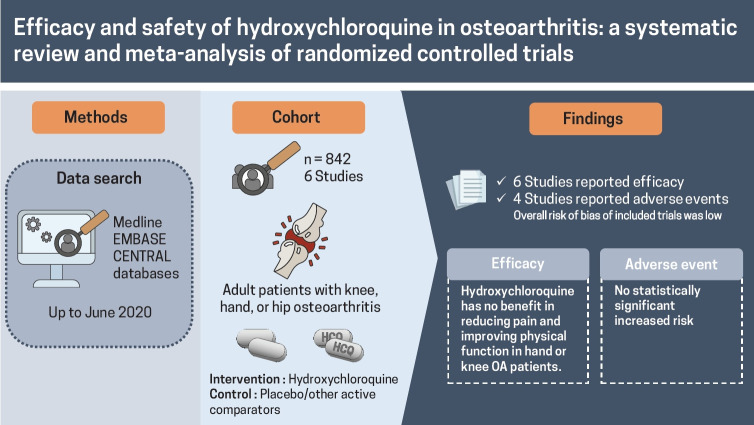
Abstract
Background/Aims
Conventional disease-modifying anti-rheumatic drugs have been trialed in osteoarthritis (OA). Hydroxychloroquine (HCQ), which has shown its effectiveness in rheumatoid arthritis, has been trialed for the treatment of OA; however, its efficacy and safety remain unclear. This systematic review and meta-analysis evaluate efficacy and safety of HCQ for the treatment of OA.
Methods
MEDLINE, EMBASE, and Cochrane Central were searched from inception through June 2020. Two reviewers independently screened for randomized controlled trials (RCTs) comparing HCQ with placebo or other active-comparators for the treatment of knee, hand, or hip OA, extracted data, and performed Cochrane risk of bias assessments.
Results
Six RCTs, four in hand OA, two in knee OA, consisting of 842 patients (436 in HCQ arm, 406 in control arm) were included. RCTs were conducted between 2012 and 2020, one each at UK, Netherlands, Germany, Italy, Iran, and Egypt; follow-up period ranged 24 to 52 weeks. High-quality evidence showed no clinically important pain reduction with HCQ compared to placebo/active-control in hand OA (standardized mean difference [SMD], 0.14; 95% confidence interval [CI], –0.20 to 0.48). Effect on pain reduction in knee and hand OA was small and non-significant (SMD, –0.09; 95% CI, –0.44 to 0.25). High-quality evidence showed no improvement in dysfunction with HCQ compared to placebo in hand OA patients (SMD, 0.08; 95% CI, –0.23 to 0.40). Effect on dysfunction improvement in knee and hand OA was modest and statistically non-significant (SMD, –0.20; 95% CI,–0.57 to 0.18). No improvement in quality of life was observed in hand OA.
Conclusions
HCQ has no benefit in reducing pain and improving physical function in hand or knee OA patients.
Keywords: Hydroxychloroquine, Osteoarthritis, Systematic review, Meta-analysis, Randomized controlled trial
Graphical abstract
INTRODUCTION
Osteoarthritis (OA) has become a silent epidemic worldwide in recent years. With the combined effect of a variety of both non-modifiable and modifiable risk factors, including ageing, genetic predisposition, gender, obesity, injury or trauma, this burdensome disease is becoming more prevalent, with the estimated number of people who are suffering from hip or knee OA to be more than 300 million worldwide [1-4]. Accordingly, OA is identified as one of the 10 most common causes of disability in older adults in developed countries, with a higher prevalence in women than men [5]. Due to the absence of a cure, it poses a substantial and increasing health burden with notable implications for individuals and the healthcare systems [6-8].
Pharmacological and non-pharmacological treatments, either individually or in combination, remains as the mainstream intervention for OA [9]. The commonly recommended first-line of treatment included exercise and patient education. Pain medications for OA include paracetamol, topical, and oral non-steroidal anti-inflammatory drugs (NSAIDs) [6,9-11]. In a recent meta-analysis, paracetamol, however, has been shown to have minimal effect compared to placebo [12] and is not recommended in the recent guidelines, including the recent Royal Australian College of General Practitioners (RACGP) and Osteoarthritis Research Society International (OARSI) guidelines for the treatment of OA [13,14]. While both topical and oral use of NSAIDs were shown to be moderately effective for pain relief compared to placebo, oral use carries an increased risk of cardiovascular toxicity [6,15] and is often contra-indicated in OA patients who usually have comorbidities. The efficacy of intra-articular corticosteroids injections remains questionable due to the short-term benefits and the overall low quality of trials [15]. Moreover, a recent randomized controlled trial (RCT) highlights that intra-articular injection reduces the cartilage volume over 2 years than placebo treatment with no difference in knee pain [16]. New therapies are therefore required for the management of OA.
Given the limited efficacy of the current pharmacological treatments and the increasing evidence of the role of inflammation in OA, several conventional disease-modifying anti-rheumatic drugs (DMARDs) have been recently trialed in the hip, knee, and hand OA. One such medication is hydroxychloroquine (HCQ), which has been shown its effectiveness in treating rheumatoid arthritis (RA) with an acceptable safety profile [10,17,18]. However, the exact mechanism of action of HCQ in RA population is poorly understood. As a form of DMARDs, HCQ is suggested to have an inhibitory action on tolllike receptor (TLR) signaling [19]. In OA cartilage lesions, the TLRs have been found to be upregulated, where it stimulates cartilage breakdown via pro-inflammatory pathways [20,21]. Considering the inflammatory response in OA, the HCQ was proposed as a promising option in the treatment of OA.
Due to the moderately acceptable safety profile of HCQ, its popularity has risen in recent years, and several studies have investigated its effect in managing knee and hand OA [22-25]. To date, however, little attention has been made to systematically evaluate its efficacy for the management of OA. While few narrative reviews shed light on the inconsistent effects of HCQ on OA [10,11], the results of these reviews, however, were not based on comprehensive sources and methodical search strategies.
Although a previous systematic review focusing on non-pharmacological, pharmacological, and surgical treatment for hand OA reported a lack of efficacy of HCQ on pain, function, grip strength, and radiographic progression in hand OA [26], it only included three RCTs published as conference abstract with unclear risk of bias. Hence, a carefully constructed systematic review and meta-analysis, with a priori protocol, is essential to assess the effect of HCQ in patients with hip, knee, and hand OA. The present systematic review and meta-analysis, therefore, aimed to evaluate the efficacy and safety of HCQ for the treatment of hip, knee, and hand OA.
METHODS
Search strategy, selection, and data extraction
A systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We followed our protocol published with a priori defined search strategy, study inclusion and exclusion criteria, outcomes, and analyses [27]. Three bibliographic databases—MEDLINE and EMBASE using the Ovid interface and the Cochrane Central Register of Controlled Trials—were searched from inception till June 2020, with English and Chinese language restriction. The search strategy included a combination of Medical Subject Heading (MeSH) terms such as (Osteoarthritis OR “Degenerative Arthritis”) AND (Hydroxychloroquine OR Plaquenil) AND (“Randomized Controlled Trials” OR “Controlled Clinical Trial” OR placebo) (Appendix 1). Hand searching of abstracts from last 2 years conference proceedings of major international associations involved in OA research such as the European League against Rheumatism (EULAR), OARSI, and American College of Rheumatology (ACR) was performed to supplement the literature search. The articles were first screened based on their title and abstracts and then fulltext for their inclusion as per prespecified inclusion criteria by two researchers independently (A.S. and A.K.). Briefly, English or Chinese language articles reporting randomized, quasi-randomized, controlled trials assessing the safety and efficacy of the HCQ in the knee, hand, or hip OA patients above 40 years of age were included. The efficacy outcomes of interest included (1) change in OA associated pain and physical dysfunction assessed using the visual analog scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index Score (WOMAC), Australian/Canadian Osteoarthritis Index (AUSCAN), numeric rating scale (NRS); (2) quality of life (QoL) assessed using EQ-5D, SF-6D, health assessment questionnaire (HAQ). Safety outcomes included adverse effects (AEs). Serious adverse effect (SAE) reported with HCQ. Other outcomes of interest included radiographic structural damage and biomarker change [27]. In the event of more than one pain measure reported in a study, we used the pain outcomes in the following order: VAS, pain subscale of WOMAC, NRS, AUSCAN, and any other reported pain measures. The relevant data, such as information on study design, population characteristics, intervention/comparator details, and change in efficacy and safety outcomes, were extracted pre-designed excel sheet. Two investigators (A.S. and Z.W.) confirmed all data entries, and any discrepancy at the screening and data extraction stages were resolved by mutual discussion or arbitration by the third reviewer (B.A.).
Quality assessment
The bias risk of the included studies was evaluated according to the Cochrane Handbook 5.0.1 RCT bias risk assessment tool [28]. The quality of the literature was assessed for items such as sequence generation, allocation concealment, blinding of participants, study personnel, outcome assessors, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Two researchers (A.S. and A.K.) evaluated the quality; any difference of opinion was resolved by discussion or arbitration by the third researcher (B.A.).
Statistical analysis
Data pertaining to mean change in continuous outcome was used to estimate the pooled effect size. Efficacy outcome data on change from baseline to follow-up was calculated as the arithmetic difference between baseline and longest reported follow-up. The corresponding reported standard deviations (SD) were used, if not reported, were calculated using reported standard error (SE) or confidence intervals (CI). The change-from-baseline SD was calculated using the methods described in the Cochrane Handbook (Chapter 6; Section 6.5.2.8) [29] and a conservative correlation coefficient value of r = 0.5 [30]. To facilitate the pooling of data, we standardized the results of the studies to a uniform scale using standardized mean difference (SMD), as the studies assessed the outcome measure in a variety of ways using different assessment tools [29]. Review Manager 5 (RevMan 5.3) and STATA version 16.0 (STATA Corp., College Station, TX, USA) were used for data analysis. Statistical heterogeneity was assessed as per Q statistics (p < 0.05 was considered heterogeneous), and I2 statistic (I2 > 50% was deemed to be heterogeneous) [31]. A meta-analysis of the included studies was performed using the generic inverse variance random-effect model.
Reproducible research statements
The study protocol is available online at https://doi.org/10.1101/2020.07.20.20157669.
RESULTS
Literature search
The literature search process is shown in the PRISMA flow diagram (Fig. 1). A thorough literature search retrieved a total of 71 articles from three databases. Overall, 39 articles were sourced from PubMed and EMBASE, 32 were from Cochrane Central, and one article was identified by hand-searching. After duplicate removal, 61 articles were screened, and six articles were included in the final analysis.
Figure 1.
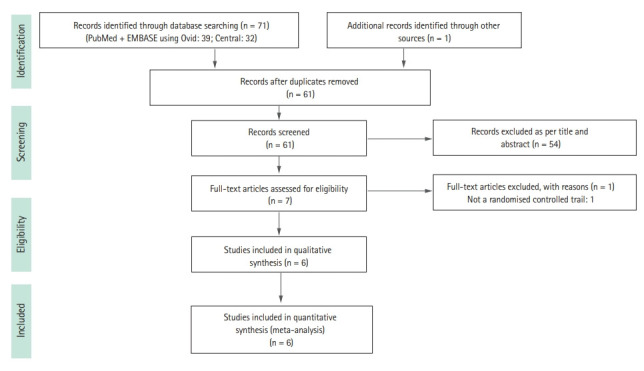
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Study characteristics
A total of 842 patients were enrolled in six included RCTs, with 436 in the experimental arm and 406 in the control arm [22-25,32,33]. Out of six studies, four were in hand OA [22,23,25,33], two were in knee OA patients [24,32] (overall: 89.6% women; mean age 55.3 years), and none of the studies assessed HCQ in hip OA. The majority of the studies compared HCQ with placebo, while one study in hand OA patients compared HCQ with an active comparator (chlodronate) [25]. The studies were conducted between 2012 and 2020, one each cross UK, Netherlands, Germany, Italy, Iran, and Egypt, and the follow-up period ranging between 24 and 52 weeks. The largest trial, including 248 hand OA patients, was conducted in the UK [23]. Table 1 describes the detailed characteristics of the included studies.
Table 1.
Characteristics of the studies included in the analysis by year of publication
| Study, country, trial name, trial registration number | Publication type | Group | Sample size, n | Women | Age, yr | BMI, kg/m2 | OA type | KL gradea/ACR | Follow-up | Key outcome measures | Details of interventions and dose | Funding | RoB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saviola et al. (2012) [25], Italy, NA, NA | Full-text | HCQ | 14 | 13 (95) | 63.5 ± 7.41 | NR | Hand OA | Radiographic criteriab/NR | 12 mo | VAS | 400 mg tablet daily for 30 days, 200 mg maintenance daily for 11 months | NR | High |
| Dreiser’s score | |||||||||||||
| Synovitis assed using UI | |||||||||||||
| CLO | 21 | 23 (95) | 60.0 ± 7.13 | Morning stiffness | 300 mg i.v. in 250 cc of physiological saline solution for 7 days, followed by 100 mg i.m. maintenance dose for 14 days/3 months | ||||||||
| Physician and Patient Global Assessment | |||||||||||||
| ESR, CRP, ALP¸ RF | |||||||||||||
| Jokar et al. (2013) [32], Iran, NA, IRCT138709121479N1 | Full-text | HCQ | 21 | 21 (100) | 48.3 ± 11.14 | 25.6 ± 3.05 | Knee OA | 2, 3/ACR | 24 wk | WOMAC | 200 mg tablets twice daily for 6 months | University | Low |
| PLB | 23 | 22 (95) | 47.6 ± 8.54 | 25.4 ± 2.79 | 2, 3/ACR | Placebo tablets of 200 mg twice daily for 6 months | |||||||
| Abou-Raya et al. (2014) [24], Egypt, NA, NA | Abstract | HCQ | 83 | NR | NR | NR | Knee OA | 3, 4/ACR | 36 wk | VAS | 400 mg daily | NR | High |
| PLB | 83 | 3, 4/ACR | WOMAC function | Placebo | |||||||||
| ADL | |||||||||||||
| Kingsbury et al. (2018) [23], UK, HERO, ISRCTN91859104/EudraCT 2011-004300-38 | Full-text | HCQ | 124 | 97 (78) | 62.8 ± 9.1 | 28.4 ± 5.4 | Hand OA | NR/ACR | 12 mo | NRS | 200, 300, or 400 mg daily with dosage calculated according to ideal body weight for a maximum of 6.5 mg/kg | Non-profit organisation | Low |
| AUSCAN pain and function | |||||||||||||
| OAQoL | |||||||||||||
| SF-12 | |||||||||||||
| Kallman score | |||||||||||||
| PLB | 124 | 106 (85) | 62.5 ± 9.2 | 29.3 ± 6.2 | Placebo | ||||||||
| Lee et al. (2018) [22], Netherlands, FABIO, NCT01148043 | Full-text | HCQ | 98 | 86 (88) | 57.7 ± 8.2 | NR | Hand OA | 1, 2, or 3/ACR | 24 wk | VAS | 400 mg daily | University | Low |
| PLB | 98 | 82 (84) | 58.3 ± 7.0 | 1, 2, or 3/ACR | AUSCAN | Placebo | |||||||
| AIMS2-SF | |||||||||||||
| Kedor et al. (2020) [33], Germany, OA-TREAT, ISRCTN46445413/EUDRA CT 2011-001689-16 | Abstract | HCQ | 75 | 68 (90.6) | 52.4 ± 8.1 | NR | Hand OA | NR/ACR | 52 wk | HAQ | 200–400 mg daily | University | Low |
| PLB | 78 | 60 (76.9) | 50.2 ± 6.6 | AUSCAN pain and function | Matching placebo | ||||||||
| Morning stiffness | |||||||||||||
| SF-36 | |||||||||||||
| ESR | |||||||||||||
| Modified Kallmann score |
Values are presented as number (%) or mean ± SD.
BMI, body mass index; OA, osteoarthritis; KL grade, Kellgren-Lawrence classification grades of radiographic OA; ACR; American College of Rheumatology; RoB, risk of bias; NA, not applicable; HCQ, hydroxychloroquine; CLO, clodronate; NR, not reported; VAS, visual analog scale; UI, ultrasound imaging; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ALP, alkaline phosphatase; RF, rheumatoid factor; i.v., intravenous; i.m., intramuscular; PLB, placebo; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index Score; ADL, activities of daily living; NRS, numeric rating scale; AUSCAN, Australian/Canadian Osteoarthritis Index; OAQoL, osteoarthritis quality of life scale; SF-12, 12-item short form survey; AIMS2-SF, Arthritis Impact Measurement Scale 2 short form; HAQ, health assessment questionnaire; SF-36, 36-item short form survey.
Criteria mention if not use KL criteria.
Radiographic criteria: sharp marginal defects, central crumbling erosions, gull-wing, or saw-tooth deformities.
Five studies reported using ACR criteria for the inclusion of the patients [22-24,32,33]. Besides, three studies, two in knee OA and one in hand OA, further employed radiographic OA (Kellgren-Lawrence grade) as the inclusion criteria of the participants [22,24,32]. Three studies, one in knee OA and two in hand OA, assessed pain using VAS [22,24,25]. Three out of four hand OA studies reported pain using the AUSCAN scale [22,23,33], while all studies (n = 2) in knee OA used WOMAC scale to assess pain and function [24,32]. Two studies, reported imaging outcomes, whereas biochemical markers were reported in one study only [23,25,33]. The daily dose of HCQ was 400 mg for knee OA patients, whereas the daily dose in hand OA patients varied from 200 to 400 mg. Four of the included studies were registered with clinical trials registry [22,23,32,33], half of the included studies were investigator-initiated [22,32,33], and 67% were academic/non-profit organization funded [22,23,32,33]. The funding was not disclosed for 33% of the studies [24,25].
Quality assessment
The overall risk of bias of included trials was low, with three trials assessed as having high quality according to the Cochrane ROB tool [22,23,33]. Three of the included studies were assessed as having a high risk for incomplete outcome data either due to loss to follow-up or not providing adequate data for missing information (Fig. 2) [25,32].
Figure 2.
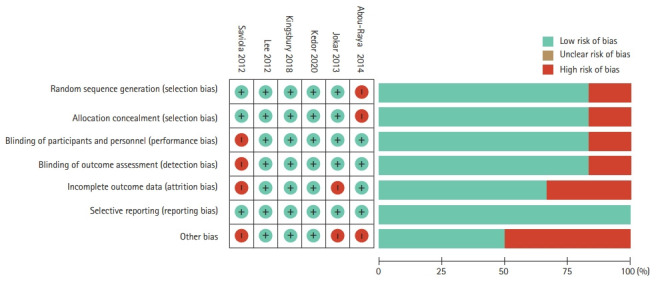
Cochrane risk of bias assessment.
Efficacy outcomes
Effect of HCQ on OA related pain
All the six included RCTs assessed pain, four in hand OA, and two in knee OA, using various patients reported outcome (PRO) instruments: VAS, NRS, AUSCAN, and WOMAC. Three RCTs evaluated pain using VAS; two in hand OA one in knee OA [22,24,25]. RCTs in knee OA patients reported pain using WOMAC and VAS each [24,32]. Three studies in hand OA assessed pain using AUSCAN [22,23,33]; whereas, one study reported NRS [23]. Overall, five trials constituting 401 participants in the HCQ groups and 406 participants in the placebo control groups contributed to the analysis of pain in hand or knee OA patients [22-24,32,33]. One trial with 14 participants in the HCQ groups and 21 in the clodronate control groups also contributed to the overall analysis of pain in hand or knee OA patients [25]. We found high-quality evidence that HCQ had no clinically important pain reduction compared to placebo/active control interventions in hand OA patients (SMD, 0.14; 95% CI, –0.20 to 0.48) [22,23,25,33]. Moderate-quality evidence from two studies showed a larger pooled effect size for pain reduction in knee OA; however, it was with a wide CI and statistically non-significant (SMD, –0.72; 95% CI, –1.57 to 0.14) [24,32]. Overall, the pooled effect on pain reduction in OA patients was small and not significant (SMD, –0.09; 95% CI, –0.44 to 0.25). An I2 statistic of 75% and 83% for studies assessing pain in hand OA and knee OA, respectively indicated a high degree of statistical heterogeneity (Fig. 3). Two studies that assessed pain using both AUSCAN and NRS [23], and AUSCAN and VAS [22] found no statistically significant improvement in pain in either of the instruments.
Figure 3.
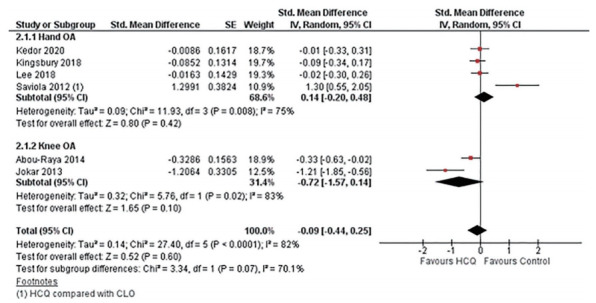
Pooled standardized mean difference (SMD) for change in osteoarthritis (OA) associated pain. Abou-Raya et al. [24] was published as an abstract in 2013 and is still not published as full-text; the author did not make the data available upon e-mail request. SE, standard error; IV, generic inverse-variance random-effect meta-analysis; CI, confidence interval; HCQ, hydroxychloroquine; CLO, clodronate.
Effect of HCQ on OA related dysfunction
Four studies assessed function limitation, two reported AUSCAN function limitation in hand OA [23,33], and two reported WOMAC function limitation in knee OA [24,32]. Overall, four trials constituting 303 participants in the HCQ groups and 308 participants in the placebo control groups contributed to the analysis of dysfunction in hand or knee OA patients [23,24,32,33]. We found high-quality evidence that HCQ had no improvement in dysfunction compared to placebo in hand OA patients (SMD, 0.08; 95% CI, –0.23 to 0.40) [23,33]. However, the moderate-quality evidence suggested a modest, although statistically significant, improvement in knee OA associated dysfunction (SMD, –0.48; 95% CI, –0.82 to –0.14) [24,32]. Overall, the pooled effect on dysfunction improvement in OA patients was modest with a wide CI and statistically non-significant (SMD, –0.20; 95% CI, –0.57 to 0.18). An I2 statistic of 58% for studies assessing dysfunction in hand OA indicated a moderate degree of statistical heterogeneity; however, I2 value of 20% demonstrated low heterogeneity between the studies assessing pain in knee OA (Fig. 4).
Figure 4.
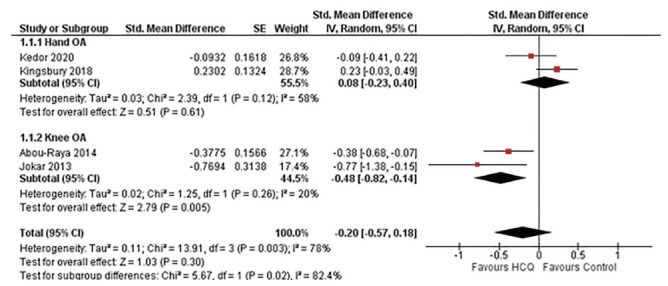
Pooled standardized mean difference (SMD) for change in osteoarthritis (OA) associated dysfunction. Abou-Raya et al. [24] was published as an abstract in 2013 and is still not published as full-text; the author did not make the data available upon e-mail request. SE, standard error; IV, generic inverse-variance random-effect meta-analysis; CI, confidence interval; HCQ, hydroxychloroquine.
Effect of HCQ on OA related quality of life
Three studies assessed the QoL in hand OA patients using PRO instruments: osteoarthritis quality of life scale (OAQoL), 12-item short form survey (SF-12), Arthritis Impact Measurement Scale 2 short form (AIMS2-SF), and 36-item short form survey (SF-36) [22,23,33]. None of the knee OA studies reported QoL outcomes; however, one study in knee OA assessed the impact of knee OA on patient’s activities of daily living (ADL) [24]. Unanimously, high-quality evidence from three studies in hand OA patients reported no systematic treatment differences between HCQ and placebo for the QoL assessed using OAQoL, SF-12, AIMS2-SF, and SF-36 (mental and physical) [22,23,33]. However, low-quality evidence from one study reported a small but statistically significant improvement in ADL (mean difference, 1.1; p < 0.05) in knee OA patients at 36 weeks [24].
Effect of HCQ on OA related imaging markers
Two studies in hand OA patients assessed radiographic progression using Kallman and modified Kallman score [23,33]. Both the studies reported no statistically significant (p > 0.24) improvement in Kallman radiographic scores at 12-month follow-up duration [23,33]. One study in knee OA assessed synovitis using ultrasound imaging and reported a clinically relevant reduction in synovitis in HCQ groups at 52 weeks; however, accompanying data was not reported [25].
Effect of HCQ on OA related biochemical markers
Two studies in hand OA patients assessed biochemical marker outcomes: erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). While one study observed no changes in ESR in both groups [25], others reported a significant difference in ESR (p < 0.01) between both groups at 52 weeks [33]. The CRP levels were reported improved initially in the first 6 months and then decreased at month 12 follow-up [25].
Safety outcomes
Four studies, two in hand and knee OA each, reported AEs [22,24,25,32] comprising 216 patients in the HCQ group and 225 patients in the placebo/active-comparator group, whereas SAEs were reported in two studies comprising 199 hand OA patients in HCQ group and 202 in the placebo group [23,33]. Fewer SAEs were observed in the HCQ group; however, the difference was non-significant (risk difference [RD], –0.04; 95% CI, –0.10 to 0.01) [23,33]. Likewise, no significant difference was observed for AEs (RD, 0.04; 95% CI, –0.02 to 0.10); however, higher AEs were reported in the HCQ arm [22,24,25,32]. Notable SAEs of prolonged QT interval with ventricular arrhythmias, erythema multiforme, and acute generalized erythematous pustulosis were being related to HCQ [23]. Overall, no difference was observed for AE/SAE between HCQ and placebo/active-comparator arm (RD, 0.00; 95% CI, –0.04 to 0.04) (Fig. 5).
Figure 5.
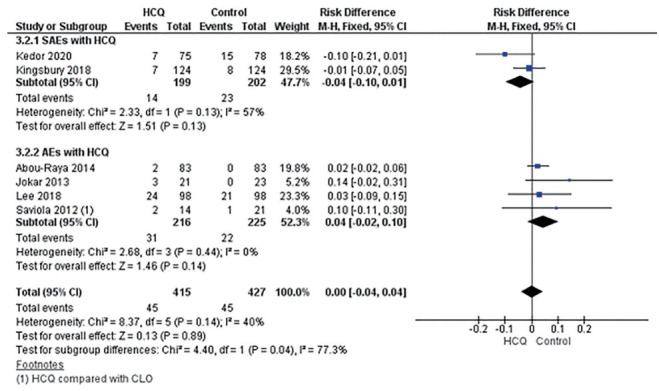
Risk difference for serious adverse effects (SAEs) and adverse effects (AEs) with hydroxychloroquine (HCQ). M-H, Mantel-Haenszel fixed-effect meta-analysis; CI, confidence interval; CLO, clodronate; HCQ, hydroxychloroquine.
DISCUSSION
To the best of our knowledge, this is the first and most comprehensive systematic review and meta-analysis assessing the efficacy and safety of HCQ for the treatment of OA. We found high-quality evidence to support that HCQ is no more effective than placebo/active control in reducing pain in knee or hand OA patients [22- 25,32,33]. Furthermore, high-quality evidence suggested that HCQ demonstrated no improvement in physical dysfunction compared to placebo for patients with hand OA [23,33]. However, moderate-quality evidence showed a modest improvement in knee OA associated dysfunction [24,32]; the benefit was small and may not have been clinically important (Table 2). The overall safety profile HCQ was acceptable with AEs/SAEs comparable to placebo/clodronate. Likewise, no favorable effect of HCQ was observed on QoL in hand and knee OA patients [22,23,33]. Limited evidence was reported assessing the effect of HCQ on radiographic markers and biomarkers in the knee and hand OA patients.
Table 2.
Meta-analysis results
| Outcome | No. of RCTs | No. of patients | Effect estimate (95% CI) |
|---|---|---|---|
| Pain | |||
| Hand OA [22,23,25,33] | 4 | 632 | SMD 0.14 (–0.20 to 0.48) |
| Knee OA [24,32]a | 2 | 210 | SMD –0.72 (–1.57 to 0.14) |
| Dysfunction | |||
| Hand OA [23,33] | 2 | 401 | SMD 0.08 (–0.23 to 0.40) |
| Knee OA [24,32]a | 2 | 210 | SMD –0.48 (–0.82 to –0.14) |
| Adverse event [22,24,25,32] | 4 | 441 | RD 0.04 (–0.02 to 0.10) |
| Serious adverse event [23,33] | 2 | 401 | RD –0.04 (–0.10 to 0.01) |
RCT, randomized controlled trial, CI, confidence interval; OA, osteoarthritis; SMD, standardized mean difference; RD, risk difference.
Abou-Raya et al. [24] was published as an abstract in 2013 and is still not published as full-text; the author did not make the data available upon email request.
The results were broadly uniform across major studies showing no beneficial effect of HCQ in for the treatment of OA, with an exception in showing improvement in function mainly derived from knee OA studies by Jokar et al. [32] and Abou-Raya et al. [24]. For hand OA associated pain outcome, Saviola et al. [25] study was the sole contributor for heterogeneity; where moderate heterogeneity was observed in hand OA associate function outcome. To be noted, Saviola et al. [25] compare HCQ with clodronate, an active comparator, and was the only open-labeled trial. Furthermore, it is essential to understand the characteristics of the two studies in knee OA [24,32]. The Joker et al. [32] study demonstrated an unusually narrow SD of symptoms and a lack of a placebo effect. RCTs in OA typically show a strong placebo effect, primarily due to alternate flare and remission in symptoms in OA patients [34,35]. The Abou-Raya et al. [24] study, which demonstrated improvement in pain and function with HCQ, was published as an abstract in 2013 and is still not published as full-text after many years; furthermore, the author did not make the data available when requested through email. Additionally, another trial by the same author team exploring the use of methotrexate in knee OA patients was retracted due to data inconsistencies [36]. Hence, any inference should be made considering these facts. Nevertheless, the pooled effect estimate for improvement in OA associated dysfunction was small and was statistically non-significant.
HCQ has a relatively acceptable safety profile and offers modest symptomatic relief in chronic immunity-mediated inflammatory rheumatic diseases [37]. However, a recent systematic review of HCQ in patients with RA demonstrated only a modest improvement in the outcome of interest (ACR20 and ACR50) when used in combination with other conventional synthetic DMARDs [38]. Nevertheless, considering the hypothesis that inflammation has a role in osteoarthritic pathogenesis, researchers have trialled HCQ in OA. Notably, the study by Saviola et al. [25] in erosive hand OA patients was stopped prematurely citing ethical concerns regarding the inefficacy of HCQ. Likewise, the results from HERO trial in nodal hand OA, OA-TREAT trial in inflammatory and erosive hand OA, and FABIO trial in primary hand OA did not support the efficacy of HCQ for pain relief and function improvement [22,23,33]. Thus, while the high-quality evidence from these methodologically rigorous trials, with long follow-up duration (6 to 12 months), put an end to the quest for exploring the efficacy of HCQ in hand OA; the current evidence also indicate that there would unlikely be any promising potential for HCQ for the treatment of knee and hip OA.
The strengths of this review include a registered protocol-oriented approach, extensive literature search, and the use of appropriate statistical techniques to pool the effect estimates. This review had few constraints as well. We restricted our research to English and Chinese language articles and may have missed studies published in other languages. Data were scarce that limited the scope for subgroup analysis and publication bias assessment. The patient population included knee and hand OA patients with varying OA phenotypes. Furthermore, studies comparing HCQ with placebo and active comparator were pooled together for the OA associated pain. However, on removing the study with the active comparator, the results did not change and remained statistically non-significant. Last, due to the inadequate data reporting in some trials, SD values were imputed; however, we used the prescribed methods and assumptions.
In conclusion, this systematic review and meta-analysis found that HCQ has no benefit in reducing pain and improving physical function in hand or knee OA patients. Off-label use of HCQ for patients with OA should be discouraged, considering no additional benefit.
KEY MESSAGE
1. Current evidence shows that, hydroxychloroquine (HCQ) has no benefit in reducing pain or improving physical function in patients with hand and knee osteoarthritis (OA).
2. However, HCQ demonstrated an acceptable safety profile in this population.
3. Off-label use of HCQ for patients with OA should be discouraged.
Acknowledgments
Ambrish Singh is supported by International Graduate Research Scholarship, University of Tasmania. Benny Antony is supported by National health and Medical Research Council of Australia Fellowship.
Appendix 1. Search strategy.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.World Health Organization . Geneva (CH): WHO; 2014. Priority diseases and reasons for inclusion: osteoarthritis [Internet] [cited 2021 Mar 17]. Available from: https://www.who.int/medicines/areas/priority_medicines/Ch6_12Osteo.pdf?ua=1. [Google Scholar]
- 2.Haq I, Murphy E, Dacre J. Osteoarthritis. Postgrad Med J. 2003;79:377–383. doi: 10.1136/pmj.79.933.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations . World Population to 2300. New York (NY): United Nations; 2004. [Google Scholar]
- 4.Safiri S, Kolahi AA, Smith E, et al. Global, regional and national burden of osteoarthritis 1990 2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79:819–828. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Geneva (CH): WHO; 2013. Chronic diseases and health promotion: chronic rheumatic conditions [Internet] [cited 2021 Mar 17]. Available from: http://www.who.int/chp/topics/rheumatic/en/ [Google Scholar]
- 6.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 7.Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 9.Steinmeyer J, Bock F, Stove J, Jerosch J, Flechtenmacher J. Pharmacological treatment of knee osteoarthritis: special considerations of the new German guideline. Orthop Rev (Pavia) 2018;10:7782. doi: 10.4081/or.2018.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghouri A, Conaghan PG. Update on novel pharmacological therapies for osteoarthritis. Ther Adv Musculoskelet Dis. 2019;11:1759720X19864492. doi: 10.1177/1759720X19864492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann W, Lambova S, Muller-Ladner U. Current treatment options for osteoarthritis. Curr Rheumatol Rev. 2018;14:108–116. doi: 10.2174/1573397113666170829155149. [DOI] [PubMed] [Google Scholar]
- 12.da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 13.Royal Australian College of General Practitioners . Guideline for the Management of Knee and Hip Osteoarthritis. Melbourne (AU): Royal Australian College of General Practitioners East Melbourne; 2018. [Google Scholar]
- 14.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;10:CD005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967–1975. doi: 10.1001/jama.2017.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haar D, Solvkjaer M, Unger B, Rasmussen KJ, Christensen L, Hansen TM. A double-blind comparative study of hydroxychloroquine and dapsone, alone and in combination, in rheumatoid arthritis. Scand J Rheumatol. 1993;22:113–118. doi: 10.3109/03009749309099254. [DOI] [PubMed] [Google Scholar]
- 18.Clark P, Casas E, Tugwell P, et al. Hydroxychloroquine compared with placebo in rheumatoid arthritis. A randomized controlled trial. Ann Intern Med. 1993;119:1067–1071. doi: 10.7326/0003-4819-119-11-199312010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol. 2006;2:458–459. doi: 10.1038/ncprheum0292. [DOI] [PubMed] [Google Scholar]
- 20.Kim HA, Cho ML, Choi HY, et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 21.Sillat T, Barreto G, Clarijs P, et al. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 2013;84:585–592. doi: 10.3109/17453674.2013.854666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee W, Ruijgrok L, Boxma-de Klerk B, et al. Efficacy of hydroxychloroquine in hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken) 2018;70:1320–1325. doi: 10.1002/acr.23471. [DOI] [PubMed] [Google Scholar]
- 23.Kingsbury SR, Tharmanathan P, Keding A, et al. Hydroxychloroquine effectiveness in reducing symptoms of hand osteoarthritis: a randomized trial. Ann Intern Med. 2018;168:385–395. doi: 10.7326/M17-1430. [DOI] [PubMed] [Google Scholar]
- 24.Abou-Raya S, Abou-Raya A, Khadrawe T. SAT0450 Efficacy of hydroxychloroquine in the treatment of symptomatic knee osteoarthritis in older adults: a randomized placebo-controlled trial. Ann Rheum Dis. 2014;73(Suppl 2):756–757. doi: 10.1136/annrheumdis-2013-204856. [DOI] [PubMed] [Google Scholar]
- 25.Saviola G, Abdi-Ali L, Campostrini L, et al. Clodronate and hydroxychloroquine in erosive osteoarthritis: a 24-month open randomized pilot study. Mod Rheumatol. 2012;22:256–263. doi: 10.1007/s10165-011-0506-8. [DOI] [PubMed] [Google Scholar]
- 26.Kroon FPB, Carmona L, Schoones JW, Kloppenburg M. Efficacy and safety of non-pharmacological, pharmacological and surgical treatment for hand osteoarthritis: a systematic literature review informing the 2018 update of the EULAR recommendations for the management of hand osteoarthritis. RMD Open. 2018;4:e000734. doi: 10.1136/rmdopen-2018-000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh A, Kotlo A, Dissanayaka T, Wang Z, Antony B. Efficacy and safety of hydroxychloroquine for the treatment of osteoarthritis: protocol for a systematic review of randomized controlled trials. medRxiv. 2021 Jul 26; doi: 10.1101/2020.07.20.20157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Savovic J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Hoboken (NJ): Wiley-Blackwell; 2019. [Google Scholar]
- 29.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- 30.Fu R, Vandermeer BW, Shamliyan TA, O’Neil ME, Yazdi F, Fox SH, et al. Rockville (MD): Agency for Healthcare Research and Quality; 2013. Handling continuous outcomes in quantitative synthesis. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. [cited 2021 Mar 17]. https://www.ncbi.nlm.nih.gov/books/NBK154408. [PubMed] [Google Scholar]
- 31.Singh A, Hussain S, Najmi AK. Number of studies, heterogeneity, generalisability, and the choice of method for meta-analysis. J Neurol Sci. 2017;381:347. doi: 10.1016/j.jns.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Jokar M, Mirfeizi Z, Keyvanpajouh K. The effect of hydroxychloroquine on symptoms of knee osteoarthritis: a double-blind randomized controlled clinical trial. Iran J Med Sci. 2013;38:221–226. [PMC free article] [PubMed] [Google Scholar]
- 33.Kedor C, Detert J, Rau R, Wassenberg S, Listing J, Klaus P, et al. OP0186 Hydroxychloroquine in patients with inflammatory and erosive osteoarthritis of the hands: results of a randomized, double-blind, placebo controlled, multi-centre, investigator-initiated trial (OA TREAT) Ann Rheum Dis. 2020;79(Suppl 1):115–116. [Google Scholar]
- 34.Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67:1716–1723. doi: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 35.Bannuru RR, Schmid CH, Sullivan MC, Kent DM, Wong JB, McAlindon TE. Differential response of placebo treatments in osteoarthritis trials: a systematic review and network meta-analysis. Osteoarthritis Cartilage. 2014;22 Suppl:S24–S25. [Google Scholar]
- 36.Retraction. Ann Rheum Dis. 2016;75:1580. doi: 10.1136/annrheumdis-2013-204856ret. [DOI] [PubMed] [Google Scholar]
- 37.Dos Reis Neto ET, Kakehasi AM, de Medeiros Pinheiro M, et al. Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv Rheumatol. 2020;60:32. doi: 10.1186/s42358-020-00134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rempenault C, Combe B, Barnetche T, et al. Clinical and structural efficacy of hydroxychloroquine in rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken) 2020;72:36–40. doi: 10.1002/acr.23826. [DOI] [PubMed] [Google Scholar]