57%): 12 (43%) | 14 (48%): 15 (52%) |
| Body mass index (kg/m2) | 29.8 (4.1) | 30.5 (4.9) |
| Smoking∗ | 1 (4%) | 6 (21%) |
| Age-adjusted Charlson comorbidity index† | ||
| 2-3 | 12 (43%) | 8 (28%) |
| 4-5 | 12 (43%) | 14 (48%) |
| 6+ | 4 (14%) | 7 (24%) |
| ASA score | ||
| I | 0 (0%) | 1 (3%) |
| II | 21 (75%) | 24 (83%) |
| III | 7 (25%) | 4 (14%) |
| VO2 at the VAT (mL/kg/min)‡ | 9.6 (1.2) | 9.1 (1.1) |
| VO2peak (mL/kg/min)§ | 14.7 (3.1) | 14.4 (2.8) |
| Haemoglobin level (mmol/L)|| | 7.9 (1.1) | 7.8 (1.2) |
| Timed up-and-go test (s)¶ | 9.7 (2.2) | 9.6 (4.0) |
| MET score on VSAQ | 4.6 (1.4) | 4.3 (1.3) |
| SNAQ score# | 0.4 (1.1) | 0.4 (0.8) |
| GFI score∗∗ | 3 (2) | 3 (3) |
| Surgical procedure | ||
| Right hemicolectomy | 15 (54%) | 12 (41%) |
| Transverse hemicolectomy | 1 (4%) | 1 (3%) |
| Left hemicolectomy | 2 (7%) | 3 (10%) |
| Sigmoid colectomy | 9 (32%) | 9 (31%) |
| APR | 1 (4%) | 0 (0%) |
| LAR | 0 (0%) | 1 (3%) |
| Subtotal colectomy | 0 (0%) | 1 (3%) |
| Other | 0 (0%) | 2 (7%) |
| Type of surgery | ||
| Open | 0 (0%) | 2 (7%) |
| Laparoscopic | 23 (82%) | 21 (72%) |
| Conversion to open | 5 (18%) | 6 (21%) |
| Time between inclusion and surgery (d) | 34.6 (28.8) | 19.0 (10.2) |
Data are number of patients (%), median (IQR) or mean (SD).
Four missing in the prehabilitation group, so in this case n = 24.
Each decade of age over 40 adds 1 point to risk (50–59 years, 1 point; 60–69 years, 2 points; 70–79 years, 3 points), and these points for age are added to the score from the Charlson comorbidity index (eg, 0, 1, 2, 3, etc).41
One missing in the usual care group, so in this case n = 28.
Ten patients (36%) in the prehabilitation group and 11 patients (38%) in the usual care group did not meet the criteria for a valid maximal effort at the baseline CPET, and 1 patient in the usual care group did not perform a CPET, so in this case n = 18 and n = 17, respectively.
Two missing in the prehabilitation group, so in this case n = 26. Hemoglobin level was assessed within three months before surgery.
One missing in the prehabilitation group and 7 missing in the usual care group, so in this case n = 27 en n = 22, respectively.
One missing in the prehabilitation group and 3 missing in the usual care group, so in this case n = 27 en n = 26, respectively.
Two missing in the prehabilitation group and 4 missing in the usual care group, so in this case n = 26 en n = 25, respectively.
APR indicates abdominal perineal resection; ASA, American Society of Anesthesiologists; GFI, Groningen frailty indicator; LAR, low anterior resection; MET, metabolic equivalent of task; N.a., not applicable; OR, odds ratio; SNAQ, short nutritional assessment questionnaire; VAT, ventilatory anaerobic threshold; VO2, oxygen uptake; VO2peak, oxygen uptake at peak exercise; VSAQ, veterans-specific activity questionnaire.
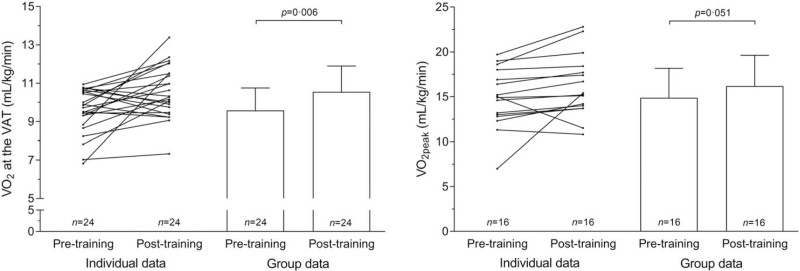
Median time between the first visit at the outpatient clinic and the baseline CPET was 4 days (interquartile range (IQR) 3–6). Mean time between the second CPET and surgery for patients in the prehabilitation group was 2.4 days (SD 1.6). In the prehabilitation group, VO2 at the VAT had improved by 10.1% [+0.97 mL/kg/min (n = 24), 95% confidence interval (CI) 0.3–1.6; P = 0.006] and VO2peak had improved by 8.8% [+1.3 mL/kg/min (n = 16), 95% CI –0.006 to 2.6; P = 0.051] after the 3-week program; see Figure 2. Ten (36%) patients showed no or minimal improvement (<+0.5 mL/kg/min VO2 at the VAT) at the second CPET. Quadriceps strength had increased on average by 2.1% [+6.6 Newton (n = 23), 95% CI –21.4 to 8.2; P = 0.37]. None of the patients reported adverse events during the exercise program and none were observed by the physical therapists. Patients attended 8.1 (SD 2.4) of the 9 supervised exercise training sessions (90%).
FIGURE 2.
Pre- and post-training VO2 at the VAT and VO2peak in patients randomized for prehabilitation. n = 24 for VO2 at the VAT, as 4 patients did not perform a post-training CPET. n = 16 for VO2peak, as 4 patients did not perform a post-training CPET and 8 patients did not perform a maximal effort on both CPETs. CPET indicates cardiopulmonary exercise test; VAT, ventilatory anaerobic threshold; VO2, oxygen uptake; VO2peak, oxygen uptake at peak exercise.
The overall complication rate in the full cohort was 58%. One patient randomized to prehabilitation died as a result of complications arising from a leaking anastomosis. However, this patient had withdrawn from the study directly after randomization because his surgery had been planned during the study period and he did not want to postpone surgery. Both patients with rectal cancer who received neoadjuvant chemoradiation and who were randomized to the prehabilitation group had grade 2 complications. The third patient with rectal cancer, who did not receive neoadjuvant therapy, was randomized to the usual care group and also had a postoperative complication (grade 1).
The complication rate in patients in the prehabilitation group (n = 12, 42.9%) was significantly lower than that of patients in the usual care group (n = 21, 72.4%, P = 0.024, Table 2). There were no differences in the type of complications between the 2 groups. Analysis showed that prehabilitation had a protective role against overall postoperative complications (relative risk 0.59, 95% CI 0.37–0.96). No differences in hospital readmission rates were found between the 2 groups (n = 4, 14.3% in the prehabilitation group vs n = 5, 17.2% in the usual care group; P > 0.99).
TABLE 2.
Postoperative Outcomes.
| Parameter | Prehabilitation Group (n = 28)‡ | Usual Care Group (n = 29)‡ | P-value§ | ||||||||||||||
| Clavien-Dindo Classification | Total | 1 | 2 | 3a | 3b | 4a | 4b | 5 | Total | 1 | 2 | 3a | 3b | 4a | 4b | 5 | |
| Number of patients with overall postoperative complication∗ | 12 (43%) | 8 (29%) | 3 (11%) | 1 (4%) | 21 (72%) | 5 (17%) | 12 (41%) | 1 (3%) | 3 (10%) | 0.02 | |||||||
| Comprehensive complication index42 | 17.3 (26.1) | 18.4 (16.5) | 0.24 | ||||||||||||||
| Surgical reintervention | 2 (7%) | 2 (7%) | >0.99 | ||||||||||||||
| Length of stay | 8.4 (7.4) | 9.1 (7.0) | 0.14 | ||||||||||||||
| ICU admission | 4 (14%) | 4 (14%)|| | >0.99 | ||||||||||||||
| Hospital readmission <30 days | 4 (14%) | 5 (17%) | >0.99 | ||||||||||||||
| Hospital readmission <90 d | 6 (21%) | 8 (28%) | 0.59 | ||||||||||||||
| Type of complication | |||||||||||||||||
| Non-surgical | 8 (29%) | 11 (38%) | 0.45 | ||||||||||||||
| Cardiovascular | 3 (11%) | 3 (11%) | 6 (21%) | 5 (17%) | 1 (3%) | ||||||||||||
| Neurological | 1 (4%) | 1 (4%) | 0 (0%) | ||||||||||||||
| Pulmonary | 5 (18%) | 4 (14%) | 1 (4%) | 4 (14%) | 1 (3%) | 3 (10%) | |||||||||||
| Renal | 4 (14%) | 3 (11%) | 1 (4%) | 1 (3%) | 1 (3%) | ||||||||||||
| Thromboembolic | 0 (0%) | 1 (3%) | 1 (3%) | ||||||||||||||
| Other† | 1 (4%) | 1 (4%) | 4 (14%) | 2 (7%) | 2 (7%) | ||||||||||||
| Surgical | 10 (36%) | 16 (55%) | 0.14 | ||||||||||||||
| Anastomotic leakage | 1 (4%) | 1 (4%) | 1 (3%) | 1 (3%) | |||||||||||||
| Intra-abdominal abscess | 1 (4%) | 1 (4%) | 1 (3%) | 1 (3%) | |||||||||||||
| Sepsis | 3 (11%) | 1 (4%) | 2 (7%) | 0 (0%) | |||||||||||||
| Ileus | 4 (14%) | 4 (14%) | 6 (21%) | 1 (3%) | 4 (14%) | 1 (3%) | |||||||||||
| Abdominal wound complication | 3 (11%) | 2 (7%) | 1 (4%) | 6 (21%) | 3 (10%) | 3 (10%) | |||||||||||
| Urological | 2 (7%) | 1 (4%) | 1 (4%) | 3 (10%) | 3 (10%) | ||||||||||||
| Bleeding | 1 (4%) | 1 (4%) | 1 (3%) | 1 (3%) | |||||||||||||
| Iatrogenic intestinal injury | 0 (0%) | 1 (3%) | 1 (3%) | ||||||||||||||
Data are number of patients (%) or mean (SD).
Some patients had multiple postoperative complications; the complication with the highest Clavien-Dindo grade26,27 is given in this row. An overall complication means any complication that occurred.
Delirium, collapse, decubitus.
P-value is given for the difference in overall complication rate between the prehabilitation and usual care group.
One out of these 4 patients was routinely admitted at the ICU postoperatively, not because of a complication.
ICU indicates intensive care unit.
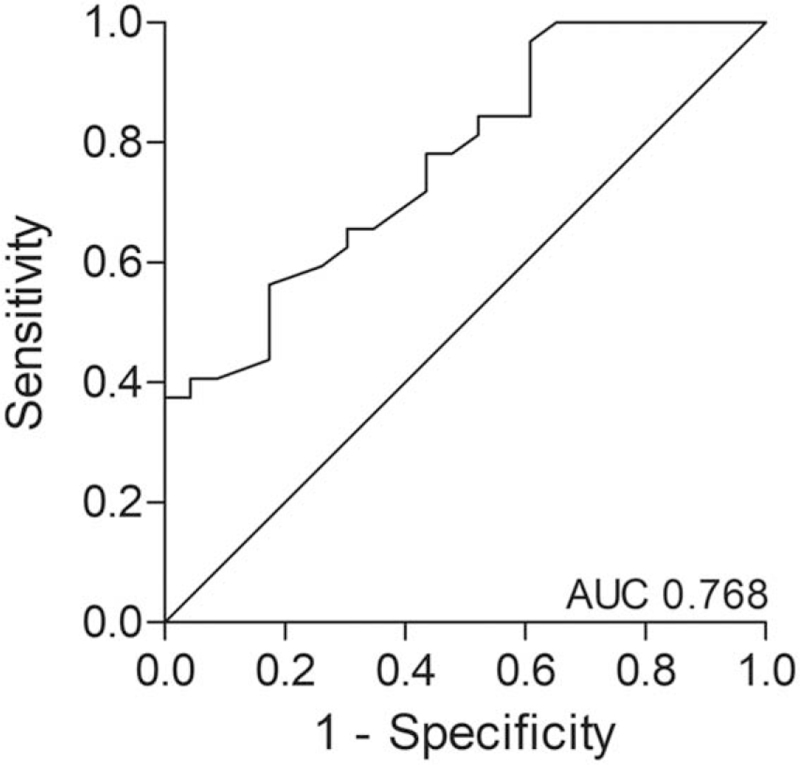
In the univariable analysis of all baseline characteristics (see Table 1), hemoglobin level (P = 0.002), age-adjusted Charlson score (P = 0.024), and SNAQ score (P = 0.084) were associated with postoperative complications. In the final multivariable model including hemoglobin level and prehabilitation, a higher preoperative hemoglobin level [odds ratio (OR) 0.37, 95% CI 0.18–0.75; P = 0.006] and prehabilitation (OR 0.21, 95% CI 0.06–0.79; P = 0.021) were individually associated with a decreased 30-day risk of postoperative complications. Patients with a 1.0 mmol/L higher hemoglobin level were 3 times less likely to have a postoperative complication than patients with a lower hemoglobin level. ROC analysis for predicting patients with a postoperative complication based on the hemoglobin level and prehabilitation gave an AUC of 0.77 (95% CI 0.65–0.89; P = 0.001; Fig. 3).
FIGURE 3.
ROC analysis for predicting patients with a postoperative complication, based on the preoperative hemoglobin level and prehabilitation. ROC indicates receiver operating characteristic.
DISCUSSION
To the best of our knowledge, this is the first state-of-the-art randomized clinical trial to evaluate the effects of a 3-week community-based and personalized prehabilitation program for patients scheduled for surgery for colorectal (pre)malignancy who were considered to be at high risk of postoperative complications, as established with a preoperative clinical decision rule. Within a relatively short period of 3 weeks, prehabilitation improved aerobic fitness by approximately 10% and patients subsequently experienced an almost 50% decrease in the incidence of postoperative complications. The program thus seems to make patients more resistant to the potentially negative consequences of some or all ingredients and perceptions of the perioperative trajectory, so that they experience fewer postoperative complications.
With regard to the overall complication rate in this study (58%), one has to bear in mind that the population under study is a high-risk population, known to demonstrate incidences of about 50% or even more. For instance, comparable studies like that of West et al9 showed an overall morbidity rate after major colorectal surgery of 65% in patients with a low preoperative aerobic fitness (oxygen consumption at the ventilatory anaerobic threshold <11 mL/kg/min). Moreover, Barberan-Garcia et al17 found an overall incidence of complications of 46% in high-risk patients (age >70 years, ASA score III/IV, and Duke activity status index score ≤46) undergoing major abdominal surgery. Although prehabilitation cannot prevent all complications, the impact of a postoperative complication might also be reduced in patients with a higher physical fitness,12,30 as previously indicated in coronary artery bypass graft surgery and pancreatic surgery.31,32
Barberan-Garcia and colleagues17 reported that prehabilitation before elective major abdominal surgery significantly improved preoperative aerobic fitness and significantly reduced the number of high-risk patients with postoperative complications (RR 0.5, 95% CI 0.3–0.8), as compared to usual care. However, they selected high-risk patients based on age, ASA score, and Duke activity status index score and not on formal (cardiopulmonary) exercise testing. Moreover, Carli et al15 recently showed that prehabilitation in (pre)frail patients (based on the Fried frailty index)33 who underwent colorectal cancer resection did not reduce postoperative complications. Patients with a low preoperative aerobic fitness would be expected to benefit the most from prehabilitation.11,12 Therefore, after making a preselection of potentially high-risk patients (eg, with the VSAQ), further selection of high-risk patients on the basis of CPET findings might be the risk screening strategy of choice. In our study, patients with a lower preoperative hemoglobin level were at greater risk of postoperative complications. This suggests that the preoperative hemoglobin level should also be taken into consideration when stratifying patients by risk. Preoptimization of iron deficiency anemia by means of iron supplements in surgical patients undergoing major abdominal surgery increases preoperative hemoglobin concentrations, decreases the need for transfusion,34 and results in a shorter hospital stay.35 Use of erythropoietin is not recommended, because it potentially increases the risk of thrombosis, mortality,36 and tumor growth.37
Our study followed current recommendations regarding the use of a clinical decision rule to minimize the risk of bias and to ensure therapeutic validity.18–20 Nevertheless, our prehabilitation approach could be improved. First, patient inclusion was challenging, and we had a low participation rate (56%) among patients who met (preliminary) inclusion criteria. The main reasons given for non-participation were no inclination to participate, inability to perform cycling exercises, considered self to be physically fit, or desire for surgery as soon as possible (Fig. 1). This suggests that it is important to present preoperative risk stratification and prehabilitation as an integral part of the perioperative care package for high-risk patients, in which patients, their relatives, and their (in)formal caregivers should be adequately informed about the importance of physical activity and physical fitness before and after surgery in relation to surgical outcomes. Moreover, the context of delivering prehabilitation should be well-considered, as patients prefer home-based preoperative physical exercise training, supervised once a week,38 ideally by a dedicated and competent community physical therapist. Second, our prehabilitation program was a unimodal program, focusing on exercise, whereas a recent systematic review by Thomas and colleagues12 reported that multimodal programs including exercise training, nutritional support, psychological support, and the interaction between these components might be most effective and should be considered in further research. However, definitive clinical evidence of the feasibility and cost-effectiveness of multimodal prehabilitation is currently very limited, and should be investigated further.12,39 Third, we did not objectively assess whether and how a patient's physical fitness (aerobic and muscular fitness) improved during prehabilitation. Instead, we adjusted the program each week in a standardized way. When measured objectively and on multiple occasions, training intensity can be adjusted according to the improvement in physical fitness, to maintain an effective training stimulus. Moreover, frequent monitoring of progress is essential to identify nonresponders or noncompliant individuals as soon as possible, because of the short time window available for the intervention before surgery.12 Strous and colleagues recently showed that prehabilitation programs can safely be extended because a prolonged treatment delay did not lead to poorer overall or cancer-free survival in patients with primary colorectal cancer who underwent curative surgical treatment.40 In the present study, 10 of the 28 patients in the prehabilitation group were considered post hoc to be nonresponders (n = 7) or minimal responders (<+0.5 mL/kg/min, n = 3), based on the VO2 at the VAT at the second CPET. Of these 10 patients, 6 had a postoperative complication (60%), whereas this was 43% of the total prehabilitation group and 72% of the usual care group. Ideally, aerobic fitness should improve in all high-risk patients, and achieving this will be a challenge for future research. If we had monitored progress to adjust the intervention as needed, we might have seen a greater effect of prehabilitation.
The study had some other limitations. Although adequately powered, the patient group was small and selective. Moreover, the ROC analysis was performed in a small cohort and might therefore not be very robust. Since only 3 patients with rectal cancer were included in the study, their findings possibly cannot be generalized to this patient group. Lastly, the decision not to include the 5 patients who were randomized but who ultimately did not undergo surgery in the intention-to-treat analysis is debatable.
In future research, attempts should be made to ensure that all eligible high-risk patients are willing and able to participate in prehabilitation programs. Moreover, as mentioned before, prehabilitation should ideally be performed in high-risk patients selected with a preoperative CPET, and must be multimodal, personalized, (partly) supervised, and home- or community-based, with frequent objective monitoring of progress and subsequent consideration of adjustment of training frequency and/or intensity. We suggest using a stepped wedge design, with modern data techniques, to implement and evaluate the cost-effectiveness of prehabilitation in real-life care of high-risk patients undergoing colorectal surgery.
In conclusion, our study showed that community-based exercise prehabilitation reduced the risk of postoperative complications in high-risk patients scheduled for elective colon resection for (pre)malignancy. Probably, this is also true for high-risk patients undergoing rectal resection; however, the small number of patients with rectal cancer included in this study makes it impossible to draw conclusions about the effectiveness of prehabilitation in this specific patient group. Prehabilitation should be considered as usual care in high-risk patients scheduled for elective colon, and probably also rectal, surgery.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank surgical residents from the Medisch Spectrum Twente S. Paas, S. Gray, S. Stokmans, and M. Leemkuil for their contribution to the inclusion of patients in the study.
Footnotes
This study was performed with funding from Amgen.
JMK received research funding from Amgen. Amgen had no role in the study design, acquisition, analysis, and/or interpretation of the data, nor in writing the report and submission. Other authors report no conflicts of interest.
Authors contribution: AEMB, BCB, HK, PW, MMME, ANW, MBB, JvdP, NLUvM, and JMK contributed to the conception and design of the study. AEMB, BCB, HK, PW, FHCdJ, and MJvD contributed to the acquisition of the data. AEMB, BCB, and JvdP contributed to the analysis and interpretation of the data. AEMB wrote the first draft of the manuscript, which was critically revised initially by BCB and JMK. AEMB, BCB, HK, PW, FHCdJ, MMME, ANW, MBB, JvdP, MJvD, NLUvM, and JMK revised the manuscript critically for important intellectual content. All authors read and approved the final version of the manuscript.
Supplemental digital content is available for this article.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Dutch institute for clinical auditing (DICA) Leiden. DICA jaarrapportage 2018: Dutch ColoRectal Audit (DCRA). Available at: https://dica.nl/jaarrapportage-2018/dcra. Accessed April 20, 2020. [Google Scholar]
- 3.Govaert JA, Fiocco M, van Dijk WA, et al. Costs of complications after colorectal cancer surgery in the Netherlands: building the business case for hospitals. Eur J Surg Oncol 2015; 41:1059–1067. [DOI] [PubMed] [Google Scholar]
- 4.Tevis SE, Kennedy GD. Postoperative complications: looking forward to a safer future. Clin Colon Rectal Surg 2016; 29:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: A systematic review and meta-analysis. Surgery 2016; 160:1189–1201. [DOI] [PubMed] [Google Scholar]
- 6. Integraal Kankercentrum Nederland (IKNL). Cambridge, United Kingdom: Cijfers over kanker. February 2, 2019; Available at: https://www.cijfersoverkanker.nl/selecties/dataset_2/img5d2314aa65ca5. Accessed July, 8, 2019. [Google Scholar]
- 7.Santa Mina D, Scheede-Bergdahl C, Gillis C, et al. Optimization of surgical outcomes with prehabilitation. Appl Physiol Nutr Metab 2015; 40:966–969. [DOI] [PubMed] [Google Scholar]
- 8.Hulzebos EH, van Meeteren NL. Making the elderly fit for surgery. Br J Surg 2016; 103:e12–e15. [DOI] [PubMed] [Google Scholar]
- 9.West MA, Asher R, Browning M, et al. Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg 2016; 103:744–752. [DOI] [PubMed] [Google Scholar]
- 10.Moran J, Wilson F, Guinan E, et al. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. Br J Anaesth 2016; 116:177–191. [DOI] [PubMed] [Google Scholar]
- 11.Bongers BC, Punt IM, van Meeteren NL. On "prehabilitation: the emperor's new clothes or a new arena for physical therapists?" Lundberg M, Archer KR, Larsson C, Rydwik E. Phys Ther 2018; 12:127–130. Phys Ther 2019;99:953-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas G, Tahir MR, Bongers BC, et al. Prehabilitation before major intra-abdominal cancer surgery: a systematic review of randomised controlled trials. Eur J Anaesthesiol 2019; 36:933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruns ER, van den Heuvel B, Buskens CJ, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis 2016; 18:O267–O277. [DOI] [PubMed] [Google Scholar]
- 14.Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg 2017; 39:156–162. [DOI] [PubMed] [Google Scholar]
- 15.Carli F, Bousquet-Dion G, Awasthi R, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg 2020; 155:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dronkers JJ, Lamberts H, Reutelingsperger IM, et al. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin Rehabil 2010; 24:614–622. [DOI] [PubMed] [Google Scholar]
- 17.Barberan-Garcia A, Ubre M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018; 267:50–56. [DOI] [PubMed] [Google Scholar]
- 18.McGinn TG, Guyatt GH, Wyer PC, et al. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 2000; 284:79–84. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoogeboom TJ, Oosting E, Vriezekolk JE, et al. Therapeutic validity and effectiveness of preoperative exercise on functional recovery after joint replacement: a systematic review and meta-analysis. PLoS One 2012; 7:e38031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snowden CP, Prentis JM, Anderson HL, et al. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg 2010; 251:535–541. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 2013; 37:259–284. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkel AEM, Bongers BC, van Kamp MS, et al. The effects of prehabilitation versus usual care to reduce postoperative complications in high-risk patients with colorectal cancer or dysplasia scheduled for elective colorectal resection: study protocol of a randomized controlled trial. BMC Gastroenterol 2018; 18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Press WH, Teukolsky S, Vetterling WT, et al. Numerical Recipes in C: the Art of Scientific Computing. Cambridge University Press; 1992:280. [Google Scholar]
- 26.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250:187–196. [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson TN, Wu DS, Pointer L, et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 2013; 206:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979; 35:549–556. [PubMed] [Google Scholar]
- 30.Bongers BC, Dejong CHC, den Dulk M. Enhanced recovery after surgery programmes in older patients undergoing hepatopancreatobiliary surgery: what benefits might prehabilitation have? Eur J Surg Oncol 2021; 47:551–559. [DOI] [PubMed] [Google Scholar]
- 31.Hulzebos EH, Helders PJ, Favie NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006; 296:1851–1857. [DOI] [PubMed] [Google Scholar]
- 32.Van Beijsterveld CAFM, Bongers BC, Den Dulk M, et al. Exploring the relation between preoperative physical functioning and the impact of major complications in patients following pancreatic resection. HPB (Oxford) 2020; 22:716–727. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 34.Munting KE, Klein AA. Optimisation of pre-operative anaemia in patients before elective major surgery - why, who, when and how? Anaesthesia 2019; 74: (Suppl 1): 49–57. [DOI] [PubMed] [Google Scholar]
- 35.Froessler B, Palm P, Weber I, et al. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg 2016; 264:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unger EF, Thompson AM, Blank MJ, et al. Erythropoiesis-stimulating agents--time for a reevaluation. N Engl J Med 2010; 362:189–192. [DOI] [PubMed] [Google Scholar]
- 37.Tankiewicz-Kwedlo A, Hermanowicz J, Surazynski A, et al. Erythropoietin accelerates tumor growth through increase of erythropoietin receptor (EpoR) as well as by the stimulation of angiogenesis in DLD-1 and Ht-29 xenografts. Mol Cell Biochem 2016; 421:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira V, Agnihotram RV, Bergdahl A, et al. Maximizing patient adherence to prehabilitation: what do the patients say? Support Care Cancer 2018; 26:2717–2723. [DOI] [PubMed] [Google Scholar]
- 39.Looijaard SMLM, Slee-Valentijn MS, Otten RHJ, et al. Physical and nutritional prehabilitation in older patients with colorectal carcinoma: a systematic review. J Geriatr Phys Ther 2018; 41:236–244. [DOI] [PubMed] [Google Scholar]
- 40.Strous MTA, Janssen-Heijnen MLG, Vogelaar FJ. Impact of therapeutic delay in colorectal cancer on overall survival and cancer recurrence - is there a safe timeframe for prehabilitation? Eur J Surg Oncol 2019; 45:2295–2301. [DOI] [PubMed] [Google Scholar]
- 41.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 42.Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013; 258:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.