Supplemental digital content is available in the text.
Keywords: anti-inflammatory, cytokines, genetics, genome-wide association studies, inflammation, psychotic disorders, schizophrenia
Abstract
Dysregulation of immunological and inflammatory processes is frequently observed in psychotic disorders. Numerous studies have examined the complex components of innate and adaptive immune processes in schizophrenia and related psychoses. Elevated inflammation in these conditions is related to neurobiological phenotypes and associated with both genetics and environmental exposures. Recent studies have utilized multivariate cytokine approaches to identify what appears to be a subset of individuals with elevated inflammation. The degree to which these findings represent a general process of dysregulated inflammation or whether there are more refined subtypes remains unclear. Brain-imaging studies have attempted to establish the link between peripheral inflammation and gray matter disruption, white matter abnormalities, and neuropsychological phenotypes. However, the interplay between peripheral inflammation and neuroinflammation, as well as the consequences of this interplay, in the context of psychosis remains unclear and requires further investigation. This Perspectives article reviews the following elements of immune dysregulation and its clinical and therapeutic implications: (1) evidence supporting inflammation and immune dysregulation in schizophrenia and related psychoses; (2) recent advances in approaches to characterizing subgroups of patients with elevated inflammation; (3) relationships between peripheral inflammation and brain-imaging indicators of neuroinflammation; (4) convergence of large-scale genetic findings and peripheral inflammation findings; and (5) therapeutic implications: anti-inflammation interventions leveraging genetic findings for drug discovery and repurposing. We offer perspectives and examples of how multiomics technologies may be useful for constructing and studying immunogenetic signatures. Advancing research in this area will facilitate biomarker discovery, disease subtyping, and the development of etiological treatments for immune dysregulation in psychosis.
Peripheral markers of dysregulated inflammation and related immunological pathways are present in psychotic disorders.1,2 This presence, alongside high antipsychotic discontinuation rates3 and a decrease in novel antipsychotic drug development efforts by industry,4 has prompted a surge of studies investigating anti-inflammation and immune modulation interventions. Several drugs with anti-inflammatory properties have demonstrated potential efficacy in psychosis but show variability in treatment response, with accumulating evidence that some patients may benefit more than others.5 One promising avenue toward advancing precision medicine approaches for these interventions is targeting their use in persons known to have higher inflammation. Doing so, however, is not as straightforward as it may initially seem. While many peripheral markers of inflammation are elevated in persons with psychotic disorders, their specificity is unclear, as their responses to particular drugs may be influenced by numerous innate or environmental factors. Recent studies have moved beyond examining individual cytokines to using groups of inflammatory markers to identify persons with higher and lower inflammation subtypes. Inflammation studies to date have not clarified whether a general inflammatory process affects a subgroup of patients with psychosis or whether more-refined subtypes of inflammation are present. Furthermore, how peripheral inflammatory measures are related to neuroinflammatory disease mechanisms remains an area of ongoing investigation. Separately, disease-risk genetic studies of psychotic disorders have identified associations linked to inflammation or immune dysregulation pathways. Yet, how these genetic contributions are connected to objectively measured pro-inflammatory markers in the periphery or central nervous system (CNS) and how they can be leveraged for the targeted use of existing therapies or for drug development remain to be clarified. This Perspectives article reviews the current knowledge of inflammation dysregulation in psychosis, strategies and opportunities for identifying subgroups of patients, and how inflammation-related findings from genome-wide association studies (GWAS) may inform treatment stratification and the identification of novel therapies for schizophrenia and related psychoses.
EVIDENCE SUPPORTING INFLAMMATION AND IMMUNE DYSREGULATION IN SCHIZOPHRENIA AND RELATED PSYCHOSES
Immune dysregulation in persons with schizophrenia and related psychotic disorders has been investigated in related contexts that may arguably require separate consideration when thinking about implications for treatment. These include (1) disease-risk implications and (2) abnormal immune response in those with established illness.
Disease-Risk Implications
An etiologic cytokine theory of psychosis proposes that in predisposed individuals, exposure to certain infections, stress, trauma, or other environmental insults during fetal or early life subsequently interferes with normal brain development, mediated through blood-brain barrier (BBB) disruption (Figure 1).6–9 The resulting imbalance of cytokines or microglial activation has direct effects on brain structure/function, behavior, and the risk for psychopathology later on.10–12 Infectious agents such as the influenza virus, herpes simplex virus–1/2, cytomegalovirus, and toxoplasma gondii are examples of infectious risk factors for psychosis,13–16 which may “prime” the immune system, leading to atypical responses to later environmental exposures.11,17 State versus trait relationships represent related considerations—that is, how inflammation may result from internally or externally mediated factors related to acute illness versus immune systems that may be chronically dysregulated as part of disease. Large disease-risk GWAS have identified multiple loci linked to the development of disease.18 While some of these genetic findings are linked to immune system genes, how these or other associated genes are related to outcomes from the aforementioned environmental exposures remains to be clarified.
Figure 1.
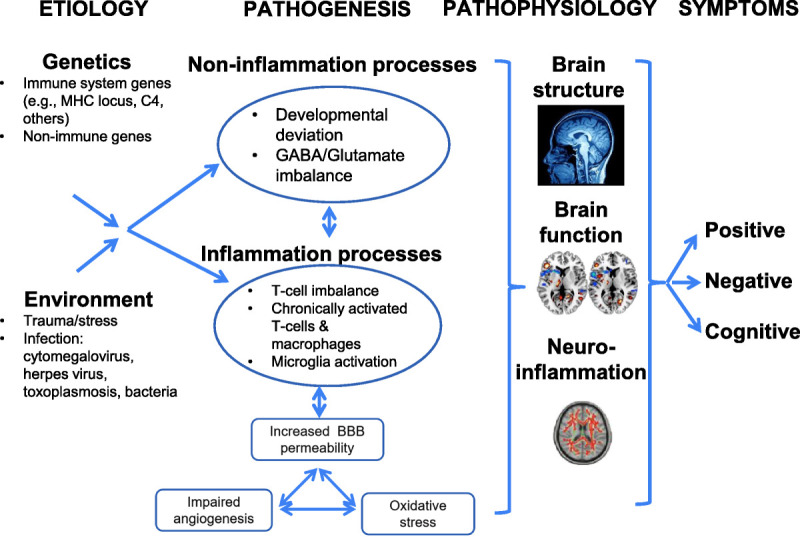
Conceptual model of immune dysregulation in psychosis. Converging evidence suggests that genetic and environmental factors increase peripheral inflammation that adversely affects the brain and clinical outcomes. BBB, blood-brain barrier; MHC, major histocompatibility complex.
Abnormal Immune Response in Persons with Established Disease
Abnormal immune responses in psychosis can be grouped into three categories: (1) chronically activated macrophages/T cells, (2) T helper (Th1 and Th2) cell imbalance, and (3) microglial activation. Chronically activated macrophages and Th1/Th2 cells release inflammatory cytokines that increase leukocyte transmigration across the BBB. These processes activate microglia, which further triggers the release of inflammatory cytokines in the brain. These cytokines bind to specific receptors on neurons19 and adversely affect neurotransmitters, synaptic plasticity, and cortisol concentrations—which subsequently affect mood, cognition, and behavior.20,21 These inflammatory alterations do not act independently and are intimately linked to changes in other pathways observable in plasma proteins, including oxidative stress, impaired angiogenesis, and increased BBB permeability.22 These pathways all have strong evidence for disruption in psychosis and for relationships with inflammation. Disruption in any or all of these pathways can result in neuronal degeneration, white matter abnormalities, and decreased neurogenesis, with proposed consequences including a hypoglutamatergic state and impaired N-methyl-D-aspartate signaling that are hallmark neurotransmitter abnormalities in psychosis.23
Peripheral Measures of Inflammation in Psychosis
Historically, studies have measured and examined how individual cytokines known to be related to inflammatory processes are related to particular diagnostic categories and related phenotypes. Individual relationships of these markers with schizophrenia and bipolar disorders in chronic states, as well as pre/post treatment, have been examined in over 34 cytokine studies,2 26 C-reactive protein (CRP) studies,24 and 15 vascular endothelial growth factor A (VEGFA) studies.25 These analyses conclude that there are reproducible plasma/serum markers of increased inflammation—in particular, interleukin 1 beta (IL-1β), IL-1 receptor antagonist (IL-1RA), serum soluble IL-2 receptor (sIL-2R), IL-4, IL-6, IL-8, IL-10, IL-12, CRP, VEGFA, interferon-gamma (IFNγ), transforming growth factor beta (TGFβ), and tumor necrosis factor alpha (TNFα)—some of which (e.g., IL-1RA, sIL-2R, IL-6, VEGFA, and CRP) decrease after antipsychotic treatment. Some of the commonly investigated cytokine measures are summarized in Table 1. When interpreting the results of individual studies, it is important to consider the extent to which other factors affecting peripheral cytokines were accounted for in specific analyses. Age, sex, ancestry, smoking, body mass index, childhood trauma, batch effects (of multiplexed assessments), sample-storage time, timing of blood draw, medical comorbidities, current medications, and current illness severity are example variables that should be but are not always included in studies and may be sources of discrepancy.27,28
Table 1.
Commonly Studied Cytokines, Vascular Markers, and Related Measures of Inflammation in Psychosis
| Protein designation | Name | Category | Description and functiona |
|---|---|---|---|
| CRP | C-reactive protein | General marker of inflammation | Acute-phase protein and peripheral marker of acute inflammation Produced in response to pro-inflammatory cytokines (IL-1 and IL-6) and lipopolysaccharides |
| FLT1 | Vascular endothelial growth factor receptor–1 | Vascular | Cell-surface receptor for vascular endothelial growth factors Involved in the development of embryonic vasculature, angiogenesis regulation, cell survival, cell migration, macrophage function, and chemotaxis |
| IFNγ | Interferon gamma | Pro-inflammatory cytokine | Produced by lymphocytes and is a potent activator of macrophages |
| IL-1β | Interleukin 1 beta | Pro-inflammatory cytokine | Induces prostaglandin synthesis, neutrophil influx and activation, T-cell activation and cytokine production, B-cell activation and antibody production, and fibroblast proliferation |
| IL-6 | Interleukin 6 | Pro-inflammatory cytokine | Activation leads to the regulation of the immune response, acute-phase reactions Plays an essential role in differentiating B cells |
| IL-8 | Interleukin 8 | Pro-inflammatory cytokine | Chemotactic factor that attracts neutrophils, basophils, and T cells involved in neutrophil activation It is released from several cell types in response to inflammatory stimuli |
| IL-10 | Interleukin 10 | Anti-inflammatory cytokine | Regulatory cytokine that has profound anti-inflammatory functions, limiting excessive tissue disruption caused by inflammation Limits macrophage and monocyte release of pro-inflammatory cytokines |
| IL-18 | Interleukin 18 | Pro-inflammatory cytokine | Pro-inflammatory cytokine that promotes the production of IFNγ from T and NK cells Enhances cytotoxic activity and proliferation of CD8+ T and NK cells and stimulates the production of IL13 and other cytokines |
| TNFα | Tumor necrosis factor alpha | Pro-inflammatory cytokine | Pro-inflammatory and apoptosis-inducing cytokine secreted by macrophages |
| TNFβ | Tumor necrosis factor beta | Pro-inflammatory cytokine | Pro-inflammatory and cytotoxic cytokine produced by lymphocytes |
| VEGFA | Vascular endothelial growth factor A | Vascular | Growth factor that induces endothelial cell proliferation, promotes cell migration, inhibits apoptosis and induces permeabilization of blood vessels |
| VEGFD | Vascular endothelial growth factor D | Vascular | Growth factor active in angiogenesis, lymphangiogenesis and endothelial cell growth, stimulating their proliferation and migration and also has effects on the permeability of blood vessels |
aAdapted from information accessed at https://www.uniprot.org/26 and reproduced here under Creative Commons Attribution (CC BY 4.0) License, https://creativecommons.org/licenses/by/4.0/.
While cytokine alterations in psychosis have been consistently observed, diagnostic specificity remains to be established. Increased peripheral inflammation has also been characterized across other psychiatric diagnoses.29–31 A common observation is that some pro-inflammatory markers (e.g., IL-6, TNFα, IL-1β, and CRP) appear to be increased across disorders. It is possible, though still uncertain, that cytokine signatures may be dimensional features across psychiatric disorders. A recent systematic review of 43 meta-analyses evaluated the reproducibility and specificity of findings for 44 inflammatory biomarkers across eight psychiatric disorders, including schizophrenia, autism spectrum disorder, major depressive disorder, bipolar disorder, posttraumatic stress disorder, obsessive-compulsive disorder, suicide, and sleeping disorders.27 These authors examined the degree to which case-control differences were observed in the same direction across studies. Their analysis conducted post hoc power calculations for each study and determined that inconstancies were commonly observed because of underpowered studies (e.g., 26% of studies). Across studies that were adequately powered, significant alterations in inflammation-related factors in psychiatric cases (versus controls) showed 84% consistency across disorders and 100% consistency in schizophrenia and bipolar disorder studies. A total of 30 inflammatory markers were found to be associated with at least one disorder, and 11 of these markers had significant alterations across a minimum of three, but not consistently across all, disorders. Cluster analysis across study findings suggested similar patterns of changes in multiple inflammatory markers shared between two disorders with clinical and genetic similarity (e.g., schizophrenia and bipolar disorder vs. posttraumatic stress disorder and sleeping disorders). Thus, dysregulated inflammatory processes exist across disorders with some similarities. Consideration of multiple markers of inflammation and their patterns of association across studies suggests that genetics may influence these relationships, with the implication that studies within illness domains (e.g., psychosis) remain important.
RECENT ADVANCES IN APPROACHES TO CHARACTERIZING SUBGROUPS OF PATIENTS WITH ELEVATED INFLAMMATION
Numerous studies have used inflammation-profiling approaches to examine differences in many peripheral cytokines between psychiatric patients and controls. However, relatively few studies have attempted to identify subgroups within patients to further parse out heterogeneity within those with psychotic disorders. In fact, a question remains whether a “subgroup” of patients actually do have higher inflammation loading as opposed to a general elevation in these measures. A recent meta-analysis of measures of central tendency, distributions, and variability ratios of individual peripheral cytokines or other markers of inflammation in antipsychotic-naive first-episode patients concluded that this represents an overall shift in the unimodal distributions in cases versus controls as opposed to a subgroup.28 Based on a premise that inflammatory processes and immunological pathways involve the collective effects of multiple components, additional recent research highlights the value of considering the multivariate effects of cytokines and related measures of inflammation when testing hypotheses regarding subgroups of patients.
To date, studies of peripheral plasma/serum/blood proteins and transcripts (i.e., mRNA) have examined inflammatory subgrouping approaches to aggregate groups of markers together to characterize different aspects of psychosis. Selected examples across disease course (i.e., high risk, first-episode psychosis, chronic illness) that incorporated methodological approaches to validate or establish the reliability of their findings are reviewed here. The North American Prodrome Longitudinal Study (NAPLS) used such an approach to determine whether 185 plasma measures of inflammation, oxidative stress, hormones, and metabolism distinguished persons who were at high risk for psychosis and eventually converted to a psychosis diagnosis versus those who did not and also controls.32,33 Using a “greedy algorithm” approach to build analyte profiles distinguishing the three groups, they identified an index of 15 analytes that achieved this goal. This metric included eight cytokines (IL-1β, growth hormone, KIT ligand, IL-7, IL-8, resistin, and chemokine ligand 8) along with other measures of BBB and HPA-axis function. The OPTiMiSE study is by far the largest investigation to date of these relationships in patients with first-episode psychosis. These researchers examined 325 patients before and after treatment, and conducted unsupervised clustering to define clinical subtypes.34 They examined clinical subtypes with 38 cytokines or infectious exposure (e.g., cytomegalovirus, herpes simplex virus, and toxoplasmosis) with response to antipsychotic treatment, and found that lower levels of IL-15, higher C-X-C motif chemokine ligand (CXCL) 12, and previous exposure to cytomegalovirus were associated with nonremission in one of their clinically defined patient subgroups. Enrico and colleagues35 utilized machine-learning approaches to classify 160 persons with first-episode psychosis, 70 with chronic psychosis (schizophrenia, bipolar disorder with psychosis, or major depressive disorder with psychotic features), and 132 controls based on 56 transcripts of immune-related genes. Using 33 of the mRNA transcripts and supervised machine learning, they demonstrated an ability to discriminate persons with first-episode psychosis, chronic psychosis, and controls with an accuracy of >80%, and a subgroup of drug-free first-episode patients and controls by >90%. Highlighting the importance of previously mentioned challenges related to confounders (e.g., age, sex, body mass index, and smoking status), the accuracy of their classifications increased when considering these factors in their analyses.
Two recent examples illustrate the potential of subgrouping approaches to categorize higher and lower inflammation status in persons with chronic illness and how these may relate to a range of disease phenotypes (e.g., symptoms, cognition, and imaging). Boerrigter and colleagues36 examined eight inflammatory cytokines in the plasma, and white blood cells (gene expression) of 97 patients with schizophrenia and 87 healthy controls. Using a two-step clustering approach, they identified higher (~48% of patients) and lower inflammation strata defined by gene expression measures of IL-1β, IL-6, IL-8, and IL-18, with higher inflammation more common in patients than controls. Additional findings from this group were consistent, showing ~40% of individuals with psychosis had high inflammation signatures based on similar cytokine clustering and that this clustering was associated with worse verbal fluency.1 Recently, our study team examined 140 patients with chronic psychotic illnesses (schizophrenia spectrum, n = 79; bipolar disorder with psychosis, n = 61) and 60 healthy controls. We examined 15 cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12/IL-23p40, CRP, complement 4A [C4A], TNFα, TNFβ, IFNγ, VEGFA, VEGFC, VEGFD, TGFβ1, and soluble fms-like kinase [a splice variant of the VEGF receptor]) in relation to psychosis and related phenotypes. The specific goals were to compare cytokines between cases and controls, describe relationships between clinical and immune phenotypes, and identify potential subgroups. An exploratory factor analysis was conducted on cytokine measures across patients and controls to identify a shared-factor model while controlling for covariates (e.g., storage days, hemolysis score, age, sex, and ancestry) and determining the effects of other potential confounding factors, such as medications and other concomitant illnesses. Our study identified significantly elevated levels of CRP, TNFα, IL-6, and VEGFA in patients with psychotic disorders compared to healthy controls (adj-p < .05), consistent with prior findings in the field. Our factor analysis resulted in a five-factor model. The primary Factor 1 had significant loading for CRP, IFNγ, IL-1β, IL-8, IL-10, TNFα, and VEGFA, indicating the activation of an inflammatory-vascular cascade in patients compared to controls. Scores on this factor distinguished healthy controls from patients as a whole and from patients with schizophrenia or bipolar disorder with psychosis.37 Cluster analysis of Factor 1 scores yielded a two-cluster solution, defining subgroups with relatively higher (36% of patients) and lower inflammatory cytokine levels. The inflammatory subgroup of probands did not have any enrichment of diagnosis and were matched on age, sex, ancestry, medications (both psychotropic and non-psychotropic medications), and cardiometabolic disorders.
RELATIONSHIPS BETWEEN PERIPHERAL INFLAMMATION AND BRAIN IMAGING INDICATORS OF NEUROINFLAMMATION
Relationships between peripheral and central inflammation remain active areas of investigation. The extent to which environmental exposures and resulting inflammation or immune dysregulation affect the brain, or inflammation originating in the CNS can be linked to cytokine measures in the blood, is a challenge to disentangle. Within the brain, whether the origin is from within the CNS or periphery, our prevailing understanding is that microglial, innate, or adaptive immune response activation mediates an inflammatory response in the brain to upregulate cytokines. Increased BBB permeability due to inherent deficits in brain microvascular endothelial cell dysfunction or inflammation-mediated damage is linked to both peripheral and CNS inflammation.22 Inflammatory processes in the brain may result in astroglial loss that progresses to further neuronal injury, which, if chronic, may affect neuron development and pruning.38 Brain imaging in living persons and postmortem analyses of inflammatory markers have provided the field with objective measures of pathophysiological immune dysregulation in psychotic disorders.
Gray Matter Disruption in Inflammation
Much of our understanding of brain anatomy in high inflammation subgroups come from postmortem studies, whereas these types of investigations represent recent developments in living persons. With respect to postmortem studies, significant contributions to our understanding of the consequences of neuroinflammation have resulted from studies defining and replicating a four-cytokine signature (e.g., IL-6, IL-8, IL-1β, and SERPINA3) used to assign higher or lower inflammation status.39–45 These studies have demonstrated how molecular indicators of neuroinflammation can distinguish cases and controls, and are related to altered expression of nuclear receptors, amino acid metabolites, and astrocyte integrity, and to reduced cortical gray matter volume.
Structural imaging in living patients reveals peripheral inflammation relationships with brain anatomy, but it also provides an opportunity to clarify the complex connections with the brain. In younger persons at high risk for developing psychosis, a summed measure of cytokine levels (e.g., summed IL-2 + IFNγ + TNFα) was negatively associated with prefrontal cortical thickness (i.e., greater cytokine scores are associated with lower thickness), particularly in individuals at risk for, and who eventually developed, psychotic disorders.46 Other findings have demonstrated that increased inflammatory subtypes as determined by four cytokine markers (IL-1β, IL-18, IL-8, and IL-2) were related to decreased Broca’s area volume in schizophrenia.1 Kindler and colleagues41 identified correlations between dorsolateral prefrontal cortex volumes and altered kynurenine (l-tryptophan metabolite) metabolism in high, but not low, inflammatory subgroups also defined by IL-1β, IL-18, IL-8, and IL-2. This is an example in living subjects of how inflammation burden may predispose patients to neurotransmitter dysregulation. Our group recently examined regional gray matter thickness and subcortical volumes based on prior studies proposing links to inflammation.47 We found the following in our study sample: (1) three regions of shared vulnerability within psychosis–high inflammation (defined by factor and cluster analyses of CRP, IFNγ, IL-1β, IL-8, IL-10, TNFα, and VEGFA) and psychosis–low inflammation groups compared to the control–low inflammation group (similar to prior studies, lower volumes in probands in right rostral middle frontal, bilateral medial orbitofrontal, and left inferior temporal cortex); (2) greater thickness and volume for the proband–high inflammation group versus the proband–low inflammation group in several brain regions; and (3) profound thinning observed in the psychosis–low inflammation group compared to the control–low inflammation group. Our findings suggested that the presence of a low-grade inflammatory signature may be associated with “pseudothickening,” whereas the absence of low-grade inflammation is related to the expected thinning observed in psychotic disorders.
As noted across the studies above, there are differences in relationships between pro-inflammatory markers and structural brain volume or thickness measures, with both positive and negative correlations identified. Yet, inflammation associations with worse cognitive performance remain consistent across studies.1,47 These findings suggest that the interactions between peripheral inflammation and brain structure alterations may represent a dynamic process. A possible interpretation for our observations of greater volumes in the hippocampus, amygdala, putamen, and left thalamus in the psychosis–high inflammation group compared to the psychosis–low inflammation group, as well as for the observed thickening in the psychosis–high inflammation group, is that peripheral inflammation is mediating swelling via altered BBB permeability and the resulting vasodilation in structures with closer proximity to the BBB. Longitudinal studies are required to adequately test hypotheses about whether increases in inflammation-mediated volume or thickness precede eventual decreases related to cell death or atrophy—or whether, perhaps, certain combinations of cytokines or related markers of inflammation (e.g., angiogenic markers such as VEGF) mediate vasodilation that may affect imaging findings. While the answers may not be immediately clear, the convergence of different imaging modalities may provide further insights.
White Matter Abnormalities in Relation to Peripheral Inflammation
Neuroinflammation in psychosis is associated with white matter pathology generally characterized by axonal degeneration, myelin breakdown, reduced density of astroglia and oligodendroglia in selected areas, and increased white matter neuronal density.48 Free-water (FW) imaging is an in vivo, diffusion-weighted, magnetic resonance imaging (dMRI) technique that extends diffusion tensor imaging (DTI) to quantify extracellular water that may be increased as a result of neuroinflammation via microglial activation, microvessel permeability, or vasodilatation.49,50 Investigation of white matter measures of fractional anisotropy and diffusivity were recently investigated in 47 patients with schizophrenia and 49 healthy controls in relation to IL-10, a regulatory cytokine that functions to maintain pro-inflammatory and anti-inflammatory balance.51 Elevated IL-10 in plasma was observed in patients and was correlated with lower fractional anisotropy and diffusivity measures in tracts with subcortical connections in patients but not controls, indicating disrupted microstructural white matter integrity in relation to this peripheral measure of inflammation. Disrupted white matter integrity may be related to neuroinflammatory processes linked to cytokine dysregulation and measurable by DTI as well as FW.52,53 A recent, large study by Di Biase and colleagues50 of 199 persons with psychosis and 109 controls underscores the importance of examining both DTI and FW. Their investigations of DTI and FW demonstrated that higher levels of IL-6 and TNFα were associated with higher FW levels in white matter but not with fractional anisotropy, and that these changes were widespread in patients with schizophrenia but not in controls.50 Relationships between multivariate categorizations and these imaging measures have not yet been established.
Convergence of Large-Scale Genetic Findings and Peripheral Inflammation Findings
As sample sizes for analyses increase over time, schizophrenia disease-risk GWAS have gained better power to determine the contributions of many genes across the genome. In 2014, the Psychiatric Genomics Consortium published results for wave 2 of the schizophrenia study, which included 36,989 cases and 113,075 controls.18 Nearly twice that sample size is anticipated for the third wave. In the primary analyses of that study, the major histocompatibility complex (MHC) locus on chromosome 6 was removed due to repetitive sequences. Separate analyses evaluated associations to 7751 polymorphisms across the extended MHC locus (chromosome 6: 25–34 Mb), C4 structural alleles, and human leukocyte antigen polymorphisms to examine haplotype relationships.54 Previous reviews and post hoc analyses of these findings have discussed implications for the immunogenetic architectures of schizophrenia and a possible relationship with autoimmune diseases.55–57
A recent overview of existing findings regarding immune implications from schizophrenia GWAS highlighted 39 schizophrenia immune gene candidates outside of the MHC region. These include 6 individual immune genes overly expressed in human brain tissues and linked to schizophrenia susceptibility,55 28 schizophrenia risk genes highly expressed in B and T lymphocytes,56 and 11 genes with dual roles in the immune and inflammation systems and in schizophrenia susceptibility that encode proteins known to be drug targets.58 Protein-protein interaction and functional enrichment analyses suggested extensive co-expression and functional interactions among the 39 schizophrenia immune gene candidates and overrepresentation of multiple immune and inflammatory pathways/processes.55 The potential genetic mechanisms of immune and inflammation processes in schizophrenia pathogenesis likely involve the coaction and interplay of multiple immune genes that contribute to regulation/dysregulation of innate and adaptive immune system mechanisms in the context of psychosis.
When one considers the totality of findings of peripheral cytokines and results of genetic studies, an intriguing observation is the lack of notable associations of genes encoding or regulating the expression of “usual suspect” peripheral markers repeatedly found to be dysregulated in psychosis, either individually or in combination. The extent to which these peripheral cytokines then represent differing or distinct biological mechanisms and are linked to the sources of inflammatory dysregulation is unclear. Additionally, how these genes and related proteins may be linked to existing drugs or leveraged to drug development efforts is a continued work in progress. To gain insights into the interrelationships between schizophrenia risk genes and commonly dysregulated peripheral measures of inflammation, we generated a functional protein-association network involving previously reported 41 schizophrenia immune gene candidates (39 non-MHC genes + C4A/C4B) and 13 psychosis-relevant inflammatory biomarker candidates (see Supplemental Table 1, http://links.lww.com/HRP/A179, for full lists of genes/biomarkers) using STRING v11 (https://string-db.org) with default parameter settings.59 The functional network (Figure 2A) represents intricate and multilateral co-expression, co-occurrence, and functional interactions within and between peripheral cytokines and immune genes.
Figure 2.
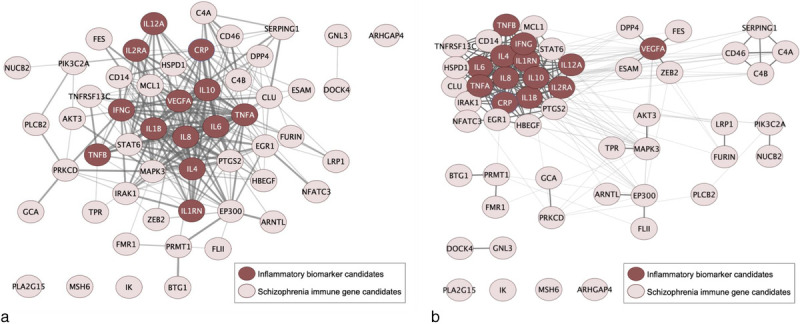
Functional protein-association network of schizophrenia immune gene and inflammatory biomarker candidates. (A) 41 previously identified schizophrenia immune gene candidates (pink nodes), including 39 non-MHC genes and C4A/C4B, and 13 psychosis-relevant inflammatory biomarkers (maroon nodes) were entered in a protein-protein interaction network analysis using STRING software59 under default settings with medium confidence (interaction score ≥ 0.4) for identified interactions. The line thickness denotes the strength of data support for a functional interaction between two molecules. (B) MCL clustering of the function network (panel A) to the inflation parameter of 5.0 was performed with clusterMaker60 and Cytoscape61 based on the interaction score. Functional interactions within a cluster and between clusters are indicated by thick and thin lines, respectively. MCL, Markov cluster; MHC, major histocompatibility complex.
Network analyses provide insights on the interconnectivity between molecules commonly measured in the periphery versus those encoded by genes in disease-risk studies. A lingering question in the field concerns the extent to which inflammation dysregulation in psychosis represents a general inflammatory process or whether distinct pathways of dysregulation have common downstream effects in measurable peripheral markers. Cluster analyses, even in the context of known limitations, may provide some insights for future hypothesis testing. As a conceptual example, we performed an exploratory Markov clustering (MCL) analysis of components of the aforementioned functional association network with clusterMaker60 and Cytoscape.61 This analysis included 41 previously published schizophrenia risk genes and 13 psychosis-relevant peripheral inflammatory markers that have been extensively studied. With an inflation parameter of 5, the MCL cluster (Figure 2B) was able to detect distinct communities of pro-inflammatory cytokines, vascular markers, and complement proteins based on the strengths of functional interactions identified from curated data sources.
To better understand the complex immune and inflammatory responses in psychosis, future efforts should focus on system-wide investigations at multiple levels of molecular biology and on signaling pathways involving gene expression and protein interaction. There have been exciting advances in multivariate marker approaches to characterize inflammation and immune dysregulation in psychosis. However, differences in the markers examined across studies necessitate further clarification to harmonize the markers for examination in future mechanistic and clinical studies. Large-scale comprehensive proteomic studies examining the “usual suspect” cytokines along with all other known immune-related markers are now feasible. Multiomics approaches will improve our understanding of the pathophysiology of inflammation and immune dysregulation. Examples include aligning findings from disease-risk genetic studies, epigenetic signatures affected by environmental exposures, and proteomic characterization of inflammation and immune dysregulation. Machine-learning approaches will facilitate analyses of multiple sources of molecular and phenotype data to better understand the molecular mechanisms of immune system regulation/disturbances and the pathogenesis of CNS dysfunction in schizophrenia and related psychoses.
THERAPEUTIC IMPLICATIONS: ANTI-INFLAMMATION INTERVENTIONS AND LEVERAGING GENETIC FINDINGS FOR DRUG DISCOVERY AND REPURPOSING
Anti-inflammation or Immune-Modulating Interventions
Antipsychotics and antidepressants—in addition to reducing psychotic and depressive symptoms, respectively—may also affect inflammatory cytokine levels (e.g., IL-1β, IL-4, IL-6, IL-10, IL-12, and TNFα) in the blood.2,62 With respect to psychosis, however, it is important to note that anti-inflammatory drug studies to date have been adjunctive to stable doses of antipsychotics in patients and may thus confer an additional benefit to these therapies. Of ~18 anti-inflammatory or immune-modulator agents studied to date, 6 (cyclooxygenase inhibitors, minocycline, neurosteroids, N-acetylcysteine, statins, and estrogens) have replicated beneficial effects, whereas others have produced mixed results.5,63–65 Within individual studies, there are indications that some groups of patients may preferentially respond. A closer look at the “anti-inflammation” mechanisms of the agents studied, as well as others that one might consider, reveals substantial pharmacological diversity, including selective cyclooxygenase inhibition (e.g., aspirin, and celecoxib), broad “antioxidant” (e.g., N-acetylcysteine), broad immunological/anti-inflammatory properties (e.g., minocycline), monoclonal antibodies (e.g., tocilizumab), neurosteroids, and statins. These drugs may have selective effects on specific cytokines (e.g., TNFα, IL-1β, CRP, and IL-6) or broader mechanisms, including links to vascular function and neurobiological pathways. Most published studies to date characterizing patterns of “inflammation” in psychosis have examined only a relatively focused group of cytokines, with broader connections to other pathways investigated separately or not at all.
Of ~70 randomized, controlled trials to date that have examined adjunctive anti-inflammatory drugs on psychosis symptoms or cognition in schizophrenia,5,63 22 publications64–85 have investigated and reported the influence of the interventions on peripheral measures of inflammation. Eight65–72 of these identified changes in these measures corresponding to clinical or cognitive outcome measures of response. Of those eight, only four65,74,81,83 have performed planned or post hoc analyses stratified by higher/lower inflammation defined by CRP or relationships of baseline markers (e.g., CRP, IL-1β, IL-4, IL-6, IL-10, IL-12, IL-17a, INFγ, and TNFα), with higher inflammation being linked to greater benefit in three of these four studies (tocilizumab, aspirin, and pravastatin).65,81,83 With only roughly one-third of anti-inflammatory interventions replicating efficacy to date, it is conceivable that treatment effects could have been stronger if they were targeted to participants with elevated markers of inflammation or disruption in related pathways.
Treatment Implications of Disease-Risk Genetic Findings for Drug Discovery and Repurposing
As previously mentioned, numerous studies have already investigated the clinical impact of drugs with anti-inflammatory or immune-modulating properties for improving psychosis symptoms.5,63 Furthermore, post hoc analyses of the wave 2 Psychiatric Genomics Consortium schizophrenia study examined links between GWAS findings and currently approved drugs and compounds in registered clinical trials.58 Among the 341 genes mapped (based on the 108 distinct significant loci associated with schizophrenia risk), Lencz and Malhotra58 identified 20 genes that code for proteins known to be targets of Food and Drug Administration–approved drugs and an additional 20 genes that have been investigated in clinical trials as treatment targets. Pouget55 further reviewed the immune implications of each druggable schizophrenia risk gene on Lencz and Malhotra’s list and found 11 “druggable” genes involved in immune function. Four of these genes (DPP4, FURIN, MAPK3, and SERPING1) encode proteins targeted by approved drugs, and seven (AKT3, C14, CLU, EGR1, HSPD1, LRP1, and TNFRSF13C) encode proteins that are potential treatment targets for clinical trials.
An additional way to gain insights into potential drugs or drug targets for future study is through functional module detection and tissue-specific enrichment analyses. Of 341 genes from the Psychiatric Genomics Consortium schizophrenia GWAS report, 103 were determined to be druggable based on the Drug Gene Interaction Database (DGIdb).86 We performed an exploratory analysis of functional interactions and module detection among these 103 genes (see Supplemental Table 2, http://links.lww.com/HRP/A180, for the gene list) within the CNS by using the HumanBase online toolkit (https://hb.flatironinstitute.org/). A total of 70 genes were clustered using the published shared-nearest-neighbor-based community-finding method,87 and 42 of them revealed significant membership to one of five clusters as shown in Figure 3. Each cluster, containing 3 to 14 genes, was examined for CNS-specific functional enrichment with genes annotated to Gene Ontology Biological Processes (GO:BP) terms, the top of which are presented in Figure 3 along with the network (full results of functional enrichment are summarized in Supplemental Table 3). Two (cluster C2 and cluster C4) out of five clusters involving 17 schizophrenia-associated druggable genes with known CNS function and statistically overrepresented immune-relevant GO:BP terms stand out with strong immune/inflammation implications. These clusters were represented by nine and three genes, respectively (Supplemental Table 3, http://links.lww.com/HRP/A181). The highest-associated GO:BP terms for cluster C2 were related to the regulation of IL-10, which is involved in regulating anti-inflammatory processes. The GO:BP terms associated with cluster C4 components were regulation of vesicle-mediated transport and immune effector process. In addition to these 12 genes being categorized as “druggable,” 8 (APH1A, ATP2A2, CLCN3, HSPD1, NEK4, PSMA4, TMX2, and FES) were identified as having direct interactions with currently approved or developmental drugs. Findings included some examples such as celcoxib and atorvastatin—which, as previously noted, already have replicated findings suggesting efficacy in schizophrenia and related psychoses. Novel examples included investigational therapies for Alzheimer’s disease (tarenflurbil, begacestat, semagacestat, and avagacestat). As expected, a number of immune-modulating biologics were also included in the findings above. These identified interactions do not necessarily translate to clinical utility but provide elements of both face validity and connections for hypothesis generation for further exploration. These findings may exemplify pathways or provide insights based on known drug mechanisms for future drug development efforts or novel application of existing pharmacological agents.
Figure 3.
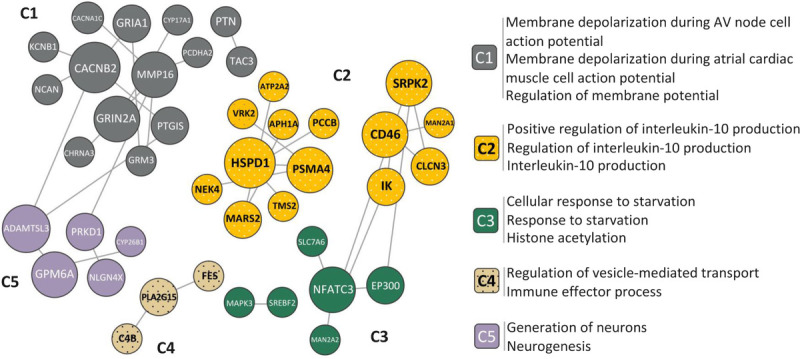
CNS-specific functional modules for druggable schizophrenia risk genes. The network is comprised of 42 out of 103 druggable genes assigned to one of five clusters (C1–C5) with significant membership. The network clustering was performed based on shared k-nearest-neighbors and community-finding algorithm.87 Functional enrichment with genes annotated to Gene Ontology Biological Process (GO:BP) terms was examined across five clusters, with the top overrepresented GO:BP terms within each cluster listed on the right (the mapped gene list and full results of pathway enrichment are available in supplemental materials). C2 and C4 containing a total of 17 genes (with polka dots in figure) and 50 enriched terms had strong immune/inflammation implications associated with central nervous system function.
CONCLUSIONS
Inflammatory and immune processes are dysregulated in schizophrenia and related psychoses. Elevated measures of peripheral and neuroinflammation within patients are associated with brain structure abnormalities and lower performance on cognitive assessments. Studies are now moving beyond univariate analyses of individual inflammatory markers to examine how discrete groups of them may define subgroups of patients with dysregulated pathways. Accumulating data support the existence of at least one subgroup of psychosis represented by alterations in inflammation-related pathways. Studies examining this phenomenon in different ways all converge at a similar proportion, which is ~40% of patients. We still need to clarify the defining components of this dysregulation and to determine whether there is one or more subgroups. This research presents opportunities to inform precision-medicine approaches to improve treatment strategies and identify novel therapies. Brain-imaging studies utilizing structural measures of gray matter as well as white matter DTI or FW provide initial links to CNS pathology that is correlated to peripheral measures of inflammation and cognitive dysfunction. Differences in the types of associations that have been reported (e.g., related increases or decreases in brain structure volume or thickness) indicate that additional studies are needed to clarify these relationships or determine the components of what may be a more dynamic process than originally hypothesized.
Anti-inflammatory interventions for psychosis have revealed promising leads for future study. Opportunities abound to better target these interventions to those with higher indices of inflammation. Information from genetic studies and examining the possible interplay of genetics with peripheral measures of inflammation represent an avenue for additional research. Genes linked to immune dysregulation may converge to common endpoints in the periphery. Groups or networks of these molecules present novel areas for further mechanistic exploration, including new drug development or targeted application of existing therapies to persons most likely to benefit. Upcoming larger-scale genetic studies will undoubtedly provide additional opportunities to clarify these relationships. Additionally, leveraging large studies to converge data spanning peripheral inflammation, illness domains, and detailed clinical, cognitive, neurophysiology, and imaging phenotyping will provide clarity on the complex relationships of immune dysregulation and psychosis pathophysiology.
Supplementary Material
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Footnotes
Supported, in part, by National Institute of Mental Health grant no. K23MH122701 (Dr. Lizano) and a One Mind Bipolar Research Award (Dr. Lizano).
Original manuscript received 12 May 2021; revised manuscript received 6 August 2021, accepted for publication subject to revision 25 August 2021; revised manuscript received 7 September 2021.
Supplemental digital contents are available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Web site (www.harvardreviewofpsychiatry.org).
Contributor Information
Lusi Zhang, Email: zhan6021@umn.edu.
Paulo Lizano, Email: lizanopl@gmail.com.
REFERENCES
- 1.Fillman SG Weickert TW Lenroot RK, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry 2016;21:1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 2016;21:1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman JA Stroup TS McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209–23. [DOI] [PubMed] [Google Scholar]
- 4.Hyman SE. Revolution stalled. Sci Transl Med 2012;4:155 cm11. [DOI] [PubMed] [Google Scholar]
- 5.Jeppesen R Christensen RHB Pedersen EMJ, et al. Efficacy and safety of anti-inflammatory agents in treatment of psychotic disorders—a comprehensive systematic review and meta-analysis. Brain Behav Immun 2020;90:364–80. [DOI] [PubMed] [Google Scholar]
- 6.Barichello T Badawy M Pitcher MR, et al. Exposure to perinatal infections and bipolar disorder: a systematic review. Curr Mol Med 2016;16:106–18. [DOI] [PubMed] [Google Scholar]
- 7.Bloise E Petropoulos S Iqbal M, et al. Acute effects of viral exposure on P-glycoprotein function in the mouse fetal blood-brain barrier. Cell Physiol Biochem 2017;41:1044–50. [DOI] [PubMed] [Google Scholar]
- 8.Simões LR Sangiogo G Tashiro MH, et al. Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats. J Psychiatr Res 2018;100:71–83. [DOI] [PubMed] [Google Scholar]
- 9.Sham PC, O’Callaghan E, Takei N, Murray GK, Hare EH, Murray RM. Schizophrenia following pre-natal exposure to influenza epidemics between 1939 and 1960. Br J Psychiatry 1992;160:461–6. [DOI] [PubMed] [Google Scholar]
- 10.Müller N, Myint A-M, Schwarz MJ. Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr Pharm Des 2011;17:130–6. [DOI] [PubMed] [Google Scholar]
- 11.Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2013;73:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monji A, Kato T, Kanba S. Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci 2009;63:257–65. [DOI] [PubMed] [Google Scholar]
- 13.Müller N, Schwarz MJ. Immune system and schizophrenia. Curr Immunol Rev 2010;6:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torrey EF, Bartko JJ, Lun Z-R, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 2007;33:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgdorf KS Trabjerg BB Pedersen MG, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun 2019;79:152–8. [DOI] [PubMed] [Google Scholar]
- 16.Dalman C Allebeck P Gunnell D, et al. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am J Psychiatry 2008;165:59–65. [DOI] [PubMed] [Google Scholar]
- 17.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 2001;58:1032–7. [DOI] [PubMed] [Google Scholar]
- 18.Schizophrenia Working Group of the Psychiatric Genomics Consortium . Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janeway C, Travers P, Walport M, Schlomchik M. Immunobiology: the immune system in health and disease. 5th ed. New York: Garland, 2001. [Google Scholar]
- 20.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009;65:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pong S, Karmacharya R, Sofman M, Bishop JR, Lizano P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry 2020;6:30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 2014;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes BS Steiner J Bernstein H-G, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry 2016;21:554–64. [DOI] [PubMed] [Google Scholar]
- 25.Misiak B, Stramecki F, Stańczykiewicz B, Frydecka D, Lubeiro A. Vascular endothelial growth factor in patients with schizophrenia: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2018;86:24–9. [DOI] [PubMed] [Google Scholar]
- 26.UniProt . UniProt: the universal protein knowledgebase in 2021. https://www.uniprot.org/. [DOI] [PMC free article] [PubMed]
- 27.Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry 2019;9:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillinger T, Osimo EF, Brugger S, Mondelli V, McCutcheon RA, Howes OD. A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophr Bull 2019;45:1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Passos IC Vasconcelos-Moreno MP Costa LG, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015;2:1002–12. [DOI] [PubMed] [Google Scholar]
- 30.Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun 2020;87:901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 2017;42:254–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins DO Jeffries CD Addington J, et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull 2015;41:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffries CD Perkins DO Fournier M, et al. Networks of blood proteins in the neuroimmunology of schizophrenia. Transl Psychiatry 2018;8:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinuzzi E Barbosa S Daoudlarian D, et al. Stratification and prediction of remission in first-episode psychosis patients: the OPTiMiSE cohort study. Transl Psychiatry 2019;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enrico P Delvecchio G Turtulici N, et al. Classification of psychoses based on immunological features: a machine learning study in a large cohort of first-episode and chronic patients. Schizophr Bull 2021;47:1141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boerrigter D Weickert TW Lenroot R, et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation 2017;14:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clementz BA Sweeney JA Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry 2016;173:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streit WJ, Mrak RE, Griffin WST. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 2004;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fillman SG, Cloonan N, Miller LC, Weickert CS. Markers of inflammation in the prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 2013;18:133. [DOI] [PubMed] [Google Scholar]
- 40.Fillman SG Cloonan N Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 2013;18:206–14. [DOI] [PubMed] [Google Scholar]
- 41.Kindler J Lim CK Weickert CS, et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry 2020;25:2860–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai S-Y, Catts VS, Fullerton JM, Corley SM, Fillman SG, Weickert CS. Nuclear receptors and neuroinflammation in schizophrenia. Mol Neuropsychiatry 2018;3:181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry 2014;4:e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catts VS, Wong J, Fillman SG, Fung SJ, Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: association with neuroinflammation. Aust N Z J Psychiatry 2014;48:722–34. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry 2016;6:e982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannon TD Chung Y He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry 2015;77:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lizano P Lutz O Xu Y, et al. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol Psychiatry 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 2015;161:102–12. [DOI] [PubMed] [Google Scholar]
- 49.Pasternak O, Westin C-F, Dahlben B, Bouix S, Kubicki M. The extent of diffusion MRI markers of neuroinflammation and white matter deterioration in chronic schizophrenia. Schizophr Res 2015;161:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Biase MA Zalesky A Cetin-Karayumak S, et al. Large-scale evidence for an association between peripheral inflammation and white matter free water in schizophrenia and healthy individuals. Schizophr Bull 2021;47:542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu G Zhang W Dai J, et al. Increased peripheral interleukin 10 relate to white matter integrity in schizophrenia. Front Neurosci 2019;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasad KM, Upton CH, Nimgaonkar VL, Keshavan MS. Differential susceptibility of white matter tracts to inflammatory mediators in schizophrenia: an integrated DTI study. Schizophr Res 2015;161:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hegde RR Kelly S Lutz O, et al. Association of white matter microstructure and extracellular free-water with cognitive performance in the early course of schizophrenia. Psychiatry Res Neuroimaging 2020;305:111159. [DOI] [PubMed] [Google Scholar]
- 54.Sekar A Bialas AR de Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016;530:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pouget JG. The emerging immunogenetic architecture of schizophrenia. Schizophr Bull 2018;44:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin J-R, Cai Y, Zhang Q, Zhang W, Nogales-Cadenas R, Zhang ZD. Integrated post-GWAS analysis sheds new light on the disease mechanisms of schizophrenia. Genetics 2016;204:1587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pouget JG Gonçalves VF, et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium . Genome-wide association studies suggest limited immune gene enrichment in schizophrenia compared to 5 autoimmune diseases. Schizophr Bull 2016;42:1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lencz T, Malhotra AK. Targeting the schizophrenia genome: a fast track strategy from GWAS to clinic. Mol Psychiatry 2015;20:820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szklarczyk D Gable AL Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris JH Apeltsin L Newman AM, et al. clusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC Bioinformatics 2011;12:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shannon P Markiel A Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu JJ Wei YB Strawbridge R, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry 2020;25:339–50. [DOI] [PubMed] [Google Scholar]
- 63.Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med 2019;49:2307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller BJ, Dias JK, Lemos HP, Buckley PF. An open-label, pilot trial of adjunctive tocilizumab in schizophrenia. J Clin Psychiatry 2016;77:275–6. [DOI] [PubMed] [Google Scholar]
- 65.Girgis RR Ciarleglio A Choo T, et al. A Randomized, double-blind, placebo-controlled clinical trial of tocilizumab, an interleukin-6 receptor antibody, for residual symptoms in schizophrenia. Neuropsychopharmacology 2018;43:1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Zheng H, Wu R, Kosten TR, Zhang X-Y, Zhao J. The effect of minocycline on amelioration of cognitive deficits and pro-inflammatory cytokines levels in patients with schizophrenia. Schizophr Res 2019;212:92–8. [DOI] [PubMed] [Google Scholar]
- 67.Tang W Wang Y Xu F, et al. Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav Immun 2020;88:529–34. [DOI] [PubMed] [Google Scholar]
- 68.Xu F Fan W Wang W, et al. Effects of omega-3 fatty acids on metabolic syndrome in patients with schizophrenia: a 12-week randomized placebo-controlled trial. Psychopharmacology 2019;236:1273–9. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L Zheng H Wu R, et al. Minocycline adjunctive treatment to risperidone for negative symptoms in schizophrenia: association with pro-inflammatory cytokine levels. Prog Neuropsychopharmacol Biol Psychiatry 2018;85:69–76. [DOI] [PubMed] [Google Scholar]
- 70.Vetlugina TP, Lobacheva OA, Sergeeva SA, Nikitina VB, Nevidimova TI, Semke AV. Adjunctive use of interferon γ inducer for treatment of patients with schizophrenia. Acta Neuropsychiatr 2016;28:149–56. [DOI] [PubMed] [Google Scholar]
- 71.Müller N Ulmschneider M Scheppach C, et al. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci 2004;254:14–22. [DOI] [PubMed] [Google Scholar]
- 72.Chen S-L Lee S-Y Chang Y-H, et al. Inflammation in patients with schizophrenia: the therapeutic benefits of risperidone plus add-on dextromethorphan. J Neuroimmune Pharmacol 2012;7:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chengappa KNR, Brar JS, Gannon JM, Schlicht PJ. Adjunctive use of a standardized extract of withania somnifera (ashwagandha) to treat symptom exacerbation in schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2018;79:17 m11826. [DOI] [PubMed] [Google Scholar]
- 74.Deakin B Suckling J Barnes TRE, et al. The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry 2018;5:885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mossaheb N Schäfer MR Schlögelhofer M, et al. Predictors of longer-term outcome in the Vienna omega-3 high-risk study. Schizophr Res 2018;193:168–72. [DOI] [PubMed] [Google Scholar]
- 76.Bresee CJ, Delrahim K, Maddux RE, Dolnak D, Ahmadpour O, Rapaport MH. The effects of celecoxib augmentation on cytokine levels in schizophrenia. Int J Neuropsychopharmacol 2006;9:343–8. [DOI] [PubMed] [Google Scholar]
- 77.Strzelecki D, Urban-Kowalczyk M, Wysokiński A. Serum levels of interleukin 6 in schizophrenic patients during treatment augmentation with sarcosine (results of the PULSAR study). Hum Psychopharmacol 2018;33:e2652. [DOI] [PubMed] [Google Scholar]
- 78.Smesny S Milleit B Schaefer MR, et al. Effects of omega-3 PUFA on immune markers in adolescent individuals at ultra-high risk for psychosis—results of the randomized controlled Vienna omega-3 study. Schizophr Res 2017;188:110–7. [DOI] [PubMed] [Google Scholar]
- 79.Behdani F Roudbaraki SN Saberi-Karimian M, et al. Assessment of the efficacy of omega-3 fatty acids on metabolic and inflammatory parameters in patients with schizophrenia taking clozapine and sodium valproate. Psychiatry Res 2018;261:243–7. [DOI] [PubMed] [Google Scholar]
- 80.Strzelecki D, Urban-Kowalczyk M, Wysokiński A. Serum levels of TNF-alpha in patients with chronic schizophrenia during treatment augmentation with sarcosine (results of the PULSAR study). Psychiatry Res 2018;268:447–53. [DOI] [PubMed] [Google Scholar]
- 81.Laan W, Grobbee DE, Selten J-P, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2010;71:520–7. [DOI] [PubMed] [Google Scholar]
- 82.Föcking M Dicker P Lopez LM, et al. Differential expression of the inflammation marker IL12p40 in the at-risk mental state for psychosis: a predictor of transition to psychotic disorder? BMC Psychiatry 2016;16:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vincenzi B Stock S Borba CPC, et al. A randomized placebo-controlled pilot study of pravastatin as an adjunctive therapy in schizophrenia patients: effect on inflammation, psychopathology, cognition and lipid metabolism. Schizophr Res 2014;159:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desta M, Tadesse A, Gebre N, Barci BM, Torrey EF, Knable MB. Controlled trial of hydroxychloroquine in schizophrenia. J Clin Psychopharmacol 2002;22:507–10. [DOI] [PubMed] [Google Scholar]
- 85.Liu F Zhang B Xie L, et al. Changes in plasma levels of nitric oxide metabolites and negative symptoms after 16-week minocycline treatment in patients with schizophrenia. Schizophr Res 2018;199:390–4. [DOI] [PubMed] [Google Scholar]
- 86.Freshour SL Kiwala S Cotto KC, et al. Integration of the Drug-Gene Interaction Database (DGIdb 4.0) with open crowdsource efforts. Nucleic Acids Res 2021;49:D1144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krishnan A Zhang R Yao V, et al. Genome-wide prediction and functional characterization of the genetic basis of autism spectrum disorder. Nat Neurosci 2016;19:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


