Abstract
Stress-associated proteins (SAPs) are zinc finger proteins involved in the regulation of various stresses in a variety of plant species. A total of nine PdSAP genes were identified in Prunus dulcis. Phylogenetic and synteny analyses were performed to analyze the homology and evolutionary relationship of PdSAP genes. The functions of PdSAP genes were assessed by further analyses, including cis-regulatory elements, gene duplication, gene ontology, gene structure, subcellular localization, and motif pattern. This study found that PdSAP genes were unevenly distributed on chromosomes 2, 3, 6, and 7. Phylogenetic analysis of PdSAP genes with Arabidopsis thaliana and Oryza sativa suggested that six subgroups have a similar pattern of AN1 and A20 domains in each subgroup. PdSAP genes lacked duplicated blocks. The majority of PdSAP genes were localized in the nucleus region. Three hormonal and five stress cis-regulatory elements were found in the upstream promoter region of the PdSAP gene family. RNA-seq analysis revealed differential gene expression of PdSAP genes at days 12, 17, 22, 27, 32, and 37 of fruitlet development after flowering. This study identifies the SAP genes in P. dulcis and also provides insights into the expression of PdSAP genes in abnormal fruitlets with diapause atrophic growth at various developmental stages.
Keywords: almonds, stress-associated protein (SAP), genome-wide identification, phylogenetic analysis, defense and stress responses, RNA sequence analysis, differential gene expression
1. Introduction
Biotic and abiotic stresses adversely affect plants’ growth and productivity [1,2]. These stresses result in great damage to global crops by reducing their average yield [3]. To eliminate the effects of biotic and abiotic stresses, plants have developed several mechanisms, including the responsive action of stress-regulating genes for the plant’s growth and maintenance [4]. Plants mediate the early stress response by the regulation of phytohormones, such as jasmonic acid (JA), abscisic acid (ABA), and salicylic acid (SA) [5,6]. Various transcription factor proteins have also been identified that play a key role in the regulation of gene expression by interacting with cis-regulatory promoter elements at the transcription level [7]. The DNA binding domain of transcription factors (TFs) determines the function of the transcription factors in the regulation of gene expression [8]. The binding of transcription factors with cis-regulatory promoter elements results in the enhanced or reduced expressions of targeted genes [9]. Transcription factors play an active role in the growth and development of plants, including hormone signaling, organ formation, secondary metabolism, and response to various stresses [10,11]. Similarly, numerous plant transcription factors also improve tolerance against abiotic stresses in plants [12].
Almond, Prunus dulcis, belongs to the Rosaceae family, and its seeds have commercial importance. Its cultivation is widely spread in hot climate regions [13]. Almond is one of the most cultivated trees in Spain and one of the most cultivated crops in the Mediterranean area [14]. Recently, the USA had a high production rate of almonds [15]. It is also being cultivated in other countries, including Spain, Italy, Iran, Syria, and Turkey [16]. The nutrient content is very high in almonds, including carbohydrates, amino acids, vitamins, proteins, lipids, and secondary metabolites. The seeds of almonds have concentrated energy due to their high lipid content [17].
Fruit drop is a major concern for growers of P. dulcis, as it occurs at three different stages of development due to various reasons [18]. Deficient pistil development leads to the first drop of malformed flowers. Unfertilized flowers are also dropped at 3–4 weeks after the bloom. The third drop or the June drop, which is basically a physiological drop, occurs at 6 or 7 weeks after the bloom [19]. Different stresses that include drought, salt, and cold greatly affect the development and yield of almonds [15]. Although almond is a drought-resistant species, irrigation is much needed to improve fruit quality and crop yield [20].
Given the role of stresses in the fruit development of P. dulcis, stress-associated protein (SAP) transcription factors should regulate stress responses. SAPs have been studied in various plant species, such as maize (11 genes), cucumber (12 genes), Arabidopsis (14 genes), rice (18 genes), cotton (37 genes), and tomato (13 genes) [3,21,22,23,24]. Stress-associated proteins (SAPs) contain one or two domains (A20 and AN1) at their N and C terminal, respectively [25]. A20 zinc finger was first identified in humans with Cys2/Cys2 finger motifs [26]. However, the AN1 zinc finger domain was identified in Xenopus laevis. A20 and AN1, both zinc finger domains, have key roles in the regulation of various stresses and exist in all eukaryotes, including several plant species [26].
One remarkable characteristic of plant SAPs is that they are the key regulators of plants’ biotic and abiotic stresses. Rice SAP1 (OsSAP1) was first identified in plants by the induction of responses against different environmental stresses, such as drought, wounding, cold, and abscisic acid (ABA) [27]. The high expression of OsSAP1 resulted in tolerance against cold, drought, and salt stresses during different developmental stages [28]. SAP genes are also involved in the regulation of the immune system, the maintenance of plant developmental stages, and the response to phytochromes [29]. Overexpressed SAP genes of rice and other plant species have been found to induce abiotic stress tolerance against pathogens, drought, salt, temperature, and cold stresses [28,30,31]. For example, overexpression of the OsSAP8 gene of rice induced tolerance to different stresses, such as cold, drought, and salt, in transgenic tobacco and rice during seed germination [30]. Similarly, OsSAP1 overexpression in transgenics showed an increase in basal resistance in tobacco leaves against the bacterial pathogen Pseudomonas syringae [27].
Limited studies have been carried out on P. dulcis. SAP genes have not been identified to date. This study is based on the identification of SAP transcription factor genes in the P. dulcis genome and their expression in the normal and abnormal fruitlets at various growth stages. As fruitlets are dropped due to various stresses, SAP must have a pivotal role in the regulation of these stresses, as these are expressed in response to stresses. This study used RNA-seq data from another study based on the analysis of carbon signaling genes in the fruit development of P. dulcis at days 12, 17, 22, 27, 32, and 37 after flowering to evaluate the expression of SAP genes in normal and abnormal fruitlets [32]. This study identified nine SAP genes in almonds at the genomic level. We performed various bioinformatics analyses, such as chromosomal position, gene structure, phylogeny, and gene duplication analyses, in addition to synteny analysis, to identify SAP genes of P. dulcis that are homologous with those of rice and Arabidopsis. This study gives insights into the functional and structural characterization of the PdSAP gene family, along with their differential expression in the abnormally developed fruitlets with diapause atrophic growth in comparison with the normally developed fruitlets in Prunus dulcis.
2. Results
2.1. Identification of PdSAP Gene Family
A total of nine SAP genes were identified in almond after the removal of 100% similar sequences. A total of seven PdSAP gene sequences contained both AN1 and A20 domains, whereas two PdSAP genes contained only the zf-AN1 domain. The protein sequences containing SAP domains are provided in Data S1. SAP gene sequences were renamed from PdSAP1 to PdSAP9 based on their position on chromosomes 2, 3, 6, and 7. The identified SAPs of P. dulcis, along with their accession numbers, domains, and chromosome numbers, are presented in Table S1. The protein sequence length of the SAP genes was between 152 amino acid residues of PdSAP3 and 292 amino acid residues of PdSAP7.
2.2. Multiple Sequence Alignment and Phylogenetic Analysis of PdSAP
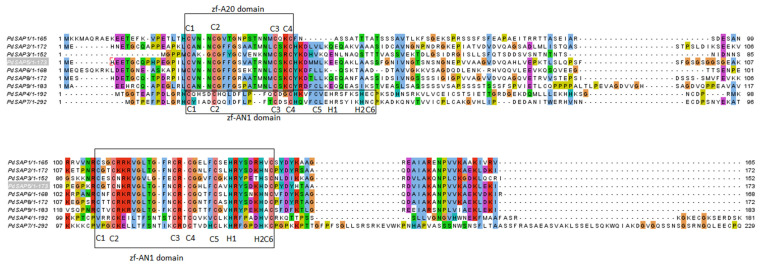
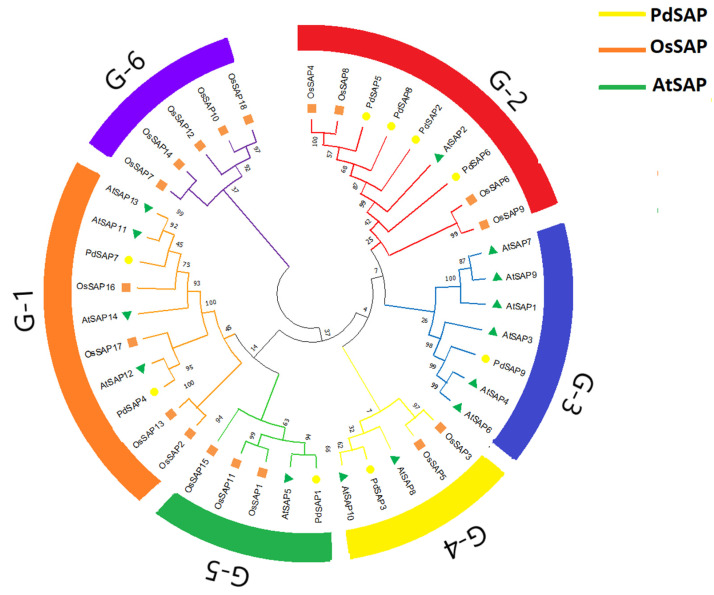
To predict the presence of zf domains in PdSAP, a multiple sequence alignment of predicted protein sequences of SAP genes was carried out using MegaX-V10.2.4 software. The zinc finger domains zf-A20/A1 are highlighted in the boxes labeled in Figure 1. The peptide sequences of AtSAP, PdSAP, and OsSAP genes were used to construct a phylogenetic tree using the neighbor-joining method, which is shown in Figure 2. Most of the PdSAP genes showed close links with the OsSAP genes. Few genes of PdSAP are distantly related to AtSAP genes. The SAP genes of P. dulcis, O. sativa, and A. thaliana are highlighted in different colors. The phylogenetic tree was divided into six subgroups (G1–G6). The G-1 subgroup was the largest group, with two members of PdSAP (PdSAP7 and PdSAP4), four members of A. thaliana (AtSAP11, AtSAP12, AtSAP13, and AtSAP14), and four members of OsSAP genes (OsSAP2, OsSAP13, OsSAP16, and OsSAP17).
Figure 1.
Multiple sequence alignment of SAP gene sequences. Multiple sequence alignment of zf-A20/AN1 domains using Jalview. Conserved zf-A20 and zf-AN1 domains are boxed.
Figure 2.
Phylogenetic tree of PdSAP genes with A. thaliana and O. sativa. PdSAP genes are classified into six subgroups (G1–G6) based on number of SAP genes present in groups. The six subgroups are marked in different colors.
Members of the G-1 subgroup mainly possessed the AN1 domain, except for OsSAP2, which harbored both AN1 and A20 domains. The G-2 subgroup possessed both AN1 and A20 domains with four PdSAP genes (PdSAP2, PdSAP5, PdSAP6, and PdSAP8), whereas the G-3 subgroup consisted of only PdSAP9. The G-4 subgroup and the G-5 subgroup consisted of five members with PdSAP3 and PdSAP1, respectively. Members of the G-4 subgroup contained only the AN1 domain, except for PdSAP3. Members of the G-5 subgroup contained both A20 and AN1 domains, except for OsSAP15, which harbored only the AN1 domain. The G-6 subgroup showed no relationship with PdSAP genes; however, it only possessed OsSAP genes with both AN1 and A20 domains.
2.3. Chromosomal Position and Cis-Acting Regulatory Analysis of PdSAP Genes
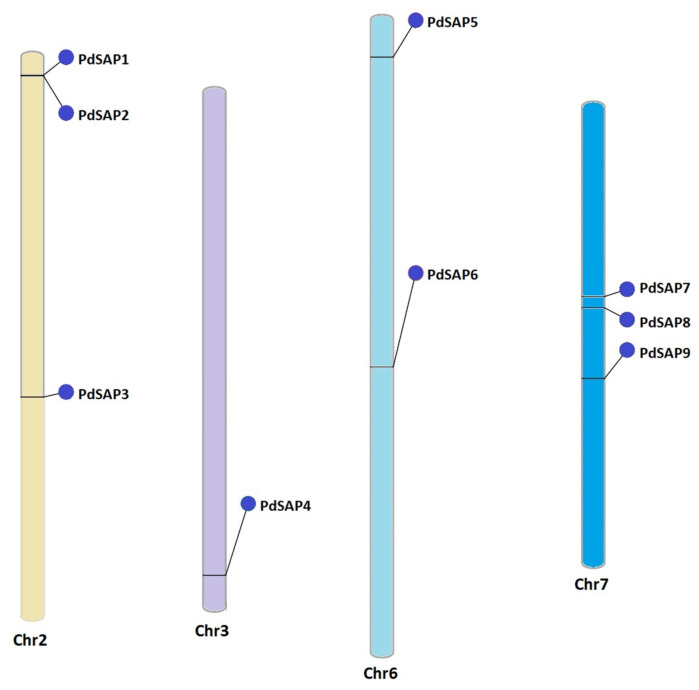
A phenogram predicted the chromosomal distribution of the nine SAP genes of almond. SAP genes were unevenly distributed on the chromosomes 2, 3, 6, and 7 of P. dulcis as shown in Figure 3. SAP genes were absent on the chromosomes 1, 4, 5, and 8 of almond. There were three SAP genes present on chromosomes 2 and 7, whereas chromosomes 3 and 6 contained one and two PdSAP genes, respectively. Chromosome 2 had only one cluster of PdSAP genes consisting of PdSAP1 and PdSAP2. Chromosome 3 contained only one PdSAP gene, which was PdSAP4.
Figure 3.
Chromosomal mapping of PdSAP genes. PdSAP genes have been mapped on the chromosomes of P. dulcis. Chromosome numbers are presented below each chromosome. Each chromosome is colored differently.
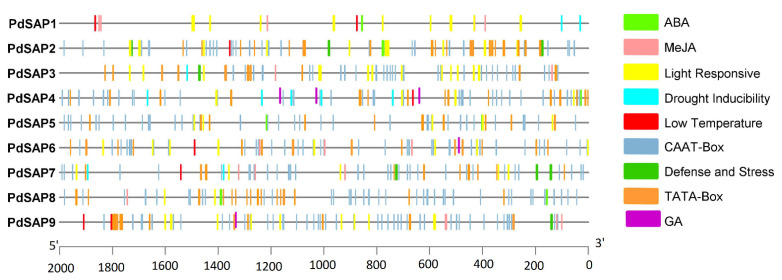
A total of three hormonal and five cis-acting regulatory elements, namely, abscisic acid (ABA), methyl jasmonate responsiveness (MeJA), gibberellic acid (GA), drought inducibility (MYB), defense and stress (TC-rich repeat), low temperature responsive element (LTR), TATA box, and CAAT box, were selected in the upstream genomic sequences of PdSAP. These cis-regulatory elements are depicted in Figure 4. TATA box was the most prominent and the most frequently occurring compared to all other elements in all PdSAP sequences. PdSAP2 contained nine ABA cis-regulatory elements that were higher in number as compared to the other PdSAP genes. CAAT box was present in all PdSAP sequences, containing 53 of the highest elements in PdSAP1. Among all, GA was the least occurring promoter element in the PdSAP sequences. MYB is responsible for drought inducibility with the highest number in PdSAP7. TC-rich elements were detected in all SAP genes that are responsible for stress responsiveness and defense. Cis-regulatory elements, with their start and end position in the upstream region of PdSAP, are provided in Data S2.
Figure 4.
PdSAP cis-acting regulatory elements. Upstream promoter regions of PdSAP contained the cis-acting regulatory elements, and these are shown in various colors in the figure, with the length of the upstream region of PdSAP genes indicated on the x-axis.
2.4. Domain Analyses, Motif Composition, and Gene Structure of PdSAP Genes
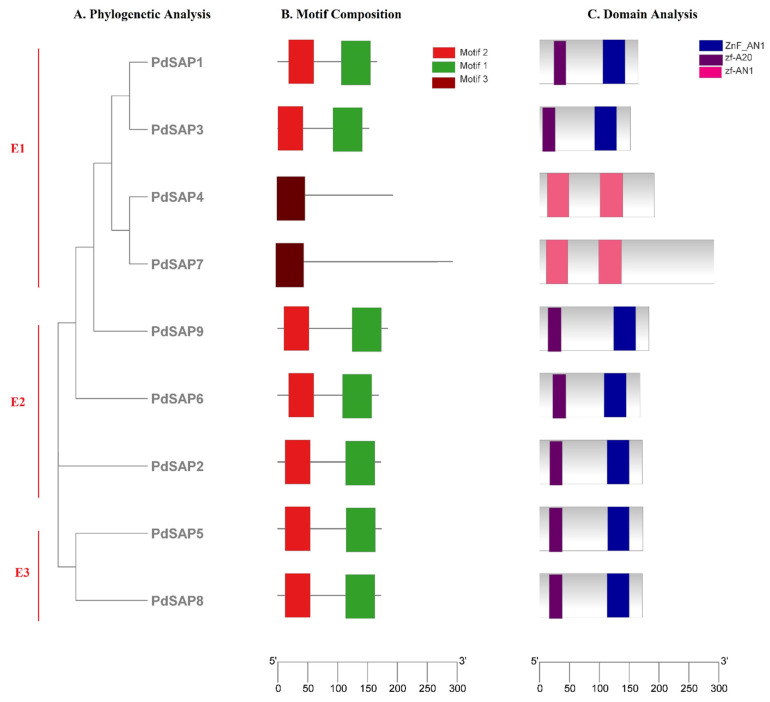
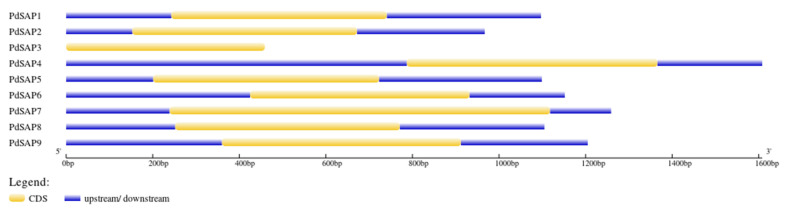
A phylogenetic tree of nine PdSAP genes was constructed consisting of three subgroups (E1–E3) as shown in Figure 5A. The E1 subgroup had four members of PdSAP genes, namely, PdSAP1, PdSAP3, PdSAP4, and PdSAP7. The E2 subgroup consisted of three PdSAP genes, namely, PdSAP2, PdSAP6, and PdSAP9. In the E3 subgroup, only two members of PdSAP genes (PdSAP5 and PdSAP8) were present. Further, motif analysis of nine PdSAP proteins was carried out as shown in Figure 5B. Three motifs were predicted in the PdSAP genes, and their sequence logos are provided in Figure S1. A total of seven members of PdSAP genes consisted of motif 1 and motif 2, whereas motif 3 was present only in PdSAP4 and PdSAP7. In seven SAP genes, both AN1 and A20 domains were present, whereas only the AN1 domain was present in the remaining two SAP genes, which were PdSAP4 and PdSAP7, as depicted in Figure 5C. All PdSAP genes were intronless. All members of PdSAP genes contained UTRs at 3′ and 5′ of the genes as shown in Figure 6.
Figure 5.
PdSAP motif analysis and gene structure prediction. In the figure, phylogenetic tree (A), motif pattern (B), and domains (C) are visualized.
Figure 6.
PdSAP gene structure prediction. Exons are in yellow. PdSAP genes lacked intronic regions. Blue represents the 5′ and 3′ untranslated regions (UTRs).
2.5. Gene Duplication and Synteny Analysis of PdSAP Genes
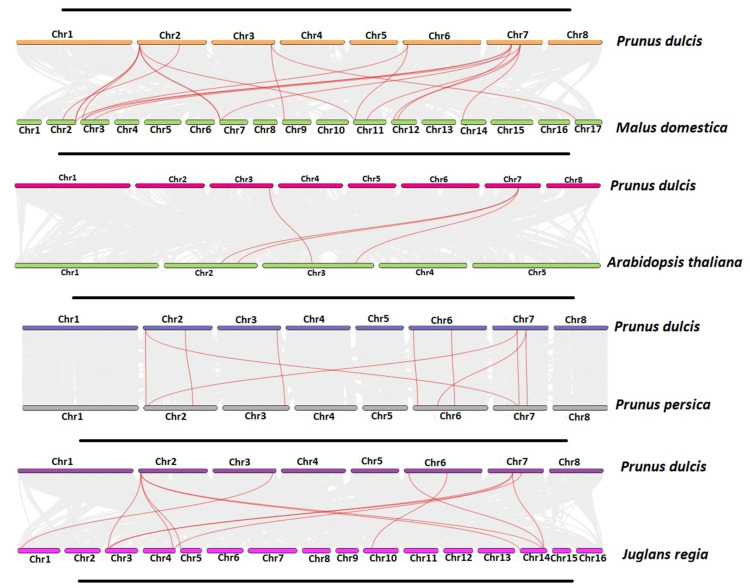
MCScanX revealed no duplication blocks in the P. dulcis genome. The TBTool was used to perform synteny analysis of P. dulcis with the genomes of P. persica, A. thaliana, J. regia, and M. domestica to analyze the presence of homologous syntenic blocks as shown in Figure 7. The synteny analysis revealed that a total of seven SAP genes in P. dulcis shared collinear gene pairs with M. domestica (19), A. thaliana (4), P. persica (10), and J. regia (12) as shown in Data S3. The total numbers of homologous gene pairs in P. dulcis with the SAPs of M. domestica, A. thaliana, P. persica, and J. regia were 15, 4, 9, and 8, respectively. Synteny maps indicated the high evolutionary homology relationship of PdSAP genes with SAP genes of the four other species.
Figure 7.
Synteny analysis of PdSAP genes. Synteny analysis of PdSAP with M. domestica, A. thaliana, J. regia, and P. persica. Red lines indicate the presence of an evolutionary relationship.
2.6. Subcellular Localization and Physicochemical Prediction of PdSAP Genes
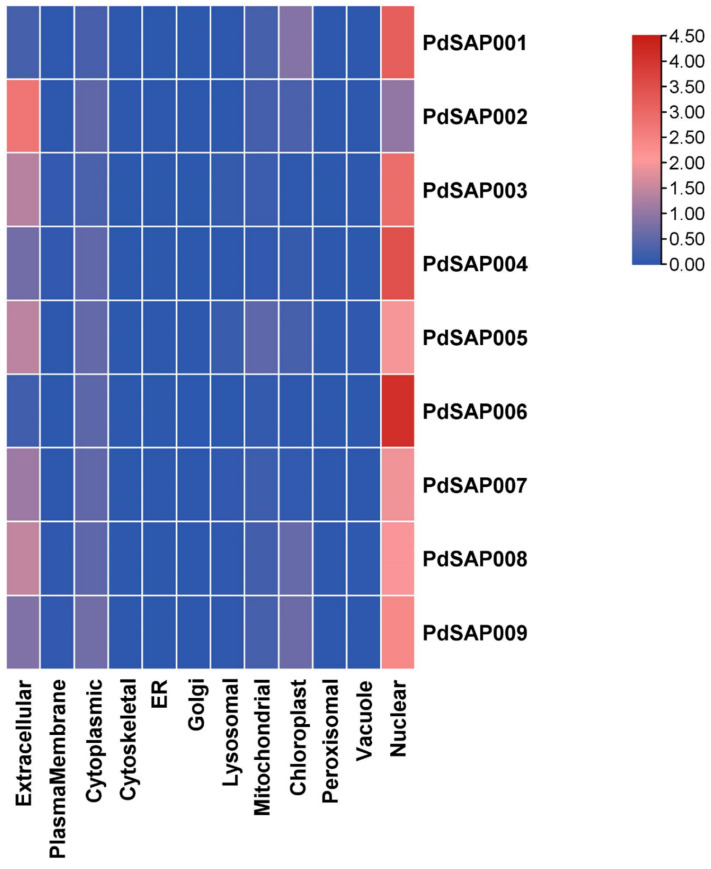
The molecular weight (MW) of PdSAP genes ranged from 16.569 kDa for PdSAP3 to 32.124 kDa for PdSAP7 as presented in Table S1. The isoelectric point (pI) varied from 7.46 for PdSAP9 to 8.98 for PdSAP4. All of the P. dulcis SAP genes were found to be localized in the nucleus region, except for PdSAP2, which was localized in the cytoplasmic region as shown in Figure 8.
Figure 8.
Subcellular localization of PdSAP gene family. Heatmap of PdSAP is shown. Majority of PdSAP genes are in nuclear region.
2.7. Gene Ontology of PdSAP Genes
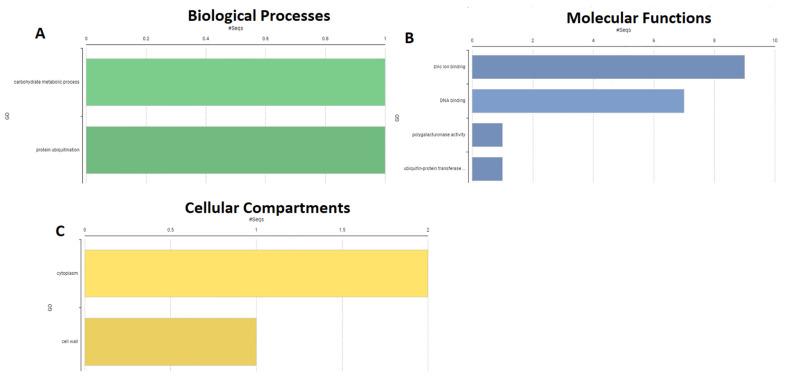
The molecular functions, cellular compartments, and biological processes of PdSAP genes were analyzed using gene ontology as presented in Figure 9. The biological processes indicated that PdSAP genes have a role in carbohydrate metabolism and the ubiquitination of proteins as predicted by Blast2Go. The molecular functions of PdSAP genes predicted their role in DNA binding and zinc ion binding, whereas the cellular compartments study revealed that the majority of PdSAP genes are present in the cytoplasm.
Figure 9.
Gene ontology of PdSAP genes by Blast2GO. Green bars indicated biological processes (A), blue bars indicate molecular functions of PdSAP (B), and yellow bars indicate the presence of PdSAP in cellular compartments (C).
2.8. Differential Gene Expression of PdSAP Genes in Normal and Abnormal Fruitlets
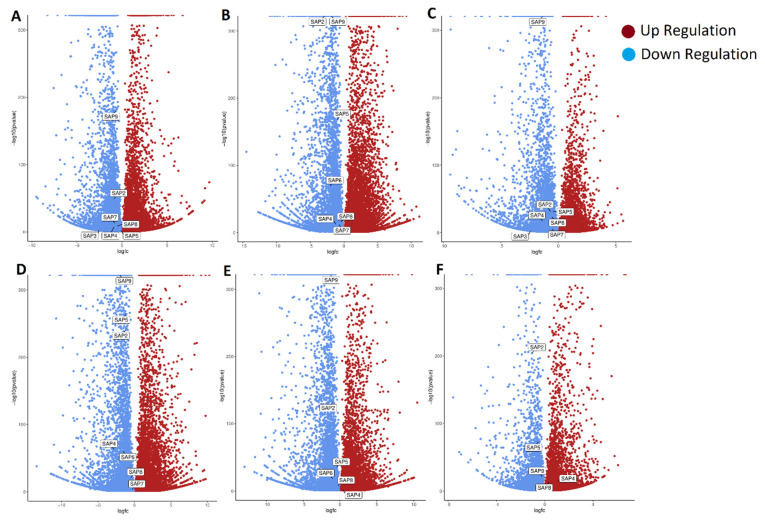
RNA-seq analyses of normal and abnormal fruitlets of P. dulcis at 12, 17, 22, 27, 32, and 37 days of development after flowering were carried out. The upregulation and downregulation of the SAP genes of normally and abnormally developed fruitlets were analyzed using DESeq2. The DESeq2 results are provided in Data S4. The DESeq2 results for only the PdSAP genes are presented in Data S5. Volcano plots with differential expression are presented in Figure 10. Individual volcano plots of PdSAP expression at 12, 17, 22, 27, 32, and 37 days of development are provided in Figures S2–S7. At day 12 of P. dulcis, PdSAP2, PdSAP3, PdSAP4, PdSAP5, PdSAP7, PdSAP8, and PdSAP9 were downregulated. Similarly, PdSAP2, PdSAP4, PdSAP5, PdSAP6, PdSAP7, PdSAP8, and PdSAP9 were downregulated at day 17. PdSAP2, PdSAP3, PdSAP4, PdSAP5, PdSAP7, PdSAP8, and PdSAP9 also had downregulated expression at day 22 of development after flowering. PdSAP2, PdSAP4, PdSAP5, PdSAP6, PdSAP7, PdSAP8, and PdSAP9 had downregulated expression at day 27. At days 12, 17, 22, and 27, no PdSAP gene had upregulated expression. PdSAP2, PdSAP5, PdSAP6, PdSAP8, and PdSAP9 had downregulated expression at day 32 of development, whereas PdSAP4 had upregulated expression at day 32. Similarly, at day 37 after flowering, PdSAP2, PdSAP5, and PdSAP9 had downregulated expression, whereas PdSAP4 and PdSAP8 had upregulated expression. PdSAP2, PdSAP5, and PdSAP9 had downregulated expression at 12, 17, 22, 27, 32, and 37 days of development, whereas the expression of the other PdSAP genes was variable.
Figure 10.
Differential gene expression of fruitlets in almonds. Volcano plots of differential expression of PdSAP genes is shown at developmental stage at 12 (A), 17 (B), 22 (C), 27 (D), 32 (E), and 37 (F) days after flowering in almonds fruitlets.
3. Discussion
Almonds are consumed worldwide, as they are a nutrition-rich source of magnesium, potassium, and vitamin E. Given their importance, SAP TFs are involved in the response to abiotic and biotic stresses, in addition to cell wall synthesis [33]. The SAP gene family has been reported in various plant species but not in P. dulcis. In this study, we identified nine PdSAP genes in the genome of P. dulcis. A high diversity of SAP genes has been reported among different plant species, ranging from 11 members in Zea mays to 57 members in Brassica napus [34,35]. All PdSAP genes were mapped to four out of eight chromosomes, and no SAP genes were found on chromosomes 1, 4, 5, and 8. Similarly, twelve CsSAP genes were present on chromosome 6, whereas chromosome 2 lacked CsSAP genes in cucumber [23]. PdSAP genes in almonds were found to be spread out on chromosomes 2, 3, 6, and 7. In addition, one cluster of PdSAP1 and PdSAP2 genes was found on chromosome 2. Similarly, three CsSAP genes were spread out on chromosomes 1, 3, 4, 5, 6, and 7, and six CsSAP genes were clustered on three chromosomes in cucumber [23]. In Medicago truncatula, chromosome 2 contained three MtSAP genes, and two MtSAP genes were spread out on chromosomes 1, 3, and 4, each [36]. Various defense and stress responsive regulatory factors were found in the upstream promoter region of P. dulcis, confirming the involvement of PdSAP genes in stress responses. In cucumber, the same cis-regulatory elements were found, indicating their role in stress responses [23]. Similarly, in the MtSAP genes of Medicago truncatula, ABRE, MBS, ARE, and TGA elements were predicted [36].
The phylogenetic tree of PdSAP genes was divided into six subgroups with respect to A. thaliana and Oryza sativa. The G-1 subgroup was the largest group with 10 members, including PdSAP4 and PdSAP7 genes with the zf-AN1 domain. Subgroups G-2 and G-3 harbored PdSAP2, PdSAP5, PdSAP6, PdSAP8, and PdSAP9 with both A20 and AN1 domains, respectively. Subgroups G-4 and G-5 contained PdSAP3 and PdSAP1 with both zinc finger domains. Subgroup G-6 had no PdSAP genes. Similarly, the phylogenetic tree of cucumber contained the seven subgroups, where group f contained two AN1 domains and groups a, b, c, d, and e contained both AN1 and A20 domains [23]. SAP genes in Solanum lycopersicum, A. thaliana, and O. sativa were divided into five groups [3]. The phylogenetic tree of the SAPs belonging to Ricinus communis, A. thaliana, Jatropha curcas, Manihot esculenta, and Hevea brasiliensis was divided into eight subgroups [37]. In addition, PdSAP genes within the same group shared a similar pattern of conserved domains, indicating that they may share similar functions. A conserved motif distribution pattern was observed in almond SAP proteins based on an evolutionary relationship. Three conserved motifs were identified, and these were distributed on PdSAP proteins, exhibiting a strong evolutionary relationship. Similar to P. dulcis motifs, CsSAP5 and CsSAP6 lacking the Znf-A20 domain also had different conserved motifs as compared to the genes containing both Znf-A20 and Znf-AN1 domains [23].
Variation in the number of exons and introns plays a pivotal role in the evolution of transcription factor protein families. Intronless SAP genes are responsible for immediate response by reducing post-transcriptional processing [38]. In the rice genome, six OsSAP genes had a single intron, whereas one OsSAP8 gene had two introns. However, all the remaining OsSAP genes were intronless [21]. In cucumber, 9 out of 12 SAP genes were intronless [23]. In the present study, PdSAP genes are also intronless, indicating their immediate expression during stress. No gene duplication was observed in the PdSAP genes of P. dulcis. However, in Medicago truncatula, two segmental duplications were identified that were between MtSAP2 and MtSAP9 in addition to MtSAP4 and MtSAP13 [36].
P. dulcis SAP genes had more synteny with P. persica as compared to M. domestica, J. regia, and Arabidopsis thaliana. However, PdSAP genes had syntenic relationships with multiple M. domestica SAP genes, as M. domestica has 14 SAP genes. Similarly, PdSAP genes were also homologous to multiple J. regia genes, whereas only two PdSAP genes were homologous to four A. thaliana SAP genes. Most of the P. dulcis SAP genes were predicted to be in the nuclear region; however, two SAPs were predicted to be in the cytoplasmic region. Previously, it had been reported that SAP genes play an important role in regulating a variety of abiotic and biotic stresses, which is because of their nuclear localization in the cell [30,39]. Similarly, SAPs were predicted to be in the cytoplasm, nucleus, and endoplasmic reticulum in Brassica napus [40]. These localization sites were also predicted for cotton [24].
Sequence read archives (SRAs) were obtained from a study based on profiling of carbon signaling genes by Guo et al. 2021 [32]. In this study, the sequence read archives of normal and abnormal fruitlets were used to check the differential expression of PdSAP genes on days 12, 17, 22, 27, 32, and 37 after flowering. All PdSAP genes had significant expression in the normal and abnormal fruitlets. Similarly, CsSAP1, CsSAP9, CsSAP11, CsSAP5, CsSAP7, and CsSAP12 had significant expression in cucumber at different stages of fruit development [23]. Some of the genes, such as PdSAP2, PdSAP5, and PdSAP9, had downregulated expression throughout the developmental stages, which were tested using RNA-seq. The downregulation of PdSAP genes suggests their expression in abnormal fruitlets. Because diapause atrophy is involved in the abnormality of fruitlets, almonds express PdSAP genes as a response to those stresses. However, there is no clear pattern of the upregulation of PdSAP genes. It is pertinent to mention that the upregulation of PdSAP4 and PdSAP8 on the 37th day of development suggests their involvement in fruit development at the final stages. Similar to this study, SAP expression was observed in response to NaCl and PEG stresses in Brassica napus [40]. In cotton, a differential expression pattern of SAP genes was reported under methyl jasmonate (MeJA), NaCl, and PEG [24]. Similarly, diapause atrophy results in abnormal fruitlets, due to which PdSAP genes are expressed to counter this stress. This study identifies P. dulcis SAP genes and provides insights into their structure as well as their evolutionary relationships. It also reports the involvement of the PdSAP genes expression in abnormally developed fruitlets at various growth stages as compared to normally developed fruitlets in P. dulcis.
4. Materials and Methods
4.1. Identification of PdSAP Gene Family in Prunus dulcis
Protein FASTA file of Prunus dulcis was retrieved from latest genome assembly (GCF_902201215.1) available on NCBI and was used as local database to perform BLASTp. To identify SAP gene family in Prunus dulcis, protein sequences of annotated SAP genes of rice and Arabidopsis were retrieved from TIGER rice database (http://blast.jcvi.org/euk-blast/index.cgi?project=osa1, accessed on 19 August 2021) and TAIR database (https://www.arabidopsis.org/, accessed on 19 August 2021). These retrieved sequences of SAP genes were used as a query to perform the local BLASTp using BLAST+ tool with BLOSUM62 matrix and 10 as E value. Pfam (http://pfam.xfam.org/, accessed on 19 August 2021) was used to scan all BLAST hits to check the presence of zinc finger domains [41]. Sequences containing A20/AN1 were selected after removing redundant sequences with 100% similarity. Putative SAP genes were renamed according to their chromosomal position.
4.2. Multiple Sequence Alignment and Phylogenetic Analysis of PdSAP
Protein sequences of SAP genes of P. dulcis were aligned in a FASTA file. For multiple sequence alignment, ClustalW-V2.1 was used on UseGalaxy webserver [42]. AN1 and A20 domains were analyzed with hidden Markov model (HMM) profile using Pfam database. Both Znf-AN1 and Znf-A20 domains were highlighted in the alignment of almond’s SAPs sequences. For phylogenetic tree construction, multiple sequence alignment was carried out using SAP peptide sequences of almonds with Arabidopsis thaliana and rice using default parameters in MegaX-V10.2.4 (www.megasoftware.net, accessed on 19 September 2021). The output file of multiple sequence alignment was used to the generate phylogenetic tree using neighbor-joining method with p-distance and 1000 bootstraps replicate. Finally, phylogenetic tree was visualized and edited in iTOL webtool (https://itol.embl.de/, accessed on 19 September 2021) [42,43].
4.3. Chromosomal Mapping and Cis-Acting Regulatory Analysis of PdSAP
Chromosomal positions of the SAP gene family were analyzed using phenogram webserver (http://visualization.ritchielab.org/phenograms/plot, accessed on 30 October 2021) [44]. Cis-acting regulatory elements and 2000 bp of genomic sequences upstream of SAP genes were retrieved using TBTool. PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 30 October 2021) was used for the prediction of cis-regulatory elements in the promoter region. TBTool (https://github.com/CJ-Chen/TBtools, accessed on 30 October 2021) was used to visualize cis-acting regulatory elements in the upstream sequences of the SAPs genes.
4.4. Gene Structure and Motif Analyses of PdSAP
For gene structure analysis of SAP genes of almonds, CDS and gene sequences of PdSAP genes were used in Gene Structure Display Server (http://gsds.gao-lab.org, accessed on 3 November 2021) [45]. Phylogenetic tree was generated using neighbor-joining method with 1000 bootstraps by MEGAX-V10.2.4 Newick. Protein domains in SAP gene sequences were analyzed in conserved domain database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 3 November 2021). Motifs were identified using the MEME webserver (https://meme-suite.org/meme/, accessed on 3 November 2021) in the PdSAP genes. The output files from these servers and Newick file were used in TBTools to generate gene motif and domain illustration in SAP gene family [46].
4.5. Gene Duplication and Synteny Analyses of PdSAP
For gene duplication in SAP genes, MCScanX with default parameters was used [47]. BLASTp output was used in MCScanX to predict duplication events in the genome. For synteny analysis, genomic FASTA files and genomic feature files (.gff files) of Prunus dulcis (almonds), Malus domestica (apple), Prunus persica (peach), Arabidopsis thaliana, and Juglans regia (walnut) were downloaded from NCBI genome database and used as input files in one-step MCScanX of TBTools. The output files of one-step MCScanX were further used in dual synteny plotter for visualization of syntenic blocks [46].
4.6. Physiochemical Properties and Subcellular Localization of PdSAP
Physiochemical properties of SAP genes were predicted using ProtParam in Expasy webserver. It depicts structural and functional values of given protein sequences. Molecular weight and isoelectric points for SAP family were predicted using the ProtParam [48]. For prediction of subcellular localization of SAP genes, WoLF PSORT (https://wolfpsort.hgc.jp/, accessed on 30 August 2021) and Cello (http://cello.life.nctu.edu.tw/, accessed on 30 August 2021) were used [49]. WoLF PSORT predicts subcellular localization by functional motifs and amino acid composition [50].
4.7. Gene Ontology Analysis of PdSAP
Blast2Go was used to carry out annotation of SAP genes. Cellular compartments, biological activities, and molecular functions of SAP genes were predicted in Blast2Go. CDS sequences of PdSAP genes were used to perform BLASTx, InterPro Scan, annotation, and mapping. For gene ontology analysis of PdSAP genes, default settings of Blast2Go were used [51].
4.8. Differential Gene Expression of PdSAP in Fruits
RNA-sequencing data of almond ZhiPi cultivate (sequenced by Illumina hiseq 2500) were retrieved from NCBI GEO database (Biosample: SAMN12855948). Thirty plants were randomly selected based on plants’ phenotypes by Guo et al. 2021 [32]. They selected the plants with diapause atrophic growth as abnormal fruits. Samples for RNA sequencing were taken at 12, 17, 22, 27, 32, and 37 days of development after flowering by Guo et al. 2021. SRA and their accessions, along with the phenotypic traits of sampled plants, are presented in Data S6. UseGalaxy webserver (https://usegalaxy.org/, accessed on 23 August 2021) was used to convert sequence read archives (SRAs) into FASTQ files with forward and reverse reads. At each step, quality of reads was checked using FASTQC [52]. For removal of adapter and low-quality sequences from each read, Cutadapt was used [53]. To remove small and low-quality reads, quality cut-off value and minimum length of sequences were set at 20. AlmondV2 assembly (Refseq: GCF_902201215.1) was selected to align the reads in RNA STAR [54]. FeatureCounts was used to determine the number of reads per gene [55]. Normalization and differential gene expression were performed using DESeq2 tool [56,57]. Insignificantly expressed genes were filtered out at the significant adjusted p-value, which was set at 0.05. DESeq2 data were used to create the volcano plots for visualization of differentially expressed genes. Finally, PdSAP genes with differential expression were highlighted in the volcano plots. Galaxy webserver was used for all these analyses [57].
5. Conclusions
This study carried out identification and a comprehensive RNA sequence analysis of SAP genes in normal and abnormal fruitlets in Prunus dulcis. Their evolutionary relationships were established with Arabidopsis thaliana and Oryza sativa. Based on genome sequence accessibility and phylogenetic analysis, nine SAP genes were identified in P. dulcis, containing A20/AN1 zinc finger domains unevenly distributed on four chromosomes. Based on the multiple sequence alignment and the phylogenetic tree, PdSAP genes were classified into six subgroups (G1–G6) corresponding to the presence of previously reported finger domains in cucumber. Gene structure and conserved motifs had a clear pattern in each subgroup. No gene duplication events were observed. Synteny analysis revealed that the almonds were homologous to P. persica. Furthermore, subcellular localization and gene ontology annotation revealed the presence of PdSAP genes in the nucleus and the function of SAP genes. This study used RNA sequence analysis and revealed the expression of SAP genes in abnormal fruits during fruit development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11010117/s1, Supplementary Data S1. Protein sequences of newly identified SAP genes of Prunus dulcis are provided. Supplementary Data S2. Cis-regulatory elements in upstream region of PdSAP genes are given. Their start and end positions are also given. Supplementary Data S3. One-to-one orthologous relationships of SAP genes with M. domestica, P. persica, J. regia, and A. thaliana. Supplementary Data S4. DESeq2 results of normal vs. abnormal fruitlets developed in P. dulcis at 12, 17, 22, 27, 32, and 37 days of development. Supplementary Data S5. DESeq2 results of normal vs. abnormal fruitlets developed in P. dulcis at 12, 17, 22, 27, 32, and 37 days of development for only SAP genes with significant expression. Supplementary Data S6. Accession of sequence read archives (SRAs) used for the RNA-seq analysis are provided. Supplementary Table S1. Name of newly identified genes, along with their accession, molecular weight, isoelectric point, and chromosome numbers, are provided. Supplementary Figure S1. Motif pattern of the three predicted motifs in the PdSAP genes. Supplementary Figure S2. Differential gene expression of fruitlets in almonds. Volcano plots of PdSAP expression in P. dulcis fruitlet are shown at day 12 after flowering. Supplementary Figure S3. DEG of SAP in fruitlets in P. dulcis. Volcano plots of DEG of PdSAP in fruitlets of P. dulcis are shown at day 17 after flowering. Supplementary Figure S4. DEG of fruitlets in almonds. Volcano plots of DEG of SAP in fruitlet of P. dulcis are shown at day 22 after flowering. Supplementary Figure S5. DEG of fruitlets in P. dulcis. Volcano plots of DEG of PdSAP in fruitlets of P. dulcis are shown at developmental stage day 27 after flowering. Supplementary Figure S6. Differential gene expression of fruitlets in almonds. Volcano plots of PdSAP expression in fruitlet of P. dulcis are shown at day 32 after flowering. Supplementary Figure S7. Differential gene expression of fruitlets in almonds. Volcano plots of differential gene expression of PdSAP in almond’s fruitlet are shown at day 37 after flowering.
Author Contributions
Conceptualization, M.F.B. and A.G.; methodology, S.F. and Z.Z.; software, S.F. and Z.Z.; validation S.F. and Z.Z.; formal analysis, S.F. and Z.Z.; investigation, S.F. and Z.Z.; resources, S.F. and Z.Z.; data curation, S.F. and Z.Z.; writing—original draft preparation, S.F.; writing—review and editing, M.F.B. and A.G.; supervision, M.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the insert article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nakashima K., Takasaki H., Mizoi J., Shinozaki K., Yamaguchi-Shinozaki K. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2012;1819:97–103. doi: 10.1016/j.bbagrm.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Yoon Y., Seo D.H., Shin H., Kim H.J., Kim C.M., Jang G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy. 2020;10:788. doi: 10.3390/agronomy10060788. [DOI] [Google Scholar]
- 3.Solanke A.U., Sharma M.K., Tyagi A.K., Sharma A.K. Characterization and phylogenetic analysis of environmental stress-responsive SAP gene family encoding A20/AN1 zinc finger proteins in tomato. Mol. Genet. Genom. 2009;282:153–164. doi: 10.1007/s00438-009-0455-5. [DOI] [PubMed] [Google Scholar]
- 4.Rushton D.L., Tripathi P., Rabara R.C., Lin J., Ringler P., Boken A.K., Langum T.J., Smidt L., Boomsma D.D., Emme N.J. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012;10:2–11. doi: 10.1111/j.1467-7652.2011.00634.x. [DOI] [PubMed] [Google Scholar]
- 5.Verma V., Ravindran P., Kumar P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016;16:86. doi: 10.1186/s12870-016-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rachappanavar V., Kumar M., Sharma J., Panday H.J. Plant hormone-mediated regulation of stress responses in fruit crops—A review. Authorea Prepr. 2020:1. doi: 10.22541/au.160466945.56576285/v1. [DOI] [Google Scholar]
- 7.Joshi R., Wani S.H., Singh B., Bohra A., Dar Z.A., Lone A.A., Pareek A., Singla-Pareek S.L. Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant Sci. 2016;7:1029. doi: 10.3389/fpls.2016.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan Thi Hoang X., Du Nhi N.H., Binh Anh Thu N., Phuong Thao N., Phan Tran L.-S. Transcription factors and their roles in signal transduction in plants under abiotic stresses. Curr. Genom. 2017;18:483–497. doi: 10.2174/1389202918666170227150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkenbihl R.P., Jach G., Saedler H., Huijser P. Functional dissection of the plant-specific SBP-domain: Overlap of the DNA-binding and nuclear localization domains. J. Mol. Biol. 2005;352:585–596. doi: 10.1016/j.jmb.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Riaño-Pachón D.M., Ruzicic S., Dreyer I., Mueller-Roeber B. PlnTFDB: An integrative plant transcription factor database. BMC Bioinform. 2007;8:42. doi: 10.1186/1471-2105-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H., Jin J., Tang L., Zhao Y., Gu X., Gao G., Luo J. PlantTFDB 2.0: Update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res. 2011;39:D1114–D1117. doi: 10.1093/nar/gkq1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puranik S., Sahu P.P., Srivastava P.S., Prasad M. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 2012;17:369–381. doi: 10.1016/j.tplants.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 13.García-Tejero I., Rubio A., Viñuela I., Hernández A., Gutiérrez-Gordillo S., Rodríguez-Pleguezuelo C., Durán-Zuazo V.H. Thermal imaging at plant level to assess the crop-water status in almond trees (cv. Guara) under deficit irrigation strategies. Agric. Water Manag. 2018;208:176–186. doi: 10.1016/j.agwat.2018.06.002. [DOI] [Google Scholar]
- 14.Egea G., González-Real M.M., Baille A., Nortes P.A., Sánchez-Bel P., Domingo R. The effects of contrasted deficit irrigation strategies on the fruit growth and kernel quality of mature almond trees. Agric. Water Manag. 2009;96:1605–1614. doi: 10.1016/j.agwat.2009.06.017. [DOI] [Google Scholar]
- 15.Küden A. Crop situation and research in almond; Proceedings of the V International Symposium on Pistachios and Almonds 912; Sanliurfa, Turkey. 6–10 October 2009; pp. 515–521. [Google Scholar]
- 16.Becerra-Tomás N., Paz-Graniel I., Kendall C.W.C., Kahleova H., Rahelić D., Sievenpiper J.L., Salas-Salvadó J. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutr. Rev. 2019;77:691–709. doi: 10.1093/nutrit/nuz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grasselly C.H. Origine et évolution de l’amandier cultivé. Cahiers Options Méditerranéennes. 1976;32:44–49. [Google Scholar]
- 18.Thomas M. Almond-Botany, Production and Uses. Cabi Publishing; Wallingford, UK: 2017. [Google Scholar]
- 19.Miarnau X., Torguet L., Batlle I., Alegre S., Rovira M. Differences in flower and fruit drop patterns in almond cultivars; Proceedings of the VII International Symposium on Almonds and Pistachios 1219; Adelaide, SA, Australia. 5–9 November 2017; pp. 37–44. [Google Scholar]
- 20.Goldhamer D.A., Viveros M., Salinas M. Regulated deficit irrigation in almonds: Effects of variations in applied water and stress timing on yield and yield components. Irrig. Sci. 2006;24:101–114. doi: 10.1007/s00271-005-0014-8. [DOI] [Google Scholar]
- 21.Vij S., Tyagi A.K. Genome-wide analysis of the stress associated protein (SAP) gene family containing A20/AN1 zinc-finger (s) in rice and their phylogenetic relationship with Arabidopsis. Mol. Genet. Genom. 2006;276:565–575. doi: 10.1007/s00438-006-0165-1. [DOI] [PubMed] [Google Scholar]
- 22.Dong Q., Duan D., Zhao S., Xu B., Luo J., Wang Q., Huang D., Liu C., Li C., Gong X., et al. Genome-wide analysis and cloning of the apple stress-associated protein gene family reveals MdSAP15, which confers tolerance to drought and osmotic stresses in transgenic Arabidopsis. Int. J. Mol. Sci. 2018;19:2478. doi: 10.3390/ijms19092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai W., Zhou Y., Pan R., Liao L., He J., Liu H., Yang Y., Liu S. Identification and expression analysis of stress-associated proteins (SAPs) containing A20/AN1 zinc finger in cucumber. Plants. 2020;9:400. doi: 10.3390/plants9030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao W., Long L., Tian X., Jin J., Liu H., Zhang H., Xu F., Song C. Genome-wide identification and expression analysis of stress-associated proteins (SAPs) containing A20/AN1 zinc finger in cotton. Mol. Genet. Genom. 2016;291:2199–2213. doi: 10.1007/s00438-016-1252-6. [DOI] [PubMed] [Google Scholar]
- 25.Giri J., Dansana P.K., Kothari K.S., Sharma G., Vij S., Tyagi A.K. SAPs as novel regulators of abiotic stress response in plants. BioEssays. 2013;35:639–648. doi: 10.1002/bies.201200181. [DOI] [PubMed] [Google Scholar]
- 26.Opipari A., Boguski M., Dixit V. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J. Biol. Chem. 1990;265:14705–14708. doi: 10.1016/S0021-9258(18)77165-2. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi H., Jha S., Sharma M., Giri J., Tyagi A.K. Rice SAPs are responsive to multiple biotic stresses and overexpression of OsSAP1, an A20/AN1 zinc-finger protein, enhances the basal resistance against pathogen infection in tobacco. Plant Sci. 2014;225:68–76. doi: 10.1016/j.plantsci.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay A., Vij S., Tyagi A.K. Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc. Natl. Acad. Sci. USA. 2004;101:6309–6314. doi: 10.1073/pnas.0401572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatti K.H., Xu C., Wu J., He C. Overexpression of rice OsLOL2 gene confers disease resistance in tobacco to Pseudomonas syringae pv. tabaci. Prog. Nat. Sci. 2008;18:807–812. doi: 10.1016/j.pnsc.2008.01.025. [DOI] [Google Scholar]
- 30.Kanneganti V., Gupta A.K. Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant Mol. Biol. 2008;66:445–462. doi: 10.1007/s11103-007-9284-2. [DOI] [PubMed] [Google Scholar]
- 31.Huang J., Wang M.-M., Jiang Y., Bao Y.-M., Huang X., Sun H., Xu D.-Q., Lan H.-X., Zhang H.-S. Expression analysis of rice A20/AN1-type zinc finger genes and characterization of ZFP177 that contributes to temperature stress tolerance. Gene. 2008;420:135–144. doi: 10.1016/j.gene.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Guo C., Wei Y., Yang B., Ayup M., Li N., Liu J., Liao K., Wang H. Developmental transcriptome profiling uncovered carbon signaling genes associated with almond fruit drop. Sci. Rep. 2021;11:3401. doi: 10.1038/s41598-020-69395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen A.N., Ernst H.A., Leggio L.L., Skriver K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 2005;10:79–87. doi: 10.1016/j.tplants.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y., Wang M., Fu J., Xuan N., Zhu Y., Lian Y., Jia Z., Zheng J., Wang G. Phylogenetic and expression analysis of ZnF-AN1 genes in plants. Genomics. 2007;90:265–275. doi: 10.1016/j.ygeno.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Mendoza-Cózatl D.G., Butko E., Springer F., Torpey J.W., Komives E.A., Kehr J., Schroeder J.I. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008;54:249–259. doi: 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Zeng L., Chen R., Wang Y., Song J. Genome-wide identification and characterization of stress-associated protein (SAP) gene family encoding A20/AN1 zinc-finger proteins in Medicago truncatula. Arch. Biol. Sci. 2018;70:087–098. doi: 10.2298/ABS170529028Z. [DOI] [Google Scholar]
- 37.Wang Z., Kuang J., Han B., Chen S., Liu A. Genomic characterization and expression profiles of stress-associated proteins (SAPs) in castor bean (Ricinus communis) Plant Divers. 2021;43:152–162. doi: 10.1016/j.pld.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia H., Li J., Zhang J., Ren Y., Hu J., Lu M. Genome-wide survey and expression analysis of the stress-associated protein gene family in desert poplar, Populus euphratica. Tree Genet. Genomes. 2016;12:78. doi: 10.1007/s11295-016-1033-8. [DOI] [Google Scholar]
- 39.Liu S., Yuan X., Wang Y., Wang H., Wang J., Shen Z., Gao Y., Cai J., Li D., Song F. Tomato stress-associated protein 4 contributes positively to immunity against necrotrophic fungus Botrytis cinerea. Mol. Plant-Microbe Interact. 2019;32:566–582. doi: 10.1094/MPMI-04-18-0097-R. [DOI] [PubMed] [Google Scholar]
- 40.He X., Xie S., Xie P., Yao M., Liu W., Qin L., Liu Z., Zheng M., Liu H., Guan M. Genome-wide identification of stress-associated proteins (SAP) with A20/AN1 zinc finger domains associated with abiotic stresses responses in Brassica napus. Environ. Exp. Bot. 2019;165:108–119. doi: 10.1016/j.envexpbot.2019.05.007. [DOI] [Google Scholar]
- 41.Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R.Y., Eddy S.R., Heger A., Hetherington K., Holm L., Mistry J., et al. Pfam: The protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 43.Dong Q., Zhao S., Duan D., Tian Y., Wang Y., Mao K., Zhou Z., Ma F. Structural and functional analyses of genes encoding VQ proteins in apple. Plant Sci. 2018;272:208–219. doi: 10.1016/j.plantsci.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe D., Dudek S., Ritchie M.D., Pendergrass S.A. Visualizing genomic information across chromosomes with PhenoGram. BioData Min. 2013;6:18. doi: 10.1186/1756-0381-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo A.-Y., Zhu Q.-H., Chen X., Luo J.-C. GSDS: A gene structure display server. Hereditas. 2007;29:1023–1026. doi: 10.1360/yc-007-1023. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Tang H., DeBarry J.D., Tan X., Li J., Wang X., Lee T.-h., Jin H., Marler B., Guo H., et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garg V.K., Avashthi H., Tiwari A., Jain P.A., Ramkete P.W., Kayastha A.M., Singh V.K. MFPPI–Multi FASTA ProtParam interface. Bioinformation. 2016;12:74. doi: 10.6026/97320630012074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Yu C.-S., Cheng C.-W., Su W.-C., Chang K.-C., Huang S.-W., Hwang J.-K., Lu C.-H. CELLO2GO: A web server for protein subCELlular LOcalization prediction with functional gene ontology annotation. PLoS ONE. 2014;9:e99368. doi: 10.1371/journal.pone.0099368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horton P., Park K.-J., Obayashi T., Fujita N., Harada H., Adams-Collier C., Nakai K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conesa A., Götz S., García-Gómez J.M., Terol J., Talón M., Robles M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 52.Bedre R.H., Avila C.A., Mandadi K. HTSeqQC: A Flexible and One-Step Quality Control Software for High-throughput Sequence Data Analysis. bioRxiv. 2021 doi: 10.1101/2020.07.23.214536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 54.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao Y., Smyth G.K., Shi W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 56.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Afgan E., Baker D., Batut B., Van Den Beek M., Bouvier D., Čech M., Chilton J., Clements D., Coraor N., Grüning B.A., et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018;46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the insert article and Supplementary Materials.