Abstract
Recent developments within the topic of biomaterials has taken hold of researchers due to the mounting concern of current environmental pollution as well as scarcity resources. Amongst all compatible biomaterials, polycaprolactone (PCL) is deemed to be a great potential biomaterial, especially to the tissue engineering sector, due to its advantages, including its biocompatibility and low bioactivity exhibition. The commercialization of PCL is deemed as infant technology despite of all its advantages. This contributed to the disadvantages of PCL, including expensive, toxic, and complex. Therefore, the shift towards the utilization of PCL as an alternative biomaterial in the development of biocomposites has been exponentially increased in recent years. PCL-based biocomposites are unique and versatile technology equipped with several importance features. In addition, the understanding on the properties of PCL and its blend is vital as it is influenced by the application of biocomposites. The superior characteristics of PCL-based green and hybrid biocomposites has expanded their applications, such as in the biomedical field, as well as in tissue engineering and medical implants. Thus, this review is aimed to critically discuss the characteristics of PCL-based biocomposites, which cover each mechanical and thermal properties and their importance towards several applications. The emergence of nanomaterials as reinforcement agent in PCL-based biocomposites was also a tackled issue within this review. On the whole, recent developments of PCL as a potential biomaterial in recent applications is reviewed.
Keywords: polycaprolactone, green biocomposites, hybrid biocomposites, mechanical properties, thermal properties
1. Introduction
Due to the increasing production of waste every day, landfill spaces have become more scarce and have a reduced capacity to intake more waste [1]. Hence, waste disposal is becoming a more problematic task. Composting and incineration are advised as ways to reduce reliance on landfills, and they are becoming increasingly essential [2]. Thus, to take advantage of this emphasis on waste conversion, degradable polymers that are tailored for various methods of waste management are required. Biopolymers, often known as biodegradable plastics, are polymeric materials that degrade, in part, through the metabolism of naturally existing microorganisms via aerobic and anaerobic processes [2,3,4,5,6]. Biodegradation causes the polymers to break or disintegrate under the right conditions of moisture, temperature, and oxygen availability, leaving no hazardous or environmentally detrimental behind [7].
Based on the origin of the raw ingredients and the manufacturing procedures, biopolymers can be categorized into several categories, including natural biopolymers and synthetic biodegradable polymers. Their characteristics are identified and applied widely in medical fields, such as implants, drug delivery, antimicrobial material, sutures and surgeries, among other things [4,5,8,9,10,11]. The example of biopolymer is listed in Table 1 [12].
Table 1.
List of biopolymers.
| Type of Biopolymer | Example | |
|---|---|---|
| Natural biopolymer | Plant carbohydrate and animal | Starch, cellulose, nanocellulose, agar, chitosan, etc. |
| Plant origin protein | Soy protein, corn zein, wheat gluten, gelatine, collagen, whey protein, casein | |
| Synthetic biodegradable polymer | Poly(L-lactide), polyglycolic acid, polycaprolactone, polybutylene succinate, polyvinyl alcohol, etc. | |
| Biopolymers produced by microbial fermentation | Polyhydroxyalkanoates including poly-β-hydroxybutyrate, poly-3- hydroxybutyrate-co-3-hydroxyvalerate, etc. | |
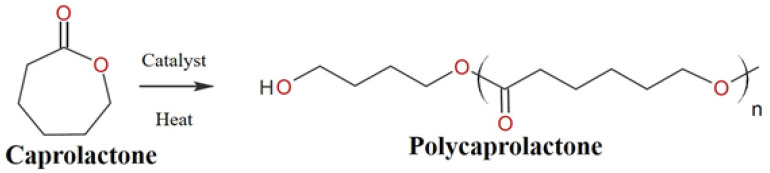
Among several types of biopolymers, polycaprolactone (PCL) has received a lot of attention due to its several advantages, such as its biodegradability, high strength, and biocompatibility. It can withstand water, oil, solvents, and chlorine. PCL is a semicrystalline ester polymer that is derived from ring-opening polymerization of ε-caprolactone monomers, as shown in Figure 1. PCL consists of a glass transition temperature (Tg) of around 60 °C and a melting point ranging between 59–64 °C, dictated by the crystalline nature of PCL which enables easy formability at relatively low temperatures [13].
Figure 1.
Synthesis of PCL.
In contrast, the average molecular weight of PCL samples can range from 3000 to 90,000 g/mol, and they can be classified based on their molecular weight. With increasing molecular weight, its crystallinity decreases. High solubility, a low melting point, and exceptional blend compatibility have sparked a lot of interest in its potential biomedical applications [14]. Table 2 summarizes the effect of PCL molecular weight on their properties [15].
Table 2.
Properties of PCL with different molecular weights.
| Molecular Weight | Melting Point, °C | Tensile Stress, N/m2 | Elongation at Break, % |
|---|---|---|---|
| 37,000 | 58–60 | 1.37 × 107 | 660 |
| 50,000 | 58–60 | 3.53 × 107 | 800 |
| 80,000 | 60–62 | 5.69 × 107 | 900 |
PCL also shows great electrospinning properties, as it can be spun into fibers at temperatures around 200 °C, without undergoing thermal degradation. Since PCL is a synthetic material, it is possible to achieve high material purity. PCL also has relatively long biodegradable time. According to the literature, PCL can biodegrade in a few months to many years, depending on its molecular weight, degree of crystallinity, shape, porosity, sample thickness, and the surrounding environment [16], unlike traditional plastics, such as polypropylene (PP) and polyethylene (PE), which take hundreds or even thousands of years to fully decay.
Recently, due to the environmental concerns, as well as energy and cost considerations, increased research efforts have been directed to the creation of bio-based materials. Bio-based polymers, which minimize reliance on petrochemical-based synthetic polymers, contribute significantly to global environmental sustainability. Recently, Gokhan et al. (2020) [17] has synthesized bio-based PCL from soybean oil-derived polyol via ring-opening polymerization. He found that the soybean-based PCL containing higher PCL molar ratio has poorer biodegradability but higher hydrophobicity and thermal characteristics compared to the others. Nonetheless, biobased polymers possess several weaknesses, including inferior mechanical properties, insufficient heat tolerance, and high moisture sensitivity relative to petroleum-derived polymers [18].
As mentioned in a previous statement, this polymer is well known for being biodegradable and have notably lower degradation. Due to its slow degradation rate, PCL is often used as a material in drug delivery devices that is active for a long time, with a period of over one year [8]. The material is also noted to be potentially used as a bioscaffold [19,20]. In terms of its biodegradability, permeability, and inability to establish an acidic environment, PCL is a better polymer than PLA or PGA in terms of possible biomedical uses [21]. Furthermore, because of the low rate of PCL degradation compared to PLA and PGA, it can be utilized to release medications over longer periods of time, even a year. The hydrolytic cleavage of ester groups is involved in PCL degradation. It is easy to predict that inserting different co-monomer units in the PCL chain will result in diverse properties, including changes in biodegradability, allowing for biodegradability control through targeted introduction of such groups [22]. Manivasagam et al. (2019) [23] have mentioned the application of PCL in dentistry by modifying the material structure with other materials (such as hydrophilic polyethylene glycol (PEG) and ceramic) and introducing co-polymers (such as polylactic acid (PLA) or polyglycolic acid (PGA)). The pKa of the degradation products; the primary mechanisms of the degradation; and the in vivo degradation rate of PCL, PLA, and PGA are summarized in Table 3.
Table 3.
The degradation behaviour of the biodegradable polyesters. Reproduce from ref. [24].
| Polyester | Degradation By-Products (pKa) | In Vivo Degradation Rate | Degradation Mechanism |
|---|---|---|---|
| PCL | Caproic acid (4.88) | 50% in 4 years 1% in 6 months |
Hydrolytic degradation |
| PLA | Lactic acid (3.85) Lactic acid (3.08) |
50% in 1–2 years 98% in 12 months 100% in >12 months 100% in 12–16 month | Hydrolysis through the action of enzymes |
| PGA | Glycolic acid (3.83) | 100% in 2–3 month 100% in 6–12 months |
Both enzymatic and non-enzymatic hydrolysis |
Unfortunately, because PCL manufacture is both complex and expensive, its wider commercialization has been limited. In addition, the material adheres poorly to cells due to its hydrophobic surface. PCL solvents are also known to be toxic, which can potentially harm human beings. The relatively low melting point is also demonstrates another drawback, since it hinders the material from being applied at higher temperatures [25,26,27]. Table 4 shows the summary of advantages and disadvantages of PCL.
Table 4.
Advantages and disadvantages of PCL.
| Advantages | Disadvantages |
|---|---|
| High biocompatibility | Adheres poorly to cells |
| Highly biodegradable | Toxic solvent |
| Great electrospinning properties | Low melting point |
| Long biodegradable time | Complex and expensive production |
| High material purity |
Nonetheless, those limitations of PCL can potentially be overcome by the use of PCL-based biocomposites. Extensive efforts have been made over the last decade in the development of PCL-based biocomposites. The properties of PCL can be improved by blending with other polymers or fibres, allowing more people to benefit from its excellent properties [28,29,30,31]. Mechanical and thermal properties are the most influential, affected by the addition of fillers to the PCL [32,33,34]. Several studies have shown that the PCL-based biocomposites can be benefited for several applications, especially in the biomedical field [26,35,36,37,38,39].
Therefore, this review opens up the potential for PCL to be used as a matrix along with natural fibers to form biocomposites. There are two types of biocomposites, i.e., green biocomposites and hybrid biocomposite, which are discussed. Moreover, this review intends to discuss the effect on mechanical and thermal properties of PCL-based biocomposites. Moreover, the potential application of blends of PCL with other materials is also investigated.
2. Overview on Natural Fiber
Humans have used natural fibers since the beginning of time, as shown by the discovery of wool and dyed flax 36,000 years ago in Georgia [40]. Natural fibers are derived from fruits, herbs, and animals. Among the engineering materials, natural fibers are used in the additive manufacturing process to create sustainable biocomposite structures [41,42]. Due to their diverse properties, such as their good mechanical behavior, low cost, eco-friendly nature, and biodegradability, they are gaining attention among scientists [43,44,45].
Additionally, natural fibers can be composed of a variety of textures, allowing them to be used in textile additive manufacturing. For example, common natural fibers, such as cotton, can absorb sweat effectively. Soft and lightweight cotton fibers can be manufactured in any size or shape, making them suitable for use with additive manufacturing techniques [46]. In contrast, natural fiber biocomposites are seen as a possible advancement in the field of material science because they are lighter and less costly than synthetic fiber composites. Furthermore, natural fibers are also biodegradable, unlike synthetic fibers, drawing the interest of environmentalists in these challenging circumstances [47].
2.1. Types of Natural Fiber
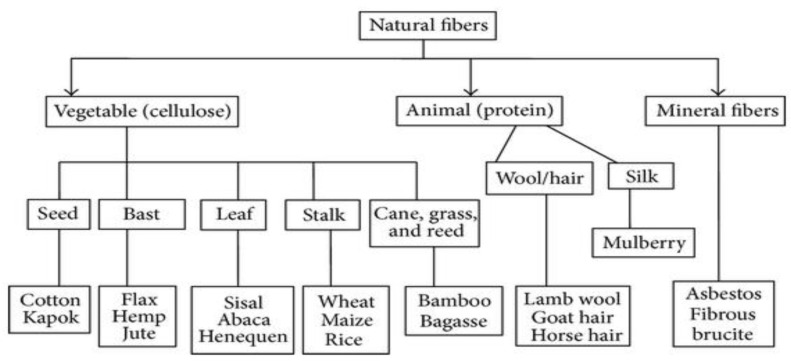
Natural fibers are those that are produced from plants or animals. It has a number of advantages over synthetic fibers, including their abundance, availability, and low cost [48,49]. These materials possess promising potential for a wide range of industries, including the biomedical, packaging, adsorption, aerospace, textile, food, and automotive industries [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. There are three main classes depending on their sources, including vegetables (cellulose), animal, and mineral fibers. Figure 2 shows the types of natural fibers. Plant fibers are the most widely acknowledged fiber within industry and the most thoroughly studied by researchers. This is owing to the short growth period, renewability, and broader availability [65,66,67,68].
Figure 2.
Type of natural fibers.
2.2. Chemical Composition of Green Fibers
The constituents of plant fibers include cellulose, lignin, hemicellulose, pectin, waxes, and water-soluble substances [69,70,71]. The presence of cellulose, which is hydrophilic in nature, affects the interfacial bonding between the polymer matrix and the fibers, since the matrix is hydrophobic [72,73]. These compositions, in turn, will affect the mechanical properties of the fibers [74]. Table 5 shows composition of cellulose, hemicellulose, lignin, and pectin in selected fibers [75].
Table 5.
Chemical composition of selected fibers.
| Fibers | Chemical Compositions (wt.%) * | |||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Pectin | |
| Abaca | 62.5 | 21 | 12 | 0.8 |
| Alfa | 45.4 | 38.5 | 14.9 | - |
| Bagasse | 37 | 21 | 22 | 10 |
| Banana | 62.5 | 12.5 | 7.5 | 4 |
| Bamboo | 34.5 | 20.5 | 26 | - |
| Coir | 46 | 0.3 | 45 | 4 |
| Cotton | 89 | 4 | 0.75 | 6 |
| Curaua | 73.6 | 5 | 7.5 | - |
| Flax | 70.5 | 16.5 | 2.5 | 0.9 |
| Hemp | 81 | 20 | 4 | 0.9 |
| Henequen | 60 | 28 | 8 | - |
| Isora | 74 | - | 23 | - |
| Jute | 67 | 16 | 9 | 0.2 |
| Kapok | 13.16 | - | - | - |
| Kenaf | 53.5 | 21 | 17 | 2 |
| Nettle | 86 | 10 | - | - |
| Phormium | 67 | 30 | 11 | - |
| Piassava | 28.6 | 25.8 | 45 | - |
| Pineapple | 80.5 | 17.5 | 8.3 | 4 |
| Ramie | 72 | 14 | 0.8 | 1.95 |
| Sisal | 60 | 11.5 | 8 | 1.2 |
* Note that the total composition in some fibers may not add up to 100% due to presence of pectin, lignin and waxes, which may exist in negligible amount and can be considered as zero.
2.3. Mechanical Properties of Natural Fibers
The mechanical properties depend on the chemical composition of the single fibre grooving (climate, soil feature, aging conditions, etc.) and processing method conditions [76]. Usually, the high cellulose content of lignocellulosic fibers is a significant contributor to their excellent tensile properties [77,78]. The mechanical properties of natural fibers can greatly affect the performance of the biocomposites [79,80]. Table 6 summarizes the mechanical properties of natural fibers from various sources [81].
Table 6.
Mechanical properties of natural fibers.
| Type of Fiber | Diameter (μm) | Density (g/cm3) | Tensile Strength (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|---|
| Abaca | 250–300 | 1.5 | 717 | 18.6 |
| Jute | 250–2500 | 1.3–1.49 | 393–800 | 13–26.5 |
| Sisal | 205–230 | 1.41 | 350–370 | 12.8 |
| Kenaf | 83.5 | 1.2 | 282.60 | 7.13 |
| Coconut | 396.98 | 1.2 | 140–225 | 3–5 |
| Bamboo | - | 1.2–1.5 | 500–575 | 27–40 |
| Date palm | - | 0.463 | 125–200 | - |
| Banana | - | 0.95–0.75 | 180–430 | - |
| Reed | - | 0.49 | 70–140 | - |
2.4. Pre-Treatment of Natural Fibers
Natural fibers have some disadvantages, such as high water absorption and thermal instability. The use of untreated natural fibers usually show decreased strength and modulus when the fibre volume fraction is increased [5,79]. These problems can be overcome by treating the natural fibers [58,82,83,84,85]. Pre-treatment can be carried out either by mechanical, chemical, and biological methods, or a combination of all of them. Chemical pre-treatment is the common method used to pretreat the lignocellulosic biomass in order to completely or partially remove the components [86,87,88]. The main goal of the pre-treatment is to alter or remove structural and compositional impediments to increase yields of intended cellulose products [89]. For example, the presence of hemicellulose and lignin in the lignocellulosic materials result in the ineffective stress transfer between the fibre and matrix, due to lower adhesion. Besides that, the presence of too high of hemicellulose content will affect the degree of crystallinity of fibre, leading to poor mechanical properties of polymer composite obtained [90]. It is necessary to bring a hydrophobic nature to the fibres by suitable chemical treatments in order to develop biocomposites with improved mechanical properties. Therefore, pre-treatment of fibres is important to achieve one or more of the following objectives [91,92]:
Removal of undesirable fibre constituents;
Roughening of the fibre surface;
Separation of individual fibre from their bundles;
Modification of the chemical nature of the fibre surface;
Reduction in the hydrophilicity of the fibre;
Cost-effectiveness;
Avoidance of degradation or loss of carbohydrate.
Pre-treatment of natural fibers can also strengthen the interaction between the fibers and the polymer matrix [59,93,94]. It increases the interfacial interaction between the matrix and the fibers by lowering the number of OH functional groups on the fibers surface while also lowering the surface roughness. Some chemical compounds, such as silane, acetic acid, sodium hydroxide, acrylic acid, isocyanates, malleated coupling agents, potassium permanganate, peroxide, and others, serve as coupling agents to form a strong interface bond between reinforcement and matrix. Thus, this minimizes the moisture absorption potential. Additionally, the alkalization process is used to physically pretreat natural fibers. This approach improves fiber mechanical properties and leads to stronger fiber interlock with the polymer matrix [95].
Meanwhile, pre-treatment of natural fibers also isolate the nanocellulose [51,96]. Nanocellulose has emerged as a new class of biobased nanofiller material to enhance the performance of biocomposites, including PCL-based biocomposites. Nanocellulose in uniform in diameter and has excellent nanofibrillar morphology [10,97,98]. Nanocellulose can be categorized into cellulose nanofiber (CNF), cellulose nanocrystals (CNC), and bacterial nanocellulose (BNC), depending on their origin and the pre-treatment approach used [60,61,99,100]. The advantages of using nanocellulose, as compared to cellulose in biocomposites, include its higher surface area, aspect ratio, and Young’s modulus. Usually, a small amount of nanocellulose is required to enhance the properties of the biocomposites [63,64,101,102].
3. Constituents and Types of Biocomposites
There are several different types of biocomposites used today. Biocomposites can be described as a material composed of two or more distinct constituent materials where one of them is naturally derived [57,63,103]. This combination helps to yield a new material with an improved performance compared to individual neat materials. Biocomposites can be classified based on three classes of reinforcement and polymer materials:
Totally renewable composites, whereby both the matrix and reinforcement are from renewable resources;
Partly renewable composites, whereby the matrix is obtained from renewable resources and reinforced with a synthetic material;
Partly renewable composites, whereby the synthetic matrix is reinforced with natural bio polymers.
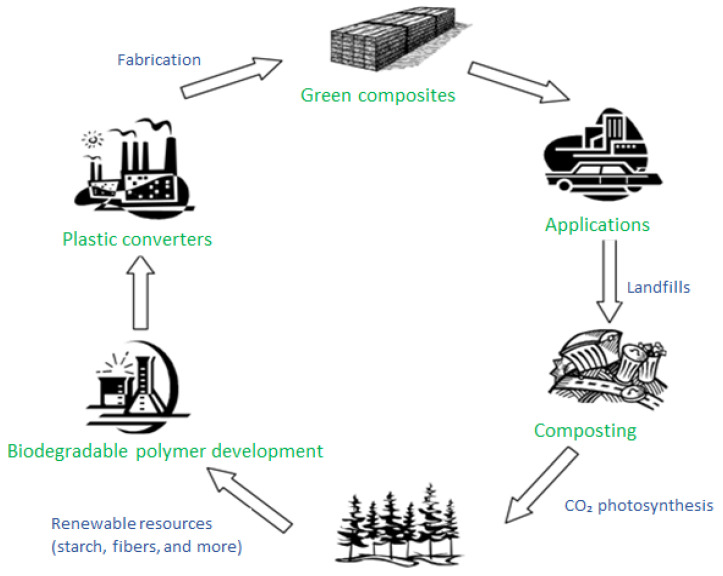
Figure 3 shows the life cycle of biocomposites. Consequently, biocomposites are a biodegradable and eco-friendly, and can be completely resolved into H2O and CO2 through microorganism’s degradation in the soil or through an incineration process without causing the emissions of toxic gases [104].
Figure 3.
Life cycle of biocomposites.
In this review, two types of biocomposites were evaluated: green biocomposites and hybrid biocomposites. Green biocomposites are known for their combination of biodegradable resins with other polymers [105]. Because of their degradable and sustainable properties, they are called green composites, as they can be easily disposed without harming the environment. Ecological concerns, such recyclability and environmental safety, have resulted in renewed interest in natural materials. In recent years, a great interest in research on more environmentally friendly materials derived from non-renewable sources has gained the attention among researchers whose primary aim is to raise public awareness of environmental sustainability [106,107]. Green biocomposites are economical and are easy to decompose. In order to reduce our dependence on fossil fuels, there are more alternatives to develop bio-based resources, such as industrial goods, wood and wood wastes, and other advanced materials. In recent years, low-density natural fibers have been used to reduce the use of expensive glass and carbon fiber, and to reduce the weight of cars. Additionally, natural fibers are less expensive, and have high thermal insulation and biodegradability. Nowadays, by using different type of natural fibers, the green biocomposites have been advanced with enhanced mechanical properties by the researchers. The properties of the natural fibers used for reinforcement can affect the performance of green composites [108]. Herrmann et al. initially reported a study of the fabrication of green composites. They developed a coir–fiber-reinforced polyhydroxybutyrate-co-volerate (PHBV) resin composite and investigated its mechanical properties [109].
Meanwhile, hybrid biocomposites are defined as fabricated materials with two or more fibers inside a single matrix. Hybridization offers new opportunities to broaden the function of the composite materials, particularly in advanced applications by improving the toughness or impact resistance [110]. In this case, PCL refers to a composite which consists of PCL matrix along with two or more fibers within it. Compared to a traditional composite, a hybrid composite offers enhanced properties and advantages. Hybrid composites materials formed by combining two or more different types of fibres in a common matrix are gaining popularity because they provide a variety of properties that cannot be accomplished with a single form of reinforcement. Simultaneously, careful selection of reinforcing fibres can dramatically reduce material costs. Several researchers have independently observed the “hybrid effect” using various types of fibres and reinforcement configurations (such as collimated fibres and fabrics). As a result, it is most likely a real impact rather than a test artifact. Composite failure under tensile load is a complicated, statistical process involving fibre power, matrix, and interfacial properties [111].
4. Polycaprolactone-Based Green Biocomposites
Many studies and scientific projects focus on green biocomposites. Green biocomposites integrate the beneficial properties of the individual constituents. Due to the production of materials with good properties and materials which are easily produced and processed, the market for these biocomposites appears to be promising and attainable for growth in the near future [45,112,113]. Biopolymers reinforced with natural fibers greatly improved the mechanical and thermal properties of green biocomposites [114,115,116]. Therefore, several mechanical and thermal properties of PCL-based green biocomposites are discussed in this section.
4.1. Mechanical Properties of Polycaprolactone-Based Green Biocomposites
For improved mechanical properties, PCL can be added with stiffer materials and is easily processed by traditional melting techniques due to its low melting temperature. PCL is often mixed with other polymers, including PP, polycarbonate (PC), polyethylene oxide (PEO), and starch, in order to create biocomposites with desired properties. The versatile and biodegradable PCL has been blended with other polymers and considered for use in biomedical applications, such as catheters, blood bags, and packaging [117]. PCL has been degraded by extracellular depolymerase or in a variety of natural environments [118]. PCL has gained popularity as a biodegradable packaging alternative to non-biodegradable products. Despite its many benefits, due to its poor thermal stability, only a few studies have assessed PCL as a biodegradable material for food packaging. PCL’s low melting point makes it easy to process, but it also restricts its applications [119].
Lu et al. (2014) [120] studied the mechanical properties of PCL-hydroxyapatite (HA) porous scaffolds created by Porogen-based solid free form a fabrication method. The ultimate compressive strength of a porous scaffold built of 80/20 polycaprolactone–hydroxyapatite (PCL–HA) composite was 3.7 ± 0.2 MPa, with a compression modulus of 61.4 ± 3.4 Mpa, which is comparable to the compressive strength of the trabecular bone. The compressive characteristics and stiffness of PCL–HA composites were considerably improved (P0.05) when the concentration of HA in the composites was increased. This shows that PCL–HA composites have the potential to be used in bone regeneration. The ultimate tensile strength and tensile modulus of solid PCL and PCL–HA composites increased as the concentration of HA in the composite increased, according to tensile tests.
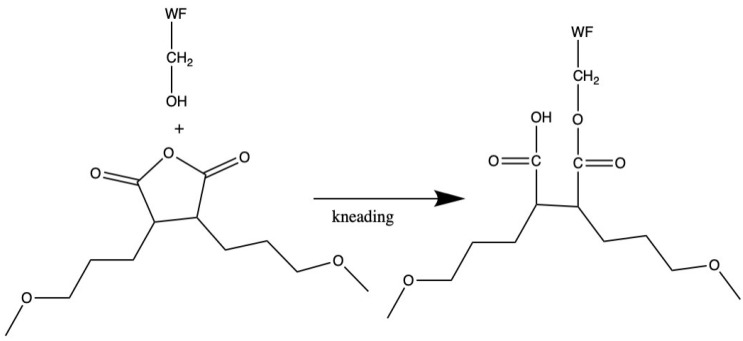
Lee et al. (2003) [121] obtained higher tensile modulus from the preparation of wood flour (WF)–PCL biocomposites by kneading process. The PCL grafted maleic anhydride (PCL-g-MA) was used as a compatibilizer. Figure 4 depicts a potential reaction between WF and PCL-g-MA. PCL-g-MA was added to the biocomposites to improve their tensile properties. Tensile strength and Young’s modulus in WF–PCL (50/50, w/w) biocomposites increased from 13 to 27 MPa and 581 to 1011 MPa, respectively, with the addition of 5% PCL-g-MA. They reported that the addition of PCL-g-MA enhanced the tensile properties. The elongation at break increased from 4 to 7%. These increments on mechanical performance may be related to chemical bonds between the anhydrous group of MA and the OH groups of WF.
Figure 4.
Possible reaction of WF and PCL-g-MA.
Combining a biobased polymeric material, such as wheat gluten (WG), with PCL is one way to cut costs and change material properties. In a study conducted by Finkenstadt et al. (2008) [122], WG was used as a filler in PCL (up to 50% w/w) to create biodegradable biocomposites. An examination under a microscope revealed a well-distributed particle-matrix system. The tensile strength of the biocomposites decreased linearly as the WG content increased from 0% to 6%. (50% WG). The tensile strength for PCL and WG is between the range of 5 to 15 MPa. Packaging materials, hygiene products, disposable consumer goods, and agricultural products may all benefit from these WG–PCL biocomposites.
In a study on the development of sustainable biodegradable lignocellulosic hemp fiber–PCL biocomposites by Dhakal et al. (2018) [123], it was shown that the biocomposites possess enhanced mechanical properties compared to PCL. The sample was first made by a twin screw extrusion machine with a different l/d fiber ratio of 19, 26, 30, and 38, respectively. The result shows that the tensile strength of biocomposites with higher aspect ratios does not differ much from PCL, apart from the biocomposites with an aspect ratio of 26, although it is significantly weaker in wet conditions compared to dry conditions. However, Young’s modulus of this composite was shown to encourage an increase from 0.14 GPa to 0.40 GPa, signifying a 186% increase from PCL. Based on the result, it is shown that the biocomposites with a fiber aspect ratio of 26 out-performed other samples in both dry and saturated conditions. Since the fiber is inherently hydrophilic, appropriate treatment on surface of fiber is suggested to be carried out to ensure that it is compatible with PCL.
Lakshmipriya et al. (2019) [124] studied the silk–PCL biocomposites using various layers of woven silk fabric as reinforcement, along with the PCL polymer matrix. The stacking of different layers of silk fabric-reinforced PCL biocomposites in this study was prepared using compression molding. The 14 layers of silk–PCL biocomposites showed higher mechanical properties when compared to other composites. This was due to its sufficient mechanical interlocking between the fabric and the matrix. However, there was a slightly decreass in the tensile properties of biocomposites, but it can still be used as a biodegradable plate due to its adequate mechanical strength.
Additionally, several researchers have successfully developed cellulose–PCL biocomposites from various sources of fibers. Theeranattapong et al. (2015) [125] developed the biocomposites of oxidized regenerated cellulose–PCL for use as a synthetic dura meter. Cellulose–PCL biocomposites were prepared with solution infiltration of a cellulose sheet with PCL solution at various concentrations ranging from 10–50 g per 100 mL. It was found that the tensile properties were initially decreased with increasing PCL concentration for up to 20%. Then, it increased with further increasing PCL concentrations. Elongation at break of all formulations was not significantly different. Interestingly, the mechanical properties of the samples were found to be similar to those of natural human dura mater.
Aguiar et al. (2016) [126] prepared a cellulose–PCL biocomposites and evaluated their mechanical performance. In their work, alkaline and acid pre-treatment were performed on fibres to remove the amorphous portion and to aid fibrillation. PCL–cellulose biocomposites were prepared in a twin screw extruder by adding different cellulose (at 2, 5, and 10 wt.%) and maintaining the processing time constant. The results suggest that increasing the cellulose crystallinity leads to an increased nucleation effect for PCL and that cellulose with low crystallinity hinders crystallization of the matrix. Moreover, shorter cellulose fibres promote a higher nucleation effect in the PCL matrix. The dynamic-mechanical analysis showed an increase in the modulus of the biocomposites containing 5 wt.% cellulose.
Moreover, Alemán-Domínguez et al. (2018) [127] manufactured porous cellulose–PCL biocomposites for application as scaffolds. The biocomposites contained 0, 2, and 5 wt.% of the carboxymethyl cellulose. Biocomposites have similar values of porosity and apparent density than the neat PCL. However, biocomposites containing 5 wt.% of carboxymethyl cellulose have micropores on the filaments due to a hindered deposition process. This characteristic affects the mechanical properties of the structures, so these ones have a mean compression modulus significantly lower than the neat PCL scaffolds. However, the mechanical properties of biocomposites containing 2 wt.% of carboxymethyl cellulose show no significant difference with the neat PCL.
Meanwhile, the use of nanocellulose in PCL-based biocomposites has also been reported in several studies. Sheng et al. (2014) [128] investigated the mechanical performance of electro-spun PCL–CNC biocomposite mats. The results showed that the mechanical properties of the biocomposite mats were improved and that the surface hydrophilicity was increased in comparison with neat PCL mats. The tensile strength of the biocomposites mats was improved following an increase in CNC loading. The maximum strength appeared at 10 wt.% CNC loading, and then it gradually decreased. This is due to the higher CNC content which led to the aggregation of CNC. Moreover, the modulus was significantly increased compared to the neat PCL mat. However, the strain at break decreased as compared to the neat PCL.
Table 7 summarizes the mechanical properties of several PCL-based green biocomposites.
Table 7.
Mechanical properties of PCL-based green biocomposites.
| Green Biocomposites | Processing Technique | Mechanical Properties | Ref. | |||
|---|---|---|---|---|---|---|
| Tensile Strength | Tensile Modulus | Flexural Strength | Flexural Modulus | |||
| Cellulose acetate–PCL | Solvent casting | 10 MPa | - | 400 MPa | - | [129] |
| Cellulose–PCL | Solution infiltration | 2.8–6.2 MPa | 15–44 MPa | - | - | [125] |
| Cellulose–PCL | Extrusion | 2.12 ± 0.15 to 2.35 ± 0.51 MPa | - | - | 18.7 ± 3.1 to 12.5 ± 4.0 MPa |
[127] |
| CNC–PCL | Electrospinning | 1.1–1.6 MPa | 7.2 MPa | - | - | [128] |
| Wood flour–PCL | Knead processing | 13–27 MPa | 581–1011 MPa | - | - | [121] |
| Wheat–PCL | Blending | 5–15 MPa | 160–500 MPa | - | - | [122] |
| Starch–PCL | Predrying of starch | 29.49 ± 2.7 MPa | [130] | |||
| Silk fabric–PCL | Compression moulding | 92.93 ± 3.273 MPa | 1.143 ± 0.108 MPa | 36.036 ± 1.903 MPa | 2.688 ± 0.0124 MPa | [124] |
| HAP–PCL | Grafted and blended techniques | 2.53 ± 0.21 MPa | 111.92 ± 3.97 MPa | - | - | [131] |
| Cellulose–PCL | Wet feeding | 31 ± 0.71 MPa | 1.85 ± 0.08 MPa | - | - | [132] |
| Cellulose–PCL | Dry-feeding | 24 ± 1.24 MPa | 0.59 ± 0.06 MPa | - | - | [132] |
| Cellulose–PCL | Electrospinning process | 4.45 ± 0.32 MPa | 19.17 ± 0.8 MPa | - | - | [133] |
| Oil palm Fiber–PCL | Melt blending | 9.8 MPa | 250 MPa | - | - | [134] |
4.2. Thermal Properties of Polycaprolactone-Based Green Biocomposites
There are several studies which have reported on the thermal properties of PCL-based green biocomposites. In 2014, Khandanlou et al. [135] investigated the effect of modified rice straw (RS) on the thermal properties of PCL biocomposites prepared via the solution–casting method. The concentration of rice straw was varied. Based on the thermal properties analysis, the TGA thermograms revealed that the biocomposites have a lower on-set temperature for thermal degradation than neat PCL. The TGA and DTG measurements of degradation temperature at 5.0, 10.0, 50.0, and 80.0 percent fibers degradation are shown in Table 8.
Table 8.
TGA and DTG measurements of degradation temperature at 5.0, 10.0, 50.0, and 80.0 percent fiber degradation. Reproduce with copyright permission from Khandanlou et al. [135].
| Sample | T5% (°C) | T10% (°C) | T80% (°C) | Tmax (°C) | T5% (°C) | Residue at 500 °C (%) |
|---|---|---|---|---|---|---|
| ORS | 222.55 | 343.11 | 452.60 | 452.60 | 404.45 | 17.2 |
| PCL | 380.01 | 404.16 | 418.50 | 418.50 | 409.04 | 5.0 |
| 1.0% | 367.95 | 400.57 | 413.62 | 413.62 | 402.27 | 5.2 |
| 3.0% | 356.29 | 393.07 | 409.08 | 409.08 | 400.10 | 6.3 |
| 5.0% | 349.94 | 388.53 | 404.37 | 404.37 | 387.76 | 8.1 |
| 7.0% | 343.13 | 381.72 | 400.15 | 400.15 | 372.95 | 10.9 |
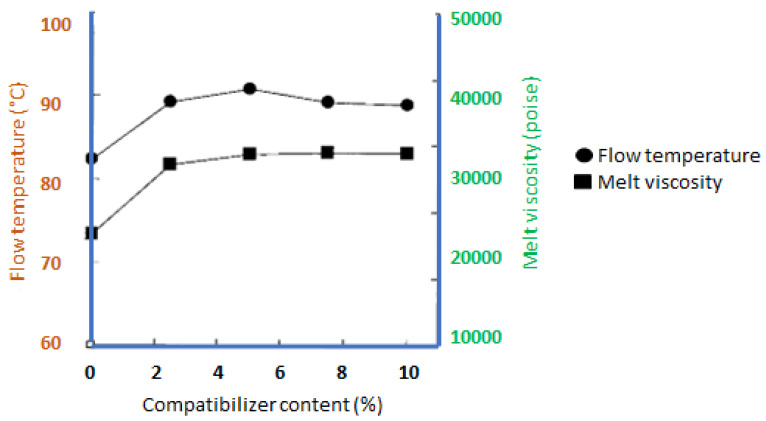
On the other hand, Lee et al. [121] has studied the thermal flow properties of wood flour (WF)–PCL biocomposites. As a compatibilizer, PCL-g-MA was used in this study. The effects of the compatibilizer material on the flow properties of the WF–PCL biocomposites are shown in Figure 5. It shows that adding 2% PCL-g-MA increased the melt viscosity from 11,600 to 27,100 poise and increased the thermal flow temperature from 82 °C to 89 °C. During the kneading reaction, esterification occurred between the acid anhydride groups of PCL-g-MA and the OH groups of WF, as shown by the variations in flow temperature and melt viscosity. When MA and WF are mixed and heated to more than 60 °C, esterification easily occurs without the use of a catalyst or a solvent [136].
Figure 5.
Effects of PCL-g-MA content on the thermal properties of WF–PCL biocomposites [121].
5. Polycaprolactone-Based Hydrid Biocomposites
Over the past few years, there has been continuous improvement in the performance of PCL by producing hybrid biocomposites. PCL hybrid biocomposites have been developed by various researchers, combining PCL with several other compounds, such as acrylic acid, epoxy, unsaturated polyester, phenolic, vinyl ester, as well as natural fibers [137,138,139,140,141,142,143]. In this section, the mechanical and thermal properties of PCL hybrid biocomposites are presented.
5.1. Mechanical Properties of Polycaprolactone Hybrid Biocomposites
The hybridization of biocomposites material is the key to designing new components with good strength at a relatively lower cost [110]. The mechanical properties of PCL hybrid biocomposites gives an advantage of good strength at a lower cost, which can be used for applications that were not possible by using the green biocomposites [144,145,146].
Wu et al. (2010) [147] investigated the structural and thermal effects of substituting a more compatible acrylic acid-grafted PCL (PCL-g-AA) for neat PCL in coconut fibre (CF) containing biocomposites. CF is commonly available as a by-product of coconut production in Taiwan’s coastal regions. A coconut weighs about 600 g and contains drinkable juice between the green outer shell and the hard nut in the centre. Within the coconut, there is a 3–5-cm-thick white fibrous content. In their study, the formation of branched and cross-linked macromolecules through reactions between carboxyl groups in PCL-g-AA and hydroxyl groups in CF resulted in significantly more homogeneous fibre dispersion in the PCL-g-AA matrix. The PCL-g-AA–CF biocomposites had a significantly higher tensile strength at break than he tPCL–CF biocomposites. Due to its lower melt viscosity, the PCL-g-AA–CF blend was also easier to process. From the study, it shows that the tensile strength of PCL–CF can range between 12 to 30 MPa.
The combination of strength and elasticity has been a particular challenge in the development of synthetic grafts, with conventional materials, such as segmented polyurethanes, which can be modified to strengthen one at the expense of the other [148]. Wise et al. (2011) [149] conducted study on multilayered synthetic human elastin/PCL hybrid vascular graft with tailored mechanical properties. The result shows that the elastin–PCL hybrid biocomposites have the lowest tensile strength within a range of 500–520 kPa due to its specific requirement. Tropoelastin was then added to a synthetic elastin–PCL conduit with mechanical properties similar to the human internal mammary artery, including permeability, compliance, elastic modulus, and burst strain. This multi-layered conduit also demonstrated suturability and mechanical durability in a small-scale rabbit carotid interposition model by presenting a synthetic elastin internal lamina to circulating blood. According to the researchers, synthetic elastin was successfully engineered into a completely synthetic vascular graft that matches the mechanical properties of the internal mammary artery.
Moreover, several other research have reported on the mechanical properties of cellulose/PCL hybrid biocomposites. Ali (2016) [150] prepared hybrid biocomposites of cellulose acetate propionate (CAP)–starch–PCL for packaging applications. CAP was used as a cellulose derivative to enhance the physical properties of biocomposites. The addition of CAP to starch and PCL blends improved the mechanical properties, even at a high content of starch. It was found that the tensile strength and elongation at break of CAP–starch–PCL biocomposites were increased. It is suggested that CAP improved the compatibility and the interfacial adhesion between PCL and starch. Moreover, it was found that the tensile yield strengths of most CAP–starch–PCL biocomposites were higher than the yield tensile strength of neat PCL. They suggested that it may be due to the existence of an interaction between this hybrid components.
Yu et al. (2018) [151] developed a graphene oxide–CNC–PCL biocomposite using quaternary ammonium salt systems to be used in ultraviolet-shielding films. The preparation of biocomposites involved grafting the CNC with PCL by ring-opening copolymerization catalysed by 4-dimethylaminopyridine in a dual tetrabutylammonium acetate–dimethyl sulfoxide solvent system. Then, a novel ultraviolet-shielding film based on CNC–PCL biocomposites was prepared by introducing graphene oxide. The results obtained showed that the introduction of graphene oxide not only obviously influenced the inherent structure of the hybrid biocomposites but remarkably changed the surface morphology of the film. Moreover, the graphene oxide–CNC–PCL hybrid biocomposites showed a significant improvement in tensile strength, from 2.63 to 4.55 MPa, as well as elongation-at-break, from 6.4 to 15.5%, compared with the CNC–PCL biocomposites, owing to the strong hydrogen-bonding interaction that physically crosslinked graphene oxide with CNC–PCL biocomposites.
Gibril et al. (2019) [152] studied on the effect CNC and zinc oxide (ZnO) to the mechanical properties of PCL hybrid biocompsites. CNC loadings at 2–8 wt.% and ZnO were added onto the PCL matrix. Increasing CNC–ZnO content enhances the tensile strength with low filler contents at 2 and 4 wt.%, due to good dispersion of the CNC within the matrix, which yields a higher reinforcement effect. However, higher CNC loadings at 6 and 8 wt.% loading resulted in a gradual decrease in the tensile strength of hybrid biocomposites due to the agglomeration of the CNC, which restrains stress movement, interrupts the matrix segmental mobility, and promotes rigidity. By adding different amounts of CNC, the elongation at break of hybrid biocomposites decreased (at loading of 6 and 8 wt.%), except for samples prepared with low content of CNC (2 and 4 wt.%). This also influenced by the dispersion of CNC within the PCL matrix.
Recently, Quilez-Molina et al. (2021) [153] developed curcumin-functionalized cellulose–magnesium and carbonate–PCL hybrid biocomposites. Magnesium carbonate (MgCO3) was used as a food additive. Meanwhile, cellulose was functionalized with ethanoic curcumin solutions to protect them against oxidative degradation. The development of this material is simple, cost-effective, non-toxic, and paper-like. Flexible bio-composites can be fabricated on large scale and can be used in several applications, ranging from sustainable packaging to medical applications and freshness indicators. They reported that the mechanical properties of hybrid biocomposites depend on different factors, such as the fiber direction, nature of the fibers, length, and the bonding strength between the fibers and polymers. It was found that functionalizing the cellulose with curcumin did not affect the mechanical properties of hybrid biocomposites. Moreover, the warp direction of hybrid biocomposites exhibited higher elastic modulus (ten times more than that of the weft direction).
Table 9 summarized the mechanical properties of several PCL-based hybrid biocomposites.
Table 9.
Mechanical properties of PCL hybrid biocomposites.
| Hybrid Biocomposites | Processing Technique | Mechanical Properties | Ref. | |||
|---|---|---|---|---|---|---|
| Tensile Strength | Tensile Modulus | Flexural Strength | Flexural Modulus | |||
| CAP–starch–PCL | Internal blending | Increased (value is not reported) | Increased (value is not reported) | - | - | [150] |
| Graphene oxide–CNC–PCL | Solvent casting and ultrasonication | 4.55 ± 0.15 MPa | - | - | - | [151] |
| Zinc oxide–CNC–PCL | Solvent casting | 12.16 ± 0.51 to 15.41 ± 0.79 MPa | 61.71 ± 0.17 to 91.71 ± 0.23 MPa | - | - | [152] |
| Elastic–PCL | - | 500–520 kPa | 257–281 kPa | - | - | [149] |
| Gelatin–acetylated CNF–PCL | Electrospun | 2.5–4.3 MPa | 21.3–24.1 MPa | - | - | [142] |
| Coconut–acrylic acid–PCL | Blending | 12–30 MPa | - | - | - | [147] |
| Alginate–PCL(electrospun)–PCL(struts) | Electrospun | 0.3–12.5 MPa | 0.9–10 MPa | - | - | [154] |
5.2. Thermal Properties of Polycaprolactone-Based Hybrid Biocomposites
The thermal stability of the hybrid biocomposites can be significantly improved as compared to the neat polymer. In recent years, there has been a lot of research to improve the thermal properties of hybrid biocomposites [110,155]. The properties of the hybrid biocomposites have been enhanced by the addition of nanomaterials, metallic, carbon, and ceramic-based fillers.
Hybrid biocomposites have comparable strength and properties to many traditional plastics as a result of the inclusion of nanoclay. Furthermore, the use of natural fibers in hybrid biocomposites lowers the cost of biodegradable polymer, expanding the range of applications for biodegradable polymer-based biocomposites. The applications of hybrid biocomposites may be more suitable for single-use materials that do not require a lot of strength, though biodegradability and environmental friendliness are also important considerations in material selection.
Abdolmohammadi et al. (2011) [156] investigated the effect of thermal properties of PCL/chitosan hybrid biocomposites when sodium montmorillonite (Na MMT) and octadecylamine montmorillonite (ODA-MMT) are used. Based on the result obtained, the degradation temperature is increased in both micro and nanocomposites when Na MMT and ODA-MMT are added to the PCL/chitosan blend, with the maximum thermal stability observed at 1 wt.% Na MMT and 3 wt.% ODA-MMT loadings. The degradation becomes faster at higher ODA-MMT loadings than 3 wt.% due to the higher amount of modifier in the matrix. Since the modifier has a lower thermal stability, it degrades more quickly. Besides that, by loading 1 wt. 100% Na MMT and 3 wt.% ODA-MMT, the onset temperature of PCL/chitosan biocomposite increases from 231.45 °C to 238.76 °C and 254.71 °C, respectively. Two stages of degradation of PCL/chitosan blend was observed in the experiment. The first step, which occurs at Tmax1 = 283 °C, is chitosan backbone degradation caused by saccharide rings dehydration, and the second stage, which occurs at Tmax2 = 382 °C, is PCL decomposition. After adding 1 wt.% Na MMT and 3 wt.% ODA-MMT, the second stage (Tmax2) rises significantly from 382.89 °C for the PCL/chitosan biocomposite to 392.55 °C and 412.72 °C, respectively. The increase in degradation temperature is due to the clay’s high thermal stability, which limits heat transmission and decreases the diffusion of volatile substances released by substances. Due to its exfoliated-intercalated structure and improved interaction between ODA-MMT and matrix, nanocomposites have a higher thermal stability than micro composites. As a result, thermal stability in nanocomposite and microcomposite materials has improved, especially in nanocomposites, where the maximum thermal stability was observed at 3 wt.% ODA-MMT loading. The broken surface morphology of the PCL–chitosan blend becomes more stretched and homogeneous in PCL–chitosan–ODA-MMT than in PCL–chitosan–Na MMT.
Moreover, Eng et al. (2014) [157] investigated the effect of adding 1 wt.% hydrophilic nanoclay on PLA–PCL–oil palm mesocarp fiber (OPMF) biocomposites towards the thermal properties. Previous study stated that adding nanoclay to PLA–PCL–OPMF biocomposites improves their thermal stability. The PLA–PCL–OPMF biocomposites have an onset temperature of 196.01 °C, which increases to 221.12 °C when clay is added. Table 10 shows the thermal properties of PLA–PCL–OPMF biocomposites and PLA–PCL/1 wt.% clay/OPMF hybrid biocomposites. By increasing the thermal stability of hybrid biocomposites, clay will minimize the permeability of volatile degradation materials. The dispersed clay, in contrast to the smooth polymer, acts as a barrier, delaying the release of thermal degradation products.
Table 10.
Thermal properties of PLA–PCL–OPMF biocomposites and PLA–PCL/1 wt.% clay/OPMF hybrid biocomposites.
| Sample | Onset Temperature (°C) | Offset Temperature (°C) | Percentage of Degradation |
|---|---|---|---|
| PLA–PCL–OPMF | 196.01 | 473.84 | 72.78 |
| PLA–PCL/1 wt.% clay–OPMF | 221.12 | 458.36 | 71.08 |
6. Applications of Polycaprolactone-Based Biocomposites
PCL has been widely applied in biomedical fields, especially in tissue engineering and medical implants, because they are biodegradable and have high biocompatibility. Manivasagam et al. (2019) [23] mentioned that polycaprolactones are being explored extensively in bone tissue engineering due to a lack of bioactivity and high degradation rates. Miller et al. (2011) [20] have stated that PCL can potentially be used as a bioscaffold. Nevertheless, when it comes to the application of tissue engineering, PCL suffers from some shortcomings, such as poor mechanical properties, slow degradation rate, and low cell adhesion. The reinforcement of bioactive glasses and calcium phosphate-based ceramics within PCL has created a class of hybrid biomaterials with improved controllable degradation rates, good mechanical properties, and enhanced bioactivity, making them suitable for bone tissue engineering [158]. PCL green biocomposites with superior mechanical properties can potentially be used as orthopaedic implants, as briefly outlined as.
The Food and Medication Administration (FDA) has approved PCL for use in specified applications in the human body as a suture, drug delivery device, or adhesion barrier [8,159,160,161]. Following the recent launch of a PCL-based microsphere dermal filler belonging to the collagen stimulator class (Ellansé), PCL is being employed in the field of human aesthetics [162,163]. PCL-based products have also been utilized to treat facial ageing indications, such as contour laxity and volume loss, by stimulating collagen formation, resulting in an immediate and long-lasting natural impact [159,163,164,165,166,167]. In addition, it is being studied as a scaffold for tissue engineering-mediated injury repair using the guided bone regeneration (GBR) membrane, which has been extensively reported by numerous researchers [168,169,170,171,172,173,174]. It has been utilized as a hydrophobic block in amphiphilic synthetic block copolymers that are used to build the vesicle membrane of polymersomes [175,176].
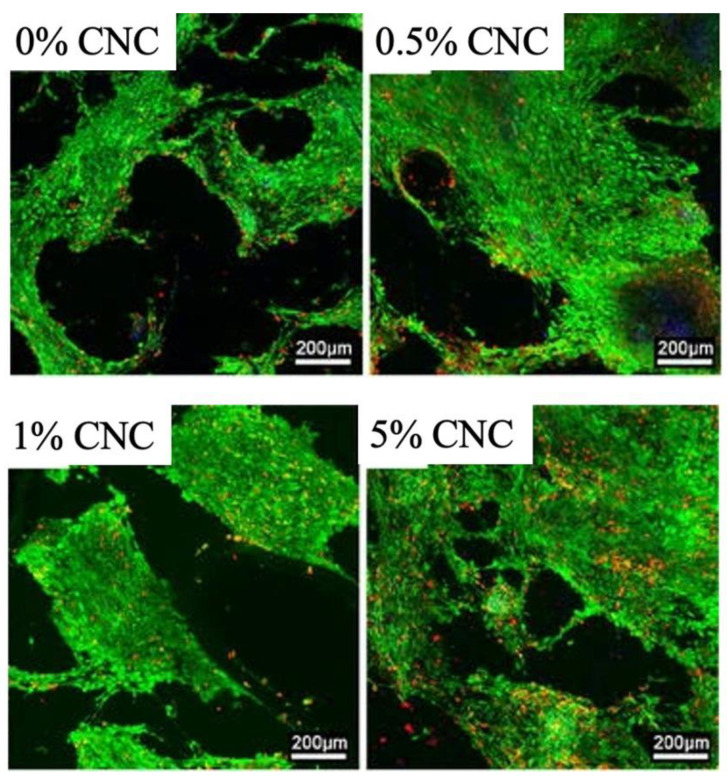
Recently, PCL green biocomposites have been developed and commercialized. The majority of PCL green biocomposites are currently in the research and development stage. To make green biocomposites at a cheaper cost, new processing techniques and technologies are being developed [177]. Hao and co-workers found that the twin screw extrusion was used to make PCL–CNC nanocomposites. Microcellular nanocomposite samples were created utilising microcellular injection moulding and a physical blowing agent of carbon dioxide (CO2). The biocompatibility of the material was examined. The result is depicted in Figure 6. The green hue in the florescence photographs symbolises live cells, whereas the red colour depicts dead cells. In comparison to bare dead cells, the clean PCL sample exhibits a substantial number of living cells. It was also found that 0.5% CNC and 1% CNC samples had mostly live cells, thus indicating good compatibility between the substrate and the cells.
Figure 6.
Florescence images with a scale bar of 200 μm of 3T3 fibroblast cell culture results at day 10 of microcellular injection moulded samples: neat PCL, 0.5% CNC, 1% CNC, and 5% CNC. Adapted with copyright permission from ref. [178].
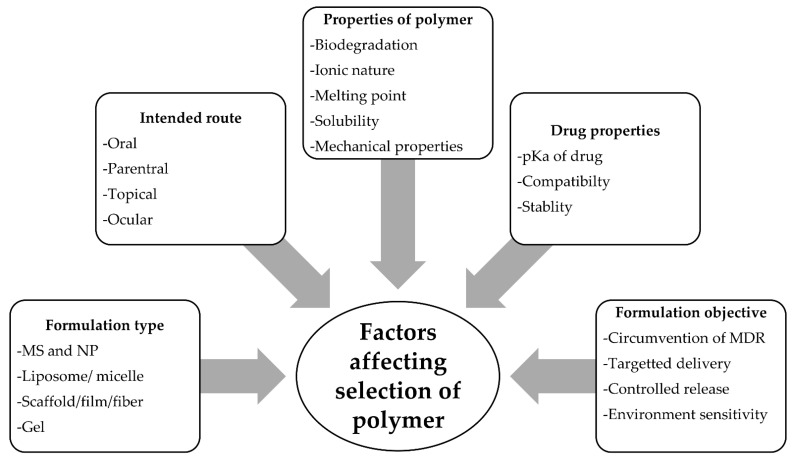
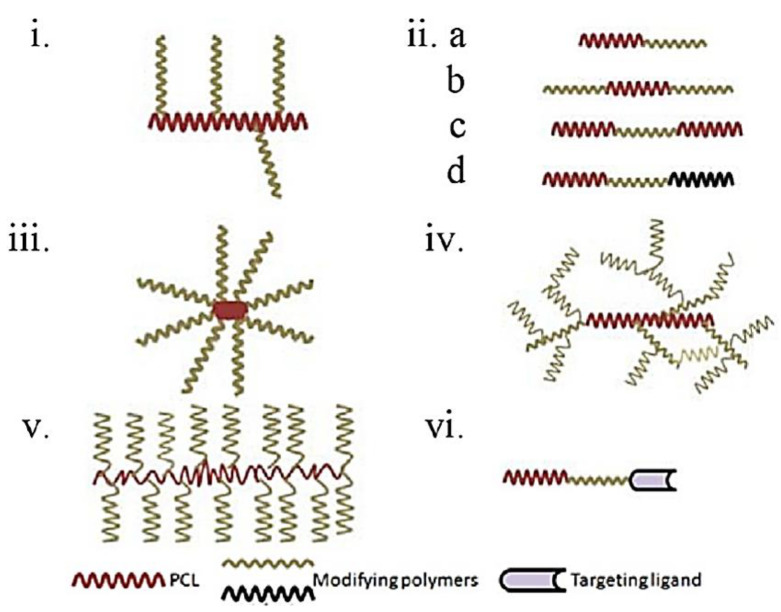
PCL beads have been used to encapsulate a range of medicines for controlled re-release and targeted drug delivery [179,180,181,182,183,184,185,186,187,188,189]. In addition to polymeric properties several other factors, such as the type and objective of formulation, the route of administration, drug or polymeric properties etc., also affect the selection of the polymer (Figure 7). Hence, selection of a polymer is an important step for the development of a successful drug delivery system or device [190]. PCL-based modified polymers demonstrated so far predominantly involve its copolymers with several other polymers in different forms (Figure 8). Nevertheless, other modifications (blends and composite) were also reported in different formulations [191,192].
Figure 7.
Factors affecting polymer selection.
Figure 8.
Different demonstrated architecture of PCL modification: (i) graft copolymer; (iia) diblock copolymer; (iib,c) triblock copolymer; (iid) tricomponent triblock copolymer; (iii) sta- shaped copolymer; (iv) hyperbranched copolymer; (v) molecular brush or comb shaped copolymer; and (vi) targeted block copolymer. Adapted with copyright permission from ref. [190].
Among biodegradable polymers modified for amelioration of properties, a special focus is made on PCL mainly due to its broad spectrum of compatibility with a wide range of other polymers. Its versatile nature, ease of fabrication, and biocompatibility establish it to be the polymer of interest by investigators worldwide for drug delivery and tissue engineering applications [193,194,195,196,197]. However, when we consider properties of unmodified PCL, there are considerable restrictions for its use. For example, its hydrophobic nature does not allow the facile release of hydrophobic drugs (from prepared formulations) and micelle formation. Furthermore, long-term degradation (ranging from weeks to months) slows down tissue replacement in the case of scaffolds, mechanical property limits its application to hard tissue engineering only, and nonreactivity is unsuitable for preparation of NC. Therefore, attempts have been made to overcome these undesirable properties by various types of modification, as mentioned in Figure 8, for successful application in pharmaceutical formulations. To great excitement, the use of modified PCL dominated over the past decade by virtue of which PCL was demonstrated in almost all novel formulations overcoming the above-mentioned restrictions. Additionally, functionalization as a result of PCL modifications is a featured advantage and is considered as another cause for this typical preference for modified PCL.
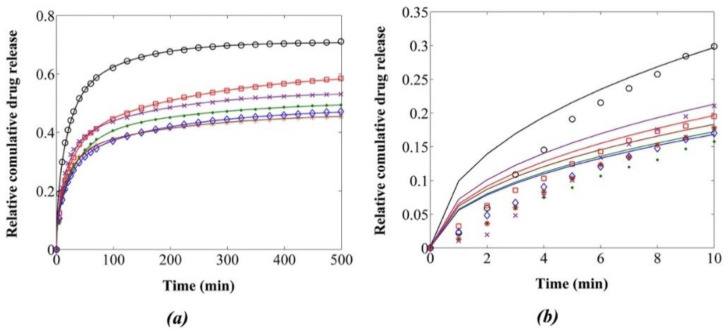
Additionally, the possibility of using PCL to be used as implants for targeted drug delivery has been explored. Boia et al. (2019) [198] have developed a novel way of using porous polycaprolactone as an intraocular implant to deliver dexamethasone to replace eye drops or intravitreal injections. The implants were made by using green supercritical carbon dioxide foaming or mixing methods, resulting in the implant having high porosity and high surface area. This, in turn, will cause a higher degradation rate than typical PCL-based implants, which have a relatively slower degradation rate. They are then inserted into adult rats for further observation. The implant is shown to have good biocompatibility, since it does not cause cell death or reduce the number of neurons [198]. In contrast, Hivechi et al. (2019) [199] investigated the regulated release of tetracycline hydrochloride using CNC-reinforced PCL nanofibers. The amount of CNC in the PCL nanofibers was increased, which resulted in a delayed release of a medication, as depicted in Figure 9a,b.
Figure 9.
Drug release diagram of drug loaded samples with various concentration of CNC (Experimental data for ○ PCL, □ PCL-CNC0.5%, × PCL-CNC1.0%, ● PCL-CNC1.5%, ▯ PCL-CNC2.5%, ◊ PCL-CNC4%. Reproduced from ref. [199].
7. Challenges and Opportunities
PCL is a unique and versatile biomaterial equipped with several importance features, including ease of processability and good stability under ambient conditions. In fact, this biomaterial has been reported to be approved by U.S Food and Drug Administration for use in several products [200]. Nevertheless, with the great potential of PCL biocomposites and its combination of desirable properties, it is deemed that this versatile biomaterial is not fully translated into a wide area of applications, especially medical sectors, such as tissue engineering and clinical application. This situation is due to several factors associated with PCL. The several challenges faced by PCL biocomposites are discussed in this section, together with its opportunities for future direction.
The main concern of neat PCL is that it is not suitable for some of tissue engineering, especially bone tissue engineering, due to the degradation and mechanical properties of this material. The most effective approach is to use it as one of the elements of blend materials. On the other hand, the incorporation of PCL with several available nanostructured filler such as nanocellulose could be a vital strategy to enhance the PCL biocomposites for its degradation and mechanical properties [201].
On the other hand, due to its hydrophobicity and insufficient wettability, PCL exhibits poor cell attachment [202]. Thus, in order to overcome this hurdle, surface functionalization is deemed to be the best approach to improve the feature of PCL biocomposites. In reviews, several efforts have been demonstrated to modify the surface of this biomaterial. Zander et al. (2010) [203] have demonstrated the functionalization of aligned and unaligned PCL electro-spun fibers using physical adsorption. The extracellular matrix proteins laminin and collagen have been utilized, resulting in the enhancement of cell attachment and further increasing neurite outgrowth. In another report, surface-initiated atom transfer radical polymerization (ATRP) has been demonstrated for its application in functionalized PCL film surfaces [204]. The results showed improved cell adhesion properties of PCL and further enhanced the cell proliferation on the incorporated collagen functionalized PCL. This finding is deemed as an important discovery to nanocomposite industries, especially for PCL-based biomaterials with widened applications in biomedical sector.
All in all, the tremendous efforts have been proposed to improve PCL-based biocomposites. With the rise in these challenges, a new path of improvement has been increasingly beneficial in various sectors.
8. Conclusions
PCL is truly a promising materiel for the future to replace the current materials that are impossible and expensive to reuse. The aim of this review is to investigate the potential of PCL biocomposites reinforced with natural fibres to enhance the quality of the produced biocomposites. Blending and processing of PCL into biocomposites has improved the materials’ properties, thus providing more area to exploit these excellent properties. In this review, green biocomposites and hybrid biocomposites of PCL were evaluated in terms of their mechanical and thermal properties. The characteristics and properties of natural fibres can greatly influence the final properties of PCL-based biocomposites. Most of the studies reported that the performance of the PCL biocomposites improved, as compared to the neat PCL after blended with natural fibres. Moreover, emerging uses of nanofillers, such as nanocellulose and MMT, also managed to greatly improve the performance of PCL-based green and hybrid biocomposites. Besides that, PC-based biocomposites have huge utilization and potential within drug delivery devices, medical devices, and tissue engineering.
Acknowledgments
The authors would like express gratitude for the financial support received from Universiti Teknologi Malaysia, project CRG 30.3: Retardant coating using graphene/bamboo aerogel mixtures on SAR robotics system, grant number PY/2020/03495—R.J130000.7351.4B534”. The research has been carried out under program Research Excellence Consortium (JPT (BPKI) 1000/016/018/25 (57)) provided by Ministry of Higher Education Malaysia (MOHE). The author also would like to thank Universiti Teknologi Malaysia (UTM) for work and facilities support. In addition, this work is also funded by Universiti Putra Malaysia, and Fundamental Research Grant Scheme FRGS/1/2021/TK0/UPM/02/21 provided by Ministry of Higher Education Malaysia (MOHE).
Author Contributions
Conceptualization, R.A.I., M.Y.M.Z.; software, M.N.F.N.; validation, M.Y.M.Z., investigation, R.A.I.; resources, M.Y.M.Z.; writing—original draft preparation, R.A.I., M.Y.M.Z., M.N.F.N., M.S.M.M., M.A.J., S.A.S., N.M.N., M.R.M.A., A.B.M.S., S.P.B., R.N., S.S., A.A.B.O., writing—review and editing, R.A.I., M.Y.M.Z., M.N.F.N., M.S.M.M., M.A.J., S.A.S., N.M.N., M.R.M.A., A.B.M.S., S.P.B., R.N., S.S., A.A.B.O.; funding acquisition, M.Y.M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like express gratitude for the financial support received from Universiti Teknologi Malaysia, project CRG 30.3: Retardant coating using graphene/bamboo aerogel mixtures on SAR robotics system, grant number PY/2020/03495—R.J130000.7351.4B534”. The research has been carried out under program Research Excellence Consortium (JPT (BPKI) 1000/016/018/25 (57)) provided by Ministry of Higher Education Malaysia (MOHE). The author also would like to thank Universiti Teknologi Malaysia (UTM) for work and facilities support. In addition, this work is also funded by Universiti Putra Malaysia, and Fundamental Research Grant Scheme FRGS/1/2021/TK0/UPM/02/21 provided by Ministry of Higher Education Malaysia (MOHE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abdel-Shafy H.I., Mansour M.S. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018;27:1275–1290. doi: 10.1016/j.ejpe.2018.07.003. [DOI] [Google Scholar]
- 2.Norrrahim M.N.F., Ariffin H., Hassan M.A., Ibrahim N.A., Nishida H. Performance evaluation and chemical recyclability of a polyethylene/poly(3- hydroxybutyrate-co-3-hydroxyvalerate) blend for sustainable packaging. RSC Adv. 2013;3:24378–24388. doi: 10.1039/c3ra43632b. [DOI] [Google Scholar]
- 3.Abhilash M., Thomas D. Biopolymers for Biocomposites and Chemical Sensor Applications. Elsevier Inc.; Amsterdam, The Netherlands: 2017. [Google Scholar]
- 4.Ilyas R.A., Sapuan S.M., Kadier A., Kalil M.S., Ibrahim R., Atikah M.S.N., Nurazzi N.M., Nazrin A., Lee C.H., Norrrahim M.N.F., et al. Properties and Characterization of PLA, PHA, and Other Types of Biopolymer Composites. In: Al-Oqla F., Sapuan S.M., editors. Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers. Elsevier; Amsterdam, The Netherlands: 2020. pp. 111–138. [Google Scholar]
- 5.Ilyas R.A., Sapuan S.M., Harussani M.M., Hakimi M.Y.A.Y., Haziq M.Z.M., Atikah M.S.N., Asyraf M.R.M., Ishak M.R., Razman M.R., Nurazzi N.M., et al. Polylactic Acid (PLA) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers. 2021;13:1326. doi: 10.3390/polym13081326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abral H., Atmajaya A., Mahardika M., Hafizulhaq F., Kadriadi, Handayani D., Sapuan S.M., Ilyas R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020;9:2477–2486. doi: 10.1016/j.jmrt.2019.12.078. [DOI] [Google Scholar]
- 7.Emadian S.M., Onay T.T., Demirel B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017;59:526–536. doi: 10.1016/j.wasman.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256. doi: 10.1016/j.progpolymsci.2010.04.002. [DOI] [Google Scholar]
- 9.Ilyas R.A., Sapuan S.M., Norrrahim M.N.F., Yasim-Anuar T.A.T., Kadier A., Kalil M.S., Atikah M.S.N., Ibrahim R., Asrofi M., Abral H., et al. Nanocellulose/starch biopolymer nanocomposites: Processing, manufacturing, and applications. In: Al-Oqla F.M., editor. Advanced Processing, Properties, and Application of Strach and Other Bio-Based Polymer. Elsevier Inc.; Amsterdam, The Netherlands: 2021. [Google Scholar]
- 10.Sharip N.S., Yasim-Anuar T.A.T., Norrrahim M.N.F., Sharip N.S., Shazleen S.S., Nurazzi N.M., Sapuan S.M., Ilyas R.A. A Review on Nanocellulose Composites in Biomedical Application. In: Sapuan S.M., Nukman Y., Osman N.A.A., Ilyas R.A., editors. Composites in Biomedical Applications. CRC Press; Boca Raton, FL, USA: 2020. pp. 162–189. [Google Scholar]
- 11.Norrrahim M.N.F., Mohd Kasim N.A., Knight V.F., Ong K.K., Mohd Noor S.A., Abdul Halim N., Ahmad Shah N.A., Jamal S.H., Janudin N., Misenan M.S.M., et al. Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes. Polymers. 2021;13:3249. doi: 10.3390/polym13193249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhim J., Park H., Ha C. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013;38:1629–1652. doi: 10.1016/j.progpolymsci.2013.05.008. [DOI] [Google Scholar]
- 13.Sisson A.L., Ekinci D., Lendlein A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer. 2013;54:4333–4350. doi: 10.1016/j.polymer.2013.04.045. [DOI] [Google Scholar]
- 14.Mohamed R.M., Yusoh K. A Review on the Recent Research of Polycaprolactone (PCL) Adv. Mater. Res. 2015;1134:249–255. doi: 10.4028/www.scientific.net/AMR.1134.249. [DOI] [Google Scholar]
- 15.Jiang L., Zhang J. 7 Biodegradable and Biobased Polymers. 2nd ed. Elsevier Inc.; Amsterdam, The Netherlands: 2017. [Google Scholar]
- 16.Nevoralová M., Koutný M., Ujčić A., Starý Z., Šerá J., Vlková H., Šlouf M., Fortelný I., Kruliš Z. Structure Characterization and Biodegradation Rate of Poly(ε-caprolactone)/Starch Blends. Front. Mater. 2020;7:141. doi: 10.3389/fmats.2020.00141. [DOI] [Google Scholar]
- 17.Acik G. Bio-based Poly(ɛ-caprolactone) from Soybean-Oil Derived Polyol via Ring-Opening Polymerization. J. Polym. Environ. 2020;28:668–675. doi: 10.1007/s10924-019-01597-7. [DOI] [Google Scholar]
- 18.Johansson C., Bras J., Mondragon I., Nechita P., Plackett D., Šimon P., Svetec D.G., Virtanen S., Baschetti M.G., Breen C., et al. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. BioResources. 2012;7:2506–2552. doi: 10.15376/biores.7.2.2506-2552. [DOI] [Google Scholar]
- 19.Patr T., Glória A. Mechanical and biological behaviour of PCL and PCL/PLA scaffolds for tissue engineering applications. Chem. Eng. Trans. 2013;32:1645–1650. [Google Scholar]
- 20.Miller K., Hsu J.E., Soslowsky L.J. Materials in Tendon and Ligament Repair. Volume 6. Elsevier Ltd.; Amsterdam, The Netherlands: 2011. [Google Scholar]
- 21.Malikmammadov E., Tanir T.E., Kiziltay A., Hasirci V., Hasirci N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018;29:863–893. doi: 10.1080/09205063.2017.1394711. [DOI] [PubMed] [Google Scholar]
- 22.Todea A., Bîtcan I., Aparaschivei D., Păușescu I., Badea V., Péter F., Gherman V.D., Rusu G., Nagy L., Kéki S. Biodegradable Oligoesters of ε-Caprolactone and 5-Hydroxymethyl-2-Furancarboxylic Acid Synthesized by Immobilized Lipases. Polymer. 2019;11:1402. doi: 10.3390/polym11091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manivasagam G., Reddy A., Sen D., Nayak S., Mathew M.T., Rajamanikam A. Dentistry: Restorative and Regenerative Approaches. Volume 1–3. Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 24.Manavitehrani I., Fathi A., Badr H., Daly S., Shirazi A.N., Dehghani F. Biomedical applications of biodegradable polyesters. Polymers. 2016;8:20. doi: 10.3390/polym8010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arunagiri V., Prasannan A., Udomsin J., Lai J.-Y., Wang C.-F., Hong P.-D., Tsai H.C. Facile fabrication of eco-friendly polycaprolactone (PCL)/Poly-D, L-Lactic acid (PDLLA) modified melamine sorbent for oil-spill cleaning and water/oil (W/O) emulsion separation. Sep. Purif. Technol. 2021;259:118081. doi: 10.1016/j.seppur.2020.118081. [DOI] [Google Scholar]
- 26.Kim S., Gwon Y., Park S., Kim W., Jeon Y., Han T., Jeong H.E., Kim J. Synergistic effects of gelatin and nanotopographical patterns on biomedical PCL patches for enhanced mechanical and adhesion properties. J. Mech. Behav. Biomed. Mater. 2021;114:104167. doi: 10.1016/j.jmbbm.2020.104167. [DOI] [PubMed] [Google Scholar]
- 27.Valerini D., Tammaro L., Vitali R., Guillot G., Rinaldi A. Sputter-Deposited Ag Nanoparticles on Electrospun PCL Scaffolds: Morphology, Wettability and Antibacterial Activity. Coatings. 2021;11:345. doi: 10.3390/coatings11030345. [DOI] [Google Scholar]
- 28.Ebrahimifar M., Taherimehr M. Evaluation of in-vitro drug release of polyvinylcyclohexane carbonate as a CO2-derived degradable polymer blended with PLA and PCL as drug carriers. J. Drug Deliv. Sci. Technol. 2021;63:102491. doi: 10.1016/j.jddst.2021.102491. [DOI] [Google Scholar]
- 29.El-Naggar M.E., Shalaby E.S., Abd-Al-Aleem A.H., Abu-Saied M.A., Youssef A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. Technol. 2021;15:3447–3460. doi: 10.1016/j.jmrt.2021.09.136. [DOI] [Google Scholar]
- 30.Herrero-Herrero M., Alberdi-Torres S., González-Fernández M.L., Vilariño-Feltrer G., Rodríguez-Hernández J.C., Vallés-Lluch A., Villar-Suárez V. Influence of chemistry and fiber diameter of electrospun PLA, PCL and their blend membranes, intended as cell supports, on their biological behavior. Polym. Test. 2021;103:107364. doi: 10.1016/j.polymertesting.2021.107364. [DOI] [Google Scholar]
- 31.Doganci M.D. Effects of star-shaped PCL having different numbers of arms on the mechanical, morphological, and thermal properties of PLA/PCL blends. J. Polym. Res. 2021;28:11. doi: 10.1007/s10965-020-02380-2. [DOI] [Google Scholar]
- 32.Ouled Ltaief A., Ghorbel N., Benhamou K., Arous M., Kaddami H., Kallel A. Impact of cellulose nanocrystals reinforcement on molecular dynamics and dielectric properties of PCL-based polyurethane. Polym. Compos. 2021;42:2737–2750. doi: 10.1002/pc.26009. [DOI] [Google Scholar]
- 33.dos Santos Filho E.A., Siqueira D.D., Araújo E.M., Luna C.B.B., de Medeiros E.P. The Impact of the Macaíba Components Addition on the Biodegradation Acceleration of Poly (Ɛ-Caprolactone) (PCL) J. Polym. Environ. 2021:1–18. doi: 10.1007/s10924-021-02215-1. [DOI] [Google Scholar]
- 34.Reis R.S., Souza D. de H.S., Marques M. de F.V., da Luz F.S., Monteiro S.N. Novel bionanocomposite of polycaprolactone reinforced with steam-exploded microfibrillated cellulose modified with ZnO. J. Mater. Res. Technol. 2021;13:1324–1335. doi: 10.1016/j.jmrt.2021.05.043. [DOI] [Google Scholar]
- 35.Ramamoorthy R., Andiappan M., Muthalagu M. Preparation and characterization of Terminalia bellerica loaded PCL nanofibrous mats for biomedical applications. Mater. Today Proc. 2021;45:7247–7252. doi: 10.1016/j.matpr.2021.03.021. [DOI] [Google Scholar]
- 36.Balan R., Gayathri V. In-vitro and antibacterial activities of novel POT/TiO2/PCL composites for tissue engineering and biomedical applications. Polym. Bull. 2021:1–18. doi: 10.1007/s00289-021-03707-9. [DOI] [Google Scholar]
- 37.El Fawal G., Hong H., Mo X., Wang H. Fabrication of scaffold based on gelatin and polycaprolactone (PCL) for wound dressing application. J. Drug Deliv. Sci. Technol. 2021;63:102501. doi: 10.1016/j.jddst.2021.102501. [DOI] [Google Scholar]
- 38.Ma S., Jiang Z., Wang M., Zhang L., Liang Y., Zhang Z., Ren L., Ren L. 4D printing of PLA/PCL shape memory composites with controllable sequential deformation. Bio-Design Manuf. 2021;4:867–878. doi: 10.1007/s42242-021-00151-6. [DOI] [Google Scholar]
- 39.Al-Kaabi W.J., Albukhaty S., Al-Fartosy A.J.M., Al-Karagoly H.K., Al-Musawi S., Sulaiman G.M., Dewir Y.H., Alwahibi M.S., Soliman D.A. Development of Inula graveolens (L.) Plant Extract Electrospun/Polycaprolactone Nanofibers: A Novel Material for Biomedical Application. Appl. Sci. 2021;11:828. doi: 10.3390/app11020828. [DOI] [Google Scholar]
- 40.Tonk R. Natural fibers for sustainable additive manufacturing: A state of the art review. Mater. Today Proc. 2020;37:3087–3090. doi: 10.1016/j.matpr.2020.09.017. [DOI] [Google Scholar]
- 41.Balla V.K., Kate K.H., Satyavolu J., Singh P., Tadimeti J.G.D. Additive manufacturing of natural fiber reinforced polymer composites: Processing and prospects. Compos. Part B Eng. 2019;174:106956. doi: 10.1016/j.compositesb.2019.106956. [DOI] [Google Scholar]
- 42.Hinchcliffe S.A., Hess K.M., Srubar W.V. Experimental and theoretical investigation of prestressed natural fiber-reinforced polylactic acid (PLA) composite materials. Compos. Part B Eng. 2016;95:346–354. doi: 10.1016/j.compositesb.2016.03.089. [DOI] [Google Scholar]
- 43.Gupta U.S., Dhamarikar M., Dharkar A., Chaturvedi S., Kumrawat A., Giri N., Tiwari S., Namdeo R. Plasma modification of natural fiber: A review. Mater. Today Proc. 2021;47:3035–3042. doi: 10.1016/j.matpr.2021.05.513. [DOI] [Google Scholar]
- 44.Asyraf M.R.M., Ishak M.R., Norrrahim M.N.F., Nurazzi N.M., Shazleen S.S., Ilyas R.A., Rafidah M., Razman M.R. Recent advances of thermal properties of sugar palm lignocellulosic fibre reinforced polymer composites. Int. J. Biol. Macromol. 2021 doi: 10.1016/j.ijbiomac.2021.10.221. [DOI] [PubMed] [Google Scholar]
- 45.Ariffin H., Norrrahim M.N.F., Yasim-Anuar T.A.T., Nishida H., Hassan M.A., Ibrahim N.A., Yunus W.M.Z.W. Oil palm biomass cellulose-fabricated polylactic acid composites for packaging applications. Bionanocomposites Packag. Appl. 2017:95–105. doi: 10.1007/978-3-319-67319-6_5. [DOI] [Google Scholar]
- 46.El-Shekeil Y.A., Salit M.S., Abdan K., Zainudin E.S. Development of a new kenaf bast fiber-reinforced thermoplastic polyurethane composite. BioResources. 2011;6:4662–4672. [Google Scholar]
- 47.Sharan Gupta U., Dharkar A., Dhamarikar M., Choudhary A., Wasnik D., Chouhan P., Tiwari S., Namdeo R. Study on the effects of fiber orientation on the mechanical properties of natural fiber reinforced epoxy composite by finite element method. Mater. Today Proc. 2021;45:7885–7893. doi: 10.1016/j.matpr.2020.12.614. [DOI] [Google Scholar]
- 48.Madhu P., Sanjay M.R., Senthamaraikannan P., Pradeep S., Saravanakumar S.S., Yogesha B. A review on synthesis and characterization of commercially available natural fibers: Part II. J. Nat. Fibers. 2019;16:25–36. doi: 10.1080/15440478.2017.1379045. [DOI] [Google Scholar]
- 49.Sari N.H., Pruncu C.I., Sapuan S.M., Ilyas R.A., Catur A.D., Suteja S., Sutaryono Y.A., Pullen G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polym. Test. 2020;91:106751. doi: 10.1016/j.polymertesting.2020.106751. [DOI] [Google Scholar]
- 50.Kamaruddin Z.H., Jumaidin R., Selamat M.Z., Ilyas R.A. Characteristics and Properties of Lemongrass (Cymbopogan Citratus): A Comprehensive Review. J. Nat. Fibers. 2021:1–18. doi: 10.1080/15440478.2021.1958439. [DOI] [Google Scholar]
- 51.Norrrahim M.N.F., Ariffin H., Yasim-Anuar T.A.T., Ghaemi F., Hassan M.A., Ibrahim N.A., Ngee J.L.H., Yunus W.M.Z.W. Superheated steam pretreatment of cellulose affects its electrospinnability for microfibrillated cellulose production. Cellulose. 2018;25:3853–3859. doi: 10.1007/s10570-018-1859-3. [DOI] [Google Scholar]
- 52.Suriani M.J., Sapuan S.M., Ruzaidi C.M., Nair D.S., Ilyas R.A. Flammability, morphological and mechanical properties of sugar palm fiber/polyester yarn-reinforced epoxy hybrid biocomposites with magnesium hydroxide flame retardant filler. Text. Res. J. 2021:004051752110086. doi: 10.1177/00405175211008615. [DOI] [Google Scholar]
- 53.Suriani M.J., Zainudin H.A., Ilyas R.A., Petrů M., Sapuan S.M., Ruzaidi C.M., Mustapha R. Kenaf Fiber/Pet Yarn Reinforced Epoxy Hybrid Polymer Composites: Morphological, Tensile, and Flammability Properties. Polymers. 2021;13:1532. doi: 10.3390/polym13091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suriani M.J., Rapi H.Z., Ilyas R.A., Petrů M., Sapuan S.M. Delamination and Manufacturing Defects in Natural Fiber-Reinforced Hybrid Composite: A Review. Polymers. 2021;13:1323. doi: 10.3390/polym13081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azman M.A., Asyraf M.R.M., Khalina A., Petrů M., Ruzaidi C.M., Sapuan S.M., Wan Nik W.B., Ishak M.R., Ilyas R.A., Suriani M.J. Natural Fiber Reinforced Composite Material for Product Design: A Short Review. Polymers. 2021;13:1917. doi: 10.3390/polym13121917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suriani M.J., Radzi F.S.M., Ilyas R.A., Petrů M., Sapuan S.M., Ruzaidi C.M. Flammability, Tensile, and Morphological Properties of Oil Palm Empty Fruit Bunches Fiber/Pet Yarn-Reinforced Epoxy Fire Retardant Hybrid Polymer Composites. Polymers. 2021;13:1282. doi: 10.3390/polym13081282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ilyas R.A., Sapuan S.M., Mohd Nurazzi N., Norrrahim M.N.F., Ibrahim R., Atikah M.S.N., Huzaifah M.R.M., Radzi A.M., Izwan S., Noor Azammi A.M., et al. Biocomposite and Synthetic Composites for Automotive Applications. Woodhead Publishing Series; Amsterdam, The Netherlands: 2020. Macro to nanocscale natural fibre composites for automotive components: Research, development, and application; pp. 51–105. [Google Scholar]
- 58.Norrrahim M.N.F., Huzaifah M.R.M., Farid M.A.A., Shazleen S.S., Misenan M.S.M., Yasim-Anuar T.A.T., Naveen J., Nurazzi N.M., Rani M.S.A., Hakimi M.I., et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers. 2021;13:2971. doi: 10.3390/polym13172971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nurazzi N.M., Harussani M.M., Aisyah H.A., Ilyas R.A., Norrrahim M.N.F., Khalina A., Abdullah N. Treatments of natural fiber as reinforcement in polymer composites—A short review. Funct. Compos. Struct. 2021;3:024002. doi: 10.1088/2631-6331/abff36. [DOI] [Google Scholar]
- 60.Norrrahim M.N.F., Kasim N.A.M., Knight V.F., Misenan M.S.M., Janudin N., Shah N.A.A., Kasim N., Yusoff W.Y.W., Noor S.A.M., Jamal S.H., et al. Nanocellulose: A bioadsorbent for chemical contaminant remediation. RSC Adv. 2021;11:7347–7368. doi: 10.1039/D0RA08005E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Norrrahim M.N.F., Kasim N.A.M., Knight V.F., Ujang F.A., Janudin N., Razak M.A.I.A., Shah N.A.A., Noor S.A.M., Jamal S.H., Ong K.K., et al. Nanocellulose: The Next Super Versatile Material for the Military. Mater. Adv. 2021;2:1485–1506. doi: 10.1039/D0MA01011A. [DOI] [Google Scholar]
- 62.Lawal A.A., Hassan M.A., Zakaria M.R., Yusoff M.Z.M., Norrrahim M.N.F., Mokhtar M.N., Shirai Y. Effect of oil palm biomass cellulosic content on nanopore structure and adsorption capacity of biochar. Bioresour. Technol. 2021;332:125070. doi: 10.1016/j.biortech.2021.125070. [DOI] [PubMed] [Google Scholar]
- 63.Norrrahim M.N.F., Yasim-Anuar T.A.T., Jenol M.A., Mohd Nurazzi N., Ilyas R.A., Sapuan S. Biocomposite and Synthetic Composites for Automotive Applications. Woodhead Publishing Series; Amsterdam, The Netherlands: 2020. Performance evaluation of cellulose nanofiber reinforced polypropylene biocomposites for automotive applications; pp. 119–215. [Google Scholar]
- 64.Norrrahim M.N.F., Kasim N.A.M., Knight V.F., Halim N.A., Shah N.A.A., Noor S.A.M., Jamal S.H., Ong K.K., Yunus W.M.Z.W., Farid M.A.A., et al. Performance Evaluation of Cellulose Nanofiber Reinforced Polymer Composites. Funct. Compos. Struct. 2021;3 doi: 10.1088/2631-6331/abeef6. [DOI] [Google Scholar]
- 65.George A., Sanjay M.R., Srisuk R., Parameswaranpillai J., Siengchin S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020;154:329–338. doi: 10.1016/j.ijbiomac.2020.03.120. [DOI] [PubMed] [Google Scholar]
- 66.Sanjay M.R., Madhu P., Jawaid M., Senthamaraikannan P., Senthil S., Pradeep S. Characterization and Properties of Natural Fiber Polymer Composites: A Comprehensive Review. J. Clean. Prod. 2017;172:566–581. doi: 10.1016/j.jclepro.2017.10.101. [DOI] [Google Scholar]
- 67.Vinod A., Vijay R., Singaravelu D.L., Sanjay M.R., Siengchin S., Yagnaraj Y., Khan S. Extraction and characterization of natural fiber from stem of cardiospermum halicababum. J. Nat. fibers. 2021;18:898–908. doi: 10.1080/15440478.2019.1669514. [DOI] [Google Scholar]
- 68.Madhu P., Sanjay M.R., Senthamaraikannan P., Pradeep S., Saravanakumar S.S., Yogesha B. A review on synthesis and characterization of commercially available natural fibers: Part-I. J. Nat. Fibers. 2018;16:1132–1144. doi: 10.1080/15440478.2018.1453433. [DOI] [Google Scholar]
- 69.Ilyas R.A., Sapuan S.M., Ibrahim R., Abral H., Ishak M.R., Zainudin E.S., Asrofi M., Atikah M.S.N., Huzaifah M.R.M., Radzi A.M., et al. Sugar palm (Arenga pinnata (Wurmb.) Merr) cellulosic fibre hierarchy: A comprehensive approach from macro to nano scale. J. Mater. Res. Technol. 2019;8:2753–2766. doi: 10.1016/j.jmrt.2019.04.011. [DOI] [Google Scholar]
- 70.Ilyas R.A., Sapuan S.M., Ishak M.R., Zainudin E.S. Effect of delignification on the physical, thermal, chemical, and structural properties of sugar palm fibre. BioResources. 2017;12:8734–8754. doi: 10.15376/biores.12.4.8734-8754. [DOI] [Google Scholar]
- 71.Ilyas R.A., Sapuan S.M., Ishak M.R., Zainudin E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019;123:379–388. doi: 10.1016/j.ijbiomac.2018.11.124. [DOI] [PubMed] [Google Scholar]
- 72.Nurazzi N.M., Asyraf M.R.M., Rayung M., Norrrahim M.N.F., Shazleen S.S., Rani M.S.A., Shafi A.R., Aisyah H.A., Radzi M.H.M., Sabaruddin F.A., et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers. 2021;13:2710. doi: 10.3390/polym13162710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roy K., Chandra Debnath S., Das A., Heinrich G., Potiyaraj P. Exploring the synergistic effect of short jute fiber and nanoclay on the mechanical, dynamic mechanical and thermal properties of natural rubber composites. Polym. Test. 2018;67:487–493. doi: 10.1016/j.polymertesting.2018.03.032. [DOI] [Google Scholar]
- 74.Komuraiah A., Kumar N.S., Prasad B.D. Chemical Composition of Natural Fibers and its Influence on their Mechanical Properties. Mech. Compos. Mater. 2014;50:359–376. doi: 10.1007/s11029-014-9422-2. [DOI] [Google Scholar]
- 75.Mwaikambo L. Review of the history, properties and application of plant fibres. African J. Sci. Technol. 2006;7:121. [Google Scholar]
- 76.Raja T., Anand P., Karthik M., Sundaraj M. Evaluation of mechanical properties of natural fibre reinforced composites: A review. Int. J. Mech. Eng. Technol. 2017;8:915–924. [Google Scholar]
- 77.Lin N., Dufresne A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014;59:302–325. doi: 10.1016/j.eurpolymj.2014.07.025. [DOI] [Google Scholar]
- 78.Ilyas R.A., Sapuan S.M., Sanyang M.L., Ishak M.R., Zainudin E.S. Nanocrystalline cellulose as reinforcement for polymeric matrix nanocomposites and its potential applications: A Review. Curr. Anal. Chem. 2018;14:203–225. doi: 10.2174/1573411013666171003155624. [DOI] [Google Scholar]
- 79.Alsubari S., Zuhri M.Y.M., Sapuan S.M., Ishak M.R., Ilyas R.A., Asyraf M.R.M. Potential of Natural Fiber Reinforced Polymer Composites in Sandwich Structures: A Review on Its Mechanical Properties. Polymers. 2021;13:423. doi: 10.3390/polym13030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ayu R.S., Khalina A., Harmaen A.S., Zaman K., Isma T., Liu Q., Ilyas R.A., Lee C.H. Characterization Study of Empty Fruit Bunch (EFB) Fibers Reinforcement in Poly(Butylene) Succinate (PBS)/Starch/Glycerol Composite Sheet. Polymers. 2020;12:1571. doi: 10.3390/polym12071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sinha A.K., Narang H.K., Bhattacharya S. Mechanical properties of natural fibre polymer composites. J. Polym. Eng. 2017;37:879–895. doi: 10.1515/polyeng-2016-0362. [DOI] [Google Scholar]
- 82.Alvarez V.A., Fraga A.N., Vázquez A. Effects of the moisture and fiber content on the mechanical properties of biodegradable polymer-sisal fiber biocomposites. J. Appl. Polym. Sci. 2004;91:4007–4016. doi: 10.1002/app.13561. [DOI] [Google Scholar]
- 83.Norrrahim M.N.F., Ilyas R.A., Nurazzi N.M., Rani M.S.A., Atikah M.S.N., Shazleen S.S. Chemical Pretreatment of Lignocellulosic Biomass for the Production of Bioproducts: An Overview. Appl. Sci. Eng. Prog. 2021;13:2971. doi: 10.14416/j.asep.2021.07.004. [DOI] [Google Scholar]
- 84.Ilyas R.A., Sapuan S.M., Atikah M.S.N., Asyraf M.R.M., Rafiqah S.A., Aisyah H.A., Nurazzi N.M., Norrrahim M.N.F. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose (Arenga pinnata (Wurmb.) Merr ) Text. Res. J. 2021;91:152–167. doi: 10.1177/0040517520932393. [DOI] [Google Scholar]
- 85.Norrrahim M.N.F., Ariffin H., Hassan M.A., Ibrahim N.A., Yunus W.M.Z.W., Nishida H. Utilisation of superheated steam in oil palm biomass pretreatment process for reduced chemical use and enhanced cellulose nanofibre production. Int. J. Nanotechnol. 2019;16:668–679. doi: 10.1504/IJNT.2019.107360. [DOI] [Google Scholar]
- 86.Nuthong W., Uawongsuwan P., Pivsa-Art W., Hamada H. Impact property of flexible epoxy treated natural fiber reinforced PLA composites. Energy Procedia. 2013;34:839–847. doi: 10.1016/j.egypro.2013.06.820. [DOI] [Google Scholar]
- 87.Premnath A.A. Impact of surface treatment on the mechanical properties of sisal and jute reinforced with epoxy resin natural fiber hybrid composites. J. Nat. Fibers. 2018;16:718–728. doi: 10.1080/15440478.2018.1432002. [DOI] [Google Scholar]
- 88.George J., Sreekala M.S., Thomas S. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001;41:1471–1485. doi: 10.1002/pen.10846. [DOI] [Google Scholar]
- 89.Zakaria M.R., Norrrahim M.N.F., Hirata S., Hassan M.A. Hydrothermal and wet disk milling pretreatment for high conversion of biosugars from oil palm mesocarp fiber. Bioresour. Technol. 2015;181:263–269. doi: 10.1016/j.biortech.2015.01.072. [DOI] [PubMed] [Google Scholar]
- 90.Berglund L., Noël M., Aitomäki Y., Öman T., Oksman K. Production potential of cellulose nanofibers from industrial residues: Efficiency and nanofiber characteristics. Ind. Crops Prod. 2016;92:84–92. doi: 10.1016/j.indcrop.2016.08.003. [DOI] [Google Scholar]
- 91.Kumar P., Barrett D.M., Delwiche M.J., Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009;48:3713–3729. doi: 10.1021/ie801542g. [DOI] [Google Scholar]
- 92.Thakur S., Shrivastava B., Ingale S., Kuhad R.C., Gupte A. Degradation and selective ligninolysis of wheat straw and banana stem for an efficient bioethanol production using fungal and chemical pretreatment. 3 Biotech. 2013;3:365–372. doi: 10.1007/s13205-012-0102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohd Nurazzi N., Khalina A., Sapuan S.M., Ilyas R.A. Mechanical properties of sugar palm yarn/woven glass fiber reinforced unsaturated polyester composites: Effect of fiber loadings and alkaline treatment. Polimery/Polymers. 2019;64 doi: 10.14314/polimery.2019.10.3. [DOI] [Google Scholar]
- 94.Ilyas R.A., Sapuan S.M. The Preparation Methods and Processing of Natural Fibre Bio-polymer Composites. Curr. Org. Synth. 2020;16:1068–1070. doi: 10.2174/157017941608200120105616. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Y., Fan M., Chen L. Interface and Bonding Mechanisms of Plant Fibre Composites: An Overview. Volume 101. Elsevier Ltd.; Amsterdam, The Netherlands: 2016. [Google Scholar]
- 96.Norrrahim M.N.F. Superheated Steam Pretreatment of Oil Palm Biomass for Improving Nanofibrillation of Cellulose and Performance of Polypropylene/Cellulose Nanofiber Composites. Universiti Putra Malaysia; Selangor, Malaysia: 2018. [Google Scholar]
- 97.Misenan M.S.M., Janudin N., Idayu M.A., Norrrahim M.N.F., Jamal S.H., Wan Yusoff W.Y., Kasim N., Yunus W.M.D.Z.W., Ernest V.F.K.V., Kasim N.A.M. Cellulose Nanofiber as Potential Absorbent Material for Chloride Ion. Solid State Phenom. 2021;317:263–269. doi: 10.4028/www.scientific.net/SSP.317.263. [DOI] [Google Scholar]
- 98.Norrrahim M.N.F., Ahmad Shah N.A., Jamal S.H., Yunus W.M.Z., Knight V.F., Kasim N.A.M. Nanocellulose-Based Filters as Novel Barrier Systems for Chemical Warfare Agents. Solid State Phenom. 2021;317:180–186. doi: 10.4028/www.scientific.net/SSP.317.180. [DOI] [Google Scholar]
- 99.Norrrahim M.N.F., Kasim N.A.M., Knight V.F., Ong K.K., Noor S.A.M., Jamal S.H., Shah N.A.A., Halim N.A., Ilyas R.A., Yunus W.M.Z.W. Cationic Nanocellulose as Promising Candidate for Filtration Material of COVID-19: A Perspective. Appl. Sci. Eng. Prog. 2021:1–8. doi: 10.14416/j.asep.2021.08.004. [DOI] [Google Scholar]
- 100.Norrrahim M.N.F., Norizan M.N., Jenol M.A., Farid M.A.A., Janudin N., Ujang F.A., Yasim-Anuar T.A.T., Najmuddin S.U.F.S., Ilyas R.A. Emerging Development on Nanocellulose as Antimicrobial Material: An Overview. Mater. Adv. 2021;11:3538–3551. doi: 10.1039/D1MA00116G. [DOI] [Google Scholar]
- 101.Fareez I.M., Jasni A.H., Norrrahim M.N.F. Phenolic Polymers Based Composite Materials. Springer; Singapore: 2020. Nanofibrillated cellulose based bio-phenolic composites; pp. 139–151. [Google Scholar]
- 102.Norrrahim M.N.F., Yasim-Anuar T.A.T., Sapuan S.M., Ilyas R.A., Hakimi M.I., Najmuddin S.U.F.S., Jenol M.A. Bio-Based Packaging: Material, Environmental and Economic Aspects. John Wiley & Sons Ltd.; West Sussex, UK: 2021. Nanocellulose reinforced polypropylene and polyethylene composite for packaging application. [Google Scholar]
- 103.Ilyas R.A., Sapuan S.M. Biopolymers and Biocomposites: Chemistry and Technology. Curr. Anal. Chem. 2020;16:500–503. doi: 10.2174/157341101605200603095311. [DOI] [Google Scholar]
- 104.Goda K., Cao Y. Research and Development of Fully Green Composites Reinforced with Natural Fibers. J. Solid Mech. Mater. Eng. 2007;1:1073–1084. doi: 10.1299/jmmp.1.1073. [DOI] [Google Scholar]
- 105.Fazeli M., Florez J.P., Simão R.A. Improvement in adhesion of cellulose fibers to the thermoplastic starch matrix by plasma treatment modification. Compos. Part B Eng. 2019;163:207–216. doi: 10.1016/j.compositesb.2018.11.048. [DOI] [Google Scholar]
- 106.Sathishkumar T.P., Naveen J., Satheeshkumar S. Hybrid fiber reinforced polymer composites—A review. J. Reinf. Plast. Compos. 2014;33:454–471. doi: 10.1177/0731684413516393. [DOI] [Google Scholar]
- 107.Garcia-Garcia D., Carbonell-Verdu A., Jordá-Vilaplana A., Balart R., Garcia-Sanoguera D. Development and characterization of green composites from bio-based polyethylene and peanut shell. J. Appl. Polym. Sci. 2016;133 doi: 10.1002/app.43940. [DOI] [Google Scholar]
- 108.La Mantia F.P., Morreale M. Green composites: A brief review. Compos. Part A Appl. Sci. Manuf. 2011;42:579–588. doi: 10.1016/j.compositesa.2011.01.017. [DOI] [Google Scholar]
- 109.Singh S., Mohanty A.K., Sugie T., Takai Y., Hamada H. Renewable resource based biocomposites from natural fiber and polyhydroxybutyrate-co-valerate (PHBV) bioplastic. Compos. Part A Appl. Sci. Manuf. 2008;39:875–886. doi: 10.1016/j.compositesa.2008.01.004. [DOI] [Google Scholar]
- 110.Nurazzi N.M., Asyraf M.R.M., Fatimah Athiyah S., Shazleen S.S., Rafiqah S.A., Harussani M.M., Kamarudin S.H., Razman M.R., Rahmah M., Zainudin E.S., et al. A Review on Mechanical Performance of Hybrid Natural Fiber Polymer Composites for Structural Applications. Polymers. 2021;13:2170. doi: 10.3390/polym13132170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zweben C. Tensile strength of hybrid composites. J. Mater. Sci. 1977;12:1325–1337. doi: 10.1007/BF00540846. [DOI] [Google Scholar]
- 112.de S. Macedo J., Costa M.F., Tavares M.I.B., Thiré R.M.S.M. Preparation and characterization of composites based on polyhydroxybutyrate and waste powder from coconut fibers processing. Polym. Eng. Sci. 2010;50:1466–1475. doi: 10.1002/pen.21669. [DOI] [Google Scholar]
- 113.Norrrahim M.N.F., Ariffin H., Yasim-Anuar T.A.T., Hassan M.A., Ibrahim N.A., Yunus W.M.Z.W., Nishida H. Performance Evaluation of Cellulose Nanofiber with Residual Hemicellulose as a Nanofiller in Polypropylene-Based Nanocomposite. Polymers. 2021;13:1064. doi: 10.3390/polym13071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ilyas R.A., Sapuan S.M., Asyraf M.R.M., Atikah M.S.N., Ibrahim R., Norrrahim M.N., Yasim-Anuar T.A., Megashah L.N. Mechanical and Dynamic Properties of Biocomposites. Wiley-VCH; Weinheim, Germany: 2021. Mechanical and dynamic mechanical analysis of bio-based composites; pp. 49–76. [Google Scholar]
- 115.Bhat A., Naveen J., Jawaid M., Norrrahim M.N.F., Rashedi A., Khan A. Advancement in fiber reinforced polymer, metal alloys and multi-layered armour systems for ballistic applications–A Review. J. Mater. Res. Technol. 2021;15:1300–1317. doi: 10.1016/j.jmrt.2021.08.150. [DOI] [Google Scholar]
- 116.Shakoor D.A., Muhammad R., Thomas N., Silberschmidt V. Mechanical and thermal characterisation of poly (l-lactide) composites reinforced with hemp fibres. J. Phys. Conf. Ser. 2013;451:12010. doi: 10.1088/1742-6596/451/1/012010. [DOI] [Google Scholar]
- 117.Rhee S.-H., Lee Y.-K., Lim B.-S., Yoo J.J., Kim H.J. Evaluation of a novel poly(epsilon-caprolactone)-organosiloxane hybrid material for the potential application as a bioactive and degradable bone substitute. Biomacromolecules. 2004;5:1575–1579. doi: 10.1021/bm049885n. [DOI] [PubMed] [Google Scholar]
- 118.Cao A., Okamura T., Ishiguro C., Nakayama K., Inoue Y., Masuda T. Studies on syntheses and physical characterization of biodegradable aliphatic poly(butylene succinate-co-ε-caprolactone)s. Polymer. 2002;43:671–679. doi: 10.1016/S0032-3861(01)00658-9. [DOI] [Google Scholar]
- 119.Sarasam A.R., Krishnaswamy R.K., Madihally S. V Blending chitosan with polycaprolactone: Effects on physicochemical and antibacterial properties. Biomacromolecules. 2006;7:1131–1138. doi: 10.1021/bm050935d. [DOI] [PubMed] [Google Scholar]
- 120.Lu L., Zhang Q., Wootton D.M., Chiou R., Li D., Lu B., Lelkes P.I., Zhou J. Mechanical study of polycaprolactone-hydroxyapatite porous scaffolds created by porogen-based solid freeform fabrication method. J. Appl. Biomater. Funct. Mater. 2014;12:145–154. doi: 10.5301/jabfm.5000163. [DOI] [PubMed] [Google Scholar]
- 121.Lee S.H., Ohkita T. Mechanical and thermal flow properties of wood flour-biodegradable polymer composites. J. Appl. Polym. Sci. 2003;90:1900–1905. doi: 10.1002/app.12864. [DOI] [Google Scholar]
- 122.Finkenstadt V.L., Mohamed A.A., Biresaw G., Willett J.L. Mechanical properties of green composites with polycaprolactone and wheat gluten. J. Appl. Polym. Sci. 2008;110:2218–2226. doi: 10.1002/app.28446. [DOI] [Google Scholar]
- 123.Dhakal H.N., Ismail S.O., Zhang Z., Barber A., Welsh E., Maigret J.E., Beaugrand J. Development of sustainable biodegradable lignocellulosic hemp fiber/polycaprolactone biocomposites for light weight applications. Compos. Part A Appl. Sci. Manuf. 2018;113:350–358. doi: 10.1016/j.compositesa.2018.08.005. [DOI] [Google Scholar]
- 124.Lakshmipriya S., Imayathamizhan N.M. Development of silk/polycaprolactone biocomposite for internal bone plate application. J. Ind. Text. 2019 doi: 10.1177/1528083719886052. [DOI] [Google Scholar]
- 125.Theeranattapong T., Luangwattanawilai T., Suwanprateeb J., Suvannapruk W., Chumnanvej S., Hemstapat W. Physical and mechanical characterizations of oxidized regenerated cellulose/polycaprolactone composite for use as a synthetic dura mater. Key Eng. Mater. 2015;659:19–23. doi: 10.4028/www.scientific.net/KEM.659.19. [DOI] [Google Scholar]
- 126.de Oliveira Aguiar V., de Fatima Vieira Marques M. Composites of Polycaprolactone with Cellulose Fibers: Morphological and Mechanical Evaluation. Macromol. Symp. 2016;367:101–112. doi: 10.1002/masy.201500142. [DOI] [Google Scholar]
- 127.Alemán-Domínguez M.E., Ortega Z., Benítez A.N., Monzón M., Garzón L.V., Ajami S., Liu C. Polycaprolactone–carboxymethyl cellulose composites for manufacturing porous scaffolds by material extrusion. Bio-Design. Manuf. 2018;1:245–253. doi: 10.1007/s42242-018-0024-z. [DOI] [Google Scholar]
- 128.Sheng L., Jiang R., Zhu Y., Ji Y. Electrospun cellulose nanocrystals/polycaprolactone nanocomposite fiber mats. J. Macromol. Sci. Part B Phys. 2014;53:820–828. doi: 10.1080/00222348.2013.861311. [DOI] [Google Scholar]
- 129.Rosa D.S., Guedes C.G.F., Bardi M.A.G. Evaluation of thermal, mechanical and morphological properties of PCL/CA and PCL/CA/PE-g-GMA blends. Polym. Test. 2007;26:209–215. doi: 10.1016/j.polymertesting.2006.10.003. [DOI] [Google Scholar]
- 130.Odusanya O.S., Manan D.M.A., Ishiaku U.S., Azemi B.M.N. Effect of starch predrying on the mechanical properties of starch/poly(ε-caprolactone) composites. J. Appl. Polym. Sci. 2002;87:877–884. doi: 10.1002/app.11295. [DOI] [Google Scholar]
- 131.Baji A., Wong S.C., Srivatsan T.S., Njus G.O., Mathur G. Processing methodologies for polycaprolactone-hydroxyapatite composites: A review. Mater. Manuf. Process. 2006;21:211–218. doi: 10.1081/AMP-200068681. [DOI] [Google Scholar]
- 132.Lo Re G., Spinella S., Boujemaoui A., Vilaseca F., Larsson P.T., Adås F., Berglund L.A. Poly(ϵ-caprolactone) Biocomposites Based on Acetylated Cellulose Fibers and Wet Compounding for Improved Mechanical Performance. ACS Sustain. Chem. Eng. 2018;6:6753–6760. doi: 10.1021/acssuschemeng.8b00551. [DOI] [Google Scholar]
- 133.Zolfagharlou Kouhi M., Behzad T., Ghasemi-Mobarakeh L., Allafchian A., Moazzami Goudarzi Z., Enayati M.S. Proceeding toward the development of poly(ɛ-caprolactone)/cellulose microfibrils electrospun biocomposites using a novel ternary solvent system. J. Text. Inst. 2020;111:249–259. doi: 10.1080/00405000.2019.1633216. [DOI] [Google Scholar]
- 134.Ahmad A.F., Abbas Z., Obaiys S.J., Zainuddin M.F. Effect of untreated fiber loading on the thermal, mechanical, dielectric, and microwave absorption properties of polycaprolactone reinforced with oil palm empty fruit bunch biocomposites. Polym. Compos. 2018;39:E1778–E1787. doi: 10.1002/pc.24792. [DOI] [Google Scholar]
- 135.Khandanlou R., Ahmad M.B., Shameli K., Hussein M.Z., Zainuddin N., Kalantari K. Mechanical and thermal stability properties of modified rice straw fiber blend with polycaprolactone composite. J. Nanomater. 2014;2014 doi: 10.1155/2014/675258. [DOI] [Google Scholar]
- 136.Matsuda H. Preparation and utilization of esterified woods bearing carboxyl groups. Wood Sci. Technol. 1987;21:75–88. doi: 10.1007/BF00349719. [DOI] [Google Scholar]
- 137.Khoee S., Moayeri S., Charsooghi M.A. Self-/Magnetic-Propelled Catalytic Nanomotors Based on a Janus SPION@PEG-Pt/PCL Hybrid Nanoarchitecture: Single-Particle versus Collective Motions. Langmuir. 2021;37:10668–10682. doi: 10.1021/acs.langmuir.1c01166. [DOI] [PubMed] [Google Scholar]
- 138.Safamehr A., Ghasemi-Mobarakeh L., Mansurnezhad R., Beigi M.-H., Kiani A. Fabrication and Characterization of a Hybrid Structure Comprising Chitosan Hydrogel and PCL Nanofibers for Potential Application in Wound Dressing. Fibers Polym. 2021:1–11. doi: 10.1007/s12221-021-0283-8. [DOI] [Google Scholar]
- 139.Brzeziński M., Socka M., Makowski T., Kost B., Cieślak M., Królewska-Golińska K. Microfluidic-assisted nanoprecipitation of biodegradable nanoparticles composed of PTMC/PCL (co)polymers, tannic acid and doxorubicin for cancer treatment. Colloids Surf. B Biointerfaces. 2021;201:111598. doi: 10.1016/j.colsurfb.2021.111598. [DOI] [PubMed] [Google Scholar]
- 140.Pour Khalili N., Moradi R., Kavehpour P., Islamzada F., Abdullayev Y. Fabrication and biocompatibility of BNNT supramolecular complexes and PCL/BNNTs nanofibers. Mater. Today Proc. 2021;42:1570–1578. doi: 10.1016/j.matpr.2020.02.782. [DOI] [Google Scholar]
- 141.Mohamady Hussein M.A., Guler E., Rayaman E., Cam M.E., Sahin A., Grinholc M., Sezgin Mansuroglu D., Sahin Y.M., Gunduz O., Muhammed M., et al. Dual-drug delivery of Ag-chitosan nanoparticles and phenytoin via core-shell PVA/PCL electrospun nanofibers. Carbohydr. Polym. 2021;270:118373. doi: 10.1016/j.carbpol.2021.118373. [DOI] [PubMed] [Google Scholar]
- 142.Moazzami Goudarzi Z., Behzad T., Ghasemi-Mobarakeh L., Kharaziha M. An investigation into influence of acetylated cellulose nanofibers on properties of PCL/Gelatin electrospun nanofibrous scaffold for soft tissue engineering. Polymer. 2021;213:123313. doi: 10.1016/j.polymer.2020.123313. [DOI] [Google Scholar]
- 143.Ensoylu M., Deliormanlı A.M., Atmaca H. Tungsten disulfide nanoparticle-containing PCL and PLGA-coated bioactive glass composite scaffolds for bone tissue engineering applications. J. Mater. Sci. 2021;56:18650–18667. doi: 10.1007/s10853-021-06494-w. [DOI] [Google Scholar]
- 144.Wang Q., Ma Z., Wang Y., Zhong L., Xie W. Fabrication and characterization of 3D printed biocomposite scaffolds based on PCL and zirconia nanoparticles. Bio-Design. Manuf. 2021;4:60–71. doi: 10.1007/s42242-020-00095-3. [DOI] [Google Scholar]
- 145.Siqueira D.D., Luna C.B.B., Araújo E.M., Barros A.B.S., Wellen R.M.R. Approaches on PCL /macaíba biocomposites—Mechanical, thermal, morphological properties and crystallization kinetics. Polym. Adv. Technol. 2021;32:3572–3587. doi: 10.1002/pat.5367. [DOI] [Google Scholar]
- 146.Chandika P., Oh G.-W., Heo S.-Y., Kim S.-C., Kim T.-H., Kim M.-S., Jung W.-K. Electrospun porous bilayer nano-fibrous fish collagen/PCL bio-composite scaffolds with covalently cross-linked chitooligosaccharides for full-thickness wound-healing applications. Mater. Sci. Eng. C. 2021;121:111871. doi: 10.1016/j.msec.2021.111871. [DOI] [PubMed] [Google Scholar]
- 147.Wu C.-S. Preparation and Characterizations of Polycaprolactone/Green Coconut Fiber Composites. J. Appl. Polym. Sci. 2010;115:948–956. doi: 10.1002/app.30955. [DOI] [Google Scholar]
- 148.Sarkar S., Schmitz-Rixen T., Hamilton G., Seifalian A.M. Achieving the ideal properties for vascular bypass grafts using a tissue engineered approach: A review. Med. Biol. Eng. Comput. 2007;45:327–336. doi: 10.1007/s11517-007-0176-z. [DOI] [PubMed] [Google Scholar]
- 149.Wise S.G., Byrom M.J., Waterhouse A., Bannon P.G., Ng M.K.C., Weiss A.S. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011;7:295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 150.Abdellah Ali S.F. Mechanical and thermal properties of promising polymer composites for food packaging applications. IOP Conf. Ser. Mater. Sci. Eng. 2016;137:012035. doi: 10.1088/1757-899X/137/1/012035. [DOI] [Google Scholar]
- 151.Yu Y., Gao X., Jiang Z., Zhang W., Ma J., Liu X., Zhang L. Homogeneous grafting of cellulose with polycaprolactone using quaternary ammonium salt systems and its application for ultraviolet-shielding composite films. RSC Adv. 2018;8:10865–10872. doi: 10.1039/C8RA00120K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gibril M.E., Ahmed K.K., Lekha P., Sithole B., Khosla A., Furukawa H. Effect of nanocrystalline cellulose and zinc oxide hybrid organic–inorganic nanofiller on the physical properties of polycaprolactone nanocomposite films. Microsyst. Technol. 2019;0123456789 doi: 10.1007/s00542-019-04497-x. [DOI] [Google Scholar]
- 153.Quilez-Molina A.I., Pasquale L., Debellis D., Tedeschi G., Athanassiou A., Bayer I.S. Responsive Bio-Composites from Magnesium Carbonate Filled Polycaprolactone and Curcumin-Functionalized Cellulose Fibers. Adv. Sustain. Syst. 2021;5:2100128. doi: 10.1002/adsu.202100128. [DOI] [Google Scholar]
- 154.Kim M.S., Kim G. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 2014;114:213–221. doi: 10.1016/j.carbpol.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 155.Nurazzi N.M., Asyraf M.R.M., Khalina A., Abdullah N., Aisyah H.A., Rafiqah S.A., Sabaruddin F.A., Kamarudin S.H., Norrrahim M.N.F., Ilyas R.A., et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers. 2021;13:646. doi: 10.3390/polym13040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abdolmohammadi S., Yunus W.M.Z.W., Rahman M.Z.A., Azowa Ibrahim N. Effect of organoclay on mechanical and thermal properties of polycaprolactone/ chitosan/montmorillonite nanocomposites. J. Reinf. Plast. Compos. 2011;30:1045–1054. doi: 10.1177/0731684411410338. [DOI] [Google Scholar]
- 157.Eng C.C., Ibrahim N.A., Zainuddin N., Ariffin H., Yunus W.M.Z.W., Then Y.Y. Enhancement of mechanical and dynamic mechanical properties of hydrophilic nanoclay reinforced polylactic acid/polycaprolactone/oil palm mesocarp fiber hybrid composites. Int. J. Polym. Sci. 2014;2014:715801. doi: 10.1155/2014/715801. [DOI] [Google Scholar]
- 158.Hajiali F., Tajbakhsh S., Shojaei A. Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polym. Rev. 2018;58:164–207. doi: 10.1080/15583724.2017.1332640. [DOI] [Google Scholar]
- 159.Moers-Carpi M.M., Sherwood S. Polycaprolactone for the Correction of Nasolabial Folds: A 24-Month, Prospective, Randomized, Controlled Clinical Trial. Dermatol. Surg. 2013;39:457–463. doi: 10.1111/dsu.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hao Y., Chen Y., He X., Yang F., Han R., Yang C., Li W., Qian Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020;5:542–552. doi: 10.1016/j.bioactmat.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Peng W., Jiang X., Zhu Y., Omari-Siaw E., Deng W., Yu J., Xu X., Zhang W. Oral delivery of capsaicin using MPEG-PCL nanoparticles. Acta Pharmacol. Sin. 2015;36:139–148. doi: 10.1038/aps.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim J.A., Van Abel D. Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. J. Cosmet. Laser Ther. 2015;17:99–101. doi: 10.3109/14764172.2014.968586. [DOI] [PubMed] [Google Scholar]
- 163.Christen M.-O., Vercesi F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020;13:31–48. doi: 10.2147/CCID.S229054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.de Melo F., Carrijo A., Hong K., Trumbic B., Vercesi F., Waldorf H.A., Zenker S. Minimally Invasive Aesthetic Treatment of the Face and Neck Using Combinations of a PCL-Based Collagen Stimulator, PLLA/PLGA Suspension Sutures, and Cross-Linked Hyaluronic Acid. Clin. Cosmet. Investig. Dermatol. 2020;13:333–344. doi: 10.2147/CCID.S248280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Melo F., Nicolau P., Piovano L., Lin S.-L., Baptista-Fernandes T., King M.I., Camporese A., Hong K., Khattar M., Christen M.-O. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®) Clin. Cosmet. Investig. Dermatol. 2017;10:431–440. doi: 10.2147/CCID.S145195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morera Serna E., Serna Benbassat M., Terré Falcón R., Murillo Martín J. Anatomy and Aging of the Perioral Region. Facial Plast. Surg. 2021;37:176–193. doi: 10.1055/s-0041-1725104. [DOI] [PubMed] [Google Scholar]
- 167.Goodwin P. Collagen stimulation with a range of polycaprolactone dermal fillers. J. Aesthetic Nurs. 2018;7:22–28. doi: 10.12968/joan.2018.7.Sup2.22. [DOI] [Google Scholar]
- 168.Fujihara K., Kotaki M., Ramakrishna S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials. 2005;26:4139–4147. doi: 10.1016/j.biomaterials.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 169.Won J.-Y., Park C.-Y., Bae J.-H., Ahn G., Kim C., Lim D.-H., Cho D.-W., Yun W.-S., Shim J.-H., Huh J.-B. Evaluation of 3D printed PCL/PLGA/ β -TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed. Mater. 2016;11:055013. doi: 10.1088/1748-6041/11/5/055013. [DOI] [PubMed] [Google Scholar]
- 170.Ji W., Yang F., Ma J., Bouma M.J., Boerman O.C., Chen Z., van den Beucken J.J.J.P., Jansen J.A. Incorporation of stromal cell-derived factor-1α in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials. 2013;34:735–745. doi: 10.1016/j.biomaterials.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 171.Shim J.-H., Yoon M.-C., Jeong C.-M., Jang J., Jeong S.-I., Cho D.-W., Huh J.-B. Efficacy of rhBMP-2 loaded PCL/PLGA/ β -TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed. Mater. 2014;9:065006. doi: 10.1088/1748-6041/9/6/065006. [DOI] [PubMed] [Google Scholar]
- 172.Türkkan S., Pazarçeviren A.E., Keskin D., Machin N.E., Duygulu Ö., Tezcaner A. Nanosized CaP-silk fibroin-PCL-PEG-PCL/PCL based bilayer membranes for guided bone regeneration. Mater. Sci. Eng. C. 2017;80:484–493. doi: 10.1016/j.msec.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 173.Ren K., Wang Y., Sun T., Yue W., Zhang H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C. 2017;78:324–332. doi: 10.1016/j.msec.2017.04.084. [DOI] [PubMed] [Google Scholar]
- 174.Castro A.G.B., Diba M., Kersten M., Jansen J.A., van den Beucken J.J.J.P., Yang F. Development of a PCL-silica nanoparticles composite membrane for Guided Bone Regeneration. Mater. Sci. Eng. C. 2018;85:154–161. doi: 10.1016/j.msec.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 175.Ahmed F., Discher D.E. Self-porating polymersomes of PEG–PLA and PEG–PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release. 2004;96:37–53. doi: 10.1016/j.jconrel.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 176.Köthe T., Martin S., Reich G., Fricker G. Dual asymmetric centrifugation as a novel method to prepare highly concentrated dispersions of PEG-b-PCL polymersomes as drug carriers. Int. J. Pharm. 2020;579:119087. doi: 10.1016/j.ijpharm.2020.119087. [DOI] [PubMed] [Google Scholar]
- 177.Zwawi M. A Review on Natural Fiber Bio-Composites, Surface Modifications and Applications. Molecules. 2021;26:404. doi: 10.3390/molecules26020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mi H.Y., Jing X., Peng J., Salick M.R., Peng X.F., Turng L.S. Poly(ε-caprolactone) (PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose. 2014;21:2727–2741. doi: 10.1007/s10570-014-0327-y. [DOI] [Google Scholar]
- 179.Grossen P., Witzigmann D., Sieber S., Huwyler J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release. 2017;260:46–60. doi: 10.1016/j.jconrel.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 180.Rai B., Teoh S.H., Hutmacher D.W., Cao T., Ho K.H. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26:3739–3748. doi: 10.1016/j.biomaterials.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 181.Nabid M.R., Tabatabaei Rezaei S.J., Sedghi R., Niknejad H., Entezami A.A., Oskooie H.A., Heravi M.M. Self-assembled micelles of well-defined pentaerythritol-centered amphiphilic A4B8 star-block copolymers based on PCL and PEG for hydrophobic drug delivery. Polymer. 2011;52:2799–2809. doi: 10.1016/j.polymer.2011.04.054. [DOI] [Google Scholar]
- 182.Holländer J., Genina N., Jukarainen H., Khajeheian M., Rosling A., Mäkilä E., Sandler N. Three-Dimensional Printed PCL-Based Implantable Prototypes of Medical Devices for Controlled Drug Delivery. J. Pharm. Sci. 2016;105:2665–2676. doi: 10.1016/j.xphs.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 183.Gong C., Shi S., Wu L., Gou M., Yin Q., Guo Q., Dong P., Zhang F., Luo F., Zhao X., et al. Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL–PEG–PCL hydrogel. Part 2: Sol–gel–sol transition and drug delivery behavior. Acta Biomater. 2009;5:3358–3370. doi: 10.1016/j.actbio.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 184.Coombes A.G.A., Rizzi S.C., Williamson M., Barralet J.E., Downes S., Wallace W.A. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials. 2004;25:315–325. doi: 10.1016/S0142-9612(03)00535-0. [DOI] [PubMed] [Google Scholar]
- 185.Chang C., Wei H., Quan C.-Y., Li Y.-Y., Liu J., Wang Z.-C., Cheng S.-X., Zhang X.-Z., Zhuo R.-X. Fabrication of thermosensitive PCL-PNIPAAm-PCL triblock copolymeric micelles for drug delivery. J. Polym. Sci. Part A Polym. Chem. 2008;46:3048–3057. doi: 10.1002/pola.22645. [DOI] [Google Scholar]
- 186.Danafar H. MPEG–PCL copolymeric nanoparticles in drug delivery systems. Cogent Med. 2016;3:1142411. doi: 10.1080/2331205X.2016.1142411. [DOI] [PubMed] [Google Scholar]
- 187.Zhang J., Zhao S., Zhu M., Zhu Y., Zhang Y., Liu Z., Zhang C. 3D-printed magnetic Fe3O4 /MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B. 2014;2:7583–7595. doi: 10.1039/C4TB01063A. [DOI] [PubMed] [Google Scholar]
- 188.Deng H., Dong A., Song J., Chen X. Injectable thermosensitive hydrogel systems based on functional PEG/PCL block polymer for local drug delivery. J. Control. Release. 2019;297:60–70. doi: 10.1016/j.jconrel.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 189.Zhao P., Liu L., Feng X., Wang C., Shuai X., Chen Y. Molecular Nanoworm with PCL Core and PEO Shell as a Non-spherical Carrier for Drug Delivery. Macromol. Rapid Commun. 2012;33:1351–1355. doi: 10.1002/marc.201200172. [DOI] [PubMed] [Google Scholar]
- 190.Dash T.K., Konkimalla V.B. Polymeric Modification and Its Implication in Drug Delivery: Poly-ε-caprolactone (PCL) as a Model Polymer. Mol. Pharm. 2012;9:2365–2379. doi: 10.1021/mp3001952. [DOI] [PubMed] [Google Scholar]
- 191.Hosseinkazemi H., Biazar E., Bonakdar S., Ebadi M.-T., Shokrgozar M.-A., Rabiee M. Modification of PCL Electrospun Nanofibrous Mat With Calendula officinalis Extract for Improved Interaction With Cells. Int. J. Polym. Mater. Polym. Biomater. 2015;64:459–464. doi: 10.1080/00914037.2014.958835. [DOI] [Google Scholar]
- 192.Hosseini Y., Emadi R., Kharaziha M. Surface modification of PCL-diopside fibrous membrane via gelatin immobilization for bone tissue engineering. Mater. Chem. Phys. 2017;194:356–366. doi: 10.1016/j.matchemphys.2017.03.051. [DOI] [Google Scholar]
- 193.Kweon H. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials. 2003;24:801–808. doi: 10.1016/S0142-9612(02)00370-8. [DOI] [PubMed] [Google Scholar]
- 194.Siddiqui N., Asawa S., Birru B., Baadhe R., Rao S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018;60:506–532. doi: 10.1007/s12033-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 195.Kundu J., Shim J.-H., Jang J., Kim S.-W., Cho D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015;9:1286–1297. doi: 10.1002/term.1682. [DOI] [PubMed] [Google Scholar]
- 196.Patrício T., Domingos M., Gloria A., Bártolo P. Characterisation of PCL and PCL/PLA Scaffolds for Tissue Engineering. Procedia CIRP. 2013;5:110–114. doi: 10.1016/j.procir.2013.01.022. [DOI] [Google Scholar]
- 197.Gautam S., Dinda A.K., Mishra N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C. 2013;33:1228–1235. doi: 10.1016/j.msec.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 198.Boia R., Dias P.A.N., Martins J.M., Galindo-Romero C., Aires I.D., Vidal-Sanz M., Agudo-Barriuso M., de Sousa H.C., Ambrósio A.F., Braga M.E.M., et al. Porous poly(ε-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control. Release. 2019;316:331–348. doi: 10.1016/j.jconrel.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 199.Hivechi A., Bahrami S.H., Siegel R.A. Drug release and biodegradability of electrospun cellulose nanocrystal reinforced polycaprolactone. Mater. Sci. Eng. C. 2019;94:929–937. doi: 10.1016/j.msec.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 200.Ciapetti G., Ambrosio L., Savarino L., Granchi D., Cenni E., Baldini N., Pagani S., Guizzardi S., Causa F., Giunti A. Osteoblast growth and function in porous poly ε-caprolactone matrices for bone repair: A preliminary study. Biomaterials. 2003;24:3815–3824. doi: 10.1016/S0142-9612(03)00263-1. [DOI] [PubMed] [Google Scholar]
- 201.Wang L., Abedalwafa M., Wang F., Li C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013;34:123–140. [Google Scholar]
- 202.Mondal D., Griffith M., Venkatraman S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016;65:255–265. doi: 10.1080/00914037.2015.1103241. [DOI] [Google Scholar]
- 203.Zander N.E., Orlicki J.A., Rawlett A.M., Beebe T.P. Surface-modified nanofibrous biomaterial bridge for the enhancement and control of neurite outgrowth. Biointerphases. 2010;5:149–158. doi: 10.1116/1.3526140. [DOI] [PubMed] [Google Scholar]
- 204.Xu F.J., Wang Z.H., Yang W.T. Surface functionalization of polycaprolactone films via surface-initiated atom transfer radical polymerization for covalently coupling cell-adhesive biomolecules. Biomaterials. 2010;31:3139–3147. doi: 10.1016/j.biomaterials.2010.01.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.