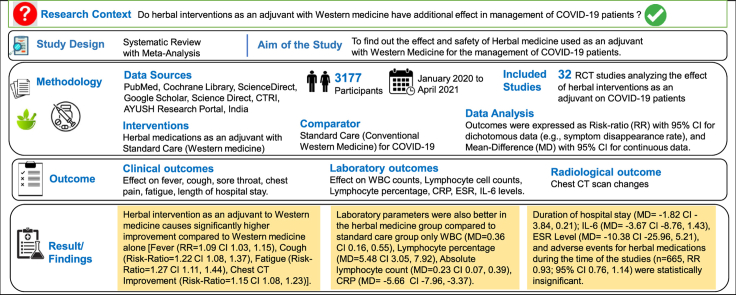
Abstract
Background and aim
The management of the worldwide spreading COVID-19 consists of amelioration of its symptoms but no cure is yet available. Herbal medicines supplemented with the Western medicine have been applied for COVID-19 treatment in India, China, Iran, and other countries. This systematic review and meta-analysis of RCTs evaluates the effect and safety of herbal intervention in the management of COVID-19.
Experimental procedure
RCTs from databases like PubMed, Cochrane Library, ScienceDirect, Google Scholar, Science Direct, CTRI, AYUSH Research Portal, India, were reviewed and the data were extracted for study sample demographics, intervention details, clinical effect, inflammatory markers and safety monitoring. Outcomes were expressed as Risk-ratio (RR) with 95% CI for dichotomous data, and Mean-Difference (MD) with 95% CI for continuous data.
Result and conclusion
From the 32 eligible studies, a total of 3177 COVID-19 patients were included in the review. Herbal intervention as an adjuvant to Western medicine causes significantly higher improvement compared to Western medicine alone [Fever (RR = 1.09 CI 1.03, 1.15), Cough (Risk-Ratio = 1.22 CI 1.08, 1.37), Fatigue (Risk-Ratio = 1.27 CI 1.11, 1.44), Chest CT Improvement (Risk-Ratio = 1.15 CI 1.08, 1.23)]. The laboratory parameters were also better in the herbal medicine group compared to standard care group only WBC (MD = 0.36 CI 0.16, 0.55), Lymphocyte percentage (MD = 5.48 CI 3.05, 7.92), Absolute lymphocyte count (MD = 0.23 CI 0.07, 0.39), CRP (MD = −5.66 CI -7.96, −3.37). However, duration of hospital stays (MD = −1.82 CI -3.84, 0.21); IL-6 (MD = −3.67 CI -8.76, 1.43), ESR Level (MD = −10.38 CI -25.96, 5.21) were statistically insignificant. No significant adverse events for herbal medications were noted in the included RCTs, during the time of the studies. (n = 665, RR 0.93; 95% CI 0.76, 1.14).
Keywords: COVID-19, Herbal medicine, Systematic review, Meta-analysis
Graphical abstract
Highlights
-
•
Herbal intervention along with Western medicine has better recovery for fever, cough, fatigue, chest CT Improvement, RT-PCR Negativity, sore-throat, and duration of hospital stay.
-
•
The laboratory parameters (e.g., WBC, Lymphocyte percentage, Absolute Lymphocyte count, CRP, IL-6, ESR levels) were also better in the herbal medicine group compared to standard care group only.
-
•
No significant adverse events for herbal medications were noted in the included RCTs.
List of abbreviations
- ADR
Adverse Drug Reaction
- AEs
Adverse Events
- CHM
Chinese Herbal Medicine
- CI
Class Interval
- CTRI
Clinical Trials Registry- India
- JHQG
Jinhua Qinggan granules
- KFT
Kidney Function Test
- LFT
Liver Function Test
- LHQW
Lianhua Qingwen granules
- MD
Mean Difference
- RCT
Randomized Controlled Trial
- RR
Risk Ratio
1. Introduction
In December 2019, an incidence of a sudden outbreak of pneumonia-like illness occurred at Wuhan, Hubei Province in China, that becomes a pandemic in a very short duration. On screening, Chinese researchers isolated a novel coronavirus by January 2020 and termed it as 2019-NCoV or SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2).1 This infectious disease has widespread symptoms that range from asymptomatic patient to normal flu symptoms such as cough, sore throat, fever, headache, body ache, dyspnea, acute respiratory distress syndrome. Some patients also reported gastrointestinal problems, abdominal pain and diarrhea. COVID-19 patients may present with a severe viral infection, a weakened immune status, and ultimately developed profound cytokine storms, pulmonary fibrosis lesion, and multiorgan failure; which ultimately become fatal that lead to death.2
Due to the severity of this outbreak and rapid outspread globally, WHO on January 31, 2020, announced it as a global health emergency.3 In February 2020, WHO termed this as coronavirus disease 2019 (COVID-19). In March 2020, WHO declared it a pandemic situation [Fei Zhou]. As per data stated by WHO on Jan 6, 2022 about 296.4 million COVID-19 cases are reported globally, including 5.46 million deaths.4
Several treatment modalities have been attempted to treat COVID-19. These consist of RNA-dependent RNA polymerase inhibitors medications that include Remdesivir, Ribavirin Favipiravir; Protease inhibitors which include Lopinavir/ritonavir; endosomal acidification inhibitors (azithromycin, Chloroquine, hydroxychloroquine); Monoclonal or polyclonal antibodies; Adjunctive treatments (Tocilizumab) for decreasing IL-6 in cytokine storm, viral exocytosis inhibitors (Interferon-a 2a, Interferon-b 1b); convalescent plasma transfusion therapy; herbal medications based on historical documents and evidence of the SARS Association.3,5
1.1. Pharmacological agents for COVID-19 management
Preliminary research suggests that some medications may be effective in preventing and improving core symptoms of COVID-19. Although Remdesivir has shown certain beneficial effects in patients with severe COVID-19, is reported for various adverse events like increased hepatic enzymes, diarrhea, rash, hypotension, renal impairment, multiple organ dysfunction syndrome, and septic shock.6 Apart from that, various health organizations among the globe including WHO have changed their guidelines regularly to include pharmacological agents which have some evidence to demonstrate effectiveness in COVID-19 management and exclude those which have not been supported by the current body of evidence. For instance, Lopinavir, ritonavir, umifenovir, hydroxychloroquine, interferon-beta etc., failed to show high quality evidences for beneficial effect in viral clearance, and disease progression.7 It is not yet known whether remdesivir, ivermectin, and other drugs will bring significant benefits to patients. However, corticosteroids and IL-6 inhibitors demonstrated significant and consistent clinical benefits in severe COVID-19 patients.8
Herbal intervention is defined as a phytopharmaceutical preparation derived exclusively from a whole plant or parts of the plant (flower, leaves, bark, stem, rhizome, root) or its exudates (resins, latex, gums) It is used either in a crude form or as a purified pharmaceutical formulation such as extracts, juice, dried powder, decoction etc., following the procedures of distillation, extraction, filtration, and so on. These are rich source active metabolites, alkaloids and flavonoids responsible for its pharmacological activity.9
Pharmacological action of the herbal formulations or drugs are due to their active phytochemical constituents such as alkaloids, flavonoids, terpenoids, phenols, polyphenols, tannins, saponins, polysaccharides, proteins, lipids and peptides. The used herbal drugs have antipyretic, anti-inflammatory, expectorant, anti-asthmatic, antitussive, and antiviral properties that holds numerous functions against invasion, penetration, reproduction and expression of the virus. For example, Allium cepa, Aloe vera, Azadirachta indica, Cannabis sativa, Curcuma longa, Glycyrrhiza glabra, Nyctanthes arbortristis, Ocimum sanctum, Withania sominifera, Zingiber officinale are potential inhibitors of SARS-CoV-2 proteases.10
Herbal drug formulations are prepared by standardized process provided in herbal drug pharmacopoeia managed by the government agency. The GMP (Good Manufacture Practice) regulations are followed to ensure the quality of these drugs.11
Herbal medicines have been actively used as complementary medicine treatments of COVID-19. Many countries have conducted clinical trials and research studies on COVID-19 to find a cure as quickly as possible. A major part of these studies also included herbal medicine as an adjuvant along with Western medicine or alone and reported somewhat better results. In India, more than half of the COVID-19 related studies registered are from herbal medications.12
The traditional system of medicine in China, India, and Iran recommends certain herbal formulation for the prevention, management, and recovery from certain diseases, including the COVID-19.3,10
It is estimated that about four billion people (80% of the world's total population) in developing countries depend on herbal medicines as primary health care.9 About 25,000 herbal formulas and extracts have been used in traditional medicines in the South Asian subcontinent.10 In China, approximately 40% of total healthcare services relies on herbal drug. Acceptance and use of herbal medicines are expanding at much faster rate in developed countries like, UK and other European nations, North America and Australia.11
Several COVID-19 Guideline's versions for Diagnosis and Treatment (COVID-19 GDT) were issued by the National Health Commission of China. In its 3rd issue, it is recommended to use Chinese herbal decoctions to treat COVID-19. Subsequently, few patent Chinese herbal medicines such as Jinhua Qinggan granules (JHQG), Lianhua Qingwen granules (LHQW), have also been suggested for COVID-19 management.13 Qingfei Paidu Decoction is another herbal formula used in China to treat patients with COVID-19. Dried Ginger, Hegan Mahuang Decoction and Qingfei Touxie Fuzheng Decoction can effectively treat SARS-CoV-2 as per trial results in China.3
Recently, the Ministry of AYUSH, India (Ayurveda, Yoga, Unani, Siddha, and Homeopathy) recommended using the herbal decoction so-called Kadha as an immune booster and relieve the symptoms caused during the COVID-19 pandemic.3 Molecular binding studies have shown that several phytochemicals found in traditional Ayurvedic kadha can have high affinity (lower binding energy) for a variety of macromolecular and viral targets, host mediators, including different SARS-CoV-2 viral proteins and human ACE2 and Furin proteins.14
The common mechanism of action of plant extracts is thought to be inhibition of viral replication, but some studies have shown that common plant extracts can bind important viral proteins associated with viral pathogenicity. Plant extracts and other active components of Rheum officinale, Polygonum multiflorum inhibit the bindings of SARS-CoV (S) spike protein with angiotensin-converting enzyme 2 (ACE2). Similarly, Methanolic extract of Dioscorea batatas and Cibotium barometz reported SARS-CoV 3CLpro inhibition activity.3
From the beginning of COVID-19 infection, a large number of clinical studies have reported and highlighted the benefits of herbal remedies for COVID-19 management. Also, numerous systematic reviews have been conducted to evaluate the efficacy of herbs in the treatment of COVID-19, including evidence from various case reports, case series, and observational studies. However, randomized controlled trials deliver the uppermost level of evidence in the case of clinical trials. This review focused on the assessment of the efficacy and side effects of herbal medicine supplementation with Western medicine in the treatment of COVID-19, through screening of available randomized controlled trials (RCT). We included study from India, Iran and China for this assessment. Also, multiple herbal formulations in the management of COVID-19 patients were evaluated for effectiveness and safety.
2. Methods
2.1. Study registration
The protocol of this review was first registered with the International prospective register of systematic reviews (PROSPERO) with the Registration number CRD42021244675 available at https://www.crd.york.ac.uk/prospero. The review was conducted according to the PRISMA guidelines.
2.2. Search strategy
Database: The search was performed in the following electronic database specified below. PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Embase and Allied and Complementary Medicine Database (AMED). We had also manually searched the National Institute of Health and Clinical Trials Database (http://www.ClinicalTrials.gov/), WHO's International Clinical Trials Registry Platform (https://www.who.int/ictrp/en/), Clinical Trials Registry - India (http://www.ctri.nic.in), Science Direct, AYUSH Research Portal (http://www.ayushportal.nic.in) and Google Scholar for any ongoing clinical trials. Search terms were reviewed constantly to ensure that they reflect any terminology changes in the topic area or the databases. The timeframe considered was January 1, 2020 to April 30, 2021.
Language: English only.
Searching Terms utilized: (“COVID-19″ OR “SARS-CoV-2″ OR “NCOV” OR ″2019 NCOV” OR “Severe Acute Respiratory Syndrome Coronavirus 2″ OR “Coronavirus disease 2019”) AND (“herbal medicine” OR “herbal” OR “alternative medicine” OR “traditional medicine” OR “complementary medicine " OR “Unani” OR “Ayurveda”)
2.3. Eligibility criteria
2.3.1. Inclusion criteria
Only RCTs and quasi-RCTs articles that reported the effect of herbal interventions on COVID-19 disease were selected.
2.3.2. Exclusion criteria
Observational, cohort, crossover trials, case reports, case series, non-RCT, preclinical studies such as in-vitro trials and studies on animal models and experimental studies, in silico drugs trials were excluded.
2.3.3. Participants/population
Participants of all ages with diagnosed COVID-19 disease confirmed through Reverse transcription-polymerase chain reaction (RT-PCR) testing. There were no restrictions for gender, age or ethnicity.
2.3.4. Intervention(s)
Clinical Trial studies with the intervention of any forms of herbal medications were included. There was no limitation for the type of herbal medications, the dosage, and duration of treatment. Studies with non-herbal medicine interventions were excluded. Non-clinical studies/reports like reviews, letters, opinions and comments were omitted.
2.3.5. Comparison
There was no restriction for the type of comparators. Included comparator groups were included: herbal intervention versus no intervention, herbal medicine versus placebo, herbal medicine versus conventional medicine/routine treatment, herbal medicine plus conventional medicine/routine treatment versus conventional medicine/routine treatment. Trials that compare herbal medicine with other forms of herbal interventions were excluded.
2.3.6. Outcome
The primary outcome was the COVID-19 symptom disappearance rate and duration for RT-PCR negativity. There were no restrictions in secondary or additional outcomes. Clinical outcomes included were effect on fever, cough, sore throat, chest pain, fatigue, length of hospital stay. Biochemical and laboratory outcomes included changes in Blood test values, e.g., WBC counts, Lymphocyte cell counts, Lymphocyte percentage, CRP level, ESR-level, IL-6 levels. Radiological outcomes include changes on chest CT scan.15 Any side effects or adverse event reported in RCTs were also assessed.
2.4. Literature screening and data extraction
An in-depth search was done as per the search strategy described above. A simultaneous search of related trials and reviews was performed for other eligible RCTs. All the irrelevant, ineligible, or duplicate articles were removed from the analysis.
2.4.1. Data extraction
All the data from the eligible studies were extracted in a Microsoft Excel sheet by the two authors autonomously (AR, ZH) and revised by the two authors (AK, MSK). The data which was extracted were following: first author's name, year of publication, country, design, sample population and sample size, patient's age, and sex, intervention and control details (forms, dosage, and duration), outcome characteristics, safety monitoring with the reporting of adverse events. All evaluations were carried out autonomously by the authors (AK, ZH), and any discrepancies if occurred were resolved through discussions with another author (AR).
2.5. Assessment of risk of Bias
The risk of bias of the RCTs included in the review was assessed by the Cochrane Risk of Bias Tool for Randomized Controlled Trials. The risk of bias assessment includes the following areas: random sequence preparation, allocation concealment, participants blinding, blinding of outcome assessor, incomplete outcome data, and selective reporting. The results were obtained using RevMan v5.4.1 software.
2.6. Data analysis
Meta-analysis for the extracted data was performed using Review Manager (RevMan) software v 5.4.1 (Nordic Cochrane Centre, Cochrane Collaboration, Denmark).
For assessment of treatment effect, the mean difference (MD) values with 95% CIs were evaluated for outcomes having continuous data (e.g., symptom scores, Lab investigation values). The risk ratios (RR) values with 95% confidence intervals (CIs) were evaluated for outcomes with dichotomous data (e.g., symptom disappearance rate and effective rate). For pooling the data, the random-effects model was used as the variability between the included studies was taken into consideration. No subgroup analysis was performed in this review.
Assessment of heterogeneity levels of the RCTs included in the review was done applying chi2 test of heterogeneity with the significance level set at p < 0.10 and applied the I2 statistic after setting significance level ≥50%.
3. Results
3.1. Literature search
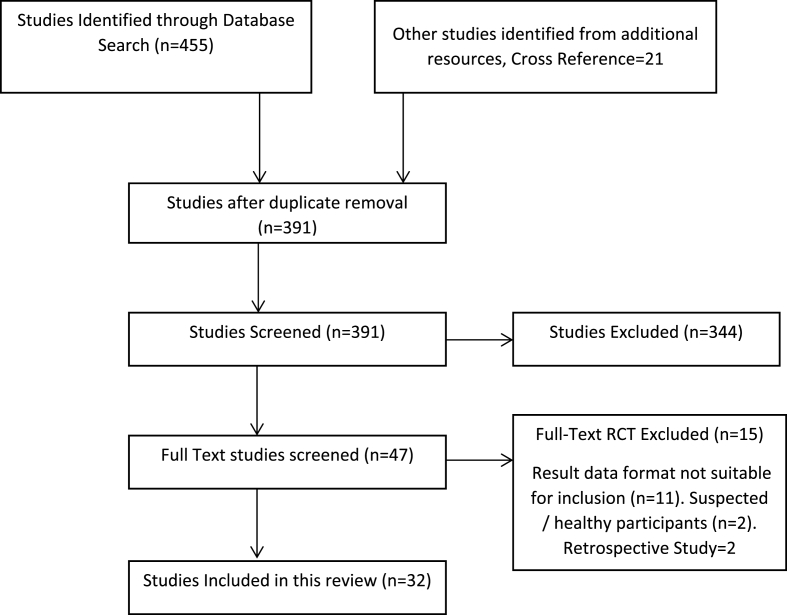
A total of 455 RCT articles for herbal intervention on COVID-19 were recovered after the databases search, with the search words described above in Search Strategy headings. Additional 21 RCTs were identified through other article references. After duplicate removal, 391 were selected for screening. Of these studies, 208 were either not valid RCTs, prophylactic study, pharmacological studies, in-silico studies, acupuncture, exercise, online health guide programs, healthy control participants, suspected COVID-19 patients, and therefore excluded from the review. Another 136 articles were abstracts-only articles, incomplete or unpublished articles, and therefore excluded. In the remaining 47 studies on COVID-19, 15 studies have inappropriate result data presentation or healthy/suspected participants included/retrospective and therefore these all are excluded. Thus, the current systematic review and meta-analysis include a total of thirty-two RCTs. [Fig. 1].
Fig. 1.
PRISMA Flow diagram for study selection.
3.2. Description of included studies
Total 32 RCTs were included in the review that were published in the year 2020 and 2021. The majority of these trials (25 studies) were conducted in China, while the five studies were from India and two from Iran. All included studies have used herbal plus Standard care (Western medicine) or herbal alone as an intervention which was compared with placebo plus Standard care (Western medicine) or only placebo. The sample sizes ranged from 30 to 517, with a total of 3177 COVID-19 patients. The treatment duration varied from 5 to 30 days. Out of 32 studies, 4 studies have not mentioned gender characteristics of the sample.16, 17, 18, 19 Overall, there were 56.96% male and 43.03% female patients. The basic characteristics of the RCTs included in the review are mentioned in Table 1.
Table 1.
Characteristics of included RCTs.
| Author | Country, Year | Treatment Duration (Days) | Disease Stage | Sample Size | Male/Female | Age (Mean ± SD) (I=Intervention, C=Control Group) | Intervention | Control | Outcome Measures | Safety Monitoring & Adverse Event Reporting |
|---|---|---|---|---|---|---|---|---|---|---|
| Ai XY et al.45 | China, 2020 | Moderate | 67 | 40/27 | I:52.33 ± 14.9 C:49.56 ± 16.3 | FeiyanYihao Chinese Medicine granules one packet BD plus Standard Care | Standard Care | Disappearance rate of clinical outcomes: Fever, Cough, Fatigue. | Reported No Adverse Events. | |
| Alireza Hashemi Shiri26 | Iran, 2021 | 7 | Mild, Moderate | 72 | 36/36 | I:41.03 ± 15.6 C:43.47 ± 11.3 | black myrobalan and mastic and sugarcane (3 g) BD with Standard treatment | Standard Care plus Placebo | Duration of Hospital Stay. | AST, ALT, BUN, Creatinine |
| Anup Thakar30 | India, 2020 | 14 | Mild, Moderate | 80 | 53/27 | I:40 ± 12.9 C:35.31 ± 11.7 | AyUSH-64 and Standard care | Standard Care | Mean Diff. for CRP, ESR Hospital Stay. | Yes (LFT, KFT) |
| Ding XJ et al.44 | China, 2020 | 10 | Mild, moderate, severe | 100 | 78/22 | I:54.7 ± 21.3 C:50.8 ± 23.25 | Qingfei Touxie Fuzheng recipe one packet 2 times a day plus Standard Care | Anti-viral medicine (α-interferon atomization inhalation BD, ribavirin 500 mg BD); cephalosporin, quinolone. | Effect Rate (RR); Disappearance rate of clinical outcomes: Fever, Cough, Fatigue; Chest CT Improvement (RR); Mean Diff. for CRP, IL-6, ESR. | Reported Adverse event |
| Duan Can et al.39 | China, 2020 | 5 | Mild | 123 | 62/67 | I:51.99 ± 13.88 C:50.29 ± 13.17 | Jinhua Qinggan granules, 2 packets TDS for 5 days plus Standard Care | Standard Care medicines (Chloroquine Phosphate 500 mg tablets, Lopinavir/Ritonavir 200 mg + α interferon, ribavirin injection BD + Arbidol 500 mg tablet TDS) | Disappearance rate of clinical outcomes: Fever, Cough, Fatigue, Sore Throat. | Reported Adverse event |
| Fu Xiaoxia et al.42 | China, 2020 | 10 | Mild, Moderate | 65 | 36/29 | Toujie Quwen granules, 1 packet BD plus Standard Care | Arbidol 200 mg Tablets + Moxifloxacin 400 mg tablets OD + Ambroxol 30 mg Tablets TDS | Effect Rate (RR); Mean Diff. for duration of Fever, Cough; Chest CT Improvement (RR); Mean Diff. for WBC, Lymphocyte Count, Lymphocyte %, CRP. | Reported No Adverse Events. | |
| Fu Xiaoxia et al. (b)43 | China, 2020 | 15 | Moderate | 73 | 38/35 | I:45.26 ± 7.25 C:44.68 ± 7.45 | Toujie Quwen granules, 1 packet BD plus Control Drug | Arbidol HCl 200 mg Tablets + Ambroxol HCl 30 mg Tablets TDS. | Effect Rate (RR); Mean Diff. for WBC, Lymphocyte Count, Lymphocyte %, CRP. | Reported No Adverse Events. |
| Gang Wang16 | China, 2020 | 7 | Mild, Moderate | 42 | I:57.1 ± 14 C:62.4 ± 12.3 | Xuanfei Baidu Decoction (XBD) (200 ml BD) for 7 days | Standard Care | Disappearance rate of clinical outcomes: Fever, Cough, Fatigue, Chest pain | Reported No Adverse Events. . | |
| Ganpat Devpura22 | India, 2021 | 7 | Asymptomatic, Mild | 95 | 77/18 | I:33.4 ± 9 C:35.4 ± 10.4 | 1 g of Giloy Ghanvati, 2 g of Swasari Ras, and 0.5 g each of Ashwagandha and Tulsi Ghanvati; orally BD for 7 days. 4 drops of Anu taila (nasal drop) OD for 7 days | Placebo | RT-PCR (Risk Ratio) | Reported No Adverse Events. . |
| Govind Ready24 | India, 2021 | 30 | Asymptomatic, mild | 52 | 36/16 | I:43.86 ± 9.97 C:35.22 ± 11.8 | AYUSH-64 and Standard care | Standard care | RT-PCR, Clinical Cure Rate, Fever, Cough, Sore throat, Chest pain; Mean diff. for WBC, Lymphocyte %, IL-6, ESR | Yes (LFT, KFT) |
| Huang H31 | China, 2020 | 10 | Moderate, Severe | 68 | 30/36 | I:60.42 ± 12.84 C:61.16 ± 13.58 | CHM plus Standard Care | Standard medicine with antiviral medication (ribavirin, lopinavir/ritonavir, Arbidol, α-interferon), antitussive, expectorant, anti-asthmatic drugs (Doxofylline, Ambroxol), moxifloxacin, with symptomatic treatment medicine. | Disappearance rate of clinical outcomes: Fever; Chest CT Improvement (RR). RT-PCR (RR); Mean Diff. for WBC, Lymphocyte Count, CRP. |
Reported Adverse event |
| Jayesh Dutt17 | India, 2021 | 7 | Mild, Moderate | 60 | I:33.87 ± 1.94 C:36.67 ± 1.81 | Aayudh Advance plus standard Care | Standard care | Hospital Stay, Mean diff. for CRP | Reported No Adverse Events. . | |
| Jin W et al.21 | China,2020 | 21 | Mild, Moderate | 38 | 20/18 | Compound Yin Chai granule + Qingqiao detoxification granule (15 g qid) plus Standard Care | Standard care medicine including antiviral, antibiotics, and symptomatic medicine. | Disappearance rate of clinical outcomes: Fever, Cough, Fatigue; Mean diff. for WBC. | NO | |
| Ke Hu29 | China, 2021 | 14 | Mild, Moderate | 284 | 150/134 | I:50.4 ± 15.2 C:51.8 ± 14.8 | Lianhuaqingwen capsules plus Standard Care | Standard Care | Effect Rate, Chest CT (RR), RT-PCR (RR); Mean Diff. for duration of Fever, Cough, Fatigue. | Elevated ALT, AST |
| Liao GR et al.37 | China, 2020 | Mild, moderate, severe | 70 | 37/33 | I:60.25 ± 10.39 C:63.16 ± 9.55 | Herbal Decoction | Standard Care | Disappearance rate of clinical outcomes: Fever, Cough, Fatigue; Chest CT Improvement (RR). | Reported Adverse event | |
| Liu XG et al.20 | China, 2020 | 9 | Moderate, Severe | 517 | 288/229 | I:48.44 ± 2.31 C:48.27 ± 2.45 | CHM plus Standard Care | Standard care medicine including antibiotics, antiviral (Ritonavir, lopinavir) | Effect Rate (RR); Mean Diff. for CRP | NO |
| Qiu M32 | China, 2020 | 10 | 50 | 27/23 | I:53.35 ± 18.35 C:51.32 ± 14.62 | CHM plus Standard Care | Standard medicine including Ritonavir, interferon-α, lopinavir. | Chest CT Improvement (RR); Mean Diff. for duration of Fever, Cough. | NO | |
| Qu XK et al.48 | China, 2020 | 10 | NA | 70 | 41/29 | I:40.65 ± 8.23 C:39.82 ± 6.4 | Capsule Shufeng Jiedu (2.08 g, TDS) plus Standard Care | Standard care drugs including antiviral medicine (Arbidol), antibiotics (moxifloxacin), expectorant, with symptomatic treatment. | RT-PCR (RR); Mean Diff. for duration of Fever, Cough, Fatigue. | Reported Adverse event |
| Saeed Sardari23 | Iran, 2020 | 7 | Not mentioned | 83 | 35/48 | I:43 ± 19.3 C:58 ± 17.7 | thyme essential oil plus conventional medicine drugs for 7 days | Standard care | Rate of disappearance for clinical outcomes: Cough, Fever, Fatigue, Chest pain, Sore Throat; Mean Diff. for WBC, Lymphocytes % | Yes (KFT) |
| Shi J et al.33 | China, 2020 | 6 | Mild, moderate, Severe | 67 | 36/31 | I:47.94 ± 14.46 C:46.72 ± 17.4 | CHM plus Standard Care | Antiviral medicine (recombinant interferon α-2b, interferon K lopinavir/ritonavir, darunavir corbita, Arbidol, HCQs), immune-modulatory, γ-globulin, anti- inflammatory drugs, with symptomatic and supportive drugs. | Chest CT Improvement (RR); Mean Diff. for duration of Hospital Stay. | NO |
| Sun Huimin et al.38 | China, 2020 | 14 | Mild, Moderate | 57 | 28/29 | I:45.4 ± 14.1 C:42 ± 11.7 | Lianhua Qingke granules, 1 packet TDS plus Standard Care | Standard Care (Lopinavir/Ritonavir + Alpha interferon injection |
Disappearance rate of clinical outcomes: Fever, Cough, Fatigue, Sore Throat, Chest CT Improvement (RR). | NO |
| Umesh Shukla27 | India, 2020 | 10 | Asymptomatic, Mild, Moderate | 30 | 23/7 | I:30.27 ± 8.83 C:32.27 ± 7.35 | Guduchighan Vati 500 mg BD | Hydroxychloroquine FOR 5 Days | Disappearance rate of clinical outcomes: Fever, Cough, Sore Throat Time to negative RT-PCR; Mean Diff. for WBC, Lymphocyte %, ESR, IL-6 | Yes (LFT, KFT) |
| WANG Jia-bo25 | China, 2020 | 14 | Mild, Moderate | 47 | 26/21 | I:46.8 ± 14.4 C:51.4 ± 17.6 | Keguan-1 plus Standard Care | Standard care | Time Period for Fever; Mean diff. for WBC. | Yes (LFT, KFT) |
| Xia WG et al.34 | China, 2020 | 10 | Moderate, Severe, Critical | 52 | 23/29 | I:54.18 ± 13.08 C:53.67 ± 12.7 | CHM plus Standard Care | Standard care medicine including antiviral medicines (lopinavir/ritonavir, Arbidol, ribavirin, oseltamivir, α-interferon), antibiotics (levofloxacin, moxifloxacin, penicillin, azithromycin, cephalosporins), with supportive treatment (γ- globulin, methylprednisolone etc.) | Effect Rate (RR); Chest CT Improvement (RR); Mean Diff. for duration of Fever, Hospital Stay. | Reported No Adverse Events. |
| Xiao Qi et al.40 | China, 2020 | 14 | Mild, Moderate | 200 | 130/70 | Shufeng Jiedu capsule, 4 capsules TDS plus Standard Care | Standard Care (Arbidol Hydrochloride tablets, 200 mg TDS) | Effect Rate (RR); Mean Diff. for duration of Fever, Cough, Fatigue; Chest CT Improvement (RR); Mean Diff. for WBC, Lymphocyte %. | Reported Adverse event | |
| Yang MB35 | China, 2020 | 7 | Moderate | 49 | 25/24 | I:50.35 ± 13.37 C:47.17 ± 16.57 | Reyanning mixture formulation (20 ml, BD/QID) plus Standard Care | Standard care medicine including antivirals (ribavirin, lopinavir/ritonavir, α-interferon, Arbidol) | Chest CT Improvement (RR). RT-PCR (RR); Mean Diff. for Lymphocyte Count, CRP. |
NO |
| Yong-an Ye28 | China, 2020 | 7 | Mild, Moderate | 42 | 6/35 | I:62.5 ± 11.5 C:57.66 ± 14.8 | Chinese herbal medicine (CHM) | Standard Care | Mean Diff. for WBC, Lymphocyte Count, CRP, ESR. | Yes (LFT, KFT) |
| Yu Ping et al.41 | China, 2020 | 7 | Mild, Moderate | 295 | 171/124 | I:48.27 ± 9.56 C:47.25 ± 8.67 | Lianhua Qingwen granules, 1 packet BD plus Control Drug | Arbidol 200 mg Tablets + Ambroxol 30 mg Tablets TDS + Moxifloxacin 400 mg tablets OD | Effect Rate (RR); Mean Diff. for duration of Fever, Cough; Chest CT Improvement (RR); Mean Diff. for WBC, Lymphocyte Count, CRP. | Reported No Adverse Events. |
| Zhang CT et al.19 | China, 2020 | Moderate | 45 | Jiaweidayuan (JW) granules granules, TDS for 7 days plus Standard Care | Standard Care | Chest CT Improvement (RR); Mean Diff. for duration of Fever, Cough, Fatigue; Mean Diff. for WBC, Lymphocyte %, CRP. | Reported No Adverse Events. . | |||
| Zhang YL et al.46 | China, 2020 | 10 | Moderate | 120 | 80/40 | I:53.4 ± 13.7 C:52 ± 14.1 | Jinyinhua oral liquid 60 mL TDS plus Standard Care, | Anti-viral (lopinavir/ritonavir 2 capsules BD, α-interferon (5 million Unit) BD, supportive treatment | Effect Rate (RR); Disappearance rate of clinical outcomes: Fever, Cough, Fatigue. | Reported Adverse event |
| Zhijian Luo18 | China, 2021 | 14 | Severe | 60 | I:60.26 ± 15.6 C:56.35 ± 18.3 | Xuebijing (XBJ) Herbal IV injection plus Standard Care | Standard Care | Mean Diff. for duration of Fever, Cough, Fatigue, Hosp Stay, Mean Diff. for WBC, Lymphocyte Count, IL-6, CRP. | Yes (LFT, KFT) | |
| Zhou WM et al.36 | China, 2020 | 14 | Moderate | 104 | 60/44 | I:52.47 ± 10.99 C:51.11 ± 9.87 | enteric-coated herbal capsules containing 150 mg of Diammonium glycyrrhizinate, TDS plus Standard Care | Antiviral drugs (ritonavir/lopinavir) 0.5 g BD | Effect Rate (RR). | Reported Adverse event |
Abbreviations Used in Table.
BD = Twice a day; C= Control Group; CHM= Chinese Herbal Medicine; I= Intervention Group; KFT=Kidney function test; LFT = Liver function test; OD=Once a day; QID = Four times per day; RR = Risk-Ratio; TDS; Thrice a day.
The standard treatment or control used in the included RCTs were antiviral drugs, antibiotics, oxygen support, and symptomatic supportive medicines. Ritonavir/Lopinavir, Arbidol, Chloroquine Phosphate, Ambroxol Hydrochloride, quinolone (Moxifloxacin) and cephalosporin, Interferon-α injections, and Ribavirin injections, methylprednisolone was the chief medications used as the Standard care medications. Herbal Intervention used in the studies included were in the form of granules, decoction, tablets/pills, nasal drops and herbal injections and were described in separate table. [See Supplementary Table 1].
3.3. Risk of Bias assessment
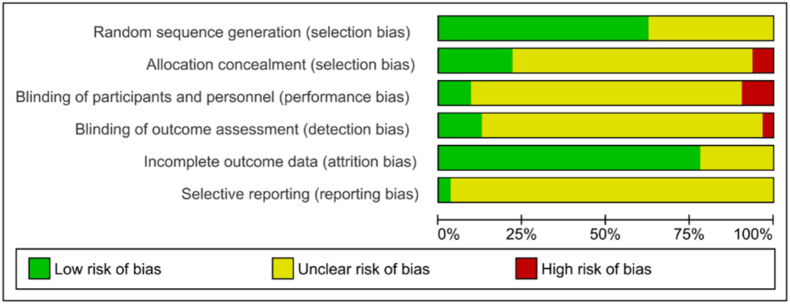
These assessment were done using the Cochrane risk of bias tool for randomized controlled trials, which includes adequate sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.47 Out of 32 studies, only 20 studies have reported adequate random sequence generation. Seven studies have clearly reported valid allocation concealment.18,25,26,28,29,39,44 In the remaining 25 studies, two RCTs have high risk because of odd-even number used in allocating groups,16,22 rest 23 have an unclear risk for allocation concealment. Only three RCTs reported proper blinding.18,22,26 Improper blinding of the patients and research-staffs were reported in three studies.19,29,38 The performance bias for 26 studies was unclear. Proper blinding of outcome assessors was reported only in four studies.18,22,29,39 One study is at high risk,38 while the other 27 were at unclear risk due to inadequate information on blinding. Risk of bias for incomplete outcome data is unclear for seven studies.19,21,36,37,43,45,46 On the grounds of having no dropouts and intention-to-treat analysis were performed, other 25 studies have a low risk for attrition bias. The risk of selective reporting was unclear for all studies, due to the lack of evidence for the judgment, and absence of the study protocol for most of the included studies [See Fig. 2 & Fig. 3].
Fig. 2.
Risk of bias - Graph.
Fig. 3.
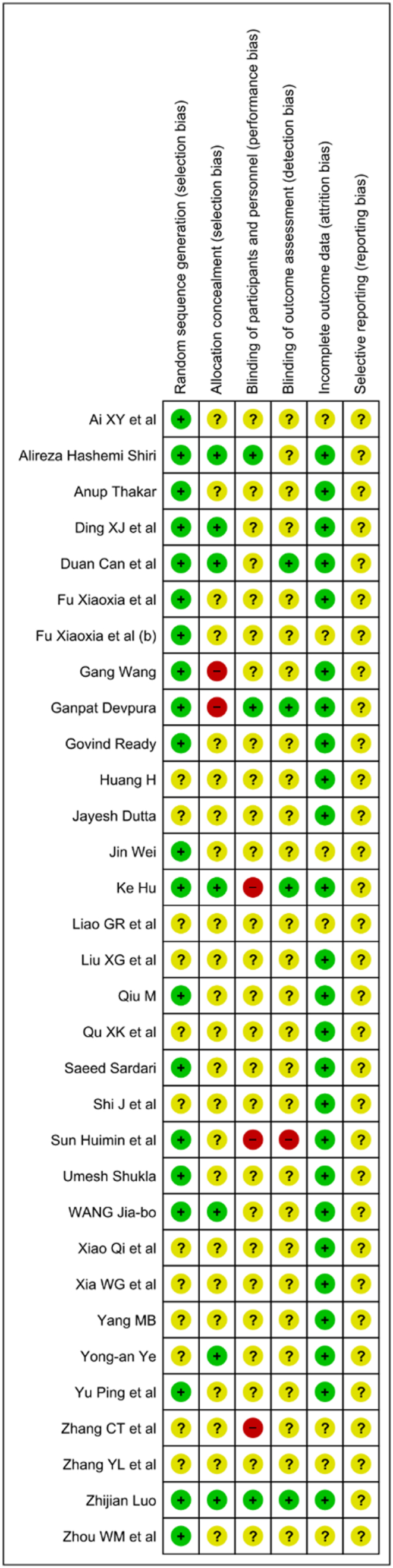
Risk of bias - Summary.
3.4. Effect of herbal intervention on COVID-19
3.4.1. Effect of herbal intervention on RT-PCR negativity
Seven studies have evaluated the effect of herbal intervention on RT-PCR negativity.22,24,27,29,31,35,48 The combined treatment with herbal medications and Western medicine showed likely a good effect regarding RT-PCR negativity with effect rate but statistically insignificant (n = 591, RR 1.22, 95% CI 1.00 to 1.44). [See Supplementary Fig. 1.].
3.4.2. Effect of herbal intervention on clinical symptoms of the COVID-19
In the present review article, the effect of herbal medication on foremost symptoms like fever, cough, fatigue, chest pain, sore throat, hospital stay of COVID-19 were assessed.
3.4.2.1. Effect on fever
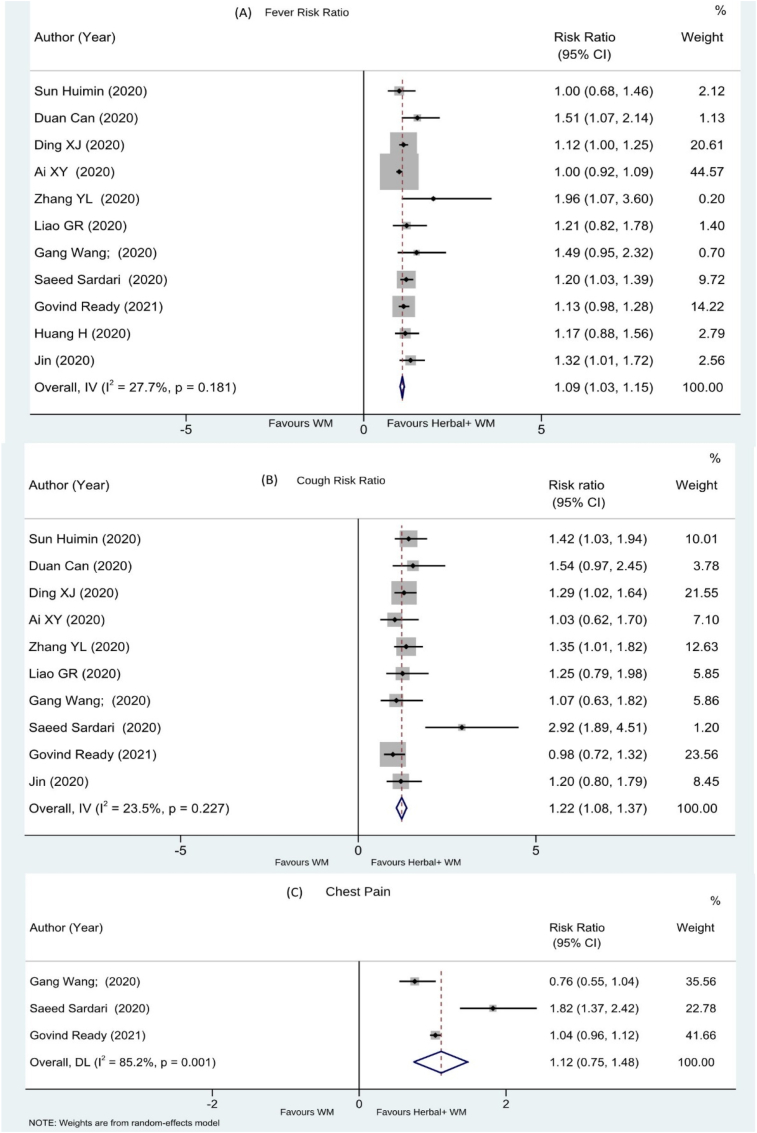
A total of 24 RCTs have evaluated effect of Herbal medicine on cough relief. Twelve studies reported the effect of herbal intervention on fever as the number of patients with decreased body temperature during the protocol treatment period, represented as Risk Ratio.16,21,23,24,27,31,37, 38, 39,44, 45, 46 The combination of herbal intervention and Western medicine showed a significant effect with the overall effect rate (n = 850, RR 1.09, 95% CI 1.03 to 1.15). [See Fig. 4 A.]. Eight studies showed the effect of herbal intervention on fever as the number of days to recover from fever18,19,25,29,32,34,40,48 represented as MD + CI. The combined herbal intervention showed a significant effect with the effect rate (n = 850, MD -1.39, 95% CI -1.76 to −1.03). [See supplement Fig 2].
Fig. 4.
(A)Fever, (B) cough, (C) chest pain.
3.4.2.2. Effect on the cough
Total of seventeen studies have reported the effect on cough. Eleven RCTs16,21,23,24,27,37, 38, 39,44, 45, 46 have shown the effect as the number of patients get relief from cough, and the data reported a significant effect as Risk Ratio. (n = 782, RR 1.22 [95% CI 1.08–1.37]; I2 = 23.5%; p = 0.227) [See Fig. 4B]. Six studies18,19,29,32,40,48 reported time for relief from cough and the effect is represented as MD + CI. (n = 706, MD -2.00, 95% CI -3.33 to −0.68) [See supplement Fig 3].
3.4.2.3. Effect on chest pain
Three studies16,23,24 have reported the effect as the number of patients without chest pain after treatment and were represented with the effect rate (n = 177, RR 1.12 95% CI 0.75–1.48) [See Fig. 4C].
3.4.2.4. Effect on sore throat
Six studies16,23,24,27,38,39 have analyzed the effect as the number of patients without sore throat after treatment (n = 387, RR 1.09 [95% CI 0.99–1.19]; I2 = 22.1%; p = 0.268). [See Supplementary Fig. 4].
3.4.2.5. Effect on fatigue
A total of fourteen studies reported the effect of herbal intervention on fatigue. Nine studies16,21,23,37, 38, 39,44, 45, 46 have represented the number of patients without fatigue after treatment. and showed a significant effect with the effect rate (n = 700, RR 1.27 [95% CI 1.11–1.44]; I2 = 19.6%; p = 0.269. Other five RCTs18,19,29,40,48 have reported the effect as the duration (in days) for relief from fatigue. The net result showed a significant effect with the effect rate represented as MD + CI (n = 656, MD -2.07; 95% CI -3.35, −0.78). [See Supplementary Fig. 5].
3.4.2.6. Effect on chest CT improvement
A total of thirteen studies19,29,31, 32, 33, 34, 35,37,38,40, 41, 42,44 have evaluated the effect of herbal intervention as the number of patients having chest CT improvement after treatment. The overall combined effect was significant and represented by Risk Ratio (n = 1402, RR 1.15 [95% CI 1.08–1.23]; I2 = 29.9%; p = 0.145). [See Supplementary Fig. 6].
3.4.2.7. Effect on the duration of hospital stay
Six studies17,18,26,30,33,34 have reported the effect of herbal intervention on improvement in number of days for hospital stay. The overall combined effect rate is represented by MD + CI (n = 388, MD -1.82; 95% CI -3.84, 0.21). [See Supplementary Fig. 7A].
3.4.2.8. Effect on the clinical effect rate
Ten studies20,29,34,36,40, 41, 42, 43, 44,46 have evaluated the clinical effect of herbal intervention as the number of patients with improved effect rate. The overall combined effect rate is significant and is represented by Risk Ratio (n = 1810, RR 1.13; 95% CI 1.08, 1.17) [See Supplementary Fig. 7B].
3.4.3. Effect on lab parameters of COVID-19 patients
Several RCTs have evaluated the effect of herbal medicine on different laboratory parameters (e.g., WBC count, Lymphocyte percentage, Absolute Lymphocyte count, CRP, IL-6, and ESR level) in COVID-19 patients.
3.4.3.1. Effect on WBC count
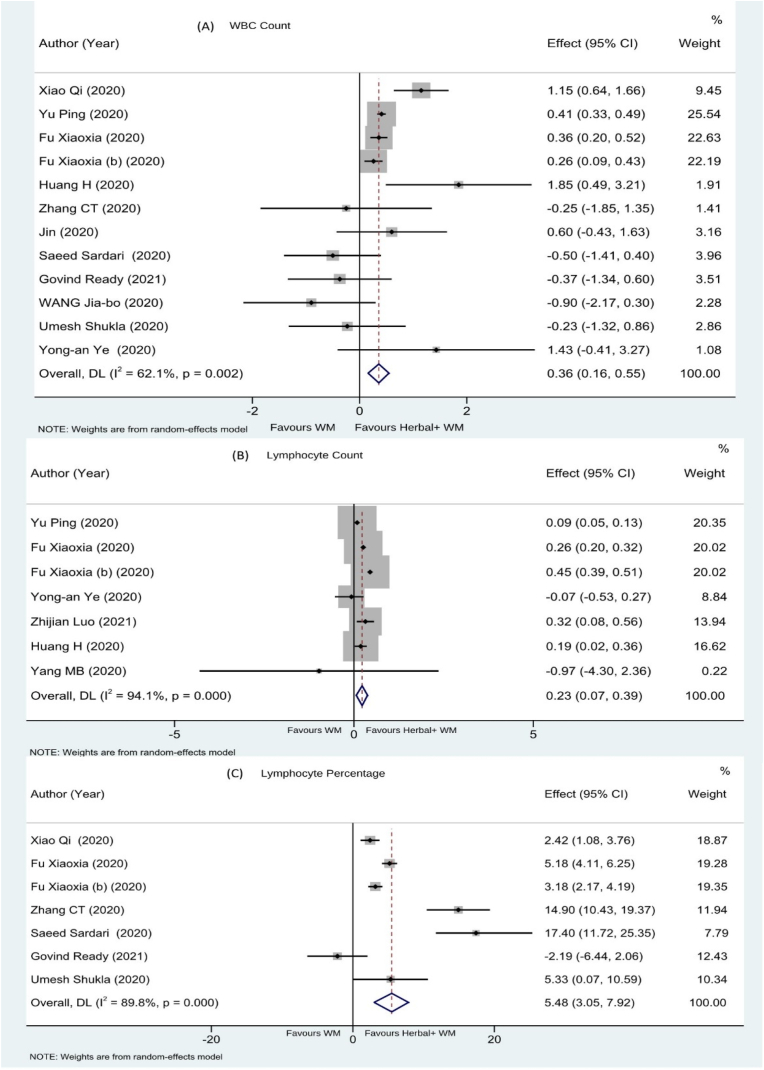
12 studies19,21,23, 24, 25,27,28,31,40, 41, 42, 43 have evaluated the effect of herbal intervention on improvement of WBC as mean increase in WBC count. The net effect is a significant increase shown by MD + CI (n = 1036, MD 0.36; 95% CI 0.16, 0.55) [See Fig. 5A].
Fig. 5.
Effect on (A) WBC, (B) Lymphocyte Count & (C) Lymphocyte percentage.
3.4.3.2. Effect on absolute lymphocyte count
Seven studies18,28,31,35,41, 42, 43 have evaluated the effect of herbal intervention on absolute lymphocyte count represented as Mean ± SD. The net effect is a significant improvement shown by MD + CI (n = 647, MD 0.23; 95% CI 0.07, 0.39) [See Fig. 5B].
3.4.3.3. Effect on lymphocyte percentage
Seven studies19,23,24,27,40,42,43 have evaluated the effect of herbal intervention on lymphocyte percentage and the result was represented as a change in Lymphocyte percentage (Mean ± SD). The net effect is a significant improvement shown by MD + CI (n = 548, MD 5.48; 95% CI 3.05, 7.92) [See Fig. 5C].
3.4.3.4. Effect on C- reactive protein
12 studies17, 18, 19, 20,28,30,31,35,41, 42, 43, 44 have reported the effect of herbal intervention on CRP level as Mean ± SD. The net effect is a significant decrease in CRP level after treatment shown by MD + CI (n = 1423, MD -5.66; 95% CI -7.96, −3.37) [See Supplementary Fig. 8].
3.4.3.5. Effect on IL-6 level
Four studies18,24,27,44 have reported the effect of herbal intervention on IL-6 as Mean ± SD. The net effect was decrease in IL-6 level after treatment but statistically insignificant, showed by MD + CI (n = 239, MD -3.67; 95% CI -8.76, 1.43) [See Supplementary Fig. 9].
3.4.3.6. Effect on ESR level
Five studies24,27,28,30,44 have evaluated the effect of herbal intervention on ESR values represented as mean ± SD. The net effect is a decrease in ESR after treatment but statistically insignificant shown by MD + CI (n = 304, MD -10.38; 95% CI -25.96, 5.21) [See Supplementary Fig. 10].
3.4.3.7. Effect on recovery time
Herbal interventions have shown better effectiveness in shortening the recovery period through early resolution of the main symptoms of COVID-19 like fever, cough, fatigue and hospital stay period as described above in section 3.4.2 of this article.
Thus, the overall result can be summarized as herbal medicine as an adjuvant with the Western medicine have better recovery rate for COVID-19 symptoms like fever, cough, fatigue, chest CT improvement. Also, the laboratory parameters like WBC count, Absolute Lymphocyte count, Lymphocyte percentage, and CRP levels were also better in the herbal medicine group compared to standard care group only. Effect on the duration of hospital stay (MD = −1.82 CI -3.84, 0.21); IL-6 (MD = −3.67 CI -8.76, 1.43), ESR Level (MD = −10.38 CI -25.96, 5.21), and adverse events for herbal medications during the time of the studies (n = 665, RR 0.93; 95% CI 0.76, 1.14) were statistically insignificant.
3.4.4. Assessment of adverse effects
Out of 32 studies, 14 studies18,29,31,34,36,37,39, 40, 41, 42, 43, 44,46,48 have provided data on adverse effects as the number of events in the treatment groups. (n = 665, RR 0.93; 95% CI 0.76, 1.14). (See Supplementary Fig. 11). Another six studies20,21,32,33,35,38 did not described about the adverse effects of herbal intervention. Six studies23, 24, 25,27,28,30 have reported no significant adverse effect of herbal intervention and also provided laboratory values of LFT, KFT [See Supplementary Table 2]. The remaining RCTs have reported insignificant adverse events in treatment groups but didn't provide any data relevant to that context. The most common occurring ADRs reported in different RCTs were diarrhea, anorexia, nausea, abdominal pain, rashes, deranged LFT and KFT values.
4. Discussion
4.1. Summary of evidence
The current review includes an assessment of thirty-two RCTs involving 3177 patients that reported the effect of the herbal interventions for the COVID-19 management (See Table 1). We have analyzed the effect of herbal intervention on RT-PCR negativity (Supplementary Fig. 1), reduction of clinical symptoms, biochemical and laboratory parameters, radiological changes (chest CT improvement), associated adverse events in COVID-19 patients. This meta-analysis showed a beneficial effect of herbal medicine supplementation with Western medicine in early resolution of fever in COVID-19 patients (Fig. 4A) and the number of cases got relieved from cough (Fig. 4B). Also, fatigue reduction time, total effect rate, WBC count (Fig. 5A), lymphocyte percentage (Fig. 5B), absolute lymphocyte count (Fig. 5C), and CRP level were also better in herbal medicine supplementation group. The various herbal intervention used for management is mentioned in Table 1. The treatment duration for herbal drugs varied from 5 to 30 days which was also a point of consideration. Whether herbal medicine can produce a significant effect in such a short time duration is a subject of concern. If the herbal medications were introduced during early onset of symptoms, or if used prophylactically, the management could be more expeditious by reducing clinical symptoms duration. As a result, in most of the cases, COVID-19 can be efficiently stopped in advancing from mild to a critical stage, thus decreasing duration of hospital stays, and mortality rate.
Regarding the side effects, six studies reported no adverse effects during the treatment period. Fourteen studies18,29,31,34,36,37,39, 40, 41, 42, 43, 44,46,48 reported some adverse events like diarrhea, anorexia, nausea, abdominal pain, rashes, deranged LFT and KFT values but statistically insignificant in the herbal group during the time of studies, which varies from 5 to 30 days for most of the included studies. Another six studies23, 24, 25,27,28,30 have reported no significant adverse effect of herbal intervention and also provided laboratory values of LFT, KFT [See Supplementary Table 2]. No follow ups were done or mentioned in included RCTs for assessment of secondary side effects. The results in the present study suggest that herbal interventions were relatively safe for the management against COVID-19. However, due to the lack of adverse event reporting in some studies, the safety of herbal medications should be recognized and included in more comprehensive evidence.
4.2. Advancement from the preceding systematic reviews
The preceding systematic reviews included studies from case reports, case-control studies, and cohort studies.49 Some reviews were focused only on case reports and case series,50 a few reviews reported only single herbal medicine or formulation,52,54,55 certain reviews focused only on Ayurvedic medicine.53 Several reviews focused only on Chinese herbal medicine.2,3,13,51,56, 57, 58, 59 In this review, we included RCTs from China, India, and Iran which assessed the effect of different herbal formulations in the COVID-19 patients.
4.3. Limitations of this review
The current systematic review possesses some limitations. First, the RCTs included weren't of superior quality following the Cochrane Review Manual. Out of 32, only 20 studies have reported adequate random sequence generation, 7 studies have reported valid allocation concealment and only 3 RCTs reported proper blinding. Second, this review included articles from the English language only that may enhance the publication bias risk. Third, different studies have reported an event as a different parameter, therefore, there is a variation in assessing the overall effect for an event. Fourth, different herbal formulations were used as the intervention in the included RCTs, and therefore, it is not easy to isolate the specific constituent/formulations which may be the main source of the efficiency of herbal formulations. Fifth, even in the outcomes like the effect on WBC, Lymphocyte count, we have different data for effect assessment such as, mean increase in WBC level, and the number of patients with increased WBC. This may affect the assessment of overall effect of the intervention in studies. Sixth, this review does not explain the pharmacological mechanism of effect of drugs separately.
4.4. Clinical implications
Based on the evidence summarized here, we have noticed more favorable effect in reducing the duration of COVID-19 symptoms (e.g., fever, cough, fatigue), improvement in the effect rate, WBC count, absolute lymphocyte count, lymphocyte percentage, C-Reactive protein level in the patients treated with herbal plus Standard care compared to the patients treated with standard care (Western medication) alone. This signifies that herbal intervention has a good supporting effect in reducing COVID-19 symptoms. Herbal medicines supplemented in combination with Western medicine improves symptoms of COVID-19 earlier than standard care alone. The sooner the symptoms disappear, the less time it will take to recover from the disease. Therefore, it could be an important substitute for better management of COVID-19 disease and reduction of the overall treatment duration.
As per observation in included RCTs, favorable response in COVID-19 management have been reported by use of Maxing Xuanfei Jiedu decoction, Shufeng Jiedu capsule, Jiaweidayuan granules, Lianhua Qingwen granules, Thyme essential oils, Xuebijing (XBJ) Herbal IV injection, Jinhua-Qinggan formulation, Lianhua-Qingke granules, AYUSH-64, Formulation for Pneumonia No. 1, Formulation for Pneumonia No. 2.
The most used single drug in these formulations are Ephedra Herb, Thorowax (Bupleuri) Root, Baical Skullcap (Scutellariae Baicalensis), Liquoric (Glycyrrhizae) Root, Forsythiae Suspensae fruit, Giant Knotweed (Polygoni Cuspidati), Caoguo (Tsaoko) Fruit, Armeniacae Amarum seed, Bitter Apricot (Armeniacae Amarum) seed, Pinellia (Pinelliae Tematae) Rhizome, Root-Rhubarb (Rhei), Honeysuckle (Lonicerae), Balloon Flower (Platycodi), Trichosanthis Fructus, Turmeric (Curcumae Longae) rhizome, Atrina Glass (Patriniae) herb.
4.5. Research implications
Further clinical trials of superior quality (study with an adequate method for random allocation to minimize selection bias, proper blinding of the participants, investigators, statisticians, outcome and result analysts to eliminate detection bias with performance bias) including a large sample of participants are needed to provide stronger evidence of the benefits of herbal intervention along with Western medicine. Additionally, the trial investigators should assess for the core outcome sets development, e.g., COS-COVID-like parameter.60 This will be enormously helpful in the standard assessment of the effect of herbal medicine. Next, the clinical trial investigating the effects of herbal medications on COVID-19 disease with varying severity of symptoms should be considered; because as the severity varies, the effect will also be quite different. Third, all clinical trials should have assessed the safety and adverse events in detail in the case of herbal interventions. Fourth, it usually requires a long duration of treatment for the effect being produced using herbal medicine; however, the treatment duration reported in the current review was 5–30 days. Therefore, trials with longer treatment courses are recommended to carry out along with extensive follow-up of the participants after the end of the treatment protocol period.
5. Conclusions
The result in this systematic review suggests that the herbal medications as an adjuvant with Western Medicine treatment have add-on beneficial effect and is likely to help in improvement of the core symptoms of COVID-19 disease like (e.g., fever, cough, fatigue), in a relatively shorter period. Improvement in the effect rate, chest CT images, WBC count, absolute lymphocyte count, lymphocyte percentage, C-Reactive protein level are also better. However, due to the lack of high-quality clinical trials and the high grade of heterogeneity in the included studies, a more definite conclusion on the effects of herbal interventions on lowering body temperature and adverse effects could not be assessed at this time. There are some variations between different herbal interventions in the obtained therapeutic effects for the same outcome of COVID-19 disease. The conclusion of this review should be further assessed by thoroughly designed, good sample-sized randomized clinical trials.
Author contributions
AK, AR, and ZH planned the study and designed the PICOs, and initiated screening of the studies. MSK, MF, GR helped in searching, screening, and selection of studies. AR, AK, and ZH has done a statistical analysis, interpreted the study results, and estimated the methodological quality of included clinical studies. AR, ZH drafted the manuscript and produced the tables, figures. AK, MSK, and AK critically revised and finalized the manuscript. The final manuscript has been read, revised, and approved for submission by all authors.
Funding
This study was supported by the fund received from the Ministry of AYUSH, India to Rajendra Institute of Medical Sciences, Ranchi.
Author disclosure statement
There is no any competing financial interest.
Declaration of interest
None.
Acknowledgments
The authors are very thankful to the authors, research team members, and all participants who have been part of the articles included in this review.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2022.01.002.
Contributor Information
Ansul Kumar, Email: docansul@gmail.com.
Arpita Rai, Email: arpita.dirims@gmail.com.
Mohd Saif Khan, Email: drsaif2k2@gmail.com.
Amit Kumar, Email: amits52003@gmail.com.
Zeya Ul Haque, Email: zeya486@gmail.com.
Mohammad Fazil, Email: fazildr@gmail.com.
Gulam Rabbani, Email: rabbani120186@gmail.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Zhou Fei, Ting Yu, Du Ronghui, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y., Wang G., Cai X., et al. An overview of COVID-19. J Zhejiang Univ - Sci B. 2020;21(5):343–360. doi: 10.1631/jzus.B2000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirzaie A., Halaji M., Dehkordi F., Ranjbar R., Noorbazargan H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19) Compl Ther Clin Pract. 2020;40 doi: 10.1016/j.ctcp.2020.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Coronavirus (COVID-19) Dashboard 2022. https://covid19.who.int/ 6th Jan.
- 5.Jean S., Lee P., Hsueh P. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou L., Wang J., Xie R., et al. The effects of traditional Chinese medicine as an auxiliary treatment for COVID-19: a systematic review and meta-analysis. J Alternative Compl Med. 2021;27(3):225–237. doi: 10.1089/acm.2020.0310. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Zhou P., Chen K., et al. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. Can Med Assoc J. 2020;192(27):E734–E744. doi: 10.1503/cmaj.200647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siemieniuk R., Bartoszko J., Ge L., et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P., Shah R., Lohidasan S., Mahadik K. Pharmacokinetic profile of phytoconstituent(s) isolated from medicinal plants—a comprehensive review. J Tradit Complement Med. 2015;5(4):207–227. doi: 10.1016/j.jtcme.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna K., Kohli S., Kaur R., et al. Herbal immune-boosters: substantial warriors of pandemic Covid-19 battle. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S., Chuang W., Lam W., Jiang Z., Cheng Y. Safety surveillance of traditional Chinese medicine: current and future. Drug Saf. 2015;38(2):117–128. doi: 10.1007/s40264-014-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maulik M., Rao M., Juneja A., et al. Emerging trends from COVID-19 research registered in the clinical trials Registry - India. Indian J Med Res. 2021;153(1):26. doi: 10.4103/ijmr.IJMR_2556_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang S., Fang M., Liang C., et al. Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: a rapid systematic review and meta-analysis of randomized controlled trials. Compl Ther Med. 2021;60 doi: 10.1016/j.ctim.2021.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurya D.K., Sharma D. Evaluation of traditional ayurvedic Kadha for prevention and management of the novel Coronavirus (SARS-CoV-2) using in silico approach. J Biomol Struct Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1852119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin X., Pang B., Zhang J., et al. Core outcome set for clinical trials on coronavirus disease 2019 (COS-Covid) Engineering. 2020;6(10):1147–1152. doi: 10.1016/j.eng.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G., Xiong W., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:A pilot randomized clinical trial. Integr Med Res. 2020;9(3) doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutt J., Ganatra B., Suthar N., et al. A randomized and comparative study to assess safety and efficacy of supplemental treatment of a herbal formulation - aayudh Advance comprising essential oils in patients with corona virus 2019 (COVID-19) Contemp Clin Trials Commun. 2021;22 doi: 10.1016/j.conctc.2021.100755. Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Z., Chen W., Xiang M., et al. The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: a prospective randomized controlled trial. Eur J Integr Med. 2021;42:101305. doi: 10.1016/j.eujim.2021.101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C.T., Yang Y., You F., et al. Clinical study on COVID- 19 from the perspective of ‘‘Yidujiashi” theory. Zhong Yao Yao Li Yu Lin Chuang. 2020;36(2):43–45. ([Chinese with abstract in English]) [Google Scholar]
- 20.Liu X., Sun P., Qu Z., Hu J., Chen C., Wu B. Efficacy of No.1 pneumonia prescription in the treatment of corona virus disease. World Chin Med. 2020 http://kns.cnki.net/kcms/detail/11.5529.R.20200506.1832.002.html 2019. [Google Scholar]
- 21.Jin W., Lu Y., Zhao W., Tang S., Sang X., Zhang L. The efficacy of recommended treatments with integrated Chinese and Western medicine on coronavirus disease 2019 (COVID-19) in Sichuan: a clinical trial observation. Pharmacol Clin Chinese Mater Med. 2020;36:6–10. doi: 10.13412/j.cnki.zyyl.20201110.006. [DOI] [Google Scholar]
- 22.Devpura G., Tomar B., Nathiya D., et al. Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients. Phytomedicine. 2021;84 doi: 10.1016/j.phymed.2021.153494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sardari S., Mobaiend A., Ghassemifard L., Kamali K., Khavasi N. Therapeutic effect of Thyme (Thymus Vulgaris) essential oil on patients with COVID19: a randomized clinical trial. 2021. J Adv Med Biomed Res. 2021;29(133):83–91. [Google Scholar]
- 24.Reddy R.G., Gosavi R.V., Yadav B., et al. AYUSH-64 as an add-on to standard care in asymptomatic and mild cases of COVID-19: a randomized controlled trial. AYU. 2020;41:107–116. doi: 10.4103/ayu.ayu_14_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J., Wang Z., Jing J., et al. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin J Integr Med. 2020;26(9):648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiri A.H., Raiatdoost E., Afkhami H., et al. 2021. The Herbal Combination of Sugarcane, Black Myrobalan, and Mastic as a Supplementary Treatment for COVID-19: A Randomized Clinical Trial. medRxiv preprint. [DOI] [Google Scholar]
- 27.Shukla U., Ujjaliya N., Gupta P., et al. Efficacy and safety of Guduchighan Vati in asymptomatic and mild to moderate cases of Covid-19: a randomized controlled pilot study. Preprint. 2021 doi: 10.31219/osf.io/c8f9h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Y., The G-CHAMPS Collaborative Group Guideline-based Chinese herbal medicine treatment plus standard care for severe coronavirus disease 2019 (G-CHAMPS): evidence from China. Front Med. 2020;7:256. doi: 10.3389/fmed.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu K., Guan W.J., Bi Y., et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020;16 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakar A., Goyal M., Bhinde S., Chhotala Y., Panara K., Chaudhary S. Efficacy of ayush 64 as add-on therapy in early stage Covid 19 - an open-label randomized controlled pilot study. Preprint. 2021 doi: 10.31219/osf.io/t8wza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H., Zhao Y., Zuo X.H., Jin J.S., Guo Y. Treatment of COVID-19 by pneumonia No.1 prescription and pneumonia No.2 prescription. Acta Chin Med. 2020:1–11. [Google Scholar]
- 32.Qiu M., Li Q.T., Zhu D.P., Wang C.H., Sun Q.Z., Qian C.F. Efficacy observation of maxing Xuanfei Jiedu Decoction on common type of novel coronavirus pneumonia. J Emerg Tradit Chin Med. 2020;29:1129–1131. [Google Scholar]
- 33.Shi J., Yang Z., Ye C., et al. Clinical observation on 49 cases of non-critical coronavirus disease 2019 in Shanghai treated by integrative traditional Chinese and western medicine. Shanghai J Tradit Chin Med. 2020;54:25–30. [Google Scholar]
- 34.Xia W.G., An C.Q., Zhen C.J., et al. Clinical study on 34 case of COVID-19 treated with integrated traditional Chinese and Western Medicine. J Tradit Chin Med. 2020;61:375–382. [Google Scholar]
- 35.Yang M.B., Dang S.S., Huang S., Li Y.J., Guo Y.L. Multi-center clinical observation of Reyanning mixture in treatment of novel coronavirus pneumonia. Chin J Exp Tradit Med Formul. 2020 doi: 10.13422/j.cnki.syfjx.20201321. [DOI] [Google Scholar]
- 36.Zhou W.M., Zhao F.M., Li B.L., Tian Z.Q. The clinical value of diamine glycyrrhizin in the treatment of moderate patients with COVID-19 [In Chinese] Chin J Virol. 2020;36:160–164. [Google Scholar]
- 37.Liao G.R. Study on the preventive effect and mortality of self-made Decoction in patients with COVID-19 [In Chinese] Int Infect Dis. 2020;9:349. [Google Scholar]
- 38.Sun H.M., Xu Feng, Zhang L., et al. Study on clinical efficacy of Lianhua Qingke Granule in treatment of mild and ordinary COVID-19 [In Chinese] Chin J Exp Tradit Med Form. 2020;26:29–34. [Google Scholar]
- 39.Duan C., Xia W.G., Zheng C.J., et al. Clinical study on treatment of cases of COVID-19 with Jinhua Qinggan granules [in Chinese] J Tradit Chin Med. 2020;61:1473–1477. [Google Scholar]
- 40.Xiao Q. Analysis on the treatment of mild novel coronavirus pneumonia by Chinese herbal medicine Shufeng Jiedu capsules combined with arbidol. J Emerg Tradit Chin Med. 2020;5:756–758. [Google Scholar]
- 41.Yu P., Li Y.Z., Wan S.B., Wang Y. Clinical observation of Huaqingwen Granules combined with Arbidol in the treatment of mild COVID-19 patients [In Chinese] Chin Pharmaceut J. 2020;55:1042–1045. [Google Scholar]
- 42.Fu X.X., Lin L.P., Tan X.H. Clinical study on treatment of cases of COVID-19 with Toujie Quwen granules. Zhongguo Shi Yan Fang Ji Xue Za Zhi. 2020 doi: 10.13422/j.cnki.syfjx.20201314. [DOI] [Google Scholar]
- 43.Fu X.X., Lin L.P., Tan X.H. Clinical study on 37 case of COVID-19 treated with integrated traditional Chinese and Western medicine [In Chinese] Tradit Chin Drug Res Clin Pharmacol. 2020;1:600–604. [Google Scholar]
- 44.Ding X.J., Zhang Y., He D.C., et al. Clinical effect and mechanism of Qingfei Touxie Fuzheng Recipe in the treatment of novel coronavirus pneumonia [In Chinese] Her Med. 2020;39:640–644. [Google Scholar]
- 45.Ai X.Y., Lin L.P., Xie M., Tan X.H. Effect of integrated traditional Chinese and Western medicine on T lymphocyte subsets of patients with normal type of COVID-19 [In Chinese] Guangdong Med J. 2020;41:1203–1206. [Google Scholar]
- 46.Zhang Y.L., Lei L., Xu Y., et al. Clinical efficacy of Jinyinhua oral liquid in the treatment of 80 patients with coronavirus disease 2019 [In Chinese] Chin Pharm. 2020;29:23–26. doi: 10.3969/j.issn.1006-4931.2020.09.006. [DOI] [Google Scholar]
- 47.Higgins J., Altman D., Gotzsche P., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(d5928) doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu X.K., Hao S.L., Ma J.H., et al. Observation on the clinical effect of Shufeng Jiedu capsule combined with arbidol hydrochloride capsules in the treatment of COVID-19. Chin Tradit Herb Drugs. 2020;57:1167–1170. [Google Scholar]
- 49.Wu Y.Q., Zou L., Yu X., et al. Clinical effects of integrated traditional Chinese and western medicine on COVID-19: a systematic review. Shanghai J Tradit Chin Med. 2020:1–8. [Google Scholar]
- 50.Zhan Z.L., Liu J., Yang W., et al. Exploratory study on evaluation criteria of Chinese medicine treatment of new coronavirus pneumonia based on case analysis. J Tradit Chin Med. 2020;1–11 http://kns.cnki.net/kcms/detail/11.2166.R.20200312.0949.002.html Available online. [Google Scholar]
- 51.Wang X., Xie P., Sun G., et al. A systematic review and meta-analysis of the efficacy and safety of western medicine routine treatment combined with Chinese herbal medicine in the treatment of COVID-19. Medicine. 2020;99:32. doi: 10.1097/MD.0000000000021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., Han L., Zhang W., Sun J. The curative effect of Reduning injection combined with Xuanfeibaidu formula on COVID-19. Medicine. 2020;99:46. doi: 10.1097/MD.0000000000022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmad A., Rai A.K., Negandhi H., et al. Efficacy and safety of ayurveda interventions as stand-alone or adjuvant therapy in management of COVID-19: a systematic review protocol. J Res Ayurvedic Sci. 2020;4(3):121–127. doi: 10.5005/jras-10064-0112. [DOI] [Google Scholar]
- 54.Liu M., Gao Y., Yuan Y., et al. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis. Integr Med Res. 2021;10(1) doi: 10.1016/j.imr.2020.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng M., Li L., Wu Z. Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019(COVID-19): meta-analysis of randomized controlled trials. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun C., Sun Y., Li X. The role of Chinese medicine in COVID-19 pneumonia: a systematic review and meta-analysis. Am J Emerg Med. 2020;38(10):2153–2159. doi: 10.1016/j.ajem.2020.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo X., Ni X., Lin J., et al. The add-on effect of Chinese herbal medicine on COVID-19: a systematic review and meta-analysis. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong X., Wang P., Su K., Cho W., Xing Y. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol Res. 2020;160 doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan A.Y., Gu S., Alemi S. Chinese herbal medicine for COVID-19: current evidence with systematic review and meta-analysis. J Integr Med. 2020;18(5):385–394. doi: 10.1016/j.joim.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin X., Pang B., Zhang J., et al. 2020. Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.