Summary:
Robotic microsurgery has emerged as a new technology with potential benefits for reconstructive surgery. We report the first-in-human use of the Symani surgical system to perform lympho-venous and arterial anastomosis for lymphatic reconstruction. In five patients, 10 robot-assisted anastomoses were performed. Next to lympho-venous anastomoses, two patients received a free vascularized lymph node transfer. Motion scaling was set to 10×. Visualization was either achieved with a 3D system or an optical microscope. All anastomoses were patent as confirmed by ICG. Despite a longer time to perform the first anastomoses with the robot, we observed a decline in duration of anastomosis. Among the advantages of the system were a high accuracy in placing the stitches even in very small and fragile vessels or when performing anastomoses with size mismatches. The challenges encountered included the lack of a touch sensation and the necessity to develop a “see-feel.” This could be achieved surprisingly well because the force necessary to close dilator and needle holder via the manipulators was perceived as comparable to using conventional micro instruments. Our data confirm feasibility and safety of the robotic system to perform lymphatic surgery. Larger patient cohorts and inclusion of surgeons at different training levels will be necessary to investigate the true potential of robotics in microsurgery. In addition, robot-assisted surgery shows a promising potential in opening up new frontiers in reconstructive microsurgery (eg, the reliable performance of anastomoses on even smaller blood and lymphatic vessels or on structures deeper within the body cavities—eg, the thoracic duct).
Takeaways
Question: Robotic surgery has emerged as technology with potential benefits for microsurgical reconstruction.
Findings: We report the first in-human use of a novel robotic microsurgical system for lymphatic surgery. All anastomoses were patent. Our data show feasibility and safety of the robotic system to perform microsurgery of vessels smaller than 1 mm.
Meaning: Robot-assisted microsurgery may open up new possibilities in reconstructive microsurgery (eg, the reliable performance of anastomoses on even smaller blood and lymphatic vessels or on structures deeper within the body cavities—eg, the thoracic duct).
INTRODUCTION
The introduction of robotic surgery has influenced surgical interventions in different fields.1,2 In reconstructive surgery, micro- and super microsurgery are the key elements that have enabled surgeons to treat complex diseases.3,4
Although the da Vinci robot was originally conceived for endoscopic interventions, its use in microsurgery remained restricted due to several limitations.5,6 The MUSA robot (Microsure) was introduced in 2021 using fixed joysticks, which are connected to a scaffold and standard microsurgical instruments.7 In contrast, the Symani robot consists of flexible robotic arms to reach into deeper anatomical regions. The system has already shown feasibility in performing microsurgical anastomosis in animal models. We hereby report on the first-in-human use of the Symani surgical system to perform lymphatic surgery.
MATERIAL AND METHODS
In July 2021, the Symani surgical system (Medical Microinstruments [MMI], Calci, Italy) was introduced at the Department of Plastic Surgery and Hand Surgery of the University Hospital Zurich. The system consists of two robotic arms and is combined with a console, that is composed of an ergonomic chair, a footswitch controller, and forceps-like joysticks (manipulators). (See Video 1 [online], which displays the handling of the remote surgical manipulators. Due to the similarity to conventional micro instruments, this task was learned quickly.) (See figure 1, Supplemental Digital Content 1, which displays (A) A Hybrid setup, Symani robot and visualization with Pentero 900 microscope. In this setup, the operating surgeon is seated on the left, and the assisting microsurgeon (on the right) can provide manual assistance with conventional microsurgery instruments. (B) The surgeon controls the robot by two manipulators and foot pedals. (C) These hand movements are converted and motion scaled onto movements of the robotic arms by a digital interface. (D) Operation field and robotic arms equipped with dilator and needle holder for lympho-venous anastomosis. http://links.lww.com/PRSGO/B878.)
Video 1. displays the handling of the remote surgical manipulators. Due to the similarity to conventional micro instruments this task was learned quickly.
Because the Symani system does not include an optical unit, visualization was either accomplished with the Pentero 900 microscope (Carl Zeiss Meditec AG, Jena, Germany) or the VITOM 3D system (Karl Storz SE & Co. KG, Tuttlingen, Germany) (Fig. 1). It features different degrees of motion scaling (ie, slowing down of the surgeon’s hand movement [7–20×] which enhances precision). Symani’s robotic arms are equipped with microinstruments comprising 3 mm wrists offering seven degrees of freedom. (See Video 2 [online], which displays the lympho-venous anastomosis. Stitching with needle holder (right) and dilator (left) with 11-0 suture. Motion scaling 10×. An intravascular stent was placed for better visualization of the vessel lumen of the sclerotic lymphatic vessel.) (See Video 3 [online], which displays the lympho-venous anastomosis. Knotting with needle holder (right) and dilator (left) with 11-0 suture. Motion scaling 10×. An intravascular stent was placed for better visualization of the vessel lumen of the sclerotic lymphatic vessel.)
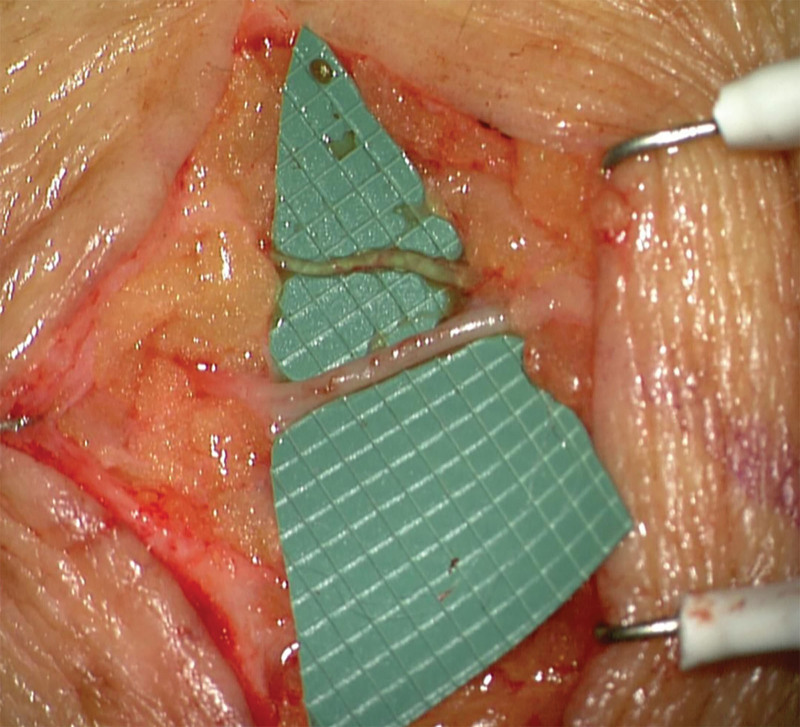
Fig. 1.
Subcutaneous 0.8 mm ICG-positive lymphatic vessel (above) and 1.5 mm vein (below).
Video 2. displays the Lympho-venous anastomosis. Stitching with needle holder (right) and dilator (left) with 11-0 suture. Motion scaling 10x. An intravascular stent was placed for better visualization of the vessel lumen of the sclerotic lymphatic vessel.
Video 3. displays the Lympho-venous anastomosis. Knotting with needle holder (right) and dilator (left) with 11-0 suture. Motion scaling 10x. An intravascular stent was placed for better visualization of the vessel lumen of the sclerotic lymphatic vessel.).
All surgeries were performed by the same surgeon (NL). To help in visualization and identification of lymphatic vessels, 0.5–1 ml indocyanine green (ICG)/patent blue was injected intradermally before surgery and ICG-lymphography using Fluobeam (Fluoptics Imaging Inc. Mass.) was performed. Lymphatic vessels and suitable veins were prepared using the Pentero 900 microscope. Lymphatic anastomoses were performed with the Symani, coupled with needle holder and dilator, using either Nylon 12-0 or 11-0 sutures. In free vascularized lymph node flaps, arterial anastomoses were performed using Nylon 10-0 sutures. Written informed consent was obtained from all patients before publication.
RESULTS
In a total of five patients, 10 robot-assisted anastomoses were performed (Table 1). Three patients had lymphedema and underwent lymphovenous anastomosis (LVA) to decrease limb volume (Figs. 1 and 2). Of those, two patients received an additional vascularized lymph node transfer and lympho-lymphatic anastomosis . (See figure 2, Supplemental Digital Content 2, which displays lympho-lymphatic anastomosis (0.8–1 mm) after tumor resection in the groin (A) with ICG flow over the anastomosis (B). Axillary lymph node flap (C). Arterial anastomosis of flap artery (2 mm) to a branch of the femoral artery (1.5 mm) deep within the groin (D). http://links.lww.com/PRSGO/B879.)
Table 1.
Patient Details and Results
| Patient Number | Age (y) | Diagnosis | Surgery | Operation Time (min) | Robot-assisted Lymphatic Anastomosis (N) | Robot-assisted Arterial Anastomosis (N) | Conventional Anastomosis (N) |
|---|---|---|---|---|---|---|---|
| 1 | 57 | Secondary lymphedema of the right leg | 2× LVA, VLNT from right axilla to right groin | 477 | 2 | 1 | — |
| 2 | 34 | Primary lymphedema of the lower extremities | 9× LVA | 366 | 2 | — | 7 |
| 3 | 49 | Secondary lymphedema of the right leg | 2× LVA, VLNT from left axilla to right groin | 313 | 2 | 1 | 1 |
| 4 | 53 | Atypical lipomatous tumor of the thigh | Tumor resection, LLA, and MLL | 167 | 1 | — | — |
| 5 | 61 | Liposarcoma of the thigh | Tumor resection, turn-over SCIP flap, LVA, and MLL | 425 | 1 | — | — |
LLA, lympho-lymphatic anastomosis; MLL, microscopic lymphatic ligation; SCIP, superficial circumflex iliac artery perforator; VLNT, vascularized lymph node transfer.
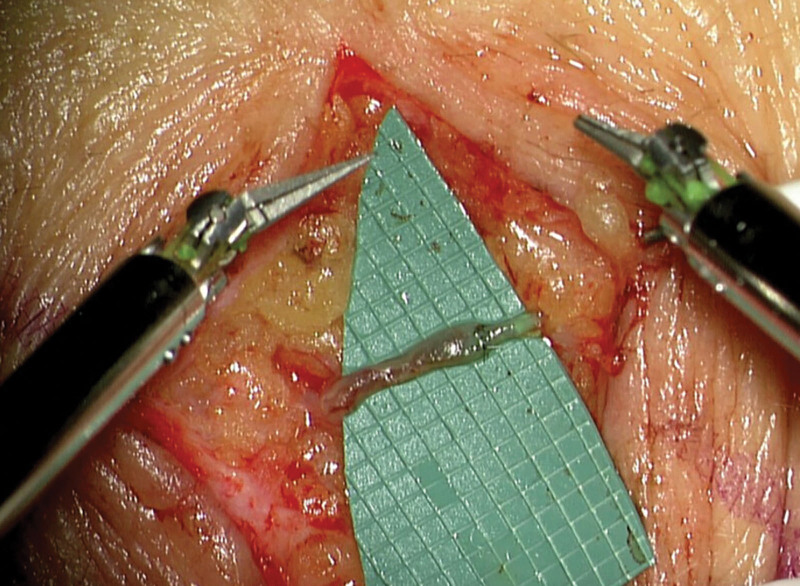
Fig. 2.
Lympho-venous anastomosis performed with the Symani and 11-0 nylon suture. The size mismatch could be handled well.
Some patients received conventional hand-sewn anastomoses in addition, due to time reasons. In the remaining patients, tumor resection at the level of the groin was combined with LVA, lympho-lymphatic anastomosis, and microscopic lymphatic ligations to prevent chronic seroma and lymphedema. In these first cases, anastomoses took considerably longer compared with hand-sewn anastomoses (on average, two to three times), mostly due to issues with set-up and a certain stickiness of the instruments. It could be overcome by rinsing and cleaning the instruments several times during the anastomosis and by active help from the assistant (eg, holding the tissue and suture ends).
Motion scaling was set to 10×. Patency of anastomoses (robotic assisted and hand-sewn) was 100%. Arterial anastomoses were checked in the surgical field by pulsation, flap perfusion, and positive milking test. Patency of LVAs was tested intraoperatively by ICG-flow over the anastomosis (Fig. 3). The Pentero 900 microscope was preferred by the surgeon as it allows for a better resolution and contrast in the visualization of thin-walled lymphatic vessels. Moreover, this set-up enables a quick switch between a conventional manual approach and robot assistance.
Fig. 3.
Good patency of the LVA as confirmed by ICG.
Among the advantages of the system were the high accuracy in placing the stitches even in very small and fragile vessels or when performing anastomoses with size mismatches (Figs. 1 and 2).
The challenges encountered in using the new system included the lack of a touch sensation and the necessity to develop a “see-feel” with the eyes to perform sutures at a microscopic level. This could be achieved surprisingly well because the force necessary to close dilator and needle holder via the manipulators was perceived as comparable to that needed using conventional micro instruments.
DISCUSSION
Our data provide first-in-human evidence that the Symani surgical system is safe and feasible for lymphatic reconstruction. The Symani robot was also used for a free flap in a posttraumatic upper limb reconstruction by Innocenti and his team recently.8
Microsurgery requires a specialized set of skills and capabilities that can only be acquired through extensive training.9 Robotic systems may provide increased controllability and precision to surgeons. Although we noted a longer time to perform the anastomoses at the beginning, we saw a steady decline in time to perform anastomoses. However, motion scaling itself slows down the movement. Further studies to evaluate this topic are ongoing within our department. In contrast to the MUSA, the Symani can also be teleoperated. Therefore a second team of surgeons would potentially have enough space to operate at a nearby anatomic region. Using the Symani, even deep structures within the groin could be reached easily by the robotic arms. It appears to be especially suitable to perform central lymphatic surgery of the thoracic duct, which was earlier reported by our group 3. On the other hand, potential limitations and downsides have to be considered. Even for experienced microsurgeons, several hours of training are necessary to adapt to the system and work with the technology. However, long-standing experience in conventional microsurgery appears to be beneficial for adaptation and the learning curve is steep. Set-up of the system, different instrument properties, learning curve, etc. may prolong operating times, and initial and running costs have to be taken into consideration (single-use instruments, sterile drapes).
Larger patient cohorts with longer investigation periods and inclusion of surgeons at different training levels will be necessary to investigate whether robotics in microsurgery will be advantageous in everyday procedures (eg, free flaps) or should be reserved for selected cases. Robot-assisted surgery may open up new frontiers in reconstructive microsurgery (eg, smaller perforator free flaps and lymphatic vessels or on structures that lie deeper in the body cavities).
Supplementary Material
Footnotes
Published online 10 January 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Gettman MT, Peschel R, Neururer R, et al. A comparison of laparoscopic pyeloplasty performed with the daVinci robotic system versus standard laparoscopic techniques: initial clinical results. Eur Urol. 2002;42:453–457; discussion 457. [DOI] [PubMed] [Google Scholar]
- 2.Gupta N, Miranda Blevins DO, Holcombe J, et al. A comparison of surgical outcomes between single-site robotic, multiport robotic and conventional laparoscopic techniques in performing hysterectomy for benign indications. Gynecol Minim Invasive Ther. 2020;9:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenblatt N, Puippe G, Broglie MA, et al. Lymphovenous anastomosis for the treatment of thoracic duct lesion: a case report and systematic review of literature. Ann Plast Surg. 2020;84:402–408. [DOI] [PubMed] [Google Scholar]
- 4.Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645–648. [DOI] [PubMed] [Google Scholar]
- 5.van der Hulst R, Sawor J, Bouvy N. Microvascular anastomosis: is there a role for robotic surgery? J Plast Reconstr Aesthet Surg. 2007;60:101–102. [DOI] [PubMed] [Google Scholar]
- 6.Katz RD, Rosson GD, Taylor JA, et al. Robotics in microsurgery: use of a surgical robot to perform a free flap in a pig. Microsurgery. 2005;25:566–569. [DOI] [PubMed] [Google Scholar]
- 7.van Mulken TJM, Schols RM, Scharmga AMJ, et al. MicroSurgical Robot Research Group. First-in-human robotic supermicrosurgery using a dedicated microsurgical robot for treating breast cancer-related lymphedema: a randomized pilot trial. Nat Commun. 2020;11:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medical Microinstruments’ Symani Surgical System Saves Patient’s Arm from Amputation. BusinessWire. 2021. Available at www.businesswire.com/news/home/20210713005292/en/
- 9.Mattos LS, Caldwell DG, Peretti G, et al. Microsurgery robots: addressing the needs of high-precision surgical interventions. Swiss Med Wkly. 2016;146:w14375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.