Abstract
Background
Herpes zoster neuralgia has a considerable impact on people’s quality of life, especially after the development of postherpetic neuralgia. There are many clinical reports on the treatment of herpes zoster neuralgia, but there have been no special reports on the treatment of herpes zoster involving the neck. Our research focuses on a posterior and upper quarter of the cervical foramina puncture approach for herpes zoster involving the cervical 3–8 (C3-8) nerve region and to consider the safety and efficacy of pulsed radiofrequency combined with steroid injection and ozone injection in this puncture path under CT guidance.
Materials and Methods
A total of 104 patients with herpes zoster neuralgia involved in the cervical 3–8 nerve region use a posterior and upper quarter of the cervical foramina puncture approach received pulsed radiofrequency combined with steroid and ozone injection to the dorsal root ganglion. The total number of injection procedures, complications, NRS collection (preprocedure, postprocedure at once, two, four and 12 weeks) and drug dose decreases were documented.
Results
During a total of 257 procedures, 254 procedures successfully completed PRF (3 cases failed to reach the C8 aim points), and the rate of puncture failure was 1.17%. Drug injection was successfully performed in 252 procedures (the injection success rate was 99.21%); the NRSs (preprocedure, postprocedure at once, two, four and twelve weeks) were 5.75 ± 0.682, 2.6 ± 1.023, 2.21 ± 0.925, 1.89 ± 1.162, and 1.43 ± 1.369, and the difference among them was statistically significant. Drug dosages decreased before and after operation and showed statistically significant differences.
Conclusion
Pulsed radiofrequency combined with steroid and ozone injection for herpes zoster neuralgia involving the C3-8 nerves under CT guidance through a posterior superior quarter approach showed safety and efficacy and had a high success rate, and the NRS decreased significantly.
Keywords: pulsed radiofrequency, herps zoster neuralgia, steroid, ozone
Introduction
Herpes zoster (HZ) is caused by the varicella-zoster virus from a latent state and is usually self-limiting, usually resolving in a few weeks.1,2 Pain often comes to the hospital as the main symptom of herpes. Continuous severe pain often affects people’s life, and some people turn into Postherpetic neuralgia (PHN) which is a common clinical complication of HZ that presents as severe, often persistent, pain that occurs after the rash has resolved.1,2 There are 5% to more than 30% patients with HZ developed to PHN, as recently reported in a systematic review of 130 studies in 26 countries.3 The PHN’s occurrence increases with age,3,4 and incidence rates appear to be increasing over time.5–7 There are several treatment strategies for herpes zoster currently, including antiviral drugs, such as acyclovir and valaciclovir combined with pain management. Pain control includes oral drug therapies such as nonsteroidal anti-inflammatory drugs, antiepileptic drugs, antidepressants and opioid drugs and interventional therapies. Interventional therapies including nerve block, epidural block and radio frequency of dorsal root ganglion. Electrical stimulation of spinal cord, morphine pump implantation, and peripheral neurotomy are also used for some refractory pain.
Currently, the study found the earlier effective intervention, the more shorten the duration and decrease the severity of herpes zoster neuralgia and also can reduce the incidence of PHN.8 Kim ED and Kim K have revealed that pulsed radiofrequency treatment of dorsal root ganglion in managing herpes zoster neuralgia has achieved ideal therapeutic effects,9,10 and nerve block with steroid injection can also reduce all types of neuralgia.11,12 It is worth mentioning that when herpes zoster is involved in the neck and upper limb, especially in the area of C3-C8, the adjacent vertebral artery and spine increase the puncture risk in this area. These injections are completed via an anterolateral approach. However, numerous publications have reported rare but significant complications using a fluoroscopic anterolateral approach.13,14 Therefore, in this study, a puncture path through the posterior and upper quarters of the foramina under CT guidance, which can close the nerve root and avoid intravascular injection, was evaluated to determine if it can satisfy the needs of both dorsal ganglion pulsed radiofrequency and drug injection and ensure adequate security. Furthermore, we also combined ozone injection with dorsal root ganglion and found that pain was significantly relieved and that the incidence of PHN was decreased.
Participants
Methods
This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Jiaxing University. A total of 104 patients with herpes zoster neuralgia involved in the cervical segment, including 65 males and 39 females aged 25–93 years with a median IQR of 72 (62–79) years, were selected at the Pain Department of the First Hospital of Jiaxing from April 2020 to April 2021. All patients’ pain remained moderate to severe. Pulsed radiofrequency treatment and steroid combined ozone injection were performed by the same doctor for all patients.
Inclusion and Exclusion
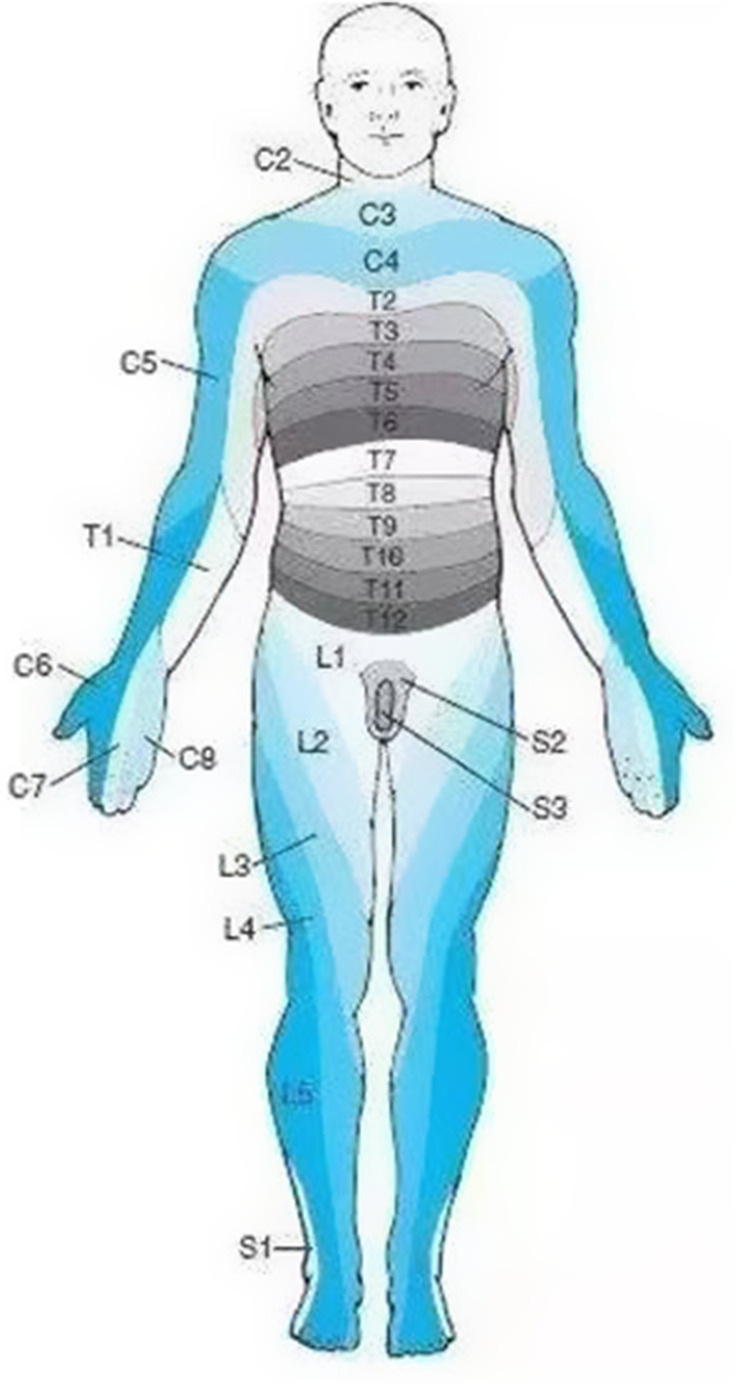
The inclusion criteria were as follows: 1) clinically diagnosed with acute zoster pain; 2) the age of the participants was ≥18 years; the herpes zoster region only involved the unilateral cervical 3–8 nerves (Figure 1); 3) the participants’ pain numerical score (NRS) was higher than four since they came to the hospital; 4) all the participants agreed to undergo pulsed radiofrequency modulation of the spinal dorsal root ganglion and steroid combine ozone injection under the CT guidance; 5) all the participants received follow-up by telephone. The exclusion criteria were as follows: 1) patients with localized infection or tumor at the treatment site; 2) those who have cardiopulmonary insufficiency or other major systemic diseases; 3) patients with severe cervical spinal stenosis; 4) those with a history of abnormal coagulation function; 5) those who are allergic to lidocaine, betamethasone, methyl cobalamin, and related drugs; 6) those with poor blood sugar control; 7) patients who are on long-term use of immunosuppressive agents or systemic failure; 8) patients who were unwilling to accept any possible operation-related complications; refusal of the operation itself. This study was conducted in accordance with the Declaration of Helsinki. Signed informed consent was obtained from all patients.
Figure 1.
The picture shows the distribution of the skin nodes in the innervated area, and the distribution of the nerve skin nodes involving C3-8 in herpes zoster patients is located in one limb.
Surgical Procedure
The patient was laid in a lateral position healthy side down on the CT imaging bed, with nasal oxygen inhalation provided, and ECG monitoring was performed continuously during the whole operation (Figure 2). Only the operation site was exposed; other body parts were covered with lead safety clothing to afford radiation shielding protection. The positioning grid was fixed on the affected neck side with adhesive tape. The puncture route involved the following steps: CT scanning was used to locate the upper 1/4 of the cervical foramen. It is ideal for the intervertebral foramen and vertebral artery foramen (transverse foramen) to be displayed on the same plane (Figure 3). The dorsal point of the foramen was selected as the target point (Figure 3). The orientation of the positional line was parallel to the pedicle of the foramen, and the intersection of the extension line and the skin was the needle insert point (Figure 3). After routine skin sterilization, draping, and local anesthesia with 0.5% lidocaine, the needle was inserted along the designated path. As the first step, we tried to place the tip on the target of the anterior nodule of the foramina, with the vertebral artery showing in the same plane (Figure 4); in the second step, we inserted the radiofrequency needle along the line path, and the depth was not deeper than 1/2 of the upper articular surface (Figure 5).
Figure 2.
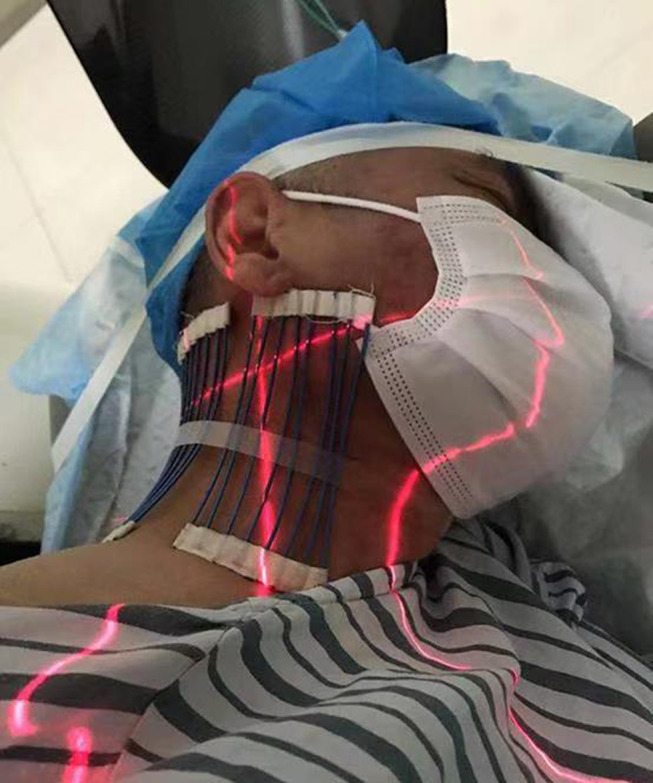
The patient was laid in a lateral position healthy side down on the CT imaging bed. The positioning grid was fixed on the neck of the affected side with adhesive tape.
Figure 3.
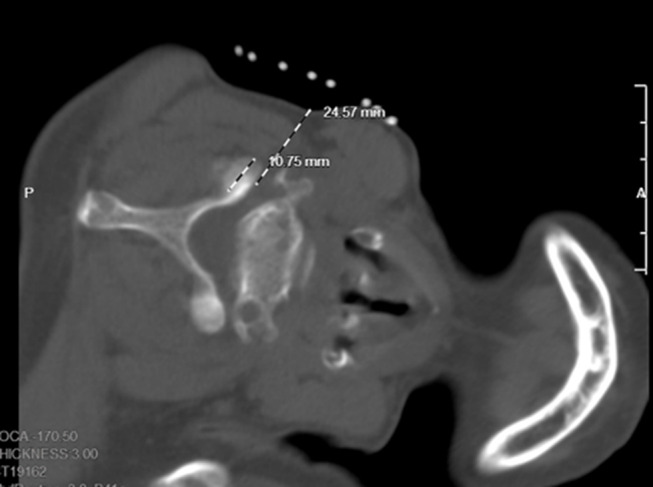
The intervertebral foramen and vertebral artery foramen can be displayed on the same plane. The dorsal point of the foramen was selected as the target point. The orientation of the positional line was parallel to the pedicle of the foramen and the intersection of the extension line and the skin is the needle insert point.
Figure 4.
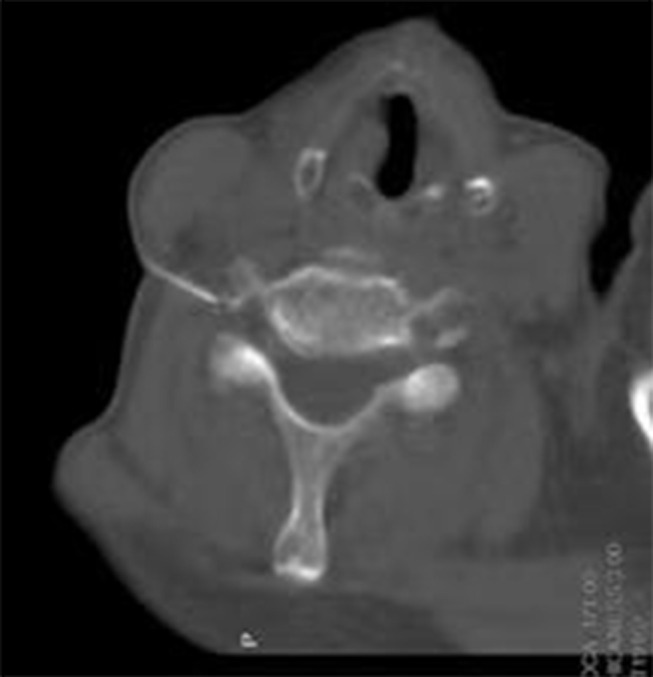
First step we try to put the tip to the target of anterior nodule of the foramina, vertebral artery showed at the same plane.
Figure 5.
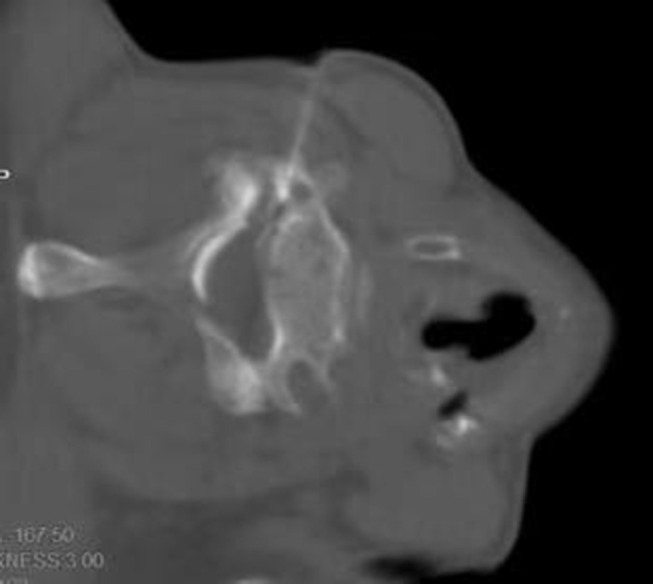
Second step we insert the Radiofrequency needle along the line path, and the depth was not deeper than 1/2 of the upper articular surface.
The parameters of the sensory test were set at a voltage of <0.5 V and a frequency of 50 Hz, which could induce soreness, swelling, numbness or tingling in the innervated area considered positive. Low-frequency current was used in the exercise test, and the parameters were set at a voltage of <0.5 V and a frequency of 2 Hz, which subsequently caused fibrillation and pulsation of the trunk muscle fibers in the corresponding segments considered positive. The temperature, time, pulse width and frequency were set at 42°C, 300 seconds, 20 msec and 2 Hz,15 the PRF voltage was no increased than 50 V, respectively16. Furthermore, we added the maximum tolerable voltage exercise stimulation on the basis of the standard parameter pulse, and the patients experienced obvious muscle contraction in the innervated area for 5 additional minutes. After the end of pulsed radiofrequency, the electrode core was pulled out, and the puncture needle was withdrawn with no blood, gas, or liquid.
Then next, 0.5 mL of contrast agent solution was injected, mixed with 0.5 mL of 0.9% normal saline, and CT scan showed no intravascular injection (Figure 5). Then, 5 mL of treatment solution was injected into each segment. The treatment solution was formulated with 100 mg of 2% lidocaine hydrochloride, 1 mg of compound betamethasone, 1 mg of mecobalamin, and 2 mL of contrast agent and was then diluted to 15 mL using 0.9% normal saline. Again, a CT scan showed that the contrast medium and drug spread well, covering the pain-related neurospinal segments, and reconfirmed no intravascular injection. After injection, ozone (40 µg/mL) was administered to 3 mL in each segment (Figure 6), and CT showed that the ozone spread well. The puncture point was compressed after the needle was pulled out. After observation for 20 minutes, the patient was wheeled back to the ward.
Figure 6.
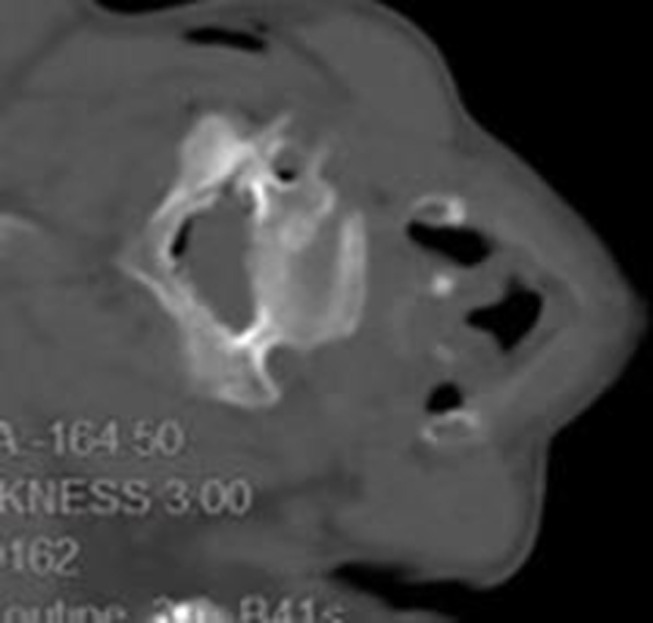
These was injected followed by ozone (40ug/mL) for 3mL each segments, this picture showed ozone spread in the epidural space and around the artery.
Observation Indexes
Data documented for each patient included the following:
• Collection of general information of patients,
• Total number of injections performed per procedure,
• Number of procedures performed all patients during the study period,
• Total number of steroid injections and ozone injections,
• NRS score collection time (preprocedure, postprocedure at once, two, four and 12 weeks),
• Dosages of drug used before and after treatment (all the patients received gabapentin before and after the operation to decrease their pain scores),
• Presence/absence of postprocedure complications,
• Surgical complications and the incidence of PHN.
Statistical Analysis
SPSS26.0 (IBM, Chicago, USA) was used to analyze the data. The Shapiro–Wilk test was used to determine whether the measurement data obeyed a normal distribution, and the results were expressed as the mean ± standard deviation (SD), while the nonnormally distributed data were expressed as the median (IQR). A paired sample t-test was used to compare the differences among measurement data, and the chi-square test was used to compare the differences between counting data. We used the Wilcoxon test to analyze the nonnormally distributed data. The Kruskal–Wallis test was used to analyze the gabapentin consumption and NRS score at each time interval after the operation. Multiple comparisons were performed by the Bonferroni test, and the significance level was corrected. A violin plot was used to describe the changing trends in NRS score and gabapentin consumption.
Results
Analysis of the Clinical Characteristics of Patients
In this study, 104 patients [65 males and 39 females; mean median IQR 72 (62–79) years, range 25–93 years] at the Pain Department of the Affiliated Hospital of Jiaxing University were enrolled, and all the patients with herpes zoster neuralgia involving the C2-8 nerve root, and every patient herps lesion involving the 3 ganglion segments (41 cases involved C2-4; 20 cases C3-5; 4 cases C4-6; 19 cases C5-7; 10 cases C6-8; 6 cases C7-T1; 4 cases C8-T2). We counted every segment as one procedure, so a total of 257 procedures (C2, T1 and T2 were not counted) involved the C3-8 nerve roots were documented. During the entire study, 1 patient was lost to follow-up due to death, and the rate of lost interviews was 0.96%. The summary statistics are shown in Table 1.
Table 1.
Baseline Characteristics of Patients
| Total Number of Patients (January 2018–December 2020) | 104 |
| Age (years) | 25–93 |
| Sex (M/F) | 65/39 |
| Duration of pain (days) | 7–43 |
| NRS (before treatment) | 4–7 |
| Medication | Yes |
| Involved dermatome | |
| C2-4 | 41 |
| C3-5 | 20 |
| C4-6 | 4 |
| C5-7 | 19 |
| C6-8 | 10 |
| C7-T1 | 6 |
| C8-T2 | 4 |
Analysis of the Puncture Effect of the Posterior and Upper Quarters of the Cervical Foramina Puncture Approach
A total of 254 procedures successfully finished PRF (3 cases failed to reach the C8 aim points), and the rate of puncture failure was 1.17%. The total number of individual injections performed was 252 (2 cases found blood when the needle was withdrawn, and the rate of failure injection was 0.79%). Each patient underwent an average of two to three procedures (2.77 individual injections) during the treatment period (Table 2).
Table 2.
Study Population Summary Statistics
| Total Number of Patients (January 2018–December 2020) | 104 |
| Total number of procedures C3-8 | 254 |
| Total number of steroid injections C3-8 | 252 |
| Total number of ozone injections C3-8 | 252 |
| Average number of procedures per patient | 2.77 |
| Average number of injections per procedure | 1.67 |
Effectiveness Analysis of NRS Score and Drug Dosage Decrease After Treatment
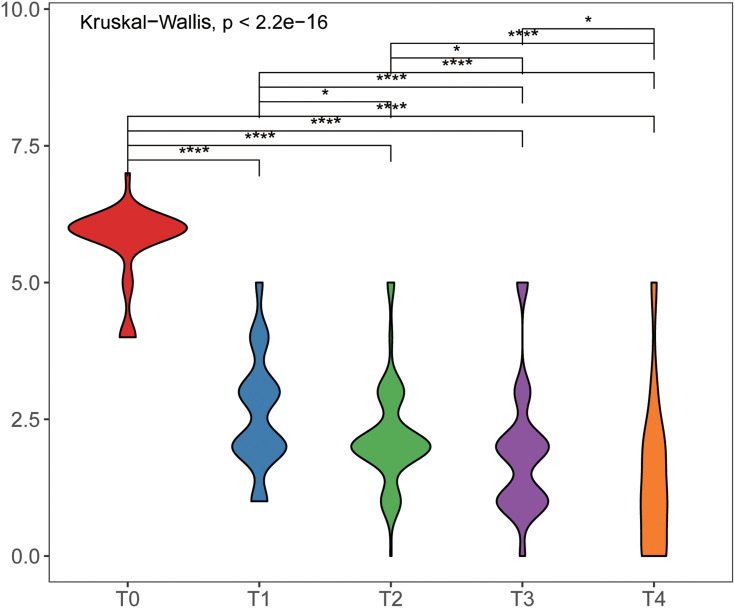
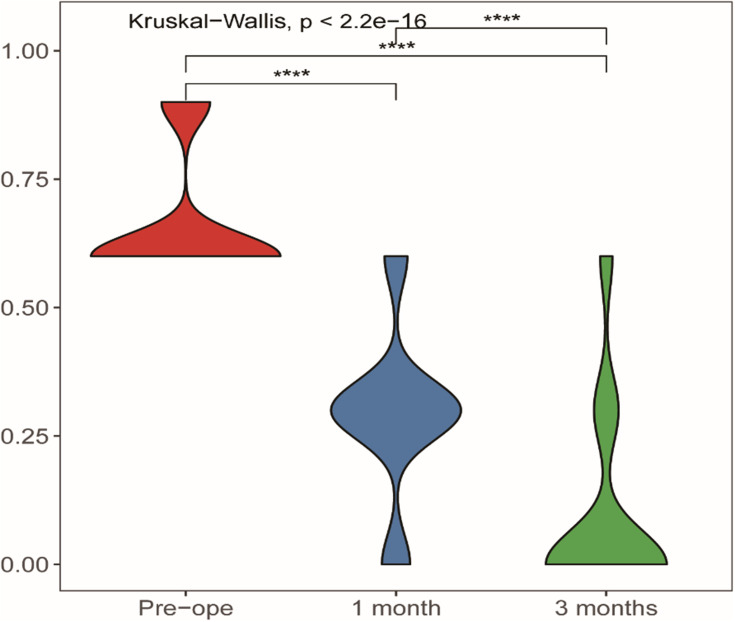
The NRS scores collection time (preprocedure, postprocedure at once, two, four and twelve weeks) were recorded (Table 3) and were 5.75±0.682, 2.6±1.023, 2.21±0.925, 1.89±1.162, and 1.43±1.369, respectively, and the differences among them were statistically significant (Table 3, Figure 7). The dosages of gabapentin before and after (one month later) surgery showed statistically significant differences (Table 4, Figure 8).
Table 3.
Comparison of Preoperative and Postoperative NRS (T0: Preoperation NRS; T1: At Once NRS; T2: 2 Week NRS; T3: 4 Week NRS; T4: 12 Week NRS)
| T0 (Preoperation) | T1 (At Once) | T2 (2 Week) | T3 (4 Week) | T4 (12 Week) | |
|---|---|---|---|---|---|
| N | 103 | 103 | 103 | 103 | 103 |
| NRS | 5.75±0.682 | 2.6±1.023 | 2.21±0.925 | 1.89±1.162 | 1.43±1.369 |
| T0 | – | Df: 177.7367278 P=0.000 Sig: **** |
Df: 187.580086 P=0.000 Sig: **** |
Df: 164.7764629 P=0.000 Sig: **** |
Df: 149.6769653 P=0.000 Sig: **** |
| T1 | – | – | Df: 201.984199706766 P=0.014 Sig: * |
Df: 200.742690407474 P=0.000 Sig: **** |
Df: 188.79908017027 P=0.000 Sig: **** |
| T2 | – | – | Df: 194.217789602244 P=0.030 Sig: * |
Df: 179.075519008888 P=0.000 Sig: **** |
|
| T3 | – | – | – | – | Df: 179.075519008888 P=0.018 Sig: * |
| T4 | – | – | – | – | – |
Notes: *P < 0.05 and ***P < 0.001.
Figure 7.
Comparison of preoperative and postoperative NRS (T0: preoperation NRS; T1: at once NRS; T2: 2 week NRS; T3:4 week NRS; T4: 12 week NRS).
Notes: *Statistically significant difference (P < 0.05), ****statistically significant difference (P < 0.0001).
Table 4.
Comparison of Preoperative and Postoperative Medication
| Drug 1 (Pre) | Drug 2 (1 Month) | Drug 3 (3 Months) | |
|---|---|---|---|
| n | 103 | 103 | 103 |
| 0.6612±0.12146 | 0.2913±0.15972 | 0.0932±0.18325 | |
| Drug 1 | – | Df: 190.40 P=0.000 Sig: **** |
Df: 177.118519389708 P=0.000 Sig: **** |
| Drug 2 | – | – | Df: 200.265254333382 P=0.000 Sig: **** |
| Drug 3 | – | – | – |
Note: ***P < 0.001.
Figure 8.
Comparison of preoperative and postoperative medication.
Note: ****Statistically significant difference (P < 0.0001).
Intraoperative and Postoperative Complications
Based on past years of experience, the severe complications of the posterior and upper quarters puncturing of the foramina were mainly including intravascular injection, nerve injury, local anesthetic poisoning, and even whole spinal anesthesia. During this research period, none of the above complications happened.
Six patients (5.77%) had facial flushing, which was noticeable by the next day. This resolved in all patients within several days and required no treatment.
Eleven patients had sleep disorders that resolved within 3 days, and one patient who had a history of mental illness experienced mental disturbance and recovered 3 days later without treatment (Table 5).
Table 5.
Intraoperative and Postoperative Complications
| Facial Flushing | 6 (6.52%) |
| Sleep disorder | 11 |
| Itch | 4 |
| Intermittent fatigue | 3 |
| Steroid intravascular injection | 2 |
| Nerve injury | 0 |
| Local anesthetic poisoning | 0 |
| Spinal anesthesia | 0 |
PHN Incidence Analysis
During telephone follow-up three months after treatment, four patients still had pain in the corresponding surgical area. The PHN of C3-8 herpes zoster neuralgia after puncturing of the posterior and upper quarters of the foramina pathway at the dorsal root ganglion was 3.85%. Radiofrequency ablation of the corresponding segment of the dorsal root ganglion was performed in four patients, and the results were the same. These patients had to control their neuralgia using oral drugs at the same dosage as before treatment (Table 3).
Discussion
In the clinic, the spinal ganglion can be intervened, which can affect the transmission of peripheral pain signals to the upper center, thereby inhibiting the release of excitatory transmitters and regulating changes in central plasticity.17,18 The early treatment of herpes zoster neuralgia is very important because as soon as postherpetic neuralgia emerges, the pathogenesis may involve many aspects, such as scarring in the local lesion area, fascial contracture in the innervated area, partial compression, central sensitization, and amplification of psychological factors. Therefore, a variety of treatment schemes have been developed. However, once the course of the disease begins three months later, pain relief becomes extremely difficult. At present, there is nerve damage, and traditional Chinese medicine acupuncture and oral medicine do not have very good curative effects. In the end, such patients are disappointed, depressed, anxious, take many analgesic drugs orally, and gradually increase their drug dose. The incidence rates of herpes in the chest and lumbar region are often high. There have been few reports about cervical nerve involvement. This is also because the area has abundant neurovasculature, and the risk of bleeding or drug infusion is increased. As the area is closer to the brain, it requires a higher response time for doctors. However, if this area is involved in the lesion area, no invasive treatment will significantly increase the rate of PHN. In our research, all participants came to our hospital with a herpes zoster history of 7 to 43 days, and the treatment results were more effective than previously reported, possibly because the patients’ lesion rash areas involved the neck and upper limbs, which affected their appearance. Worry about the recovery of upper limb function and fear of physical disability are also reasons patients seek medical treatment as soon as possible. In addition, the success rate of our treatment methods was high (99.2%), and the overall efficiency was good (100%). In the clinic, dorsal root ganglion which have activation of the varicella-zoster virus that lurks the dorsal root ganglion, causing damage to the nerve axons is used as a therapeutic target, which can affect the transmission of peripheral pain signals to the upper center, thereby inhibiting the release of excitatory transmitters and regulating changes in central plasticity.18 Based on the above results, CT-guided puncturing of the dorsal root ganglion of C3-8 using the posterior and upper quarters of the foramina pathway was safer and more successful than CT-guided puncturing of the dorsal root ganglion. Our research also showed that four patients still had left neuralgia in the corresponding area after surgery, and they had pain histories of 34 days, 43 days, 40 days, and 35 days. The incidence rate of PHN was 3.85%, which was slightly lower than that in previous reports both at home and abroad.8 Early treatment may decrease the incidence.
PRF is widely believed to act through a temperature-independent, neuromodulatory process, altering synaptic transmission and pain signaling via the emission of electric fields, with no to minimal resultant tissue destruction. There are some basic science studies support the hypothesized neuromodulatory effects of PRF. Research believes that PRF can have disruptive effects on axonal microtubules, microfilaments, and mitochondria, with the greatest disruption evident in nociception mediating unmyelinated type C fibers, followed by myelinated type A-delta and type A-beta fibers. Furthermore, PRF also may modulate neuropathic pain through the enhancement of descending noradrenergic and serotonergic inhibitory pathways. Thus, the mechanism of PRF in the treatment of herpes zoster neuralgia may be realized by neuroregulation.
In our study, we found that when the needle tip was located in the upper quarter of the intervertebral foramen in some patients, higher parameter settings were needed to increase the muscle beat or tremor. Therefore, we added the maximum tolerable voltage exercise stimulation on the basis of the standard parameter pulse, and the patients experienced obvious muscle contraction in the innervated area. Our considerations for this stimulation are as follows: 1. the beating of the herpes zoster region may lead to the exercise of local muscle function; 2. the rhythmic contraction of muscle may lead to the release of peripheral adhesion; and 3. rhythmic contraction of muscle and discharge of nerves may lead to the acceleration of peripheral microcirculation and the disappearance of inflammation.
Glucocorticoid injection therapy has been used for a long time in the treatment of herpes zoster. Glucocorticoids can eliminate neuroinflammation and reduce sensitization. Granular and nongranular glucocorticoids can be chosen. Bensler et al studied about 594 patients following lumbar epidural steroid injections demonstrated significantly lower pain scores at 1 week and 1 month postinjection with particulate steroids compared to nonparticulate steroids.18 Moreover, the local deposition effect of a granular preparation is much greater than that of a nongranular preparation. Nonparticulate agents are likely to increase the absorption of the surrounding circulation, leading to systemic effects. Therefore, they are more effective for the application of granular preparations, but the complications of granular preparations can be fatal if they enter the blood. There have been 6 reported cases of spinal cord infarction and 1 brainstem infarction.15
Therefore, the safety of the treatment technology selected is particularly important. In our report, only 2 cases were found after intravascular injection.
In radiology practice, CT-guided procedures are the preferred technique which have demonstrated their safety and efficacy compared with fluoroscopy.19–21 Anterolateral and posterior approaches have been described to access the cervical foramen before.22 While pulsed radiofrequency combined with particulate steroids and ozone injection not only seeks closure of the dorsal root ganglion but also aims to reduce the possibility of accidental injection into the vertebral artery and its branches, our group has predominantly completed puncture into cervical dorsal root ganglion and injections through a posterior and upper quarter of the cervical foramina approach. This approach is closer to the dorsal root ganglion, and the intervertebral foramen and transverse foramen (contain vertebral artery) are shown in the same plane (Figure 3), which can avoid vascular injection and increase the safety of the procedure. In the process of needle insertion, we adopted two-step positioning and two-step needle insertion to ensure the safety of the puncture path and the needle insertion process, and the use of a contrast agent also further reduced the risk of the drug entering the blood. The results of this study support this procedure. Two cases found blood when the needle was withdrawn, and the rate of injection failure was 0.79%. A total of 252 procedures had successfully completed punctures, and 252 procedures had successfully completed steroid and ozone injections.
CT-guided pulsed radiofrequency combined with particulate steroids and ozone injection-treated C3-C8 herpes zoster neuralgia using a posterior and upper quarter of the cervical foramina puncture approach were safe and effective in our research.
Medical ozone is a mixture gas, typically contain 1–5% O3 and 95–99% O2; ozone is the strongest naturally occurring oxidant. Scripps’ research has determined that human neutrophils can generate ozone in the body by a catalytic reaction of immune cells endogenously creating singlet oxygen and antibodies. Ozone has a high-energy, variable molecular structure under normal temperature and is quickly and spontaneously decomposed into O2 and a single oxygen atom (O). It has strong oxidation activity and strong antimicrobial effects on bacteria and viruses.23,24 Ozone is very safe and can be injected into blood which inactivates bacteria by disrupting their cell envelope through the oxidation of phospholipids and lipoproteins, inhibits fungal growth, damages the capsid of viruses, and upsets the viral reproductive cycle by disrupting virus-to-cell contact with peroxidation.25–27 Oxygen-ozone therapy increases the production of interferon, tumor necrosis factor, and interleukin-2, activating the immune system.28 Currently, many studies have reported on available scientific evidence concerning the beneficial properties of ozone therapy for pain.31,32 It is extensively used via local injections to joints29 and discs30 for the resolution of arthritis and pain and is extremely safe. Intradiscal ozone injection was first proposed in Italy in the 1980s as a treatment for herniated discs.33 A mixture of ozone and oxygen (O2O3) can be injected directly into the disc or indirectly into the paravertebral muscles to reduce herniation, relieve nerve root compression, and have potential analgesic and anti-inflammatory effects.30,32 In our study, we want to use the concentration of ozone for best efficacy and security, that is Maximum safe concentration. Viebahn reported that the nontoxic concentration of ozone varies from one to 40 microgram per milliliter of oxygen and concentration should not exceed 40 ug/mL.34 In previous studies, we also found that intrathecal injection of high concentration ozone (40–60 μg/mL) could induce neurotoxicity, whereas intrathecal injection of low concentration ozone (<40ug/mL) is a rarely induced neurotoxicity.35 We administered 40 µg/mL ozone through a needle to the dorsal root ganglion. CT guidance and contrast confirmation can provide procedural security, and ozone can provide better effects. Specifically, pain NRS decreased immediately and at one month and three months, and all of the differences were statistically significant. The drug dose decrease also showed statistical significance. These data revealed surprising levels of pain relief. In our next study, we will compare the pain score reduction of the ozone group and the nonozone treatment group to confirm the significance of ozone in the application of herpes zoster.
In summary, CT-guided pulsed radiofrequency combined with steroid and ozone injection to treat C3-C8 herpes zoster neuralgia using a posterior and upper quarter of the cervical foramina puncture approach is safe and effective. Our study provides safety techniques for CT-guided puncture technology and can serve as an effective reference for PRF combined with steroid and ozone injection in the clinical treatment of patients with herpes zoster involving the C3-C8 area.
Conclusion
CT-guided puncture using a posterior and upper quarter of the cervical foramina approach into the dorsal root ganglion with PRF treatment for C3-8 herpes zoster neuralgia demonstrated a high successful rate, and its combination with steroid and ozone injection is considered to be safe and effective.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (81901124), the Natural Science Foundation of Zhejiang Province, China (LQ19H090007), the Medical Scientific Research Foundation of Zhejiang Province, China (2020RC122), the Construction Project of Anesthesiology Discipline Special Disease Center in Zhejiang North Region (201524), the Key Medical Subjects Established by Zhejiang Province and Jiaxing City Jointly – Pain Medicine (2019-ss-ttyx), and Medical Scientific Research Foundation of Zhejiang Province, China (2020KY949).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Johnson RW, Rice AS, Solomon CG. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371:1526–1533. doi: 10.1056/NEJMcp1403062 [DOI] [PubMed] [Google Scholar]
- 2.John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31:811–826. doi: 10.1016/j.idc.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi: 10.1136/bmjopen-2014-004833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92:1806–1821. doi: 10.1016/j.mayocp.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 5.Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18(1):55–78. doi: 10.1111/ddg.14013 [DOI] [PubMed] [Google Scholar]
- 6.Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018;31(4):235–243. doi: 10.3344/kjp.2018.31.4.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer S, Baeumler P, Geber C, et al. Somatosensory profiles in acute herpes zoster and predictors of postherpetic neuralgia. Pain. 2019;160(4):882–894. doi: 10.1097/j.pain.0000000000001467 [DOI] [PubMed] [Google Scholar]
- 8.Esposito MF, Malayil R, Hanes M, Deer T. Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med. 2019;20(Suppl 1):S23–S30. doi: 10.1093/pm/pnz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim ED, Lee YI, Park HJ. Comparison of efficacy of continuous epidural block and pulsed radiofrequency to the dorsal root ganglion for management of pain persisting beyond the acute phase of herpes zoster. PLoS One. 2017;12(8):e0183559. doi: 10.1371/journal.pone.0183559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K, Jo D, Kim E. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia. Pain Physician. 2017;20(3):E411–E418. doi: 10.36076/ppj.2017.E418 [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Chen Z, Ren H, Luo F. The effectiveness and safety of 42 degrees C pulsed radiofrequency combined with 60 degrees C continuous radiofrequency for refractory infraorbital neuralgia: a prospective study. Pain Physician. 2019;22(3):E171–E179. [PubMed] [Google Scholar]
- 12.Lazzari ZT, Palmisani S, Hill B, Al-Kaisy A, Lambru G. A prospective case series of sphenopalatine ganglion pulsed radiofrequency therapy for refractory chronic cluster headache. Eur J Neurol. 2020;27(7):1190–1196. doi: 10.1111/ene.14176 [DOI] [PubMed] [Google Scholar]
- 13.Ludwig MA, Burns SP. Spinal cord infarction following cervical transforaminal epidural injection: a case report. Spine. 2005;30:E266–E268. doi: 10.1097/01.brs.0000162401.47054.00 [DOI] [PubMed] [Google Scholar]
- 14.McMillan MR, Crumpton C. Cortical blindness and neurologic injury complicating cervical transforaminal injection for cervical radiculopathy. Anesthesiology. 2003;99:509–511. doi: 10.1097/00000542-200308000-00038 [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Fei Y, Deng J, et al. Application and therapeutic effect of puncturing of the costal transverse process for pulsed radiofrequency treated T1-T3 herpes zoster neuralgia. J Pain Res. 2020;13:2519–2527. doi: 10.2147/JPR.S266481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Z, Hong T, Ding Y, Wang S, Yao P. CT-guided pulsed radiofrequency at different voltages in the treatment of postherpetic neuralgia. Front Neurosci. 2020. doi: 10.3389/fnins.2020.579486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gierthmuhlen J, Braig O, Rehm S, Hellriegel J, Binder A, Baron R. Dynamic of the somatosensory system in postherpetic neuralgia. Pain Rep. 2018;3(6):e668. doi: 10.1097/PR9.0000000000000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bensler S, Sutter R, Pfrrmann CWA, et al. Particulate versus non-particulate corticosteroids for transforaminal nerve root blocks: comparison of outcomes in 494 patients with lumbar radiculopathy. Eur Radiol. 2018;28:946–952. doi: 10.1007/s00330-017-5045-z [DOI] [PubMed] [Google Scholar]
- 19.Wolter T, Knoeller S, Berlis A, et al. CT-guided cervical selective nerve root block with a dorsal approach. AJNR Am J Neuroradiol. 2010;31:1831–1836. doi: 10.3174/ajnr.A2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cyteval C, Thomas E, Decoux E, et al. Cervical radiculopathy: open study on percutaneous periradicular foraminal steroid infiltration performed under CT control in 30 patients. AJNR Am J Neuroradiol. 2004;25:441–445. [PMC free article] [PubMed] [Google Scholar]
- 21.Lasbleiz J, Siegfried D, Chales G, et al. Evaluation of CT guided cervical epidural injections in patients with mechanical cervicobrachial neuralgia [in French]. J Radiol. 2008;89(3 pt 1):317–323. doi: 10.1016/S0221-0363(08)93006-7 [DOI] [PubMed] [Google Scholar]
- 22.Tiso RL, Cutler T, Catania JA, et al. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4:468–474. doi: 10.1016/j.spinee.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 23.Elvis AM, Ekta JS. Ozone therapy: a clinical review. J Nat Sci Biol Med. 2011;2(1):66–70. doi: 10.4103/0976-9668.82319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogales CG, Ferrari PH, Kantorovich EO, Lage-Marques JL. Ozone therapy in medicine and dentistry. J Contemp Dent Pract. 2008;9(4):75–84. doi: 10.5005/jcdp-9-4-75 [DOI] [PubMed] [Google Scholar]
- 25.Đuričić D, Valpotić H, Samardžija M. Prophylaxis and therapeutic potential of ozone in buiatrics: current knowledge. Anim Reprod Sci. 2015;159:1–7. doi: 10.1016/j.anireprosci.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 26.Artis AS, Aydogan S, Sahin MG. The effects of colorectally insufflated oxygen-ozone on red blood cell rheology in rabbits. Clin Hemorheol Microcirc. 2010;45(2–4):329–336. doi: 10.3233/CH-2010-1316 [DOI] [PubMed] [Google Scholar]
- 27.Aydos TR, Başar MM, Kul O, et al. Effects of ozone therapy and taurine on ischemia/reperfusion-induced testicular injury in a rat testicular torsion model. Turk J Med Sci. 2014;44(5):749–755. doi: 10.3906/sag-1308-20 [DOI] [PubMed] [Google Scholar]
- 28.Pendino KJ, Shuler RL, Laskin JD, Laskin DL. Enhanced production of interleukin-1, tumor necrosis factor-alpha, and fibronectin by rat lung phagocytes following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol. 1994;11(3):279–286. doi: 10.1165/ajrcmb.11.3.8086166 [DOI] [PubMed] [Google Scholar]
- 29.Jesus C, Trevisani V, Santos F. Comparison between intra articular ozone and placebo in the treatment of knee osteoarthritis: a multicentric, comparative, randomized and double-blinded clinical trial [abstract]. Arthritis Rheumatol. 2015;67(suppl 10). Available from: https://www.acrabstracts.org/abstract/comparisonbetween-intraarticular-ozone-and-placebo-in-the-treatment-of-kneeosteoarthritisa-multicentric-comparative-randomized-and-doubleblindedclinical-trial/. [Google Scholar]
- 30.Andreula CF, Simonetti L, de Santis F, Agati R, Ricci R, Leonardi M. Minimally invasive oxygen-ozone therapy for lumbar disk herniation. Am J Neuroradiol. 2003;24(5):996–1000. [PMC free article] [PubMed] [Google Scholar]
- 31.Muto M, Giurazza F, Silva RP, Guarnieri G. Rational approach, technique and selection criteria treating lumbar disk herniations by oxygen–ozone therapy. Interv Neuroradiol. 2016;22(6):736–740. doi: 10.1177/1591019916659266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bocci V, Borrelli E, Zanardi I, Travagli V. The usefulness of ozone treatment in spinal pain. Drug Des Devel Ther. 2015;9:2677–2685. doi: 10.2147/DDDT.S74518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buric J, Rigobello L, Hooper D. Five and ten year follow up on intradiscal ozone injection for disc herniation. Int J Spine Surg Int J Spine Surg. 2014;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das G, Ray S, Ishwarari S, Roy M, Ghosh P. Ozone nucleolysis for management of pain and disability in prolapsed lumber intervertebral disc A prospective cohort study. Interv Neuroradiol. 2009;15:330–334. doi: 10.1177/159101990901500311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Lin X, Zhao X, et al. Ozone (O3) elicits neurotoxicity in spinal cord neurons (SCNs) by inducing ER Ca2+ release and activating the CaMKII/MAPK signaling pathway. Toxicol Appl Pharmacol. 2014;280(3):493–501. doi: 10.1016/j.taap.2014.08.024 [DOI] [PubMed] [Google Scholar]