Abstract
Purpose
Type 2 diabetes (T2D) medication adherence is poor and is impacted by individual drug characteristics. Treatment-associated weight change can affect medication-taking behavior. This review aimed to explore weight change on T2D therapy and consider its impact on adherence and discontinuation.
Methods
Searches were conducted in MEDLINE and EMBASE (2005 to September 2020), and among recent congress abstract books for studies providing data on medication adherence or discontinuation and weight change in people with T2D (PwD).
Results
Nine studies meeting the inclusion criteria were identified from 9188 bibliographic records. All three studies exploring weight change and discontinuation reported weight loss to be associated with higher persistence. Seven studies of varying design explored weight change and adherence. Four reported absolute weight change (kg) and adherence: one pooled data from different diabetes medications and demonstrated that self-reported adherence was significantly associated with weight loss; however, three studies found that weight change in adherent PwD was in the direction of the known weight profile (loss/gain) of the evaluated drug. Categorical weight loss (≥3%) and adherence were reported in two studies: one reported that numerically more adherent versus non-adherent PwD lost ≥3% weight regardless of the drug’s weight profile, the other showed that early weight loss with a glucagon-like peptide-1 agonist was significantly associated with better adherence. One study reported adherence by categorical weight change; as weight loss increased, adherence scores improved, regardless of drug type.
Conclusion
Findings suggest that discontinuation rates may be lower in PwD who lose as compared to those who gain weight on T2D treatment. The evidence base on adherence and weight change is more challenging to interpret due to the range of study designs. Given the importance of weight control in T2D, further research exploring the individual’s treatment, weight journey, and behaviors over time should be undertaken.
Keywords: adherence, discontinuation, type 2 diabetes, weight
Introduction
A strong relationship exists between diabetes and overweight or obesity.1 Estimates suggest that ~90% of people with type 2 diabetes (T2D) have overweight or obesity.2,3 People with T2D and high BMI have worse glycemic control.4–6 Furthermore, as BMI increases, achievement of target levels of glycated hemoglobin (HbA1c) declines.4–7 In addition, high BMI has been reported to be a major contributory factor in the development of T2D-related complications including neuropathy, nephropathy, cardiovascular disease, and peripheral vascular disease.8–10 Overweight and obesity among individuals with T2D are also strongly predictive of increasing health-care costs.9,11–13
Given these observations, weight reduction is recognized as a key component of T2D management as outlined in international clinical guidelines.14,15 However, weight management in people with T2D is complicated by the fact that many commonly used glucose-lowering agents (GLAs) are themselves associated with weight-altering properties including weight gain (eg, thiazolidinediones [TZD], sulfonylureas [SU], and insulin), or weight loss (eg, metformin, glucagon-like peptide-1 receptor agonists [GLP-1 RA], and sodium-glucose co-transporter-2 inhibitors [SGLT-2i]).16,17 As such, guidelines recommend that if lifestyle changes need to be supplemented with a GLA in T2D, then a medication with proven weight-loss benefits is preferred.14,15
Despite the range of treatments available for the management of hyperglycemia in T2D and their known benefits in reducing the risk of diabetes-related complications, adherence rates are often poor.18,19 A wealth of evidence suggests that medication non-adherence in T2D has a negative impact on several outcomes including morbidity, mortality, and health-care costs.20,21 This may be particularly pertinent among people with T2D and obesity, since it has been demonstrated that such individuals are twice as likely to have low-to-moderate medication compliance compared with individuals without obesity.22 The characteristics of T2D medications vary considerably with respect to route and frequency of administration, at what stage of diabetes they are initiated, their adverse event profile, and other clinical features such as their impact on weight. These individual treatment characteristics can impact medication-taking behaviors among people with diabetes (PwD).23
Medication taking can be viewed at the simplest level as three stages at which PwD exhibit different drug-taking behaviors.24 The process begins with initiation, when an individual and their health-care provider (HCP) must decide whether to start a new treatment. This is followed by implementation/on-treatment adherence, which describes how a PwD doses their medication from initiation to the last dose: do they strictly adhere to their prescribed regimen, or are they non-compliant with recommendations? Finally, there is discontinuation, which may occur in some cases when an individual or their HCP decides to stop treatment or switch to an alternative.24
One of the features of diabetes therapies that might influence how an individual takes their medication at each stage of the treatment journey is the impact of different drugs on weight. For example, weight gain is a commonly cited barrier to treatment initiation or intensification and is a particular problem with respect to insulin therapy.25–27 Multiple quantitative28–34 and qualitative34–36 studies have evaluated the extent of the issue, which is widespread.
A comprehensive literature review on the association between weight and medication-taking behavior beyond treatment initiation has not previously been conducted. Therefore, the aim of this paper is to further explore weight gain or loss whilst on T2D therapy and to consider its association with adherence and persistence/discontinuation with therapy. The content of the review is informed by data captured from a literature search. It aims to provide a useful resource for researchers by collating evidence from individual studies on weight change and adherence into a single paper.
Materials and Methods
A literature review identified studies providing objective data on weight change and adherence or discontinuation in adults with T2D treated with diabetes medications that was quantifiable. The focus of this search was on studies where there was a formal assessment of the association between weight change and adherence or discontinuation/persistence. The search syntax for the literature review is provided in the online supplementary materials (Tables S1 and S2).
A protocol for the literature search was developed that detailed the proposed approach, objectives, search strategy, study selection criteria, methods for data extraction and synthesis, and outcomes of interest that were specified a priori. Development of the protocol reduced the impact of reviewer bias, ensured transparency and accountability, and maximized the chances of accurate data extraction.
Data Sources and Study Eligibility
Searches were conducted in MEDLINE via Ovid and EMBASE via Ovid between January 2005 and September 3, 2020. In addition, searches of abstract books for the following congresses were also undertaken: European Association for the Study of Diabetes (EASD) 2020, American Diabetes Association (ADA) 2020, International Society for Pharmacoeconomics and Outcomes Research (ISPOR; all meetings) 2020, European and International Congress on Obesity (ECOICO) 2020, and World Obesity Federation (WOF) 2020. Study eligibility criteria are shown in Table 1. There was no geographic focus for the literature review.
Table 1.
Study Eligibility Criteria
| Study Characteristic | Eligible | Ineligible |
|---|---|---|
| Patient population | - Adults (≥18 years) with T2D | - Pediatric (<18 years) people with T2D - People without T2D (eg, T1D, gestational diabetes) - Pregnant women |
| Intervention | - Pharmacologic treatment for T2D | - Non-pharmacologic treatment for T2D - Treatments for the management of conditions other than T2D |
| Outcomes | - Adherence - Compliance - Persistence - Discontinuation |
- Other outcomes - Adherence/discontinuation measures that were not quantified |
| Measures of weight change | - Weight - BMI |
- Other measures (such as hip-to-waist ratio) - Subjective/perceived weight change - Weight change clearly associated or caused by something other than treatment for T2D (eg, cancer, after weight-loss surgery, post-partum) |
| Study type | - Real-world cross-sectional study - Real-world case control study - Real-world cohort study - Administrative or claims database study - Real-world EHR - Registry study representing real-world clinical practice - Questionnaires and surveys relating to real-world clinical practice - Clinical trial (Phase III or IV) - Pragmatic trial |
- Case studies - Utility studies - Preference or satisfaction studies based on hypothetical profiles - Reviews - Editorials/comments - Economic evaluations - Literature reviews - Clinical trials earlier than Phase III |
| Language | - English | - Non-English |
Abbreviations: BMI, body mass index; EHR, electronic health records; T1D, type 1 diabetes; T2D, type 2 diabetes.
Search Strategy and Study Selection
The overall search strategy consisted of four broad concepts: T2D AND weight change AND (non-specific adherence OR specific named adherence measures). Please refer to the online supplementary materials (Tables S1 and S2) to review the actual search syntax that was used in the databases. The strategy excluded records indexed as news, editorial, case reports, letter or comment publication types, or that included the phrase “case report” in the title. The strategy was designed to retrieve records that explicitly referred to either non-specific adherence terms or the specific named adherence/discontinuation measures or questionnaires (eg, the eight-Item Morisky Medication Adherence Scale [MMAS-8]), a full list of which is provided in the supplementary materials (Table S3). Furthermore, the strategy was developed to identify records in which weight change was objectively reported.
Search results were assessed by two reviewers independently. A broad review of the title/abstract of search results was conducted initially followed by full-text review of records identified as potentially eligible. Studies failing to meet study criteria after full-text review were excluded and the reason for exclusion recorded. Disagreements between reviewers regarding study inclusion were resolved by discussion until consensus was met.
Results
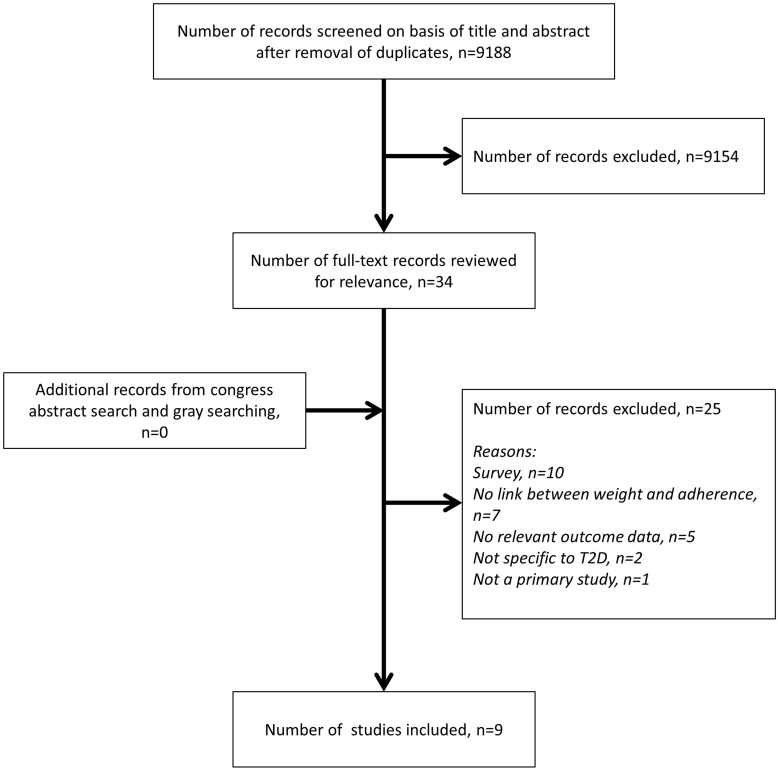
Figure 1 provides an overview of the search results. After de-duplication, the literature search yielded 9188 records. Review of the title/abstracts resulted in the identification of 34 potentially eligible records. There were 9154 articles that were excluded at abstract review stage as they did not meet the inclusion criteria for the review. Following full-text review, 25 records were excluded and nine included in the final review. No congress abstracts of relevance were identified. Of the nine included studies, six reported on the association between weight change and adherence,37–42 two on the relationship between weight change and discontinuation,43,44 and one on weight and both adherence and discontinuation.45 Presentation of results is reported separately for studies on adherence and for those on discontinuation.
Figure 1.
Literature review: study selection.
Studies Associated with Adherence
Overview of Adherence Studies
An overview of study objectives, methods, populations, and measures in the seven adherence studies is provided in Table 2. Six studies were conducted in the USA and one in the UK. Most were retrospective cohort studies (n = 5) using electronic medical record (EMR) and administrative claims data.37,38,40,41,45 Two studies were prospective and included a 5-year, survey-based study, SHIELD, conducted in a representative US population39 and a pragmatic, randomized, open-label, parallel group trial (SIMPLE).42
Table 2.
Overview of Studies Identified in the Literature Review Evaluating the Association Between Weight and Medication Adherence or Discontinuation
| Author/Year (Country) | Objective (as it Relates to this Review) | Study Type and Data Source | PwD Population | Study Duration | Drugs Evaluated | Adherence/Discontinuation Measure |
|---|---|---|---|---|---|---|
| Adherence | ||||||
| Carls et al, 2017 (USA)37 | To examine real-world weight change and the role of medication adherence among PwD initiating 1 of 3 drug classes | Retrospective cohort study using Optum/ Humedica SmartFile database (administrative claims and EMR) | Adults with T2D (N=5818) | 12 months (+ 12 weeks baseline) | GLP-1 RA; SU DPP-4i (disaggregated) | PDC: very adherent, ≥90%; adherent, ≥80%; poorly adherent, <80%; very poorly adherent, <50% |
| Gordon et al, 2018 (UK)38 | To examine relationships between medication adherence and clinical outcomes | Retrospective cohort study using UK CPRD | Adults with T2D (N=33,849) | 12 months | OADs (grouped by mono, dual, and triple therapy) | MPR: adherence, ≥80% (data excluded from PwD with MPR >120%) |
| Grandy et al, 2013 (USA)39 | To investigate whether individuals who lost weight had better medication adherence than those who gained weight | Prospective survey-based study (SHIELD); weight change evaluated between 2007 and 2008; adherence captured in 2008 | Adults with T2D enrolled in the SHIELD study (N=2209) | 12 months | SU; GLP-1 RA; insulin; TZD (grouped by weight change profile)a | MMAS |
| McAdam-Marx et al, 2014 (USA)40 | To examine the association between weight loss and adherence with glycemic goal attainment in PwD with inadequately controlled T2D | Retrospective cohort study using EMR data from GHS and PCP and self-reported adherence surveys | Adults with T2D initiated on drug class not previously received (N=477) | 6 months | Metformin; GLP-1 RA; SU; TZP; DPP-4i; insulin (disaggregated) | MARS-5 |
| McAdam-Marx et al, 2014 (USA)41 | To describe the relationships between medication adherence, weight change, and glycemic control in people with T2D | Retrospective cohort study using EMR data from GHS and PCP and self-reported adherence surveys | Adults with T2D initiated on drug class not previously received (N=166) | 6 months | Metformin; GLP-1 RA; SU; TZP; DPP-4i; insulin (pooled) | MARS-5 (9–15 months after index date); MPR |
| Patel et al, 2019 (USA)42 | To evaluate adherence with GLP-1 RA + BI vs BBI and effects of adherence on clinical and PRO, and baseline predictors of adherence | Prospective randomized pragmatic trial (SIMPLE) | Adults with T2D (N=120) | 6 months | GLP-1 RA; insulin | Adherence defined as amount of product (for the treatment arms) used vs that expected at each study visit |
| Adherence and discontinuation | ||||||
| Durden et al, 2018 (USA)45 | To analyze how outcomes affect adherence and persistence | Retrospective cohort study using EMRs from the IBM Watson Health Explorys Universe Dataset | Adults with T2D initiating GLP-1 RA (N=8329) | 18 months (within 3–6 months = early responders) | GLP-1 RA | Adherence: PDC >0.80 Persistence: index line of therapy sustained over 18 months |
| Discontinuation | ||||||
| Bell et al, 2014 (USA)43 | To assess the impact of weight change on treatment discontinuation among metformin-treated people with T2D | Retrospective cohort study using administrative data in EMRs and progress notes from the Health Alliance Plan | Adults with T2D treated with metformin (N=2110) | 18 months + 6 months pre-index | NIAD (90.6% metformin monotherapy) | >30 days elapsed without drugs belonging to index class |
| Melzer-Cohen et al, 2019 (Israel)44 | To compare outcomes in people with T2D who continued liraglutide for 12 months vs discontinuers | Retrospective cohort study using EMR data from Maccabi Healthcare Services | Adults with T2D initiating liraglutide (N=3580) | 24 months | Liraglutide | Gap of ≥120 days between dispenses (after refill date) |
Notes: aTwo drug groups were defined based on the weight association for each antidiabetic drug class: (1) drugs associated with weight loss, including GLP-1 RA and metformin; and (2) drugs associated with weight gain, including TZDs, insulin, and SUs. Respondents who received a diabetes treatment regimen with 41 antidiabetic drugs that included any weight-gain drug (TZDs, insulin, SUs) were grouped into the weight-gain drug group regardless of other antidiabetes drugs (GLP-1 RAs, metformin, DPP-4i) in that treatment regimen. Respondents who received DPP-4 inhibitors and no other antidiabetes drug (monotherapy) were not included in the analysis of adherence by drug group because DPP-4 inhibitors are weight neutral.
Abbreviations: BBI, basal-bolus insulin; BI, basal insulin; CPRD, Clinical Practice Research Datalink; DPP-4i, dipeptidyl peptidase 4 inhibitor; EMR, electronic medical record; GHS, Geisinger Health System (an integrated health system in central Pennsylvania); GLP-1 RA, glucagon-like peptide-1 receptor agonist; MARS-5, 5-item Medication Adherence Report Scale; MMAS, Morisky Medication Adherence Survey; MPR, medication possession ratio; NIAD, non-insulin antidiabetes drug; OAD, oral antidiabetes drug; PCP, primary care physician; PDC, proportion of days covered; PRO, patient-reported outcomes; PwD, person/people with diabetes; SHIELD, Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes; SIMPLE, Simple basal Insulin titration, Metformin Plus Liraglutide for type 2 diabetes with very Elevated HbA1c; SU, sulfonylurea; T2D, type 2 diabetes; TZD, thiazolidinedione.
Methods for the measurement of adherence were highly variable across studies, with five different approaches used (Table 2). Three studies employed objective and widely used claims-based approaches (eg, proportion of days covered [PDC] or medication possession ratio [MPR]).37,38,45 Another three studies used subjective measures that rely on patient self-report of adherence (eg, MMAS and the 5-item Medication Adherence Report Scale [MARS-5]).39–41 In the SIMPLE study, adherence was directly assessed at each clinic encounter by dividing the amount of each drug used by the expected use over the interval between visits.42
Several T2D medications were evaluated across studies (Table 2). In one study, weight data were aggregated for PwD initiating any one of six different treatments (metformin, GLP-1 RA, SU, TZD, dipeptidyl peptidase 4 inhibitor [DPP-4i], or insulin).41 A second study aggregated data according to whether PwD initiated a drug associated with weight loss (eg, GLP-1 RA and metformin) or weight gain (eg, TZD, insulin, and SU).39 A third reported adherence across cohorts of PwD receiving oral antidiabetes drug (OAD) mono-, dual, or triple therapy.38 Four studies reported weight and adherence data for individual drugs including metformin, GLP-1 RA, SU, TZD, DPP-4i, or insulin.37,40,42,45
Study populations varied widely across the studies with respect to both numbers (N = 120 to N = 33,849) and characteristics. Briefly, ~50% of study participants were male, with most being around 50–60 years of age. Mean HbA1c was generally in the range of 7.8–9.1%. Where mean BMI was reported, it was high (>32 kg/m2). One study, in particular, appeared to include a less representative study population (ie, included a higher proportion of individuals from ethnic minorities, fewer male subjects, younger PwD, and PwD with a higher mean HbA1c reflective of the study inclusion criterion requiring individuals to have very uncontrolled T2D).42 An overview of baseline demographics in the included studies is provided in Supplementary Table S4.
Adherence Study Results
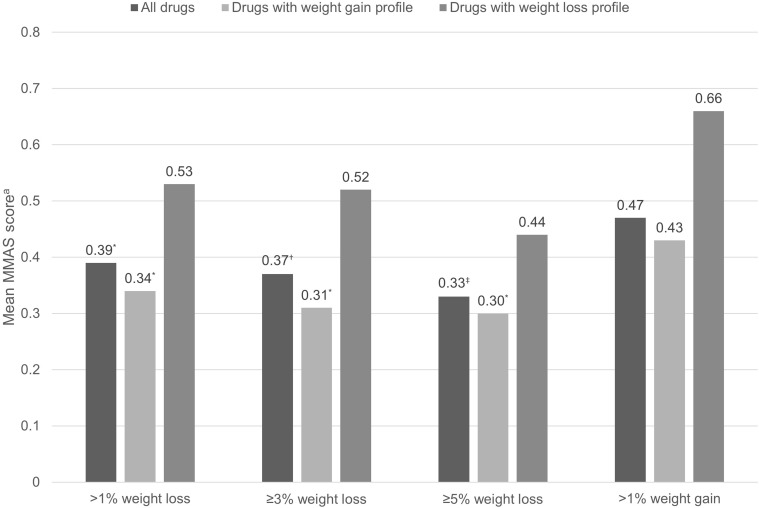
Seven studies were identified that reported data on weight change and adherence. Across the included adherence studies, six reported weight change according to medication adherence (Table 3). Of these, four reported absolute weight change (in kg) in adherent versus non-adherent PwD,37,38,41,42 one reported the proportion of adherent versus non-adherent PwD achieving ≥3% body weight reduction from baseline,40 and one reported the odds of adherence in individuals with early (at 3–6 months) body weight loss compared with those with no early response45 (definition of medication adherence varied depending on the measure used). One study reported adherence according to categorical weight change; mean MMAS scores were determined for PwD achieving a ≥1%, ≥3%, or ≥5% weight loss from baseline, or a >1% weight gain from baseline (Figure 2).39
Table 3.
Overview of Main Study Findings with Respect to Weight and Adherence
| Author/Year | Study Drug(s) | Weight Measure | Adherence | Absolute Weight Change/ Proportion of PwD with Weight Change from Baseline (95% CI where Reported) | Weight Loss Associated with Better Adherence | Weight Gain Associated with Better Adherence | |
|---|---|---|---|---|---|---|---|
| Adherent | Non-adherent | ||||||
| Studies reporting absolute weight loss (kg) and adherence | |||||||
| Patel et al, 201942 | Liraglutide plus BI | Baseline to 6 months | Amount of medication used/amount expected to be used over days between clinic visits (adherent: measure assessed at 2/3 clinic visits and time-adjusted adherence rate for study duration ≥80%) | –1.3 kg (–0.32, 0.7) | +0.3 kg (–2.2, 2.9) | ✔ | |
| BBI | +4.6 kg (2.2, 7.0) | +2.1 kg (0.1, 4.0) | ✔ | ||||
| Study author observation: “Those with ≥80% adherence, compared with those who had lower adherence, had numerically greater effects on … weight” | |||||||
| Carls et al, 201737 | GLP-1 RA | Baseline to 1 year | PDC (adherent: ≥0.80) | –3.77 kg | –2.04 kg | ✔ (p<0.01)a | |
| DPP-4i | –1.18 kg | –1.29 kg | No effect | ||||
| SU | +0.85 kg | –0.26 kg | ✔ (p<0.01)a | ||||
| Study author observations: “Adherence to GLP-1 RA appears to enhance its weight-loss effect and adherence to SU appears to amplify weight gain. In contrast, patients treated with DPP4 experience small weight changes and adherence to DPP4 treatment had no effect on weight” | |||||||
| Gordon et al, 201838 | OAD monotherapy | Baseline to 1 year | MPR (adherent: ≥0.80) | –2.65 kg (–2.80, –2.50) | –1.64 kg (–1.94, –1.34) | ✔ (p<0.001)a | |
| OAD dual therapy | +0.67 kg (0.46, 0.88) | +0.31 kg (–0.22, 0.83) | ✔ | ||||
| OAD triple therapy | +0.50 kg (0.03, 0.97) | +0.26 (–0.65, 1.17) | ✔ | ||||
| Study author observations: “Within each OAD cohort, adherent patients tended to lose more weight (OAD monotherapy) or gain more weight (OAD dual and triple therapy) compared with non-adherent patients” | |||||||
| McAdam-Marx et al, 201441 | Pooled metformin, GLP-1 RA, SU, TZD, DPP-4i, and insulin | Baseline to 6 months | MPR (adherent: ≥0.80) | –1.6 kg | –1.3 kg | ✔ | |
| MARS-5 (adherent: 25) | –1.7 kg | –1.1 kg | ✔ (p=0.016)b | ||||
| Study author observations: “Adherent patients had significant changes in body weight from baseline, but non-adherent patients did not” “Self-reported adherence to diabetes medication was associated with weight loss ≥3%” | |||||||
| Studies reporting categorical weight loss and adherence | |||||||
| Durden et al, 201945 | GLP-1 RA | >3% body weight loss at 3–6 months | PDC (adherent: ≥0.80) | Early weight-loss responders: 43.3% No early weight-loss effect: 38.0% (p<0.001) |
✔ (OR [95% CI], 1.18 [1.02, 1.36])c |
||
| Study author observations: “Early response was associated with a higher likelihood of medication adherence … over a period of up to 18 months compared with those patients who did not achieve changes of … >3% reduction in body weight within 3–6 months” | |||||||
| McAdam-Marx et al, 201440 | All | ≥3% body weight loss at 6 months | MARS-5 (adherent: 25) | 29.9% | 24.2% | ✔ | |
| Metformin | 47.1% | 42.1% | ✔ | ||||
| GLP-1 RA | 50.0% | 40.0% | ✔ | ||||
| SU | 16.5% | 11.5% | ✔ | ||||
| TZD | 23.8% | 16.7% | ✔ | ||||
| DPP-4i | 20.8% | 16.7% | ✔ | ||||
| Insulin | 27.5% | 12.9% | ✔ | ||||
| Other | 25.0% | 0% | ✔ | ||||
| Study author observations: “This study showed that medication adherence is associated with … weight loss” | |||||||
Notes: ✔: Numerical (not statistically significant) difference or difference based on author conclusions; ✔ (p-value)a: statistically significant difference between adherent and non-adherent PwD reported; ✔ (p-value)b: from SEM, adherent PwD were more likely to experience weight loss compared with non-adherent PwD when assessed by MARS-5 (OR 1.70, 95% CI 1.11, 2.61); however, no significant association found with adherence assessed by MPR (OR 1.59, 95% CI 0.66, 3.83; p = 0.305); ✔c: odds of early weight-loss responders being adherent versus those with no early weight-loss response.
Abbreviations: BBI, basal-bolus insulin; BI, basal insulin; CI, confidence interval; DPP-4[i], dipeptidyl peptidase 4 [inhibitor]; GLP-1 RA, glucagon-like peptide-1 receptor agonist; MARS-5, 5-item Medication Adherence Report Scale; MPR, medication possession ratio; NS, not statistically significant; OAD, oral antidiabetes drug; PDC, proportion of days covered; PwD, people/person with diabetes; SU, sulfonylurea; TZD, thiazolidinedione.
Figure 2.
Adherence with T2D medications according to categorical weight change from baseline to 12 months. Data from Grandy et al.39
Notes: aThe MMAS is a 4-item questionnaire with yes/no responses (no = 0; yes = 1). Scores range from 0 to 4, with higher scores indicating poorer adherence. *p ≤ 0.05; †p = 0.026; ‡p = 0.014 vs >1% weight gain. Drugs associated with weight gain include thiazolidinediones, insulin, and sulfonylureas; drugs associated with weight loss include glucagon-like peptide-1 receptor agonists and metformin.
Abbreviations: MMAS, Morisky Medication Adherence Scale; T2D, type 2 diabetes.
Absolute Weight Change According to Level of Medication Adherence
In all four studies that reported absolute weight change (kg) according to level of adherence, the trend for weight loss or gain from baseline was associated with better medication adherence (Table 3).37,38,41,42 Of these four studies, three demonstrated that the direction of weight change in more adherent PwD appeared to follow the known weight profile of the drug under evaluation such that PwD who were more adherent put on weight if taking a drug associated with weight gain or lost weight if taking a drug associated with weight loss (Table 3). For example, Carls et al (2017)37 reported that study participants who were more adherent with GLP-1 RAs, which are known to be associated with weight loss, experienced a statistically significantly greater weight reduction compared with individuals who were less adherent (p < 0.01). Conversely, individuals who were more adherent with SU, known to be associated with weight gain, experienced statistically significantly greater weight increases compared with those who were less adherent (p < 0.01). No significant difference in weight change was noted between people adherent or non-adherent with DPP-4i, which have a weight-neutral profile.
In a study that reported weight change among people with T2D initiating basal-bolus insulin or liraglutide plus basal insulin, the authors suggested that PwD with ≥80% adherence experienced numerically greater weight changes compared with those with lower adherence.42 Specifically, weight loss from baseline to 6 months was observed in individuals who were adherent with liraglutide plus basal insulin, while those who were non-adherent experienced little weight change (Table 3); PwD who were adherent to basal-bolus insulin gained numerically more weight compared with PwD non-adherent with this regimen.42 A retrospective cohort study that included people with T2D initiating different OAD regimens found that absolute weight loss at 1 year was greater among individuals who were adherent to OAD monotherapy versus those who were non-adherent, with the difference reaching statistical significance (−1.01 kg; p < 0.001) (Table 3).38 In people receiving OAD dual- or triple-therapy regimens, however, weight tended to increase, and to a greater extent in adherent versus non-adherent PwD, although the trend toward greater weight gain in non-adherent individuals was not statistically significant. These findings appear to be driven by the specific OADs included in the regimens, with monotherapy largely consisting of treatment with metformin, known to be associated with weight loss, and results in the dual- and triple-therapy cohorts influenced by trends for weight gain among people on SU- and TZD-based regimens.38
One of the four studies reporting absolute weight change by adherence failed to demonstrate that the direction of change followed that of the known weight effects of the drugs under investigation.41 Rather, this analysis pooled data from study participants taking six different T2D medications with varying known weight effects and still demonstrated that PwD who were adherent with their medication (according to two different methods, MARS-5 self-report and MPR) experienced significant weight loss from baseline (p < 0.001), but that non-adherent PwD did not. While weight loss was numerically greater in adherent PwD, there was no significant difference in changes from baseline between adherent and non-adherent PwD. Using a structural equation model (SEM), it was reported that PwD who were adherent according to MARS-5 self-report (score=25) were 70% more likely to experience a ≥3% body weight loss compared with non-adherent PwD (OR 1.70, 95% CI 1.11, 2.61; p = 0.016) (Table 3). However, no significant association was found with adherence assessed by MPR (OR 1.59, 95% CI 0.66, 3.83; p = 0.305).41
Categorical Weight Loss According to Level of Medication Adherence
Two studies reported on the relationship between adherence and categorical weight loss, specifically ≥3% body weight loss from baseline (Table 3).40,45 The first of these demonstrated that significantly more people with T2D who were considered adherent (PDC ≥0.80) with GLP-1 RAs over 18 months achieved an early weight-loss response, losing >3% body weight within 3–6 months of treatment initiation (p < 0.001), compared to those without early weight loss.45 Using multivariable logistic regression, it was also reported that early weight-loss responders were nearly 20% more likely to be adherent with GLP-1 RAs over 18 months compared with individuals with no early weight-loss response (OR 1.18, 95% CI 1.02, 1.36).45 The second study included cohorts of people with T2D treated with six different medications (Table 3).40 Regardless of treatment, a higher proportion of adherent PwD (MARS-5=25) achieved ≥3% body weight loss from baseline to 6 months versus PwD considered non-adherent to treatment. Assessment of the association between index date adherence and weight change by SEM (adjusted for drug and baseline characteristics) demonstrated a trend for adherent subjects to be more likely to lose weight compared with non-adherent subjects (OR 1.56, 95% CI 0.97, 2.49), but this trend did not reach statistical significance (p = 0.06).40
Medication Adherence According to Categorical Weight Change
Figure 2 shows the main findings from the prospective survey-based study by Grandy et al (2013),39 in which adherence was reported according to categorical weight change associated with T2D medications grouped by known weight effect (gain or loss). MMAS scores were lower (better adherence) in study participants receiving TZDs, insulin, or SU and who experienced >1%, ≥3%, or ≥5% body weight loss from baseline to 12 months compared with those receiving these medications and who experienced a >1% weight gain. Differences in MMAS scores were statistically significant (p < 0.05) in all three weight-loss categories compared with the weight-gain category for the whole study population and for those receiving drugs with known weight-gain properties. Similarly, study participants who lost weight and received a GLP-1 RA or metformin had lower MMAS scores compared with individuals who gained weight while receiving these medications, but the difference failed to reach statistical significance.39
Studies Associated with Discontinuation
Overview of Discontinuation Studies
The three studies reporting on discontinuation and weight were conducted in the USA (n = 2) and Israel (n = 1), and all were retrospective cohort studies using EMR data.43–45 Discontinuation was measured by a prolonged gap in therapy, the duration of which varied by study (Table 2). One study included PwD mostly treated with metformin monotherapy43 and two included individuals receiving GLP-1 RAs.44,45 Baseline demographics from discontinuation studies are outlined in Supplementary Table S4.
Discontinuation Study Results
Absolute weight and BMI change were reported according to discontinuation in one study44 and discontinuation rates were reported according to categorical weight change in two studies43,45 (Table 4).
Table 4.
Overview of Main Study Findings with Respect to Weight and Discontinuation
| Author/Year | Study Drug(s) | Weight Measure | Discontinuation | Absolute Weight Change | Weight Loss Associated with Lower Discontinuation | Weight Neutrality or Gain Associated with Lower Discontinuation | |
| Continuers | Discontinuers | ||||||
| Studies reporting absolute weight change and discontinuation | |||||||
| Melzer-Cohen et al, 201944 | Liraglutide | Baseline to 24 months | Gap of ≥120 days between dispensing before 12 months | Weight, −3.57 kg† BMI, −1.29 kg/m2† |
Weight, −1.25 kg BMI, −0.45 kg/m2 |
✔ | |
| Study author observations: “Patients with type 2 diabetes who persist with liraglutide treatment are characterized by … greater reductions in body weight … compared with patients who discontinue liraglutide therapy” | |||||||
| Author/Year | Study Drug(s) | Weight Measure | Discontinuation | Discontinuation rates, % | Weight Loss Associated with Lower Discontinuation | Weight Neutrality or Gain Associated with Lower Discontinuation | |
| Weight Loss | Weight Neutrality or Gain | ||||||
| Studies reporting discontinuation by categorical weight change | |||||||
| Bell et al, 201443 | NIAD (90.6% metformin monotherapy) | >3% body weight loss, >3% gain, or ≤3% loss of gain (neutral) at 6 months | >30-day lapse without index drug or switch | 43%*, a | 50% (neutral)a 53% (gain)a |
✔ | |
| Study author observations: “Overall, the results suggest that modest weight loss is associated with … lower rates of treatment discontinuation compared with no weight change” | |||||||
| Durden et al, 201945 | GLP-1 RA | >3% body weight loss at 3–6 months (early response) | Index therapy lasting <18 months | 61.9%** | 67.5% | ✔ | |
| Study author observations: “Early response was associated with a … lower likelihood of discontinuation over a period of up to 18 months compared with those patients who did not achieve changes of … >3% reduction in body weight within 3–6 months” | |||||||
Notes: *p < 0.001 vs weight neutral; **p < 0.001 early weight-loss response (3–6 months) vs no early response; †p < 0.001 vs discontinuers. aPercentages from Figure 4 in Bell et al (2014).43
Abbreviations: BMI, body mass index; GLP-1 RA, glucagon-like peptide-1 receptor agonist; NIAD, non-insulin antidiabetes drug.
Absolute Weight Change According to Discontinuation
In a retrospective cohort study conducted in Israel, people with T2D initiating liraglutide were categorized into two propensity score-matched cohorts depending on whether they completed ≥12 months of therapy (continuers) or stopped therapy within 12 months (discontinuers; 50.8% stopped after ≤3 months and 11.2% completed 9–12 months).44 A significantly greater reduction in both weight and BMI was observed from baseline to 24 months in the matched cohort of continuers compared with discontinuers (p < 0.001) (Table 4).
Discontinuation Rates According to Categorical Weight Change
Bell et al (2014)43 categorized participants into three groups based on change in body weight from baseline to 6 months: weight loss (>3% loss); weight gain (>3% gain); and weight neutral (≤3% loss or gain). Most PwD (90.6%) were receiving metformin monotherapy for the duration of the study. After controlling for baseline characteristics, PwD in the weight-loss cohort had lower rates of discontinuation over 18 months compared with the weight-neutral cohort (Table 4). Time-to-event analysis demonstrated that individuals who lost >3% weight in the first 6 months of therapy were 21% less likely to discontinue treatment compared with those in the weight-neutral cohort (hazard ratio [HR] 0.785; 95% CI 0.688, 0.896; p < 0.001), while PwD who experienced weight gain were equally likely to discontinue therapy compared with the weight-neutral cohort (HR 1.061; 95% CI 0.845, 1.334; p = 0.609).43 A second study that evaluated PwD initiating a GLP-1 RA reported that significantly lower proportions of individuals with early weight-loss (>3% loss from baseline to 3–6 months) discontinued therapy over 18 months compared with those who did not have an early weight loss (p < 0.001) (Table 4).45 In addition, logistic regression demonstrated that the likelihood of discontinuation over 18 months was significantly lower among early weight responders versus those with no early response (OR 0.81; 95% CI 0.70, 0.94).45
Discussion
The highly complex relationship between weight change and medication-taking behaviors embraces many different elements, including attributes of the medication itself; PwD demographics and disease characteristics; and the attitudes, concerns, and experiences of PwD.46 Overall, the evidence base suggests that weight concerns or actual weight change associated with T2D medication can impact medication-taking behavior at different stages of treatment: initiation, implementation/on-treatment, and discontinuation. Evidence from the broader literature regarding PwD attitudes and concerns at initiation of therapy and from the quantitative studies identified by the current literature review on discontinuation appear to present a clear story with respect to the impact of weight change.
Timely initiation and intensification of T2D treatment is crucial to maintain glycemic control,14,15 yet therapeutic inertia is a common phenomenon and its negative impact on outcomes is well established.25,47 Concerns around weight change appear to be a commonly cited barrier to the initiation of treatment, particularly insulin.26,27 For example, a multinational study found that 53% of people with T2D considering insulin initiation had concerns about weight gain in diabetes in general, and with OADs and insulin specifically.36 Furthermore, in this study weight change was rated as 6.1 on a scale of the most important factors influencing the decision to start insulin (1 = not at all important; 10 = extremely important).36 Similarly, a more recent US survey reported that in 52.6% of individuals initiating basal insulin, potential weight gain was a major worry prior to commencement of therapy.32 Such data suggest that medications with a no weight gain or potential weight loss profile could be preferred by people with T2D.
Weight and Discontinuation
The current review identified only three studies that quantified the relationship between weight change and discontinuation,43–45 but all demonstrated that people with T2D who lose weight while on therapies including metformin and GLP-1 RAs are less likely to discontinue treatment. These findings appear to suggest that successful weight control or weight loss could be a motivating factor for the continuation of therapy. Durden et al (2019)45 demonstrated that people with T2D who experienced weight reduction soon after initiation of GLP-1 RAs were more likely to be persistent with medication compared with those who did not achieve an early weight-loss. It is likely that PwD are discouraged if they do not rapidly achieve the anticipated results, and so will seek alternatives. Indeed, the findings from another literature review based on meta-ethnography of qualitative studies found that the key motivations for PwD in accepting their medication are instant success and benefits of both weight loss and glycemic control.48 The quantitative findings from the discontinuation studies reported herein are also consistent with the qualitative evidence from various surveys where weight gain was reported to be a common reason for treatment discontinuation among people with T2D.49–51 For example, in a multinational online survey evaluating persistence with basal insulin, it was reported that weight gain was one of the most common reasons for interruption or discontinuation of therapy in 44.2% and 37.6% of survey respondents, respectively.50 Another survey in an online patient community found that 18% of respondents in the USA and UK who had discontinued at least one medication in the previous 6 months (including OADs, insulin, and GLP-1 RAs) did so because of weight gain.49 Furthermore, in a European cross-sectional survey, 25% of individuals who had discontinued GLP-1 RAs in the previous 6 months indicated that they did so because treatment “did not help me lose weight”.51 It should be noted, however, that there is also evidence for treatment discontinuation due to weight loss.52 In a US retrospective cohort study, weight loss as reported in EMR was the reason for HCP-countenanced discontinuation of insulin in 18.5% of study participants with T2D, implying that better disease management results in insulin no longer being required.52
Weight and On-treatment Adherence
The current review also identified several studies that attempted to quantify the link between weight change and medication adherence following initiation of various therapies. Findings from these investigations indicate that the relationship between weight and adherence might not be as straightforward during this part of the treatment journey as at initiation or discontinuation. The direction of the adherence–weight relationship was generally demonstrated to depend on the known weight-loss or weight-gain properties of the drug under study. For example, greater adherence with GLP-1 RAs and metformin was associated with more weight loss in some analyses,37,38,40,42,45 while greater adherence with insulin, SU, and TZD was associated with more weight gain.37,38,42
Assuming that weight loss is a desirable outcome, as it may be for many PwD, it is perhaps intuitive that individuals who experience a greater on-therapy weight reduction would be more likely to comply with treatment and continue to take medication as recommended. Indeed, there is plenty of evidence to suggest that weight loss or the avoidance of weight gain is valued by PwD. Discrete choice experiments have revealed that avoiding weight gain is an important medication attribute,53–55 with PwD in one study indicating that avoiding weight gain was even more important to them than achieving moderate blood glucose control.54 Using a standard-gamble approach, it has been previously demonstrated that weight reduction of 3% or 5% was associated with increased health state utilities while utilities decreased with equivalent weight gains in people with T2D.56 In this study, weight change appeared to have a stronger effect on utility among individuals with obesity. Weight gain was also cited as one of the main reasons for insulin omission/non-adherence in a multinational telephone survey among individuals with T1D or T2D.57 What is perhaps more counterintuitive is that a PwD would remain adherent with a medication that increases weight when weight loss is considered a key goal of overall diabetes management.14,15 The included studies do not provide any insight regarding this observation, but it could be hypothesized that some PwD are not bothered by weight gain, do not perceive it as important, or are even totally unaware that it is occurring, so adherence is not impacted. Alternatively, a PwD could be concerned about weight gain but willing to accept it either as an anticipated side effect, because they value the drug benefits on glycemic control and other outcomes as more important, or because they are unaware of any therapeutic alternatives.58–60 Assuming that PwD know the particular weight properties of a medication, it could even be that there is unmeasured selection bias in these studies toward individuals who are inherently less concerned about weight gain, or they would not have agreed to initiate the treatment in the first place.
It is noteworthy that in some of the adherence studies reviewed, when weight or adherence data were pooled across several different drug classes with mixed weight effects, the results also indicated that more adherent PwD experienced greater weight loss.39–41 Possibly, in these studies, it could be that the weight-loss effects of drugs known to be associated with weight reduction were simply greater than the weight-gain effect of those drugs known to increase weight (certainly, in studies where data were disaggregated, the extent of weight loss appeared to be greater than weight gain). It was, however, also reported by Grandy et al (2013)39 and McAdam-Marx et al (2014)40 that greater medication adherence with individual drugs with a known weight-gain profile such as SU, TZD, and insulin was still associated with weight loss. Furthermore, results from Grandy et al (2013)39 appear to indicate that adherence is generally better with drugs that have a known weight-gain profile. The reasons for all these observations are unclear and are not discussed or interpreted by study authors. However, it could be that PwD who exhibit good medication adherence are intrinsically more inclined to adopt other positive behaviors with respect to diabetes management, including making dietary and lifestyle changes to avoid medication-related weight gain and promote weight loss. Although beyond the scope of the current literature review, there is qualitative evidence from the broader literature that when PwD begin to perceive the seriousness of their diabetes, such as when they initiate a new therapy, particularly insulin, this may motivate them to place greater importance on lifestyle measures that could ultimately result in weight loss regardless of medication type.48,60–63 Patient motivation might also have an important role in the adherence–weight relationship: knowing that a drug has the potential to cause weight gain could encourage PwD to make efforts to counteract the anticipated effect. Finally, it is also possible that individuals initiating a T2D medication with known weight-gain potential receive additional help with weight management from their clinical team.
Limitations Within the Evidence Base
This review raises almost as many questions as it answers regarding the relationship between adherence and weight. Unfortunately, the designs of the included studies do not allow us to unpick the data or untangle the relationship. Interpretation of the data is challenging on account of several issues including unmeasured confounding, differences in the measurement of adherence and duration of assessment, and amount of weight change.
Unmeasured confounders could include the influence of other lifestyle interventions that are not captured in the studies; it is also unknown whether participants were receiving additional support and counseling for weight management. In addition, individual patient characteristics (eg, baseline weight or BMI), expectations, and previous experience could impact the results. For example, there is evidence that women may value weight loss more highly than men and that previous treatment experience can influence subsequent behaviors, with PwD who experienced weight gain when starting their current medication indicating that they would be more likely to be non-adherent with an alternative.53,64 Obesity research also indicates that pre-treatment weight-loss expectations can have an impact on treatment outcomes,65,66 and this might also apply in people with T2D and overweight and obesity. Furthermore, adherence itself is impacted by multiple other factors, including hypoglycemia, patient–provider relationships, ability to pay, and regimen complexity.37,40,45,46
Methods for the measurement of adherence were also highly variable across the included studies, which further complicates interpretation and comparison of the various findings. For example, some studies used self-reported adherence measures (MMAS or MARS-5) that could be subject to recall bias and the consequent over- or under-estimation of adherence behaviors.39–41 Indeed, in one of these analyses, weight loss was reported to be associated with adherence using the MARS-5 self-report but not when a claims-based method (modified MPR) was used.41 Most of the included studies had a relatively short follow-up period after drug initiation and did not measure adherence at different time points. The relationship between adherence and weight change is inevitably dynamic and changes over time; yet, the data do not allow evaluation of how adherence impacts weight, or vice versa, over the longer term or at different stages of treatment. For example, in one study, PwD were not followed up from drug initiation; instead, weight change was reported from year to year in an ongoing survey, rendering it even more challenging to understand the relationship.39
Among the studies, the most common threshold for weight loss or gain was 3%.40,41,43,45 However, it is generally accepted that weight loss of ≥5% is considered clinically meaningful (although this is subject to debate).67 It may be that a 3% loss or gain is simply not a large enough change to robustly impact adherence, or that an individual is unaware of small changes. Indeed, Grandy et al (2013)39 demonstrated a “dose response” with respect to adherence and weight loss, with individuals with >1%, ≥3%, and ≥5% weight loss having MMAS scores that were 17.8%, 22.8%, and 29.4% better, respectively, compared with the weight-gain group.
It should be noted that the majority of the studies (seven out of nine) identified in this review were undertaken in the USA and this may limit the generalizability of the findings to other countries.
Another major challenge associated with the evidence base is that it is impossible to demonstrate the directionality of the weight–adherence relationship: does better adherence drive weight loss, or does weight loss promote better adherence? Key to better understanding the impact of weight change on drug-taking behaviors is the development of studies to explore the hypothesis that PwD are less adherent to medications that mediate weight gain and more adherent to those that promote weight loss. Demonstration of causality would require that PwD reduce the dose of their treatment (assuming adherence is related to the amount of drug taken) after experiencing weight gain, but that there was no change in dosing following weight loss. Study participants would need to accurately record their reasons for changing drug-taking behaviors as they relate to changes in their weight at all stages of the treatment journey. These various stages of decision-making and behavior are not captured within any of the studies identified by the current search.
Limitations of the Literature Search
The current literature search is subject to some limitations. While the search was conducted using a robust and reproducible protocol, the approach was largely pragmatic, and so other relevant studies might have been published. In addition, a two-stage approach was adopted for the review of search results, with the decision to include or exclude a publication made based on review of the title/abstract and not on a comprehensive review of the full text of the article. It cannot, therefore, be ruled out that potentially relevant studies were excluded at this stage due to lack of detail in the title or abstract. Certain restrictions were applied to the search syntax to focus the search, but some publications relevant to the research questions could have been overlooked. For example, the syntax was designed to retrieve records that explicitly referred to either weight or BMI but did not include additional terms that might indicate a weight-change context (eg, obesity, obese, or overweight). With respect to adherence concepts, the syntax was designed to retrieve records that explicitly referred to adherence, compliance, persistence, or discontinuation. Variant descriptions for discontinuation (eg, “stopped taking” or “did not continue taking”) or adherence were not captured. Since the review explicitly searched for studies evaluating the link between adherence and weight change, it is possible that studies with a broader focus than just adherence or weight were missed.
Conclusions
This review identified studies that quantified the relationship between weight change and adherence during drug use and at discontinuation. Although only a limited number of studies on discontinuation were identified, it appeared that weight loss was associated with lower discontinuation. The relationship between on-treatment adherence and weight change was more complex. For the most part, the direction of the relationship depended on the known weight properties of the medication under evaluation, with greater adherence generally reported to enhance the weight-loss or weight-gain effects of T2D therapies compared with non-adherence. There were, however, studies that found better adherence was associated with weight loss regardless of the medication under study.
The findings from this review add to the broader literature that includes qualitative studies and surveys showing that weight change associated with different medications is an important concern for people with T2D at all stages of their treatment journey – from drug initiation, during drug use, and at discontinuation. Taken together, the evidence base indicates that if adherence either enhances or undermines the weight-loss efforts of PwD depending on medication class, then they would benefit most from therapies that have intrinsic weight-loss properties in addition to ongoing education and support to minimize or avoid weight gain.
Robustly designed studies that can objectively track an individual’s weight and medication-taking behavior at key points in the treatment journey, and document the reasons for any changes in use, are needed to provide valuable new insights regarding the complicated relationship between adherence and weight in T2D.
Acknowledgments
The authors thank Mick Arber (York Health Economic Consortium [YHEC]) for assistance with the literature search, and Alison Terry for assistance with editing the manuscript.
Funding Statement
This study (including development of study design, conduct of the research, and medical writing services) was funded by Eli Lilly and Company (Indianapolis, IN, USA).
Data Sharing Statement
Data sharing is not applicable to this article as, since this is a review, no datasets were generated or analyzed.
Ethics Approval and Informed Consent
This article is a review and analysis of previously published studies and does not include any new studies on human or animal subjects performed by any of the authors.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Kristina S Boye, Shraddha Shinde, and Vivian T Thieu are employees and minor shareholders of Eli Lilly and Company. Tessa Kennedy-Martin and Susan Robinson are employees of KMHO, who received funding from Eli Lilly for time spent conducting this research.
References
- 1.Leitner DR, Frühbeck G, Yumuk V, et al. Obesity and type 2 diabetes: two disease with a need for combined treatment strategies – EASO can lead the way. Obes Facts. 2017;10(5):483–492. doi: 10.1159/000480525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatineau M, Hancock C, Holman N, et al. Public Health England. Adult obesity and type 2 diabetes; 2014. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/338934/Adult_obesity_and_type_2_diabetes_.pdf. Accessed March 18, 2021.
- 3.Centers for Disease Control and Prevention. National diabetes statistics report; 2020. Available from: https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html. Accessed November 15, 2021. 18, 2021. [Google Scholar]
- 4.Salinero-Fort MA, San Andrés-Rebollo FJ, Gómez-Campelo P, et al. Body mass index and all-cause mortality among type 2 diabetes mellitus patients: findings from the 5-year follow-up of the MADIABETES cohort. Eur J Intern Med. 2017;43:46–52. doi: 10.1016/j.ejim.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 5.Bae JP, Lage MJ, Mo D, et al. Obesity and glycemic control in patients with diabetes mellitus: analysis of physician electronic health records in the US from 2009–2011. J Diabetes Complications. 2016;30(12):212–220. doi: 10.1016/j.jdiacomp.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 6.Boye KS, Lage MJ, Thieu V, et al. Obesity and glycemic control among people with type 2 diabetes in the United States: a retrospective cohort study using insurance claims data. J Diabetes Complications. 2021;35(9):107975. doi: 10.1016/j.jdiacomp.2021.107975 [DOI] [PubMed] [Google Scholar]
- 7.Weng W, Tian Y, Kimball ES, et al. Treatment patterns and clinical characteristics of patients with type 2 diabetes mellitus according to body mass index: findings from an electronic medical records database. BMJ Open Diabetes Res Care. 2017;5(1):e000382. doi: 10.1136/bmjdrc-2016-000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray N, Picone G, Sloan F, et al. The relationship between BMI and onset of diabetes mellitus and its complications. South Med J. 2015;108(1):29–36. doi: 10.14423/SMJ.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boye KS, Lage MJ, Terrell K. Healthcare outcomes for patients with type 2 diabetes with and without comorbid obesity. J Diabetes Complications. 2020;34(12):107730. doi: 10.1016/j.jdiacomp.2020.107730 [DOI] [PubMed] [Google Scholar]
- 10.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston SS, Ammann EM, Kashyap SR, et al. Body mass index and insulin as identifiers of high-cost patients with type 2 diabetes: a retrospective analysis of electronic health records linked to insurance claims data. Diabetes Obes Metab. 2019;21(6):1419–1428. doi: 10.1111/dom.13671 [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Blume SW, Huang JC, Hammer M, Graf TR. The economic burden of obesity by glycemic stage in the United States. PharmacoEconomics. 2015;33(7):735–748. doi: 10.1007/s40273-014-0248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyers JL, Parasuraman S, Bell KF, et al. The high-cost, type 2 diabetes mellitus patient: an analysis of managed care administrative data. Arch Public Health. 2014;72(1):6. doi: 10.1186/2049-3258-72-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association (ADA). Obesity management for the treatment of type 2 diabetes. Standards of Medical Care in Diabetes – 2021. Diabetes Care. 2021;44(suppl1):S100–110. doi: 10.2337/dc21-S008 [DOI] [PubMed] [Google Scholar]
- 15.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurren KM, Dunham MW. Understanding the impact of commonly utilized, non-insulin, glucose-lowering drugs on body weight in patients with type 2 diabetes. Expert Opin Pharmacother. 2018;19(10):1087–1095. doi: 10.1080/14656566.2018.1494727 [DOI] [PubMed] [Google Scholar]
- 17.Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36(1):44–58. doi: 10.1007/s12325-018-0824-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGovern A, Tippu Z, Hinton W, et al. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20(4):1040–1043. doi: 10.1111/dom.13160 [DOI] [PubMed] [Google Scholar]
- 19.Weiss T, Carr RD, Pal S, et al. Real-world adherence and discontinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer Adherence. 2020;14:2337–2345. doi: 10.2147/PPA.S277676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giorgino F, Penfornis A, Pechtner V, et al. Adherence to antihyperglycemic medications and glucagon-like peptide-1-receptor agonists in type 2 diabetes: clinical consequences and strategies for improvement. Patient Prefer Adherence. 2018;12:707–719. doi: 10.2147/PPA.S151736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy-Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. 2017;11:1103–1117. doi: 10.2147/PPA.S136639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dilla T, Costi M, Boye KS, et al. [The impact of obesity in the management and evolution of diabetes mellitus]. Rev Clin Esp. 2008;208(9):437–443. doi: 10.1157/13127604 [DOI] [PubMed] [Google Scholar]
- 23.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299–1307. doi: 10.2147/PPA.S106821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcome them. Diabetes Obes Metab. 2018;20(3):488–496. doi: 10.1111/dom.13132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng CJ, Lai PS, Lee YK, et al. Barriers and facilitators to starting insulin in patients with type 2 diabetes: a systematic review. Int J Clin Pract. 2015;69(10):1050–1070. doi: 10.1111/ijcp.12691 [DOI] [PubMed] [Google Scholar]
- 27.Berard L, Antonishyn N, Arcudi K, et al. Insulin matters: a practical approach to basal insulin management in type 2 diabetes. Diabetes Ther. 2018;9(2):501–519. doi: 10.1007/s13300-018-0375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan H, Lasker SS, Chowdhury TA. Prevalence and reasons for insulin refusal in Bangladeshi patients with poorly controlled Type 2 diabetes in East London. Diabet Med. 2008;25(9):1108–1111. doi: 10.1111/j.1464-5491.2008.02538.x [DOI] [PubMed] [Google Scholar]
- 29.Lakkis NA, Maalouf GJ, Mahmassani DM, Hamadeh GN. Insulin therapy attitudes and beliefs of physicians in Middle Eastern Arab countries. Fam Pract. 2013;30(5):560–567. doi: 10.1093/fampra/cmt022 [DOI] [PubMed] [Google Scholar]
- 30.Nakar S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in Type 2 diabetes: family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complications. 2007;21(4):220–226. doi: 10.1016/j.jdiacomp.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 31.Campbell MD, Babic D, Bolcina U, et al. High level of clinical inertia in insulin initiation in type 2 diabetes across Central and South-Eastern Europe: insights from SITIP study. Acta Diabetol. 2019;56:1045–1049. doi: 10.1007/s00592-019-01346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalirai S, Ivanova JI, Perez-Nieves M, et al. Basal insulin initiation and maintenance in adults with type 2 diabetes mellitus in the United States. Diabetes Metab Syndr Obesity. 2020;13:1023–1033. doi: 10.2147/DMSO.S237948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peyrot M, Skovlund SE, Landgraf R. Epidemiology and correlates of weight worry in the multinational Diabetes Attitudes, Wishes and Needs study. Curr Med Res Opin. 2009;25(8):1985–1993. doi: 10.1185/03007990903073654 [DOI] [PubMed] [Google Scholar]
- 34.Brod M, Lessard Alolga S, Meneghini L. Barriers to initiating insulin in type 2 diabetes patients: development of a new patient education tool to address myths, misconceptions and clinical realities. Patient. 2014;7(4):437–450. doi: 10.1007/s40271-014-0068-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee YK, Lee PY, Ng CJ. A qualitative study on healthcare professionals’ perceived barriers to insulin initiation in a multi-ethnic population. BMC Fam Pract. 2012;13:28. doi: 10.1186/1471-2296-13-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan AM, Muthusamy L, Ng CC, Phoon KY, Ow JH, Tan NC. Initiation of insulin for type 2 diabetes mellitus patients: what are the issues? A qualitative study. Singapore Med J. 2011;52(11):801–809. [PubMed] [Google Scholar]
- 37.Carls GS, Tan R, Zhu JY, et al. Real-world weight change among patients treated with glucagon-like peptide-1 receptor agonist, dipeptidyl peptidase-4 inhibitor and sulfonylureas for type 2 diabetes and the influence of medication adherence. Obes Sci Pract. 2017;3(3):342–351. doi: 10.1002/osp4.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon J, McEwan P, Idris I, et al. Treatment choice, medication adherence and glycemic efficacy in people with type 2 diabetes: a UK clinical practice database study. BMJ Open Diabetes Res Care. 2018;6(1):e000512. doi: 10.1136/bmjdrc-2018-000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandy S, Fox KM, Hardy E. Association of weight loss and medication adherence among adults with type 2 diabetes mellitus: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes). Curr Ther Res Clin Exp. 2013;75:77–82. doi: 10.1016/j.curtheres.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAdam-Marx C, Bellows BK, Unni S, et al. Determinants of glycaemic control in a practice setting: the role of weight loss and treatment adherence (the DELTA Study). Int J Clin Pract. 2014;68(11):1309–1317. doi: 10.1111/ijcp.12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAdam-Marx C, Bellows BK, Unni S, et al. Impact of adherence and weight loss on glycemic control in patients with type 2 diabetes: cohort analyses of integrated medical record, pharmacy claims, and patient-reported data. J Manag Care Spec Pharm. 2014;20(7):691–700. doi: 10.18553/jmcp.2014.20.7.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel S, Abreu M, Tumyan A, et al. Effect of medication adherence on clinical outcomes in type 2 diabetes: analysis of the SIMPLE study. BMJ Open Diabetes Res Care. 2019;7(1):e000761. doi: 10.1136/bmjdrc-2019-000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell K, Parasuraman S, Shah M, et al. Economic implications of weight change in patients with type 2 diabetes mellitus. Am J Manag Care. 2014;20(8):e320–329. [PubMed] [Google Scholar]
- 44.Melzer-Cohen C, Chodick G, Husemoen LLN, et al. A retrospective database study of liraglutide persistence associated with glycemic and body weight control in patients with type 2 diabetes. Diabetes Ther. 2019;10(2):683–696. doi: 10.1007/s13300-019-0583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durden E, Liang M, Fowler R, Panton UH, Mocevic E. The effect of early response to GLP-1 RA therapy on long-term adherence and persistence among type 2 diabetes patients in the United States. J Manag Care Spec Pharm. 2019;25(6):669–680. doi: 10.18553/jmcp.2019.18429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fridman M, Lucas ME, Paprocki Y, et al. Impact of weight change in adults with type 2 diabetes mellitus: a literature review and critical analysis. Clinicoecon Outcomes Res. 2020;12:555–566. doi: 10.2147/CEOR.S266873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12. doi: 10.1016/j.pcd.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 48.Psarou A, Cooper H, Wilding JPH. Patients’ perspectives of oral and injectable type 2 diabetes medicines, their body weight and medicine-taking behavior in the UK: a systematic review and meta-ethnography. Diabetes Ther. 2018;9(5):1791–1810. doi: 10.1007/s13300-018-0490-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Climens AR, Pain E, Boss A, Shaunik A. Understanding reasons for treatment discontinuation, attitudes and education needs among people who discontinue type 2 diabetes treatment: results from an online patient survey in the USA and UK. Diabetes Ther. 2020;11(8):1873–1881. doi: 10.1007/s13300-020-00843-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peyrot M, Perez-Nieves M, Ivanova J, et al. Correlates of basal insulin persistence among insulin-naïve people with type 2 diabetes: results from a multinational survey. Curr Med Res Opin. 2017;33(10):1843–1851. doi: 10.1080/03007995.2017.1341868 [DOI] [PubMed] [Google Scholar]
- 51.Sikirica MV, Martin AA, Wood R, et al. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403–412. doi: 10.2147/DMSO.S141235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Morrison F, Zhao Z, et al. Reasons for discontinuing insulin and factors associated with insulin discontinuation in patients with type 2 diabetes mellitus: a real-world evidence study. Clin Diabetes Endocrinol. 2021;7(1):1. doi: 10.1186/s40842-020-00115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauber AB, Mohamed AF, Johnson FR, et al. Treatment preferences and medication adherence of people with Type 2 diabetes using oral glucose-lowering agents. Diabet Med. 2009;26(4):416–424. doi: 10.1111/j.1464-5491.2009.02696.x [DOI] [PubMed] [Google Scholar]
- 54.Mohamed AF, Zhang J, Johnson FR, et al. Avoidance of weight gain is important for oral type 2 diabetes treatments in Sweden and Germany: patient preferences. Diabetes Metab. 2013;39(5):397–403. doi: 10.1016/j.diabet.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 55.Morillas C, Feliciano R, Catalina PF, et al. Patients’ and physicians’ preferences for type 2 diabetes mellitus treatments in Spain and Portugal: a discrete choice experiment. Patient Prefer Adherence. 2015;9:1443–1458. doi: 10.2147/PPA.S88022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matza LS, Yurgin N, Boye KS, et al. Obese versus non-obese patients with type 2 diabetes: patient-reported outcomes and utility of weight change. Curr Med Res Opin. 2007;23(9):2051–2062. doi: 10.1185/030079907X219454 [DOI] [PubMed] [Google Scholar]
- 57.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger P-M. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetic Med. 2012;29(5):682–689. doi: 10.1111/j.1464-5491.2012.03605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alatawi YM, Kavookjian J, Ekong G, Alrayees MM. The association between health beliefs and medication adherence among patients with type 2 diabetes. Res Social Adm Pharm. 2016;12(6):914–925. doi: 10.1016/j.sapharm.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 59.Farmer AJ, Rodgers LR, Lonergan M, et al. Adherence to oral glucose-lowering therapies and associations with 1-year HbA1c: a retrospective cohort analysis in a large primary care database. Diabetes Care. 2016;39(2):258–263. doi: 10.2337/dc15-1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu Hassan H, Tohid H, Mohd Amin R, Long Bidin MB, Muthupalaniappen L, Omar K. Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: a qualitative exploration. BMC Fam Pract. 2013;14:164. doi: 10.1186/1471-2296-14-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parry O, Peel E, Douglas M, Lawton J. Issues of cause and control in patient accounts of Type 2 diabetes. Health Educ Res. 2006;21(1):97–107. doi: 10.1093/her/cyh044 [DOI] [PubMed] [Google Scholar]
- 62.Phillips A. Experiences of patients with type 2 diabetes starting therapy. Nurse Stand. 2007;21(30):35–41. doi: 10.7748/ns.21.23.35.s54 [DOI] [PubMed] [Google Scholar]
- 63.Noakes H. Perceptions of black African and African-Caribbean people regarding insulin. J Diabetes Nurs. 2010;14:148–156. [Google Scholar]
- 64.Ross SA, Dzida G, Vora J, et al. Impact of weight gain on outcomes in type 2 diabetes. Curr Med Res Opin. 2011;27(7):1431–1438. doi: 10.1185/03007995.2011.585396 [DOI] [PubMed] [Google Scholar]
- 65.Crawford R, Glover L. The impact of pre-treatment weight-loss expectations on weight loss, weight regain, and attrition in people who are overweight and obese: a systematic review of the literature. Br J Health Psychol. 2012;17(3):609–630. doi: 10.1111/j.2044-8287.2011.02059.x [DOI] [PubMed] [Google Scholar]
- 66.Calugi S, Marchesini G, El Ghoch M, Gavasso I, Dalle Grave R. The influence of weight-loss expectations on weight loss and of weight-loss satisfaction on weight maintenance in severe obesity. J Acad Nutr Diet. 2017;117(1):32–38. doi: 10.1016/j.jand.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 67.Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity. 2015;23(12):2319–2320. doi: 10.1002/oby.21358 [DOI] [PubMed] [Google Scholar]