Abstract
Background.
Neurodegenerative disorders have been reported in elite athletes who participated in contact sports. The incidence of neurodegenerative disease in former professional soccer players has not been well characterized.
Methods.
In a retrospective cohort study, causes of death from death certificates and medications dispensed for the treatment of dementia were obtained from national registries for 7,676 former professional soccer players and compared to 23,028 population controls matched for sex, age and degree of social deprivation.
Results.
Over a median of 18 years, 1,180 (15.4%) former soccer players and 3,807 (16.5%) controls died. All-cause mortality was lower in former players compared to controls up to age 70, and higher thereafter. Former soccer players had lower mortality from ischemic heart disease (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.66–0.97; P=0.02) and from lung cancer (HR, 0.53; 95% CI, 0.40–0.70; P<0.001) compared to controls. Mortality from neurodegenerative disease was higher in former soccer players than controls (HR, 3.45; 95% CI, 2.11–5.62; P<0.001). For neurodegenerative disorders coded as either primary or contributory causes of death, rates were highest for Alzheimer’s disease (HR, 5.07; 95% CI 2.92–8.82; P<0.001) and lowest for Parkinson’s disease (HR, 2.15, 95% CI, 1.17–3.96; P=0.01). Prescriptions for dementia-associated medications were more frequent in former players than controls (odds ratio, 4.90; 95% CI, 3.81–6.31; P<0.001). The incidence of deaths with neurodegenerative disease was not different in goalkeepers compared to outfield players (HR, 0.73; 95% CI, 0.43–1.24, P=0.24) but rates of prescriptions for dementia-associated medications were lower in goalkeepers (HR, 0.41; 95% CI, 0.19–0.89; P=0.02).
Conclusions.
In this retrospective epidemiologic analysis former Scottish professional soccer players had higher rates of death from neurodegenerative diseases and of prescriptions for dementia-associated medications compared to matched controls, and lower rates of death with other common diseases. These data need to be confirmed in prospective matched cohort studies.
Introduction
There has been concern regarding risk of several neurodegenerative diseases such as Alzheimer’s disease, amyotrophic lateral sclerosis, and chronic traumatic encephalopathy (CTE) associated with participation in contact sports1–3. This has been partly the result of growing recognition of the pathology of CTE in former participants of a range of contact sports4–8, including American football4,5 and soccer6,9–13. There are limited data regarding risk of neurodegenerative disease in former professional soccer players.
The neurocognitive consequences of participation in contact sports are uncertain, but overall health benefits of physical activity have been established in prevention of chronic diseases including dementia14, and in reducing mortality15,16. Evidence from participation in elite level sports has also shown lifelong health benefits17 including reduced all-cause mortality18 and reduced risk of cardiovascular disease19 compared to the general population.
Soccer is played in over 200 countries, with in excess of a quarter of a billion active participants20. While casual and amateur play is not comparable to professional soccer, information on health outcomes in former professional players may be valuable to informing management of risks in the sport. We conducted a retrospective cohort study to compare rates of death from neurodegenerative disease in former professional soccer players to matched population controls.
Methods
This study was approved by the University of Glasgow College of Medical, Veterinary and Life Sciences Ethics Committee (Project Number 200160147), with protocol and data governance procedures reviewed and approved by National Health Service Scotland’s Public Benefit and Privacy Panel for Health and Social Care. All health records data were anonymised by an independent information analyst working within the electronic Data Research and Innovation Service of National Health Service Scotland and participant level consent was not required. The protocol for the study, “Football’s InfluencE on Lifelong outcomes and Dementia risk” (FIELD), has been published 21. The analysis and reporting of results from this study are consistent with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidleines22.
Cohort inclusion criteria
We accessed electronic health records to compare data on death certification and medications that are typically dispensed for dementia in a cohort of former professional soccer players. Former professional soccer players were identified from databases of all Scottish professional soccer players23,24, compiled from the archives of the Scottish Soccer Museum and the individual league clubs. Available data included player full name and date of birth and career information including dates of first signing and retirement, number of match appearances and player position. These databases were merged and duplicate entries deleted. Study inclusion was restricted to individuals born prior to 1st January 1977, with study follow-up commencing from age 40. All former soccer players were male.
Probabilistic matching was applied to the full name and date of birth of former soccer players to link them to their unique Community Health Index (CHI). Variables such as date of birth or full name were compared between the former player and Community Health Index datasets and a score was attached to each variable being matched reflecting the level of agreement. The scores for each variable were summated to generate an overall score, which correlated with the likelihood of the two records belonging to the same individual. An automated linkage agent computer programme identified matched population controls as men with the same year of birth and social deprivation score as former soccer players, in a ratio of three persons in the general population to each former player. To match social status and deprivation between cohorts we used the National Health Service (NHS) Information Services Division, which contains the last known postcode of residence for all individuals, from which area socioeconomic deprivation is derived using postcode level data on income, employment, health, education, housing, access to local amenities and crime and expressed as the Scottish Index of Multiple Deprivation (SIMD)25. The SIMD is categorised into quintiles ranging from 1 (most deprived) to 5 (least deprived).
The Community Health Index provides exact matching to information on death certificates and to the national Prescribing Information System. Death certificates include date of death and the primary and contributory causes of death, coded using the International Classification of Diseases ICD9/10. The Prescribing Information System records every prescription dispensed in the community, with complete CHI data since 2009 and with medication coded using the British National Formulary26. The ICD9/10 codes used to identify outcomes as neurodegenerative disease (all neurodegenerative diseases; dementia, not otherwise specified; Alzheimer’s disease; non-Alzheimer’s dementias; motor neuron disease; Parkinson’s disease), and the remaining most common causes of death in the Scottish adult male population using codes for: diseases of the circulatory system (all; ischemic heart disease; stroke/cerebrovascular disease); diseases of the respiratory system; or cancer (all; lung) are shown in Table S1 in the supplementary appendix available with the full text of this article at NEJM.org. As no ICD9/10 codes exist for CTE or its synonym, dementia pugilistica, these diagnoses were not identifiable from death certificate records. The drugs included in the analysis of drugs for dementia are in Section 4.11 of the British National Formulary and are shown in Table S2 in the supplementary appendix. All analyses included data up to Dec. 31, 2016 and the database interrogation was performed on Dec. 10, 2018.
Statistical analysis
We used Cox proportional hazard regression to model time to death and tested the assumption of proportional hazards using Schoenfeld residuals27. Where the assumption of non-proportional hazards did not hold, a time-varying model was used to derive hazard ratios over different periods of follow-up28. Age was used as the time covariate, with follow up from age 40 to the date of censor, either as date of death or end of follow-up (Dec. 31, 2016), whichever occurred first. In a sensitivity analysis, the outcome of death from neurodegenerative disease was subjected to a competing risks regression analysis to ascertain whether the estimated hazard ratio was sensitive to competing risks from deaths due to ischemic heart disease or cancer29. We tested the assumption of proportional sub-hazards by including an interaction term between analysis time and a dummy variable for former soccer players status and testing for a P value less than 0.05. The mortality models were repeated in the former soccer player sub-group using player position (outfield or goalkeeper) as a surrogate of exposure. Because prescribing outcomes were only available from 2009 onwards, a nested case-control design was employed and analysed using conditional logistic regression for the whole cohort (matched data)30 and standard logistic regression for the former soccer player sub-group analysis. All statistical analyses were undertaken using Stata MP v14.131 with statistical significance set at two-sided P<0.05.
Results
Cohort characteristics
A total of 9,670 former professional soccer player records were identified from the player databases, of which 7,676 were successfully matched to their Community Health Index, permitting the identification of 23,028 matched population controls (Table 1). Of the remaining 1,994 records identified from the master datasets of former soccer players, failure to match to their Community Health Index reflected duplicate entries (170) or incomplete or inaccurate demographic information (1,824) (Figure S1). Over a median 18.0 years of follow-up from entry to study at age 40, 1,180 (15.4%) former soccer players and 3,807 (16.5%) matched population controls died, with a mean age at death of 67.9 (standard deviation 13.0) years and 64.7 (14.0) years, respectively.
Table 1:
Cohort demographic information
| Former professional soccer players | Matched population controls | |
|---|---|---|
| Number | 7,676 | 23,028 |
| Deprivation (SIMD quintile) | ||
| 1 = high | 1,206 (15.7%) |
3,617 (15.7%) |
| 2 | 1,371 (17.9%) |
4,112 (17.9%) |
| 3 | 1,449 (18.9%) |
4,349 (18.9%) |
| 4 | 1,613 (21.0%) |
4,839 (21.0%) |
| 5 = low | 2,037 (26.5%) |
6,111 (26.5%) |
| Player position | ||
| Goalkeeper | 598 (7.8%) |
NA |
| Outfield | 6,024 (78.5%) |
NA |
| Unknown | 1,054 (13.7%) |
NA |
SIMD, Scottish Index of Multiple Deprivation.
Primary cause of death from death certificates
All-cause mortality was lower in former soccer players than their matched controls (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.80 to 0.93; P<0.001), however, the proportional hazards assumption was not met, with mortality lower in former soccer players than matched controls up to 70 years of age and greater thereafter (Figure 1). Deaths due to ischemic heart disease (HR, 0.80; 95% CI, 0.66 to 0.97; P=0.02) and lung cancer (HR, 0.53; 95% CI, 0.40 to 0.70; P<0.001) fulfilled the proportional hazards assumption and were lower in former soccer players than matched controls (Table 2).
Figure 1: Pattern of time varying hazard for overall, all-cause mortality for ages 40 through 90 years.
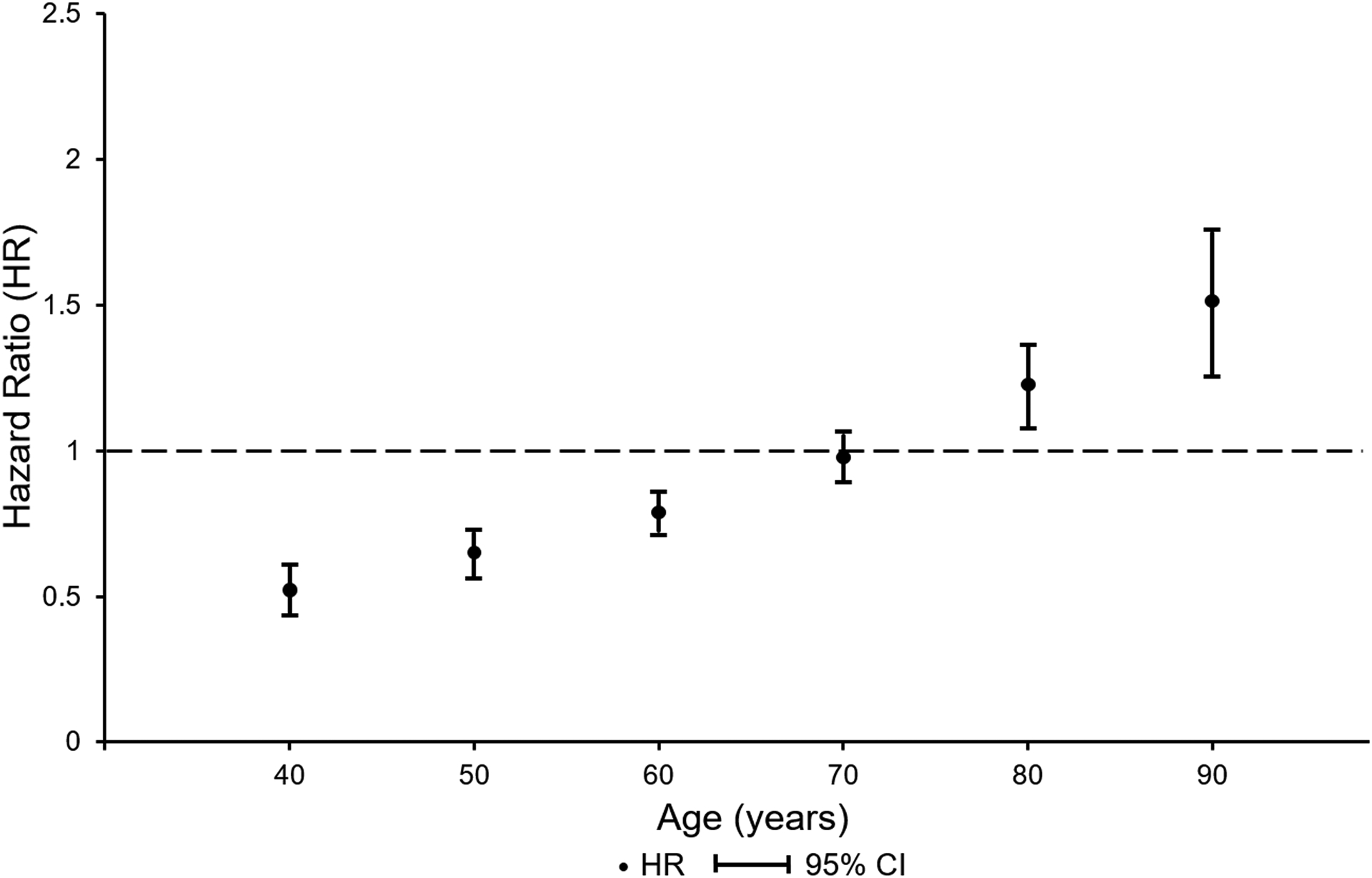
As the assumption of proportional hazards did not hold for overall, all-cause mortality in former professional soccer players versus matched population controls a time dependent analysis was performed. This shows reduced all-cause mortality in former professional soccer players to age 70, increased thereafter. Time varying hazard ratios and 95% CIs were calculated using Stata’s “lincom” command.
Table 2:
Primary cause of death outcomes
| Primary cause of death | FSP n |
MPC n |
Hazard ratio (95% CI) |
P* |
|---|---|---|---|---|
| All causes ** | 1180 | 3807 | 0.87 (0.80, 0.93) |
<0.001 |
| Ischemic heart disease | 173 | 568 | 0.80 (0.66, 0.97) |
0.02 |
| Lung cancer | 74 | 362 | 0.53 (0.40, 0.70) |
<0.001 |
| Adjusted+ | 3.45 (2.11, 5.62) |
<0.001 |
Cox proportional hazard regression;
Overall, all-cause mortality does not fulfil the proportional hazards assumption, showing time dependent variability (see Figure 1);
Adjusted for competing risks of ischemic heart disease and all cancer deaths; FSP, former professional soccer players; MPC, matched population controls.
Mortality with neurodegenerative disease as the primary diagnosis on the death certificate was higher among former soccer players than matched controls (HR 4.10, 2.88 to 5.83; P<0.001) with similar but attenuated risk after adjusting for the competing risks of ischemic heart disease and cancer deaths (adjusted HR, 3.45, 2.11 to 5.62; P<0.001) (Table 2). There was no significant difference between groups in rates of deaths due to stroke/cerebrovascular disease (HR 0.88, 0.78 to 1.00; P=0.06). Deaths due to all cancers combined (HR 0.94, 0.71 to 1.25) and respiratory disease (HR, 0.61, 0.46 to 0.82) failed the proportional hazards assumption.
Primary and contributory causes of death and medication prescribing for dementia
Neurodegenerative disease was recorded as either the primary or contributory cause of death in 222 former soccer players and 228 controls (HR, 3.53; 95% CI, 2.72 to 4.57; P<0.001). Estimates of rates of combined primary and contributory causes of death for neurodegenerative disease in the two cohorts varied according to disease subtype (Table 3) and were highest for Alzheimer’s disease (HR, 5.07; 95% CI, 2.92 to 8.82; P<0.001) and lowest for Parkinson’s disease (HR, 2.15; 95% CI, 1.17 to 3.96; P=0.01). There was no difference in age at death with neurodegenerative disease or its subtypes between former soccer players and their matched controls, other than for non-Alzheimer’s dementia (former soccer players age at death 77.5±7.5 years versus controls 81.3±7.3 years; P=0.005) (Figure S2). Former soccer players were more likely to receive medication for dementia than controls (odds ratio, 4.90; 95% CI 3.81 to 6.31; P<0.001) (Table 4).
Table 3:
Combined primary and contributory cause of death outcomes for neurodegenerative disease subtypes
| FSP n (%) |
MPC n (%) |
Hazard ratio (95% CI) |
P* | |
|---|---|---|---|---|
| All neurodegenerative diseases | 222 (2.89%) |
228 (0.99%) |
3.53 (2.72, 4.57) |
<0.001 |
| Dementia, not otherwise specified | 180 (2.34%) |
178 (0.77%) |
3.87 (2.86, 5.24) |
<0.001 |
| Alzheimer’s disease | 64 (0.83%) |
47 (0.20%) |
5.07 (2.92, 8.82) |
<0.001 |
| Non-Alzheimer’s dementias | 121 (1.58%) |
133 (0.58%) |
3.48 (2.42, 5.00) |
<0.001 |
| Motor neuron disease | 22 (0.29%) |
17 (0.07%) |
4.33 (2.05, 9.15) |
<0.001 |
| Parkinson’s disease | 28 (0.36%) |
44 (0.19%) |
2.15 (1.17, 3.96) |
0.01 |
Cox proportional hazard regression; FSP, former professional soccer players; MPC, matched population controls.
Table 4:
Prescribing for dementia and influence of player position on outcomes
| Mortality | Hazard ratio (95% CI) |
P+ | |
|---|---|---|---|
| Outfield | 180/873 (20.6%) | (0.43, 1.24) | |
| Outfield | 148/873 (17%) | (0.31, 1.12) | |
| Prescribing for dementia | Odds ratio (95% CI) |
P | |
| Matched population controls | 144/20,914 (0.7%) | (3.81, 6.31) | |
| Outfield | 171/5,581 (3.1%) | (0.19, 0.89) | |
Cox proportional hazard regression;
Conditional logistic regression;
Standard logistic regression; Prescribing data available from 2009, thus for 7,058/7,676 (92%) former soccer players, for which position data available for 6,126/7,058 (87%)
Player position and neurodegenerative mortality and prescribing for dementia
There was no difference in the proportions of goalkeepers compared to outfield players coded as primary or contributory causes of death with neurodegenerative disease (HR, 0.73; 95% 0.43 to 1.24; P=0.24) or dying with dementia, not otherwise specified (HR, 0.59; 95% CI 0.31 to 1.12; P=0.10) (Table 4). Dispensed prescriptions for dementia were less common among goalkeepers than outfield players (odds ratio, 0.41; 0.19 to 0.89; P=0.02) (Table 4).
Discussion
Our data show that overall mortality was lower in a cohort of Scottish former professional soccer players compared to age and deprivation matched population controls. This association was age-dependent, with mortality in former soccer players lower until age 70 years, higher thereafter. There were higher rates of mortality with neurodegenerative disease among the former soccer players, a finding that was independent of the competing risks of mortality from ischemic heart disease and cancer, but was attenuated when these risks were incorporated into the analysis. The difference from matched controls in neurodegenerative disease incidence among former professional soccer players was highest for Alzheimer’s disease and lowest for Parkinson’s disease, when considering combined primary and secondary causes of death from death certificates. These observations are consistent with our finding of higher rates of prescribing of medications for dementia in the former player cohort compared to controls. Mortality due to neurodegenerative disease was not different between outfield players and goalkeepers, however, rates of prescribing of medications for dementia were higher among outfield players.
The observation of reduced overall mortality up to 70 years of age in former professional soccer players is comparable to previous studies on elite athletes who participated a range of sports17 including soccer32 and may be consistent with higher levels of physical activity and lower levels of obesity and smoking than the general population. In contrast, deaths from neurodegenerative disease were higher in former soccer players, similar to findings in former National (American) Football League (NFL) players18,33. Neither chronic traumatic encephalopathy nor dementia pugilistica were coded items in ICD9/10 and inquiry for these causes of death was not possible in our study. Studies reporting clinicopathological diagnoses from autopsies in former soccer players report a range of dementia subtypes. In some series, CTE-neuropathologic change has been described as occurring in 75% of former soccer and rugby players, however, it was infrequently the primary pathology recorded as the cause of dementia13. In the absence of specific diagnostic coding for CTE we are unable to make conclusions from our study regarding its presence as either primary diagnosis or co-morbid disease, but might anticipate it would have been coded among other disorders, if diagnosed.
Death certificates are subject to errors in reporting. We sought to corroborate our findings on mortality from neurodegenerative disease and dementia by investigating rates of prescribing of medications that are typically used for Alzheimer’s disease and related dementias. This analysis supported observations from death certificates, with higher prescribing of drugs for dementia in former soccer players than matched controls.
A strength of this study is the use of a comprehensive cohort of former Scottish professional soccer players. We assessed their outcomes in comparison to matched population controls and adjusted for competing causes of death when calculating the risk of death from neurodegenerative causes. However, not all former players identified from the master datasets of professional footballers could be matched to their Community Health Index. It is possible that differences between this group and those players who were included in the study introduced bias in the results. Studies to corroborate or refute our findings and to identify specific risk factors for neurodegenerative disease among former professional soccer players would ideally be designed around prospectively studied cohorts.
In summary, our data show increased rates of death with neurodegenerative disease and of prescribing of medications used to treat dementia in former professional soccer players compared to matched men in the Scottish population. The risk of neurodegenerative disease varied by disease subtype. There was lower mortality in former soccer players from other common causes of death. The relevance of these findings to recreational soccer participation is not known.
Supplementary Material
Acknowledgements
This work is supported by funding from: The Football Association and Professional Footballers Association; and an NHS Research Scotland Career Researcher Fellowship (WS).
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Wilson L, Stewart W, Dams-O’Connor K et al. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol 2017; 16: 813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DH, Johnson VE, Trojanowski JQ, Stewart W. Chronic traumatic encephalopathy – confusion and controversies. Nat Rev Neurol 2019; 15: 179–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart W. Allinson K, Al-Sarraj S et al. Primum non nocere: a call for balance when reporting on CTE. Lancet Neurol 2019; 18: 231–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005; 57: 128–34 [DOI] [PubMed] [Google Scholar]
- 5.McKee AC, Stein TD, Nowinski CJ et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2012; 136: 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol 2014; 127: 29–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart W, McNamara PH, Lawlor B, Hutchinson S, Farrell M. Chronic traumatic encephalopathy: a potential late and under recognized consequence of rugby union? QJM 2016; 109: 11–15. [DOI] [PubMed] [Google Scholar]
- 8.Hay J, Johnson VE, Smith DH, Stewart W. Chronic traumatic encephalopathy: the neuropathological legacy of traumatic brain injury. Ann Rev Pathol 2016; 11: 21–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hales C, Neill S, Gearing M, Cooper D, Glass J, Lah J Late-stage CTE pathology in a retired soccer player with dementia. Neurology 2014; 83:2307–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bieniek KF, Ross OA, Cormier KA et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 2015;130: 877–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinberg LT, Anghinah R, Nascimento CF et al. Chronic traumatic encephalopathy presenting as Alzheimer’s disease in a retired soccer player. J Alzheimers Dis 2016; 54: 169–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling H, Holton JL, Shaw K, et al. Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 2017; 133: 337–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol 2019; 138: 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol 2005; 4: 705–11 [DOI] [PubMed] [Google Scholar]
- 15.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ 2006; 174: 801–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celis-Morales CA, Lyall DM, Welsh P et al. Association between active commuting and incident cardiovascular disease, cancer, and mortality: prospective cohort study. BMJ 2017; 357: j1456. [DOI] [PubMed] [Google Scholar]
- 17.Teramoto M, Bungum TJ. Mortality and longevity of elite athletes. J Sci Med Sport 2010; 13: 410–16 [DOI] [PubMed] [Google Scholar]
- 18.Lehman EJ, Hein MJ, Baron SL, Gersie CM. Neurodegenerative causes of death among retired National Football League players. Neurology 2012; 79: 1970–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillan TM, McConnachie A,Hay J, et al. Long Term Health Outcomes after Exposure to Repeated Concussion in Elite Level Rugby Union Players. J Neurol Neurosurg Psychiatry 2017; 88: 505–11 [DOI] [PubMed] [Google Scholar]
- 20.Kunz M. 265 Million Playing Football. FIFA Magazine 2007; 10–15 [Google Scholar]
- 21.Russell ER, Stewart K, Mackay DF, MacLean J, Pell JP, Stewart W. Football’s InfluencE on Lifelong health and Dementia risk (FIELD): protocol for a retrospective cohort study of former professional footballers. BMJ Open 2019; 9: e028654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Elm E,Altman DG,Egger M et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies Lancet 2007; 370: 1453–57 [DOI] [PubMed] [Google Scholar]
- 23.Litster J. A record of pre-war Scottish league players: version 2. http://www.pmfc.co.uk/prewar.html
- 24.Litster J. A record of post-war Scottish league players: version 6. http://www.pmfc.co.uk/postwar.html
- 25.Scottish Executive (2016). The Scottish Index of Multiple Deprivation. The Scottish Government, Edinburgh. https://www2.gov.scot/Topics/Statistics/SIMD [Google Scholar]
- 26.Joint Formulary Committee. British National Formulary (online) London. BMJ Group and Pharmaceutical Press; http://www.medicinescomplete.com [Google Scholar]
- 27.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526. [Google Scholar]
- 28.Dupont W (2009) Statistical Modelling for Biomedical Researchers, Cambridge University Press, cambridge [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 30.Hosmer D,Lemeshow S, Sturdivant R (2013)Applied Logistic Regression, 3rdEd,John Wiley & Sons, New Jersey [Google Scholar]
- 31.StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP [Google Scholar]
- 32.Taioli E. All causes mortality in male professional soccer players. Eur J Public Health 2007; 17: 600–04 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen VT, Zafonte RD, Chen JT et al. Mortality Among Professional American-Style Football Players and Professional American Baseball Players. JAMA Network Open 2019; 2: e194223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


