Abstract
Parasitologic confirmation of cutaneous leishmaniasis is obligatory before chemotherapy can be considered. Direct microscopic examination of scrapings taken from indurated borders of ulcers has been routinely used as primary method of diagnosis. In this report we compared the sensitivity of examination of dermal scrapings taken from the bottoms of ulcers (BDS) with that of dermal scrapings taken from indurated active margins of lesions (MDS) in a total of 115 patients. The sensitivities of the microscopic examination were 90.4 and 78.3% for BDS and MDS samples, respectively. When the PCR method was used with a group of 40 patients, we also observed a higher sensitivity when BDS samples were examined (80.8% in BDS samples versus 57.7% in MDS samples). The improvement of the diagnostic sensitivity in the BDS samples appears to be related to the higher parasite load and more easily detectable morphology of amastigotes in the centers of the ulcers. Other parasitologic diagnostic methods, such as culture and histopathologic examination of biopsies, are less sensitive (67.5 and 64.3%, respectively). Aspirate culture, however, was shown to be the most sensitive method for the diagnosis of patients with chronic ulcers. When microscopic examinations of both MDS and BDS samples are combined, the sensitivity of diagnosis may rise up to 94%. We therefore recommend this method as a primary routine procedure for diagnosis of cutaneous leishmaniasis.
Fourteen New World-specific Leishmania species have been reported to cause leishmaniasis in the Americas. Typically, a wide spectrum of clinical forms of the disease can be observed in this area (22, 27). In Colombia, the most common clinical presentation is the cutaneous form, representing more than 90% of symptomatic infections (5). The Leishmania species most frequently isolated from cutaneous leishmaniasis (CL) lesions are Leishmania (Viannia) panamensis and Leishmania (Viannia) braziliensis, both belonging to the Viannia subgenus (6, 23). In areas of endemicity without sufficient laboratory infrastructure, CL is often diagnosed on the basis of the clinical characteristics of the lesions. Parasitologic confirmation of a Leishmania infection is absolutely critical in order to exclude an erroneous diagnosis, which may easily occur due to (i) the wide spectrum of cutaneous presentations caused by Leishmania (12, 16) and (ii) confusion with other dermal lesions which mimic the presentation of CL, such as sporotrichosis and bacterial ulcers, both of which are frequent in regions where leishmaniasis is endemic (1). In addition, treatment of leishmaniasis is expensive, toxic, and difficult to administer (4), and cutaneous lesions caused by Leishmania species of the Viannia subgenus may reactivate and produce the progressive and defiguring mucosal form if not adequately treated (11, 18). Parasitologic diagnosis of CL relies on two major methods: (i) visualization of amastigotes by direct microscopic examination of tissue samples and (ii) isolation of parasites (16, 20). Novel methods, including the PCR technique, have gained increasing importance over the last few years and have been successfully applied in order to detect parasite DNA (3, 10, 13, 19).
Several studies in which different conventional parasitologic methods were evaluated showed heterogenous and sometimes conflicting results (2, 7, 8, 14, 17, 21, 26). However, due to the low cost, ease of performance, speed, and lack of a need for sophisticated laboratory equipment, the direct microscopic examination of Giemsa-stained scrapings of lesions still represents the most suitable method for the definitive diagnosis of leishmaniasis. It has usually been recommended that scrapings should be taken from the indurated margins of the lesions (28). In work on the optimization and improvement of this simple method of diagnosis, we performed a detailed study aimed to compare the sensitivities of parasite detection by microscopic examination and PCR in samples collected from two different sites within lesions. Our results provide evidence that the sensitivity of the dermal scraping technique may significantly increase when a different site of sample extraction is used. We found that samples taken from the bottom of the lesion allow parasitologic confirmation with higher sensitivity than the routinely used technique of sample extraction from the margin of the lesion and therefore recommend that both sample extraction sites be considered for the routine diagnosis of leishmaniasis.
MATERIALS AND METHODS
Patients.
A total of 115 patients with skin lesions compatible with CL were included in this study. All patients live in or have visited areas in different regions of Colombia where CL is endemic. They were attended in the outpatient service of the Programa de Estudio y Control de Enfermedades Tropicales. The patients' diagnosis and treatment were supervised by medically qualified persons. A total of 14.8% (17 of 115) of patients were 0 to 17 years old, 59.1% (68 of 115) were between 18 and 35 years old, and 26.1% (30 of 115) were older than 35 years; 81.7% were male. Seventy-four patients (64.3%) had one lesion, 15 patients (13%) had two lesions, and 24 patients (20.8%) had three or more lesions. Additionally, two patients showed symptoms of dissiminated CL, with at least 30 lesions per patient. Lesions were localized as follows: 59.1% (68 of 115) of patients had at least one lesion in the superior extremities, 34.8% (40 of 115) had at least one lesion in the inferior extremities, and the remaining 7 patients (6.1%) had lesions distributed on different parts of the body, such as the head, neck, face, and trunk. The majority of lesions were typical indurated ulcers with well-defined margins. Patients with nonulcerated skin lesions (plaques, nodules, verrucous, and papular lesions) were excluded from this study.
Montenegro skin test (MST).
A 0.1-ml portion of Montenegro antigen (leishmanin) was injected intradermally into the right forearms of the patients, and 48 h later, the diameter of induration was read by the ballpoint pen method (28). A diameter of 5 mm or larger was considered positive. The preparation of Montenegro antigen was performed as follows: after large-scale culturing of L. panamensis (MHOM/CO/87/UA140) in NNN medium, the promastigotes were washed three times with phosphate-buffered saline and resuspended at 2 × 106 parasites/ml in autoclaved solution of Cocas (NaCl, 5 g/liter; NaHCO3, 2.75 g/liter; phenol, 4.0 g/liter). The antigen was subsequently tested for sterility and infectivity and refrigerated until use.
Samples and diagnostic procedures.
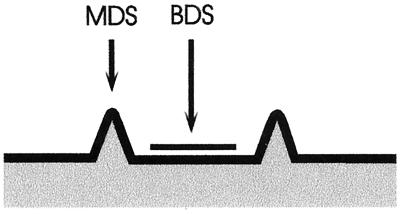
For patients exhibiting more than one lesion, a detailed examination of each lesion was performed in order to choose the site of sample extraction. Generally, samples were obtained only from those sites which showed the most indurated margin. The lesion was cleaned of debris with saline solution. Purulent or necrotic ulcers were treated with particular care, and debris was removed. None of the patients had received any antileishmanial chemotherapy treatment prior to diagnostic examination. In two cases where bacterial infections present in the ulcers complicated adequate collection of the samples and led to painful open sores, patients were treated with antibiotics during a period of 5 days before sample extraction. Once CL had been diagnosed by one of the parasitologic methods used, patients received treatment with Glucantime at the dosage internationally recommended (28). Samples for parasitologic diagnosis included dermal scrapings of the active indurated margins of lesions (margin dermal scrapings [MDS]) (25) (Fig. 1), dermal scrapings of the bottoms of the ulcers (bottom dermal scrapings [BDS]) (Fig. 1), fine-needle aspirate cultures, and biopsies. All BDS and MDS samples were taken by the same person in order to avoid individual variation. For the MDS, a thorough cleaning of the indurated active margin of the lesion with 70% alcohol was performed. The selected site of the margin was then subjected to pressure with the forefinger and thumb in order to achieve hemostasis. A no. 15 sterile surgical blade was used to make a slit, 3 mm in length and 3 mm in depth, and the blood was cleaned by using sterile gauze. Once the bleeding had stopped, dermal tissue from the wall of the slit was scraped with a blade and smeared onto a glass slide. A total of three scrapings from the slit were smeared on a single slide. For the BDS, the central area of the bottom of the lesion (Fig. 1) was cleaned, and fibrinic material was eliminated by using gauze and saline solution in order to expose the granular ground of the ulcer. Hemostasis was achieved in the same way as for MDS, with pressure maintained with the fingers. Three scrapings were collected and smeared onto the glass slide. The MDS and BDS slides were finally air dried, fixed with methanol, and Giemsa stained. The whole slide was analyzed with a ×100 immersion objective. All of the slides were examined by the same person. This person had no previous knowledge of the patients or of the sample registration code in order to avoid subjective interpretation of results. A semiquantitative scaling of the amount of amastigotes in each slide was performed based on the following criteria: −, amastigotes could not be observed in the whole slide; +, 1 amastigote in the whole slide up to one amastigote per field in a total of at least 100 fields; ++, 2 to 10 amastigotes per field in a total of at least 50 fields; +++, 11 to 20 amastigotes per field in a total of at least 50 fields; and ++++, 21 or more amastigotes per field in a total of at least 10 fields. In addition, analysis of parasite DNA in the MDS and BDS samples by PCR was performed for a group of 40 patients. Scraping material was collected in a 1.5-ml Eppendorf tube containing 200 μl of lysis solution (10 mM NaCl, 10 mM EDTA, 10 mM Tris-HCl, 1% sodium dodecyl sulfate) and stored at −20°C until final processing. The aspirate culture and biopsy samples were taken from the active indurated margins of lesions as previously described (20, 25, 28). Briefly, a 26-gauge needle is used on disposable tuberculin syringes with 0.4 ml of 0.1 M phosphate-buffered saline at pH 7.2. The needle is inserted intradermally into the outer border of the lesion and rotated several times, and tissue fluid is gently aspirated. A 0.2-ml portion of this material is used to inoculate two tubes with the biphasic culture medium NNN, and the tubes are incubated at 27°C. Every 2 to 3 days, the liquid phases of cultures are examined in order to observe motile promastigotes (28). For the biopsy samples, after cleansing with 70% alcohol, 2% xylocaine is injected into the dermis and a sample is obtained from the indurated margin of the lesion using a sterile 4-mm disposable biopsy punch. The sample is subsequently fixed in 10% buffered formaldehyde and processed for routine histologic studies (20).
FIG. 1.
Schematic representation of a typical CL ulcer. The sites where MDS and BDS samples were collected are indicated (for details, see Materials and Methods).
PCR.
A total of 80 samples collected from 40 patients were analyzed by PCR using primers highly specific for minicircle DNA sequences present in members of the Viannia subgenus of Leishmania, such as L. panamensis, L. braziliensis, L. guyanenesis, and L. peruviana (9). Oligonucleotide primer B1 recognizes a highly conserved sequence in the minicircle DNA which is thought to form part of the replication origin of all Leishmania species. Oligonucleotide primer B2 hybridizes to an adjacent sequence which is found exclusively within the L. braziliensis complex (9). The amplification product is a 750-bp DNA band which represents full-length minicircles. Using an annealing temperature of 60°C, we observed the highly specific amplification product only in DNAs isolated from Leishmania species of the subgenus Viannia, whereas minicircle DNAs from other Leishmania species used as controls failed to amplify. The usefulness of this PCR method for the diagnosis of CL has also been demonstrated elsewhere (10). Purification of DNA in the MDS and BDS sample material was performed by using a QIAmp tissue kit (Qiagen, Hilden, Germany). The DNA was eluted in a final volume of 50 μl of H2O, and 1 μl was used for PCR amplification in a master mix containing 1 pmol of B1 primer (5′GGG GTT GGT GTA ATA TAG TGG3′) and B2 primer (5′CTA ATT GTG CAC GGG GAG G3′) per ml, 0.2 mM deoxynucleoside triphosphates, 2.5 mM MgCl2, and 0.05 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer, Roche Molecular Systems, Branchburg, N.J.) per μl, adjusted with water to a final volume of 25 μl. The following protocol for PCR cycling in the GeneAmp PCR System 9700 (Perkin-Elmer) was used: initial heat activation of the enzyme at 95°C for 5 min, followed by 35 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 60.5°C, and polymerization for 1 min at 72°C. A final extension step (72°C for 10 min) was included at the end of the reaction. Fifteen microliters of the PCR product was run in 1% agarose gels, stained with ethidium bromide, and visualized on a UV transilluminator. The intensity of the amplification product of 750 bp was quantitatively determined by one-dimensional image analysis (Digital Science System DC 120; Kodak, Rochester, N.Y.), calculated on the basis of the known amounts of DNA fragments produced by the DNA marker. According to the image analysis results, we assigned ++++ to a DNA amount of >250 ng, +++ to an amount in the range of 100 to 250 ng, ++ to an amount in the range of 50 to 100 ng, and + to an amount of <50 ng. In cases where no amplification product was observed, we assigned a negative result (−).
Statistics.
Due to the lack of a “gold standard” test for the parasitologic diagnosis of leishmaniasis, different diagnostic approaches are difficult to compare. For our studies, we defined as the reference diagnostic method the positivity obtained by at least one of the applied methods. The sensitivity of each method was then calculated based on this criterion. To compare the MDS and BDS methods, the Cohen's kappa (κ) and weighted kappa coefficients were calculated based on the percentage of positivity of the total analyzed samples. Fisher's exact test was performed in order to determine the association between positivity of methods and duration of lesions. All of these tests were calculated by using the Statxact 3.0 computer program.
RESULTS
Table 1 summarizes the results of the parasitologic diagnosis for a total of 115 patients with symptoms of CL. The MST was applied for 92 patients; 76 individuals (82.6%) were MST positive. Leishmaniasis was confirmed in 72.2% of the initial patient group (83 of 115) and in 85.5% of the MST-positive individuals (65 of 76) by at least one of the methods used for parasitologic diagnosis. Only one patient from the group of 16 MST-negative patients gave a positive result in the parasitologic diagnosis.
TABLE 1.
Comparison of sensitivities of conventional parasitologic methods and PCR for the diagnosis of CL
| Patients analyzed (n) | No. positive/total (% positive) as determined by:
|
|||||
|---|---|---|---|---|---|---|
| Biopsy | Culture | Microscopya:
|
PCRa
|
|||
| BDS samples | MDS samples | BDS samples | MDS samples | |||
| Total (115) | 18/37 (48.6) | 56/115 (48.7) | 75/115 (65.2) | 65/115 (56.5) | 21/40 (52.5) | 15/40 (37.5) |
| Leishmaniasis (83) | 18/28 (64.3) | 56/83 (67.5) | 75/83 (90.4) | 65/83 (78.3) | 21/26 (80.8) | 15/26 (57.7) |
Comparison between BDS and MDS samples always showed κ values higher than 0.29 (P < 0.05).
Both the MDS- and BDS-based microscopic examinations as well as culture aspirates were performed for a total of 115 patients. PCR was performed on the samples collected from the MDS and BDS sites of 40 patients (34.8%). From this group of patients, leishmaniasis in 65% (26 of 40) was parasitologically confirmed by at least one of the parasitologic methods. Histopathologic examination of biopsies was performed for 37 patients. Among the 19 negative biopsies, 4 showed a granulomatose reaction compatible with leishmaniasis, but amastigote forms were not observed and the patients were therefore registered as negative. However, these four patients were positively diagnosed by other methods. Bacterial or fungal contamination of NNN cultures was observed in three patients; one had a negative MST and negative parasitologic diagnosis, and two were positive for both.
As shown in Table 1, the most sensitive method of parasitologic diagnosis is the microscopic examination of BDS samples (90.4% of confirmed patients). Applying the classical MDS-based microscopic examination, a significant drop in sensitivity, down to 78.3%, could be observed. Applying the PCR technique, we detected infection in 80.8 or 57.7% of the demonstrated cases in BDS or MDS samples, respectively. One patient diagnosed as positive by BDS-based PCR was negative by all other methods, including MDS-based PCR. The biopsy was positive in 64.3% of confirmed cases. One positive sample as diagnosed by this method remained negative in microscopic examination and culture. Aspirate culture was positive in 67.5% of the confirmed cases. A total of 56 Leishmania isolates were obtained. From these, 54 were identified as L. panamensis and 2 were identified as L. braziliensis by means of isoenzyme analysis or monoclonal antibody reactivity patterns (29).
Subsequently, we performed a detailed semiquantitative analysis in order to compare the level of positivity of the microscopic examination in samples collected either from the margins or the bottoms of the lesions in a group of 75 patients. The results are shown in Table 2. Results for 66.7% of the cases (50 patients) were concordant between the two methods. Discordance of results was observed in 25 samples. In this group 22 samples (88%) appear to be better diagnosed by the BDS-based microscopic method due to the larger amount of parasites observed, while only 3 samples (12%) were better diagnosed by the MDS-based microscopic examination. A parasite load equivalent to more than the + level was observed in 21 patients when samples were obtained from BDS, compared with only 9 patients in the MDS group (Table 2). In Giemsa-stained BDS samples we also observed consistently sharper and larger amastigotes with nuclei and kinetoplastids which could be more easily distinguished than those typically obtained from the MDS samples. In addition, mitotic figures and intracellular forms are more frequently observed in the BDS samples (not shown).
TABLE 2.
Semiquantitative comparison of BDS and MDS samples for parasitologic diagnosis of CL
| Test | Result for MDS samples | No. of BDS samples with the following resulta:
|
|||||
|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | ++++ | Total | ||
| Microscopic examinationb | − | 21 | 7 | 0 | 0 | 0 | 28 |
| + | 1 | 24 | 7 | 5 | 1 | 38 | |
| ++ | 0 | 0 | 3 | 2 | 0 | 5 | |
| +++ | 0 | 1 | 1 | 1 | 0 | 3 | |
| ++++ | 0 | 0 | 0 | 0 | 1 | 1 | |
| Total | 22 | 32 | 11 | 8 | 2 | 75 | |
| PCRc | − | 19 | 5 | 1 | 0 | 0 | 25 |
| + | 0 | 1 | 5 | 2 | 0 | 8 | |
| ++ | 0 | 0 | 1 | 3 | 0 | 4 | |
| +++ | 0 | 1 | 0 | 2 | 0 | 3 | |
| ++++ | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 19 | 7 | 7 | 7 | 0 | 40 | |
Boldface indicates concordant results.
κ = 0.64 (P < 0.001).
κ = 0.70 (P < 0.001).
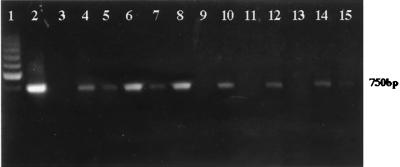
The tendency to discover parasites in larger amounts in the BDS compared with the MDS samples was also evident in the group of 40 patients for whom the PCR method was used. DNA was extracted from MDS and BDS samples from the 40 patients analyzed, and PCR amplification was performed on all 80 samples. Figure 2 shows a typical result of the PCR amplification. The intensities of the diagnostic 750-bp band corresponding to the full-length minicircle sequence (9) were comparable in approximately 50% of the MDS and BDS sample pairs. However, in the rest of the patients a reproducible stronger signal was generally observed in BDS compared with MDS samples (Fig. 2). The intensity of the 750-bp band in each patient's sample was analyzed by a digital image system and semiquantitatively interpreted using a grading scale described in Materials and Methods. In a similar way as for the microscopic examination, PCR results for both sample sites were compared (Table 2). Equal amounts of the PCR product in both the BDS and MDS samples were obtained with 57.5% of patients (23 of 40), while 42.5% of samples showed discordance; 94.1% (16 of 17) of these discordant samples produced larger amounts of PCR amplification products when DNA was extracted from the BDS site, compared with 5.9% (1 of 17) when DNA was obtained from the MDS site. In 14 patients the intensity of the 750-bp diagnostic band was equivalent to more than the + level in BDS samples, compared with 7 patients in the MDS group (Table 2). This stronger signal reflects a major amount of parasite DNA which was systematically observed in BDS samples.
FIG. 2.
PCR amplification of the diagnostic 750-bp DNA fragment representing kinetoplastid minicircle DNA. Lanes 1, molecular size marker (200-bp ladder); 2, positive control with 50 ng of purified L. panamensis DNA; 3, negative control (no DNA template); 4 to 15, samples from six CL patients. BDS samples (even-numbered lanes) and MDS samples (odd-numbered lanes) of each patient are directly compared.
Subsequently, we correlated the sensitivity of the applied diagnosis methods with the chronicity of lesions in the patients with parasitologically confirmed leishmaniasis. The majority of the diagnostic methods for Leishmania appeared to be less sensitive when the lesion evolution time exceeded 3 months (Table 3). However, the techniques of aspirate culture and PCR amplification of BDS samples led to similar percentages of positivity in recent and chronic lesions, and these appear to be the most sensitive methods for the detection of Leishmania in lesions with more than 3 months of evolution (76.9 and 75%, respectively) (Table 3). When the groups of patients with recent and chronic lesions were separately observed, the tendency to a higher positivity in BDS samples, by both microscopy and PCR, was again evident (Table 3). Of the 13 chronic CL patients, 9 cases were confirmed by microscopic examination of BDS samples and 10 were confirmed by culture methods. Aspirate culture confirmed two CL cases that had not been previously confirmed by microscopic examination of BDS samples. In one case culture gave a negative result, while microscopic examination of the BDS sample was positive. Of the eight chronic patients evaluated by PCR, only two cases were confirmed using MDS samples, while six were positive using BDS samples (Table 3). Both microscopically and by PCR, all patients in the chronic group detected with MDS samples were also detected using BDS samples.
TABLE 3.
Sensitivities of methods for parasitologic diagnosis of CL as related to chronicity of lesionsa
| Time of lesion evolution (mo) | No. positive/total (% positive) as determined by:
|
||||
|---|---|---|---|---|---|
| Microscopy
|
PCR
|
Culture | |||
| BDS samplesb | MDS samplesb | BDS samples | MDS samplesb | ||
| ≤3 | 66/70 (94.3) | 58/70 (82.9) | 15/18 (83.3) | 14/18 (77.8) | 46/70 (65.7) |
| >3 | 9/13 (69.2) | 7/13 (53.8) | 6/8 (75) | 2/8 (25) | 10/13 (76.9) |
Calculated for the parasitologically confirmed patients (n = 83 for microscopy and culture; n = 26 for PCR).
P < 0.05 (Fisher's exact test).
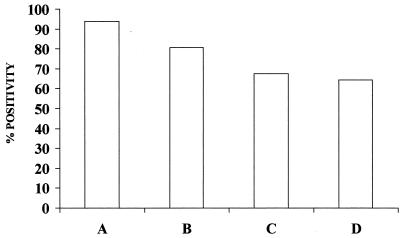
A total of 13 patients confirmed positive by microscopic BDS proved to be negative by microscopic MDS, whereas in only 3 patients was the contrary observation made. For this reason, when both samples are combined, the sensitivity of microscopic examination rises to 94% (78 of 83) of confirmed patients (Fig. 3). Since all patients showing a positive PCR result in MDS samples were also detected by PCR with BDS samples, the PCR sensitivity when MDS and BDS are combined is the same as that with BDS alone (Table 1; Fig. 3). If microscopy and PCR at the MDS site are combined, the sensitivity is 76.9% (20 of 26), compared with 92.3% (24 of 26) at the BDS site. The total combination of microscopy and PCR at both sites leads to a sensitivity of 96.1% (25 of 26). Combined microscopic examination failed to diagnose Leishmania infection in only 3 patients who had been previously confirmed by aspirate culture, while 25 cases of CL with negative results in culture were diagnosed by combined microscopy.
FIG. 3.
Comparison of the sensitivities of different methods for parasitologic diagnosis of CL. The relative positivity of each analyzed method with respect to the total number of parasitologically diagnosed patients is indicated. Bars: A, combined microscopic examination of BDS and MDS samples; B, PCR detection of parasite DNA by combining BDS and MDS samples; C, aspirate cultures; D, biopsy.
DISCUSSION
Ulcerated skin lesions account for more than 90% of clinical manifestations of CL (5, 15, 24). However, the relatively wide range of morphological variations of the skin lesions, which are particularly frequent in New World leishmaniasis, as well as the prevalence of other microbial infections in areas where leishmaniasis is endemic which may mimic the symptoms of a Leishmania infection, often complicate the diagnosis of leishmaniasis. Parasitologic confirmation of Leishmania infection is therefore necessary before the relatively toxic chemotherapy should be applied (5, 28). Among the diagnostic methods available at present, the fine-needle aspirate culture has been reported to be the most sensitive method (17, 26), although the direct microscopic examination of lesion scrapings still continues to be the diagnostic method most widely applied due to the ease of performance, low cost, and speed of this technique.
In this report we provide evidence that variations in the technique of direct microscopic examination, with special emphasis on the site of sample collection, influence the sensitivity of this method. Our results suggest that sample extraction from the central region of the bottom of the ulcer significantly increases the sensitivity of direct microscopic examination compared to the routinely recommended extraction of samples from the margin of the lesion (28). This also holds true when applying the PCR-based diagnostic method. When we analyzed the parasite loads in both sites of sample collection using microscopy and PCR, it could be observed that around 90% of discordant cases were due to a major quantity of amastigotes in BDS samples, confirming that the larger amount of parasites present in the bottoms of lesions accounts for this increased sensitivity. In addition to this overall higher number of parasites found in BDS samples, by microscopy we observed that a better morphology of amastigotes allowed easier and faster identification of Leishmania. We therefore believe that these observations may have major implications for routine laboratory diagnosis. For example, in areas of endemicity, where health care personnel are not always sufficiently well trained and experienced to take adequate MDS samples and to identify the parasite by microscopy, the abundant and easily detectable amastigotes in BDS samples could significantly improve the diagnosis.
A previous study that aimed to compare the sensitivities of the microscopic examination technique in samples obtained from Guatemalan CL patients did not show significant differences which correlated with different sampling sites (17). However, we think that the discrepancy between their and our results can be explained by the larger group of subjects in our study and the technical details of sample extraction, as described in Materials and Methods.
The diagnostic sensitivity of the microscopic examination observed in our study is the highest reported so far. Weigle et al. (26) reported an extremely low sensitivity (32.7% of confirmed patients) with conventional microscopic MDS. In other reports, however, the diagnostic sensitivity was significantly higher (2, 21), and in one case it reached 84% of confirmed patients (17). Different modifications of sample extraction have been recommended. Navin et al. (17) found a substantial increase in the sensitivity of this method, from 40 up to 80%, when the number of samples collected from each lesion was increased from one to four. In our study, in contrast, only a single sample was taken for each applied method of parasite diagnosis, and the sensitivity was 90.4% when the BDS-based technique was used.
The aspirate culture of lesions is an easy and nontraumatic method of parasitologic diagnosis. However, it requires special equipment, it is time-consuming, and contamination is often observed under field work conditions. In contrast to our studies, this method was previously reported to be the most sensitive for the parasitologic diagnosis (17, 26). In this study we found a significant number of patients (25 of 78) who were diagnosed by microscopy but had a negative result by aspirate culture. The low sensitivity of the microscopic method observed previously could significantly underestimate the total number of patients suffering from CL and therefore overestimate the sensitivity of others methods, such as culture. We think that this is the most plausible explanation for this discrepancy.
Consistent with other studies, the least sensitive method reported for the diagnosis of CL was the histopathologic analysis. However, in one case this test was the only method which allowed diagnosis of a Leishmania infection, while it failed in 10 of 28 patients (36%) parasitologically confirmed by any other method. We therefore agree with other authors that histopathologic examination is more helpful in the diagnosis of pathologies which are not related to leishmaniasis and that it is not recommended as the primary method (26).
PCR is at present the most widely used molecular method for the study of clinical and epidemiological aspects of infectious diseases, due to its high sensitivity. In leishmaniasis, although many target sequences for PCR amplification have been characterized over the last few years, this technique is more routinely used in studies related to epidemiological aspects than in clinical tests. This may be partly due to the prerequisite of a specialized laboratory infrastructure and to the relatively high cost in developing countries. In the present study the PCR technique detected Leishmania parasite DNA in 81% of the confirmed cases. In three patients where PCR failed, direct microscopic examination demonstrated the presence of only a single amastigote on the whole slide, thus indicating that the overall sensitivity of the PCR technique may be less than that of the traditional microscopic method at least under the conditions used in our study. So far, most of the PCR studies have been performed with biopsy material from lesions, as they were shown to allow parasite detection with highest sensitivity (unpublished observation). Since we observed a similar or even superior sensitivity of parasite DNA amplification in BDS-extracted samples compared with biopsy material (unpublished observation), we think that BDS-based PCR could be a noninvasive alternative option for PCR diagnosis.
Our observations that (i) most of the parasitologic methods analyzed in this study are significantly less sensitive in lesions with more than 3 months of evolution time and (ii) culture appears to be the most sensitive method in this group of chronic patients are not completely novel and have already been demonstrated elsewhere (26). However, this study additionally demonstrates that, in both recent and chronic patients, the sensitivity of microscopy and PCR is consistently higher in BDS than in MDS samples. The observed shift in sensitivity between MDS and BDS samples is more pronounced in patients with chronic lesions than in patients with recent lesions, both by microscopy (11.4 and 15.4% for recent and chronic patients, respectively) and by PCR (5.5 and 50% for recent and chronic patients, respectively). This observation emphasizes the need to collect BDS samples in this hard-to-diagnose group of chronic patients.
Taken together, our results show that use of the combination of BDS- and MDS-extracted samples may significantly enhance the sensitivity of the microscopic examination to a value of 94% of the diagnosed patients. We therefore recommend taking both sampling sites into consideration for microscopic examination as the primary technique of diagnosis. For local health services with limited laboratory equipment, we give the following suggestions for direct microscopic examination: (i) to take samples from both the active margin and the bottom of the lesion; (ii) to achieve a strict hemostasis at the site of sample extraction in order to avoid contamination with hemoglobin and red blood cells in the slides, which may make interpretation of the sample difficult; and (iii) always to examine the whole slide in order to detect at least one amastigote. In view of its having the highest sensitivity in chronic patients, we recommend performing, if possible, the aspirate culture method in addition to BDS- and MDS-based microscopy. We similarly recommend the use of MDS and BDS samples for PCR-based diagnosis instead of the invasive biopsy.
ACKNOWLEDGMENTS
This work was supported in part by grants CIM 9821 (CODI, Universidad de Antioquia) and Colciencias 1115-04-306-96. S. Agudelo is the recipient of a Ph.D. fellowship from Colciencias. C. Muskus is recipient of a Ph.D. fellowship from the University of Antioquia.
We are grateful to Juan Alberto Puerta, Diana L. Muñoz, and Soraya Diez for technical assistance.
REFERENCES
- 1.Agudelo S, Restrepo S, Velez I D. Cutaneous New World leishmanisis-sporotrichosis co-infection: report of 3 cases. J Am Acad Dermatol. 1999;40:1002–1004. doi: 10.1016/s0190-9622(99)70093-9. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jitawi S A, Farraj S E, Ramahi S A. Conventional scraping versus fine needle aspiration cytology in the diagnosis of cutaneous leishmaniasis. Acta Cytol. 1995;39:82–84. [PubMed] [Google Scholar]
- 3.Belli A, Rodriguez B, Avilés H, Harris E. Simplified PCR detection of New World Leishmania from clinical specimen. Am J Trop Med Hyg. 1998;58:102–109. doi: 10.4269/ajtmh.1998.58.102. [DOI] [PubMed] [Google Scholar]
- 4.Berman J. Treatment of New World cutaneous and mucosal leishmaniasis. Clin Dermatol. 1996;14:519–522. doi: 10.1016/0738-081x(96)00048-x. [DOI] [PubMed] [Google Scholar]
- 5.Colombian Health Ministry. Leishmaniasis, plan nacional de control. Manual de normas tecnico-administrativas. Santafé de Bogotá, Colombia: Trazo Ltda; 1994. [Google Scholar]
- 6.Corredor A, Kreutzer R, Tesh R, Boshell J, Palau M, Caceres E, Duque S, Pelaez D, Rodriguez G, Nichols S, Hernandez C, Morales A, Young D, Ferro C. Distribution and etiology of leishmaniasis in Colombia. Am J Trop Med Hyg. 1990;42:206–214. doi: 10.4269/ajtmh.1990.42.206. [DOI] [PubMed] [Google Scholar]
- 7.Cuba-Cuba C A, Llanos-Cuentas A, Barreto A, Magalhaes A, Lago E, Reed S, Marsden P. Human mucocutaneous leishmaniasis in Tres Bracos, Bahia, Brazil. An area of Leishmania braziliensis braziliensis transmission. I. Laboratory diagnosis. Rev Soc Brasil Med Trop. 1984;17:161–167. [Google Scholar]
- 8.Cuba-Cuba C A, Masden P, Barreto A C, Rocha R, Sanpaio R R, Padzlaff L. Parasitologic and immunologic diagnosis of American (mucocutaneous) leishmaniasis. Bull Pan Am Health Organ. 1981;15:249–259. [PubMed] [Google Scholar]
- 9.DeBruijn M, Barker D C. Diagnosis of New World leishmaniasis: specific detection of the Leishmania braziliensis complex by amplification of kinetoplastid DNA. Acta Trop. 1992;52:45–58. doi: 10.1016/0001-706x(92)90006-j. [DOI] [PubMed] [Google Scholar]
- 10.DeBruijn M, Labrada L A, Smyth A J, Barker D C. A comparative study of diagnosis by the polymerase chain reaction and by current clinical methods using biopsies from Colombian patients with suspected leishmaniasis. Trop Med Parasitol. 1993;44:201–207. [PubMed] [Google Scholar]
- 11.Desjeux P. Leishmaniasis: public health aspects and control. Clin Dematol. 1996;14:417–423. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- 12.Dowlati Y. Cutaneous leishmaniasis. Clinical aspects. Clin Dermatol. 1996;14:425–431. doi: 10.1016/0738-081x(96)00058-2. [DOI] [PubMed] [Google Scholar]
- 13.Harris E, Kropp G, Belli A, Rodriguez B, Agabian N. Single-step multiplex PCR assay for characterization of New World Leishmania complexes. J Clin Microbiol. 1998;36:1989–1995. doi: 10.1128/jcm.36.7.1989-1995.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendricks L, Wright N. Diagnosis of cutaneous leishmaniasis by in vitro cultivation of saline aspirates in Schneider's drosophila medium. Am J Trop Med Hyg. 1979;28:962–964. doi: 10.4269/ajtmh.1979.28.962. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickx E P, Agudelo S, Muñoz D L, Puerta J A, Velez I D. Lack of efficacy of mefloquine in the treatment of New World cutaneous leishmaniasis in Colombia. Am J Trop Med Hyg. 1998;59:889–892. doi: 10.4269/ajtmh.1998.59.889. [DOI] [PubMed] [Google Scholar]
- 16.Kalter D C. Laboratory tests for the diagnosis and evaluation of leishmaniasis. Dermatol Clin. 1994;12:37–50. [PubMed] [Google Scholar]
- 17.Navin T, Arana F, deMérida A, Arana B, Castillo L, Silvers D. Cutaneous leishmaniasis in Guatemala: comparison of diagnostic methods. Am J Trop Med Hyg. 1990;42:36–42. doi: 10.4269/ajtmh.1990.42.36. [DOI] [PubMed] [Google Scholar]
- 18.Organización Panamericana de la Salud/Organización Mundial de la Salud. Epidemiología, diagnóstico, tratamiento y control de la leishmaniasis en América Latina. Washington, D.C.: Organización Panamericana de la Salud/Organización Mundial de la Salud; 1994. [Google Scholar]
- 19.Osman O F, Oskam L, Kroon C M, Schoone G J, Khalil E A G, El-Hassan A M, Zijlstra E, Krager P. Use of PCR for diagnosis of post-kala-azar dermal leishmaniasis. J Clin Microbiol. 1998;36:1621–1624. doi: 10.1128/jcm.36.6.1621-1624.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palma G, Gutierrez Y. Laboratory diagnosis of Leishmania. Clin Lab Med. 1991;11:909–922. [PubMed] [Google Scholar]
- 21.Restrepo M, Gomez M E. La reacción de inmunofluorescencia indirecta en el diagnóstico de la leishmaniasis tegumentaria americana. Biomedica (Colombia) 1983;3:15–21. [Google Scholar]
- 22.Rioux J A, Lanotte G, Serres E, Pratlong F, Bastien P, Perieres J. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann Parasitol Hum Comp. 1990;65:111–125. doi: 10.1051/parasite/1990653111. [DOI] [PubMed] [Google Scholar]
- 23.Saravia N G, Segura I, Holguin A F, Santrich C, Valderrama L, Ocampo C. Epidemiological, genetic and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. Am J Trop Med Hyg. 1998;59:86–94. doi: 10.4269/ajtmh.1998.59.86. [DOI] [PubMed] [Google Scholar]
- 24.Velez I D, Agudelo S, Hendrickx E P, Puerta J A, Grogl M, Modabber F, Berman J. Inefficacy of allopurinol as monotherapy for Colombian cutaneous leishmaniasis. Ann Intern Med. 1997;126:232–236. doi: 10.7326/0003-4819-126-3-199702010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Velez I D, Agudelo S, editors. Leishmaniasis: manual para el diagnostico de la leishmaniasis cutanea americana. Medellin, Colombia: Universidad de Antioquia; 1996. [Google Scholar]
- 26.Weigle K, Dedavalos M, Heredia P, Molineros R, Saravia N, D'Alessandro A. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg. 1987;36:489–496. doi: 10.4269/ajtmh.1987.36.489. [DOI] [PubMed] [Google Scholar]
- 27.Weigle K, Saravia N. Natural history, clinical evolution and the host-parasite interactions in New World leishmaniasis. Clin Dermatol. 1996;14:433–450. doi: 10.1016/0738-081x(96)00036-3. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Control of leishmaniasis. Technical report series no. 793. Geneva, Switzerland: World Health Organization; 1990. [PubMed] [Google Scholar]
- 29.World Health Organization. Workshop on the application of monoclonal antibodies for the rapid diagnosis/identification of Leishmania species. Prog/rep/86. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]