Graphical abstract
Keywords: Coronavirus disease-2019, Interleukin-6, Cytokine storm, Tocilizumab, Janus-kinase inhibitor
Abstract
Since 2019, COVID-19 has become the most important health dilemma around the world. The dysregulated immune response which results in ARDS and cytokine storm has an outstanding role in the progression of pulmonary damage in COVID-19. IL-6, through induction of pro-inflammatory chemokines and cytokines, is the pioneer of the hyperinflammatory condition and cytokine storm in severe COVID-19. Therefore, IL-6 pathway blockade is considered an emerging approach with high efficacy to reduce lung damage in COVID-19. This article aims to review the pleiotropic roles of the IL-6 pathway in lung damage and ARDS in severe COVID-19, and the rationale for IL-6 signaling blockade at different levels, including IL-6 soluble and membrane receptor pathways, IL-6 downstream signaling (such as JAK-STAT) inhibition, and non-specific anti-inflammatory therapeutic approaches. Recent clinical data of each method, with specific concentration on tocilizumab, along with other new drugs, such as sarilumab and siltuximab, have been discussed. Challenges of IL-6 signaling inhibition, such as the risk of superinfection and hepatic injury, and possible solutions have also been explained. Moreover, to achieve the highest efficacy, ongoing clinical trials and special clinical considerations of using different IL-6 inhibitors have been discussed in detail. Special considerations, including the appropriate timing and dosage, monotherapy or combination therapy, and proper side effect managment must be noticed regarding the clinical administration of these drugs. Future studies are still necessary to improve the productivity and unknown aspects of IL-6 signaling blockade for personalized treatment of severe COVID-19.
1. Introduction
In December 2019, a new viral respiratory infection, named coronavirus disease 2019 (COVID-19), was emerged. On March 11th, COVID-19 was announced pandemic by the world health organization (WHO) and as of 24th October 2021 has infected more than 243 million individuals around the world (https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---26-october-2021). COVID-19 is an acute respiratory disease that is caused by severe acute respiratory coronavirus-2 (SARS-CoV-2) [1], [2].The symptomatology of the disease can be divided into three main categories, severe, moderate, and mild infection. Most COVID-19 cases present with mild/moderate symptoms that only need symptomatic treatment. Nevertheless, less than 10% of patients need advanced intensive care-level treatments. The clinical manifestations of COVID-19 include dry cough, headache, fever, coryza, myalgia, pharyngitis, diarrhea, vomiting, anosmia, ageusia, and other upper respiratory symptoms in mild cases. Moderate cases can present with symptoms of lung involvement, such as dyspnea, tachypnea, and coarse crackle in the base of the lung. Severe COVID-19 infection can lead to loss of consciousness, reduced O2 saturation (hypoxia), respiratory distress, organ failure, and even shock [3]. The gold-standard test for diagnosis of COVID-19 is the identification of the viral genetic sequence by reverse transcription-polymerase chain reaction (RT-PCR) test. Laboratory studies may show increased D-dimer, lactate-dehydrogenase (LDH), C-reactive protein (CRP), lymphopenia, imbalance in platelet/white blood cell count, and increase in inflammatory markers; however, these laboratory findings are not specific for COVID-19 and must not be used as diagnostic criteria [2]. The radiologic findings may show bilateral ground-glass opacifications (GGO) in the peripheral sites of the lung in moderate cases. Moreover, consolidation and\or white lung can be observed in lung CT of severe cases [4], [5]. The prognosis of COVID-19 is worsened as age increases. Moreover, the prognosis is worse in patients with cardiovascular and chronic respiratory conditions [6].
SARS-CoV-2 employs its spike protein to bind the angiotensin-converting enzyme 2 (ACE-2) receptor and enter the alveolar type 2 pneumocytes [7]. ACE-2 is overexpressed by nasal, laryngeal, tracheal, intestinal, and alveolar epithelial cells [8]. After binding to ACE-2R and entering the target cell, the virus replicates its genome in the cell nuclei to produce viral particles. This causes cytotoxic effects to the host cell and the production of local cytokines and chemokines. Secretion of these cytokines from damaged cells eventually leads to the activation of the immune response to inhibit the viral infection [9], [10]. Studies have shown that dysregulation of the immune response and disruption of the auto-regulatory immune mechanisms play an important role in the worsening of pulmonary damage in COVID-19 [11]. Hyper-activated immune response secondary to the SARS-CoV2-associated inflammation is the major cause of multiorgan failure, acute respiratory distress syndrome (ARDS), and respiratory dysfunction in severe cases of COVID-19 who need intensive care [12]. The hyperactivity of the immune response is mediated by the uncontrolled release of pro-inflammatory cytokines and chemokines, such as IL-6 and IL-1β, and pro-inflammatory immune cells. Previous studies have demonstrated that suppression of the hyperinflammatory immune response reduces the COVID-19-associated mortality and morbidity [13]. Considering the important role of cytokine storm and hyperinflammatory response in the progression of COVID-19, understanding the underlying immunopathogenesis is necessary to control the tissue injury and develop novel strategies to improve patient outcomes and reduce COVID-19 associated mortalities. Recently, there have been huge global efforts to investigate the therapeutic efficacy of IL-6 inhibition in COVID-19. In this article, we aim to review the IL-6 signaling pathways, the role of IL-6 in the pathomechanism of CRS/HIS in COVID-19, strategies for blocking IL-6 at different levels, current clinical data of using IL-6 signaling inhibitors in COVID-19, and clinical considerations of using the IL-6 inhibitors. Moreover, data from ongoing clinical trials have been used to improve future prospects of designing personalized treatment in severe COVID-19 through IL-6 pathway blockade.
2. Pathophysiology of COVID-19
Infection of the airway epithelial cells by SARS-CoV-2 causes cytologic harmful damage to the target cells that activate the local immune response. M1 macrophages and epithelial cells are the initial cells that recognize the viral single/double-stranded RNA of the virus and pathogen-associated molecular patterns (PAMPs) through toll-like receptor 3, 7 or RIG1/MDA5 pathway. This mechanism causes the induction of the inflammasome and IRF3/NFκB pathway and induces the production of pro-inflammatory mediators, such as IFNγ, IL-1β, IL-6, TNF-α, and IL-18 by macrophages [14]. IL-1β acts on endothelial and vascular smooth muscle cells and induces the production of IL-6 [15]. Moreover, dendritic cells (DC) of the respiratory tissue contribute to the primary immune response by secreting IL-6 and type I IFNs. Infiltrated monocytes and pathogenic T cells are other immune cells that promote the immune response by secreting large amounts of IL-6 into the serum. These immune effectors also act as antigen-presenting cells (APCs) by presenting SARS-CoV-2 to the type I helper T cells (Th1). Th1s induce the adaptive immune response by secreting immune-stimulating cytokines, including IFNγ, IL-2, and TNF-α [16]. These procedures stimulate a further immune response to eliminate the virus and contaminated cells. Nevertheless, in some cases, the immune response can not effectively destroy the virus and leads to a dysfunctional immune response. This deficient immune response can lead to an unrestrained release of pro-inflammatory cytokines and chemokines, which is called cytokine release syndrome (CRS) or hyperinflammatory syndrome (HIS). CRS/HIS is the result of uncontrolled immune response and is the major underlying mechanism of end-organ damage in severe COVID-19. The severity of this hyper-inflammatory condition predicts the prognosis and severity of lung damage [17]. Therefore, to control the hyperinflammatory condition in severe cases, it is necessary to define the underlying mechanisms. IL-6 is one of the hallmarks of hyperinflammation and CRS and has a crucial role in the COVID-19 severity. Here, we aim to discuss the role of IL-6 in the immunopathogenesis of CRS, lung damage, and ARDS in severe COVID-19.
3. IL-6 signaling pathway and its role in CRS pathogenesis
Interleukin-6 (IL-6) Is a 22 KDa weighted protein that has 212 amino acids in its structure. The gene encoding IL-6 is on chromosome 7 and its protein has an up-up-down-down 4-helical construction which includes disulfide bonds [18]. IL-6 is one of the cytokines of the IL-6-family, which include IL-11, IL-6, IL-27, ciliary neutrophilic factor, leukemia inhibitory factor, etc. The receptor of the IL-6 family is gp130 which is the same for all family members [19]. IL-6 entity in the body is directly related to the body index which means fat persons have a higher level of serum IL-6 [20]. Higher IL-6 level increases muscles sensitivity to insulin, play a role as a myokine in long-time exercises, and is effective in adaptation to exercises [21]. IL-6 also has various systemic physiologic functions. Examples are the stimulation of acute-phase protein production, activation of T cells, induction of the differentiation and proliferation of hematopoietic stem cells and hepatic cells, and advancement of atherosclerotic plaque formation [22]. According to the crucial contribution of IL-6 in the progression of COVID-19 induced ARDS, it is critical to study the IL-6 and its signaling in COVID-19.
Upon binding to its receptor, IL-6 can exert two diverse pro-inflammatory and anti-inflammatory roles [23]. As an anti-inflammatory cytokine in the gastrointestinal system, it induces the expansion of intestinal cells and inhibits the apoptosis of intestinal epithelial cells [24], [25]. It also has positive impacts on the regeneration of the liver and pancreas [26]. IL-6 confronts bacterial infections by its stimulating effects on the innate immune system and the production of antibodies from B lymphocytes. IL-6 increases antibody production by inducing B cells to trans-shape to immunoglobulin-producing cells and induce T helper follicles to produce more IL-21 [25]. Moreover, IL-6 has a major part in the induction of the tissue inflammatory process by regulation of neutrophil tissue infiltration [27]. IL-6 affects the lifespan of the T cells by up-regulating the anti-apoptotic factors [28]. As an inflammatory mediator, IL-6 induces hepatocytes to secrete acute-phase markers such as α1-antichymotrypsin, fibrinogen, CRP, and serum amyloid A [29]. It also adversely reduces albumin, transferrin, and fibronectin production. In conclusion, the pro-inflammatory roles of IL-6 in viral infections are exerted by mononuclear cell recruitment, endothelial cell stimulation, inhibition of T cells apoptosis, stimulation of acute-phase protein production, and inhibition of T-reg differentiation [30].
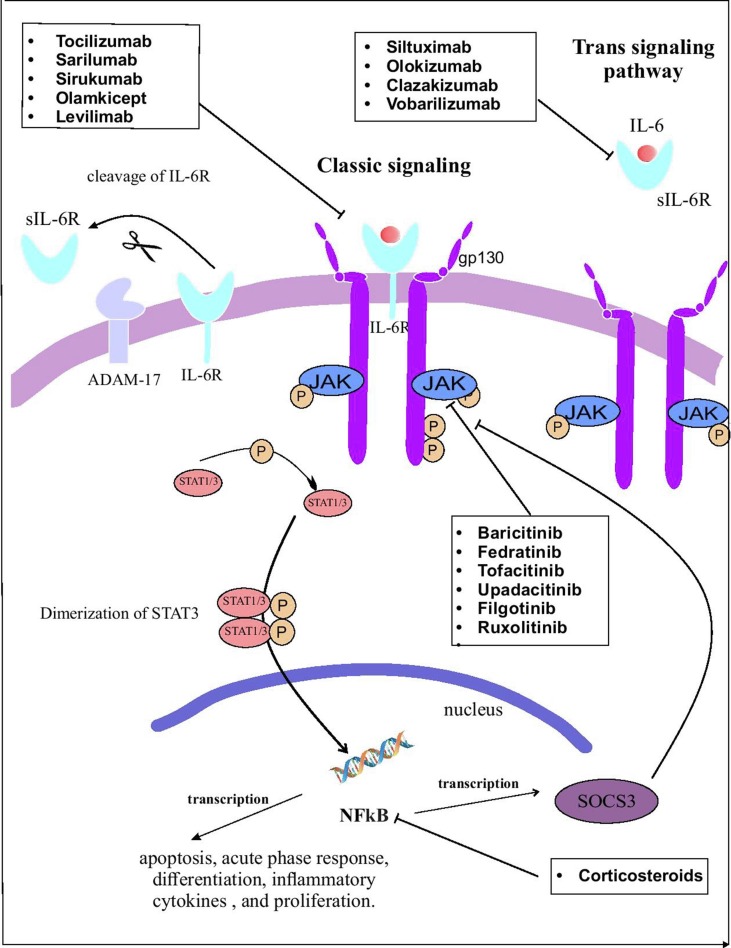
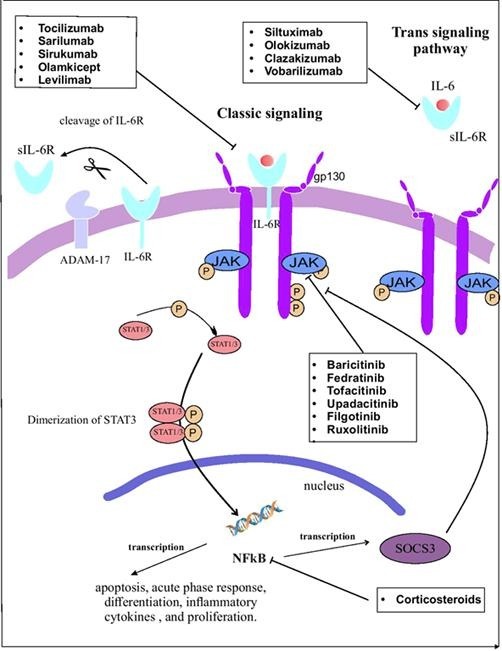
IL-6 effects begin by attaching IL-6 to its receptor (IL-6R) (Fig. 1 ). IL-6R is only expressed on the cell surface of neutrophils, hepatocytes, monocytes/macrophages, and some lymphocytes. This type of receptor is called the IL-6 classic signaling receptor [28]. The attachment of IL-6 to IL6-R and gp130 forms a hexameric complex which drives the classic signaling of IL-6. There is another pathway that IL-6 could affect other tissues without interacting with its receptor. To do this, IL-6 attaches to the soluble form of IL-6R (sIL-6R). This complex binds to the membrane-bound gp130 on endothelium, muscle cells, etc, and the IL-6 downstream signaling initiates [31]. After the integration of IL-6 with these receptors, they combine and attach to the gp130 membranous section. Intramembranous receptor regulates the classic pathway and plasma solved receptor regulates the trans-signaling pathway. ADAM-17 switches these two paths to each other by cleaving intramembranous IL-6R to sIL-6R. Coupling of IL-6 with IL-6R initiates gp130 homodimerization that induces the signal transducer and activator of transcription factor 3 (STAT3), Janus kinase (JAK), and JAK–SHP-2–mitogen-activated protein kinase (MAPK) pathways. STAT3 is phosphorylated to di-dimer form and trespass the nucleus membrane. In the nucleus, it turns on the NFKB gene expression that exits the nucleus for translating the IL-6 mRNA. STAT3 suppresses the gene expression of phosphorylated JAK and gp130 suppressors [29], [32]. These pathways induce the anti-apoptotic, acute-phase, and expression of proliferative protein genes [33].
Fig. 1.
The IL-6 pathway and the mechanisms of drugs inhibiting IL-6 pathway at different stages. Created by Esamaeilzdeh et al.
Although the anti-inflammatory effects of IL-6 exert a coping role against bacterial and viral infections, it has been shown that IL-6 level, in COVID-19 patients with ARDS and severe cases who have died from COVID-19, was tremendously elevated [34], [35]. Moreover, the production of IL-6 in COVID-19 patients was higher compared to SARS and influenza patients [36]. On the other hand, the SOC3 pathway which has a negative feedback on the IL-6 production is downregulated by COVID-19. This renders the production of IL-6 in COVID-19 out of control [37]. IL-6 has been shown to cause damage to the alveolar membrane. IL-6 affects endothelial cells by induction of vascular endothelial growth factor (VEGF) production that results in increased vessel permeability and induces the chemotaxis of monocytes and neutrophils in the alveolus. These processes lead to cytokine storm in the infection site [38]. Another proposed pathomechanism of ARDS is decreased endothelial cell E-cadherin which makes vessels wall permeable and increases vascular leakage. This results in the accumulation of interstitial fluid in the alveolus that leads to hypoxia [39].
Three pathways are proposed for the increased production of IL-6. At the beginning of virus entry to the body environment, innate responses activate TLR4 that inactivates Regnase-1 (an IL-6 inhibitor). This leads to an increase in IL-6 production by reducing SOC3, a suppressor of the JAK-STAT-3. In COVID-19, production of SOC3 is decreased which leads to uncontrolled IL-6 production [40]. SARS-CoV-2 utilizes the ACE2-R to penetrate the host cells [41]. It also occupies the Angiotensin-2 (ang-2) accumulates in the bloodstream. Increased ang-2 induces NFKB gene expression by TNF-α and sIL-6R [42]. This finally increases the IL-6 production. The outcome is increased IL-6 which affects the immune system and increases the production of pro-inflammatory cytokines which end up in CRS [43].
IL-6 has various effects on the immune system. IL-6 decreases the Th 1 and increases Th2 subtypes by inducing IL-4 production through the JAK-STAT pathway [44]. It also produces SOC-1 that inhibits STAT-1 phosphorylation which leads to lower production of Interferon-γ (IFN-γ) [45]. Finally, this process leads to reduced Th1 polarization. IFN-γ induces CD8+ T cells and NK cells that leads to viral contaminated cells cytolysis [46]. IL-6 also decreases IFN-γ production through upregulating SOC3, an IL-6 down-regulating pathway [45]. Thus, IL-6 enhances viral contaminated cells cytolysis by suppressing IFN-γ levels [47]. IL-6 elevates PDL-1 expression on viral infected cells surfaces [48] and increases PDL-1 expression on virally infected cells surface by IFN-γ. PDL-1 matches to PD-I receptor on CD8 T cells and switches off the apoptosis of the infected cell [49]. Moreover, IL-6 decreases T cell's granzymes production. IL-6 and TGF-β polarize Th17 that produce IL-17. IL-17 induces BCL-2 and BCLx that inhibit cytokine-c production in T cells [50], [51]. Cytokine-c inhibits active caspases production in T cells and therefore these T cells lose their power to eliminate infected cells. This ends in uncontrolled viral replication and increased viral load that makes the disease more severe and destructive [52]. IL-6 causes the production of other pro-inflammatory cytokines by stimulating the chemotaxis of infiltrating innate immune cells and T cells [53]. These cells produce pro-inflammatory chemokines and cytokines such as TNF-α, IL-1β, VEGF, etc. Increased cytokine production leads to vascular leakage in the infected organ and excess water absorption in the alveolus due to cytokine storm [54], [55]. CRS is the result of the uncontrolled production of pro-inflammatory cytokines, such as IFN-γ, TNF-α, IL-6, IL-18, IL-1β, etc [56]. CRS is the underlying mechanism of the progression of the hyperinflammatory response in sepsis, ARDS, and multiple inflammatory conditions, and can lead to multi-organ failure. Blockade of the progression of CRS has been shown to improve the clinical situation of severe patients with hyperinflammatory conditions, such as ARDS [57]. Since IL-6 is a major factor that contributes to driving the CRS in COVID-19, inhibition of its signaling pathway has been considered an effective therapeutic strategy.
In conclusion, IL-6 is an important cytokine with a critical role in the progression of mild inflammation to hyperinflammatory conditions, CRS, ARDS, and corresponding lung damage that causes mortality in critical COVID-19 patients. Studies have shown that IL-6 serum level is associated with the severity of respiratory involvement [58], [59] and IL-6 could be used as a predictive factor for the requirement of ventilatory support and intensive anti-inflammatory treatment for the patients [60]. Therefore, inhibition of the IL-6 pathway is a potential immunotherapeutic approach to inhibit the CRS and ARDS in severe COVID-19.
4. Inhibition of IL-6 in COVID-19
IL-6 has a crucial function in the advancement of mild respiratory inflammation to respiratory failure and ARDS in COVID-19. Therefore, inhibition of IL-6 and its downstream pathway has can decrease inflammation and improve the outcome in severe cases [30]. IL-6 pathway blockade can be conducted through multiple strategies which are described in Table 1 . The most studied strategy is inhibition of its receptor, IL-6-R, by monoclonal antibodies. Another strategy is using monoclonal antibodies to directly neutralize soluble IL-6. The third approach is inhibition of its downstream signaling, JAK-1, 2, which has a significant role in exerting the pro-inflammatory effects of IL-6. Inhibition of sIL-6R with monoclonal antibodies is another method of blocking IL-6. Moreover, non-specific immune-regulatory strategies, such as injection of systemic glucocorticosteroids, intravenous immunoglobulins (IVIG), and convalescent serum therapy can also be administered. Pilot studies have shown the life-saving efficacy of IL-6-blocking monoclonal antibodies and reducing the hospitalization period in severe COVID-19 patients with ARDS [61] and several clinical trials are evaluating the safety and efficacy of IL-6 blocking agents to repress the hyperinflammatory condition in hospitalized COVID-19 patients, which are described in Table 1. Here, we discuss different methods for inhibiting the IL-6 pathway at different stages based on recently published data and clinical trials to develop bench-to-bedside guidance to improve the clinical outcome of the patients.
Table 1.
Different methods of inhibiting IL-6 signaling and corresponding clinical trials registered in clinicaltrials.gov.
| Drug class | Number of patients | Phase | NCT |
|---|---|---|---|
| IL-6R inhibitors | |||
| Tocilizumab | 500 | 4 | NCT04377750 |
| 120 | 2 | NCT04412291 | |
| 30 | – | NCT04359667 | |
| 40 | 2 | NCT04377659 | |
| 50 | 1 | NCT04560205 | |
| 500 | 2 | NCT04445272 | |
| 32 | 2 | NCT04331795 | |
| 30 | 3 | NCT04423042 | |
| 400 | 2 | NCT04317092 | |
| 38 | 2 | NCT04315480 | |
| 100 | 2 | NCT04363736 | |
| 78 | 2 | NCT04435717 | |
| 228 | 2 | NCT04331808 | |
| 120 | – | NCT04306705 | |
| 9 | 2 | NCT04377659 | |
| 100 | 2 | NCT04363736 | |
| 400 | 2 | NCT04317092 | |
| 129 | 3 | NCT04403685 | |
| 126 | 2 | NCT04346355 | |
| 228 | 2 | NCT04331808 | |
| 332 | 2 | NCT04479358 | |
| 60 | 3 | NCT05002517 | |
| 150 | 4 | NCT04779047 | |
| 200 | 3 | NCT04690920 | |
| 106 | – | NCT04380818 | |
| 93 | 4 | NCT04730323 | |
| 310 | 3 | NCT04345445 | |
| Sarilumab (kevzara) | 1912 | 2/3 | NCT04315298 |
| 40 | 1 | NCT04386239 | |
| 239 | 2/3 | NCT04324073 | |
| 30 | 2 | NCT04357808 | |
| 30 | 2 | NCT04357808 | |
| 420 | 3 | NCT04327388 | |
| 239 | 2/3 | NCT04324073 | |
| 60 | 2 | NCT04661527 | |
| 120 | 2 | NCT04359901 | |
| 1912 | 2/3 | NCT04315298 | |
| 30 | 2 | NCT04357808 | |
| 120 | 2 | NCT04357860 | |
| 20 | 2 | NCT04322773 | |
| 7100 | 4 | NCT02735707 | |
| Sirukumab (plivensia) | 111 | 2 | NCT04380961 |
| Levilimab (BCD-089) | 206 | 3 | NCT04397562 |
| Olamkicept | – | – | – |
| JAK-STAT inhibitors | |||
| Ruxolitinib | 200 | 2/3 | NCT04477993 |
| 13 | – | NCT04361903 | |
| 15 | – | NCT04359290 | |
| 20 | 1/2 | NCT04334044 | |
| 100 | 2 | NCT04414098 | |
| NA | 2/ 3 | NCT04348071 | |
| NA | – | NCT04355793 | |
| NA | 2/3 | NCT04354714 | |
| 211 | 3 | NCT04377620 | |
| 432 | 3 | NCT04362137 | |
| 64 | – | NCT04331665 | |
| 200 | 2 | NCT04338958 | |
| 20 | 2 | NCT04374149 | |
| 456 | 1/2 | NCT04581954 | |
| Tofacitinib | NA | 2 | NCT04412252 |
| 60 | 2 | NCT04415151 | |
| 414 | 2 | NCT04750317 | |
| 260 | 2 | NCT04469114 | |
| 50 | 2 | NCT04332042 | |
| Baricitinib (olumiant) | 126 | 2 | NCT04393051 |
| 13 | 2 | NCT04399798 | |
| 1400 | 3 | NCT04421027 | |
| 12 | 2/ 3 | NCT04358614 | |
| 200 | 2/3 | NCT04320277 | |
| 168 | 2 | NCT04346147 | |
| 1033 | 3 | NCT04401579 | |
| 1010 | 3 | NCT04640168 | |
| 1167 | 4 | NCT04390464 | |
| 382 | 3 | NCT04970719 | |
| Fedratinib | – | – | – |
| Upadacitinib | – | – | – |
| Nezulcitinib | – | – | – |
| Filgotinib | – | – | – |
| Soluble IL-6 inhibitor | |||
| Clazakizumab | 30 | 2 | NCT04381052 |
| 17 | 2 | NCT04348500 | |
| 30 | 2 | NCT04659772 | |
| 60 | 2 | NCT04494724 | |
| 30 | 2 | NCT04363502 | |
| 180 | 2 | NCT04343989 | |
| Siltuximab | 200 | 2 | NCT04329650 |
| 342 | 3 | NCT04330638 | |
| 220 | – | NCT04322188 | |
| Olokizumab | 376 | 2/3 | NCT04452474 |
| 372 | 2/3 | NCT04380519 | |
| 200 | – | NCT04854941 | |
| Vobarilizumab | – | – | – |
| Non-specific inflammatory drugs | |||
| Corticosteroids | 20 | 2 | NCT04355247 |
| 20 | 2 | NCT04355247 | |
| 550 | – | NCT04344730 | |
| 1000 | 3 | NCT04348305 | |
| 300 | 4 | NCT04663555 | |
| 200 | – | NCT04586114 | |
| 11 | 2 | NCT04344288 | |
| Convalescent Plasma | 10 | 1 | NCT04407208 |
| 60 | 2 | NCT04528368 | |
| 10 | – | NCT04365439 | |
| 30 | – | NCT04327349 | |
| 30 | 2 | NCT04644198 | |
| 160 | 3 | NCT04547660 | |
| 60 | 2 | NCT04385186 | |
| 474 | 2 | NCT04621123 | |
| 920 | 2/3 | NCT04649879 | |
| 15 | 1/2 | NCT04452812 | |
| 20 | 1 | NCT04345679 | |
| 575 | 2 | NCT04347681 | |
| 10 | 1 | NCT04592705 | |
| 20 | 4 | NCT04441996 | |
| Intravenous Immunoglobulins (IVIG) | 60 | 3 | NCT04548557 |
| 310 | 2/3 | NCT04891172 | |
| 10 | 4 | NCT04616001 | |
| 100 | – | NCT04383548 | |
| 820 | 3 | NCT04910269 | |
| 35 | 2 | NCT04403269 | |
| 34 | 4 | NCT04411667 | |
| 100 | 2 | NCT04480424 | |
| 60 | 3 | NCT04548557 | |
| Combination Therapy | |||
| Tocilizumab + Anakinra + Ruxolitinib | 216 | 3 | NCT04424056 |
| Tofaitinib + Hydroxycloroquine | 116 | 2 | NCT04390061 |
| Tocilizumab + Dexamethasone | 120 | 2 | NCT04476979 |
| Sarilumab + Azithromycin + Hydroxychloroquine | 27 | 2/3 | NCT04341870 |
| Baricitinib + Remdesivir | 150 | 3 | NCT04693026 |
| Tocilizumab + Remdesivir | 649 | 3 | NCT04409262 |
| Baricitinib + Remdesivir + Dexamethasone | 4000 | 3 | NCT04832880 |
| Tofacitinib + Hydroxycloroquine | 116 | 2 | NCT04390061 |
| Ruxolitinib + Simvastatin | 94 | 2 | NCT04348695 |
| Ruxolitinib + Secukinumab vs Colchicine | 70 | 2 | NCT04403243 |
| Tocilizumab, Remdesivir, TPE, convalescent plasma | 600 | – | NCT04492501 |
| Tocilizumab + Dexamethasone | 120 | 2 | NCT04476979 |
| Tocilizumab + Corticosteroids + Siltuximab | 860 | – | NCT04486521 |
IL-6R: IL-6 receptor; JAK: Janus-kinase; STAT: signal transducer and activator of transcription factor 3; TPE: therapeutic plasm exchange, NCT: national clinical trial number.
4.1. IL-6 receptor inhibitors
The most studied strategy to block the pro-inflammatory function of IL-6 in the progression of COVID-19-associated respiratory damage is inhibiting the IL-6 receptor. IL-6 receptor is divided into two subsets, sIL-6R and mIL-6R. Most developed IL-6 receptor inhibitors block mIL-6-R; however, some drugs inhibit both receptors or can just inhibit sIL-6-R. Tocilizumab is a food and drug administration (FDA)-approved IL-6 inhibitor for rheumatoid diseases and is the most commonly studied IL-6 inhibitor in COVID-19. Other IL-6R inhibitors such as sarilumab, sirukumab, and olamkicept, are among other drugs under study.
4.1.1. Tocilizumab
Tocilizumab (TCZ) is a recombinant murine IgG1 mAb that is humanized. It includes two human light chains that are attached to two human heavy chains. TCZ inhibits downstream pro-inflammatory effects of IL-6 by binding to sIL-6R and mIL-6R and thus TCZ blocks both signaling and trans-signaling downstream pathways of IL-6 [62]. TCZ has received FDA approval to be used in rheumatologic diseases, such as Rheumatoid arthritis [63], and has been studied to suppress the CAR T-induced cytokine storm in previous studies [64]. Several articles have investigated the injection of TCZ to the COVID-19 patients before and after ICU admission. In a study, 21 patients with severe/critical COVID-19 were treated with 4–8 mg/kg TCZ with a maximum of two doses. The results indicated that 5 days after treatment, the oxygenation of the patients was significantly improved, the inflammatory markers were decreased, and no major side effects were observed. All the patients were discharged 15 days after receiving TCZ [65]. In another study, 15 moderately, severely, or critically ill COVID-19 patients were enrolled. All patients received one or two doses of 80–600 mg TCZ, in combination with methylprednisolone or alone. Despite receiving TCZ, three critically ill patients died and one patient did not respond to treatment. The rest of the patients responded well and were healed. CRP levels were shown to reduce in 11 patients after TCZ administration [66]. Similar results showed improvement of respiratory status in patients with severe COVID-19 after administration of TCZ [67], [68], [69], [70], [71].
Studies have also shown the benefits of TCZ therapy in decreasing mortality of severe COVID-19 [72], [73], [74]. Recently published systematic reviews have concluded that TCZ treatment reduces fatality, need for mechanical ventilation, and has promising positive effects on the improvement of severe COVID-19 [75], [76]. In an off-label case-control study, treatment with 400 mg or 8 mg/kg TCZ with a maximum dose of 800 mg resulted in a 75% reduction in inpatient mortality [77]. Another study was conducted to investigate the advantages of TCZ in reducing the risk of mortality in 154 intubated COVID-19 patients, of whom 78 received TCZ. This study demonstrated that the mortality risk was 45% reduced in the treatment group. In this study, bacterial superinfection with Staphylococcus aureus was the most common side effect associated with TCZ therapy; however, this did not affect the final fatality rate of the patients [78]. Other studies have similarly reported the reduction in mortality of TCZ-treated patients in comparison to the control group who were treated with the COVID-19 standard-of-care regimen [79], [80]. Moreover, TCZ therapy has been reported to reduce respiratory failure, ICU admission, the need for mechanical ventilation, and improve the total outcome of the patients in several studies [80], [81], [82], [83], [84], [85], [86], [87]. A retrospective study on 25 patients revealed that treatment with TCZ reduced the need for ventilatory support, improved the radiological condition of the lungs, and reduced inflammatory serum markers [88]. Similar results were observed in other retrospective studies [89], [90]. In a pilot multicenter study on 63 severe patients, treatment with TCZ was safe, reduced inflammatory markers including CRP and ferritin, improved oxygenation, and enhanced survival [91].
In contrast to the promising results of treatment with TCZ in reducing mortality and ICU admissions, contradictory results have been obtained in other studies [92], [93]. A study by Colaneri et al. enrolled 21 matched patients from the case group who received 8 mg/kg (maximum of 800 mg) TCZ and a second dose if no important side effects were detected. Despite treatment with TCZ, no reduction in the 7-day mortality rate or ICU admission was observed. The main limitation of this study was the small number of patients and the authors recommended further large-scale studies to investigate the efficacy of TCZ in the treatment of COVID19 [94]. Moreover, in another study by Canziani et al, TCZ did not reduce the 30-day mortality but diminished the need for mechanical ventilatory support and ameliorated the outcome in patients who survived for more than 5 days [74]. Similarly, TCZ did not improve the 28-day clinical outcome of the ICU-admitted patients in a study by Campochiaro et al. [95]. In a quasi-experimental study, Carvalho et al. showed that injection of two doses of 400 mg TCZ improved the oxygenation and facilitated the reduction of inflammatory markers and white blood cell (WBC) count, but did not affect the mortality of the treatment group [96]. Consistently, a meta-analysis demonstrated that treatment with TCZ showed no benefit in reducing the mortality and need for intubation of the treated patients [97].
TCZ can be injected both intravenously and subcutaneously. Intravenous injection has been used in most studies; however, in an observational study, Mastroianni et al. investigated the benefits of subcutaneous TCZ injection in 12 COVID-19 patients. The results indicated that similar to intravenous injection, subcutaneous injection of TCZ suppressed the CRS, improved lung CT manifestations, and improved the outcome [98]. The benefits of subcutaneous injection of TCZ were also shown in reducing mechanical ventilation and mortality in another retrospective cohort study [99]. In another study, no difference in the outcome was observed between intravenously and subcutaneously injected patients. To conclude, these data show that the route of TCZ injection, whether intravenous or subcutaneous, does not affect the efficacy of TCZ in severe COVID-19 infection.
Multiple theories have been proposed to explain the contradictory results of TCZ efficacy in reducing COVID-associated mortality. Demographic characteristics of the patients such as the age, sex, and underlying comorbidities, the clinical stage of the disease at admission, the timing of admission, higher risk of secondary bacterial/fungal infections in patients receiving TCZ, and possible unknown confounding factors are the most important factors that have been proposed [100]. To achieve the highest efficacy and best results, special clinical considerations and future perspectives should be considered which have been discussed in further sections.
4.1.2. Sarilumab (kevzara)
Sarilumab is a monoclonal antibody of human origin against sIL-6 and mIL-6 receptors. The mechanism of action of sarilumab is identical to TCZ and restricts the matching of IL-6 to gp130. In comparison to TCZ, sarilumab has a lesser dissociation constant and therefore has a higher capacity and affinity for IL-6 receptor and therefore can be administered in lower doses than TCZ [101], [102]. Sarilumab is the second most studied drug for blocking the IL-6 pathway in COVID-19 patients. In a study by Della-Torre et al., 28 cases with severe COVID-19-associated respiratory disease and hyperinflammatory condition received 400 mg of IV sarilumab infusion. The patients were followed up for 4 weeks. Sarilumab improved the time needed for recovery in the treatment group. Nevertheless, the overall improvement and mortality were not statistically distinct between sarilumab and the standard of care-treated patients [103]. Gremese et al. investigated the therapeutic efficacy of sarilumab on 53 patients, of whom 39 were treated in the COVID-19 ward and 14 patients were in the ICU. The results of this study demonstrated that after treatment with 400 mg intravenous sarilumab (92.6 % received a second infusion), 89.7% of the ward-treated patients improved after a maximum of 3 days and 85.7% did not require oxygen supplementation anymore. Also, 64.2% of the ICU-treated patients were transmitted to the ward. This study demonstrated that intravenous administration of sarilumab could be a promising treatment choice for severe COVID-19-associated pneumonia [104]. Another study by Benucci et al. demonstrated that 7 of the 8 sarilumab-treated patients experienced respiratory recovery [101].
Results of a retrospective study introduced sarilumab to have a possible clinical benefit and the serum CRP levels as a biomarker for treatment response in sarilumab-treated patients [105]. Nevertheless, a phase III study by Lescure et al. on 420 patients did not demonstrate the clinical effectiveness of treatment with sarilumab in hospitalized patients [106]. In conclusion, despite improving the recovery time, current studies do not support the efficacy of sarilumab in improving the outcome and survival of severe COVID-19-associated respiratory failure [107]. However, further large-scale studies are still required to show the efficacy of sarilumab in severe COVID-19 patients as in a phase II multicenter study, 120 patients will be treated with two different doses of sarilumab (200 mg and 400 mg) to evaluate the efficacy and dosing of sarilumab in severe and critical patients (NCT04357860.) Multiple other clinical trials (NCT04386239, NCT04315298, NCT04661527) are investigating the efficacy of sarilumab in severe hospitalized COVID-19 patients (Table 1).
4.1.3. Sirukumab (plivensia)
Sirukumab (plivensia) is a human monoclonal antibody against the IL-6R. Previous studies have described the use of sirukumab in improving the clinical manifestations of RA patients [108], [109]. Nevertheless, since the application of sirukumab was associated with serious side effects and superinfections, it has not received FDA approval for the treatment of RA (https://www.fda.gov/media/106879/download). Since sirukumab suppresses the hyperinflammatory process by inhibiting IL-6, it has been considered to be used to suppress hyperinflammation in severe COVID-19 infection [110]. No studies have yet reported the results of treatment of COVID-19 patients with sirukumab. A phase II clinical trial (NCT04380961) has been designed to investigate the efficacy and safety of sirukumab administration in severe/critical COVID-19 patients.
4.1.4. Olamkicept
Olamkicept (sgp130Fc) is a decoy protein that inhibits the trans-signaling of the pro-inflammatory IL-6 pathway [111] and has shown potent anti-inflammatory effects and remission in a preclinical mice model and phase II clinical trial in inflammatory bowel disease (IBD) [112], [113]. To our knowledge, no studies or clinical trials have yet investigated the immunomodulatory efficacy of olamkicept in COVID-19; however, according to its anti-inflammatory potential, it could be considered for the therapy of COVID-19 in future studies.
4.1.5. Levilimab
Levilimab is a mAb against IL-6 and has shown superior efficacy in improving RA symptoms in combination with methotrexate in phase II randomized clinical trials [114]. A phase III clinical trial, CORONA, demonstrated that a combination of levilimab and SOC resulted in improvement of the COVID-19 patients who needed oxygen supplementation, but not ventilator support [115]. In this study, the dynamic levels of inflammatory markers, including ESR and CRP, were significantly reduced after treatment with levilimab. Interestingly, this study showed an increase in IL-6 serum levels after levilimab treatment which could be the result of IL-6R inhibition [116]. A comparative study that intended to study the efficacy and side effects of TCZ, levilimab, and olokizumab, reported no significant difference in death rate between groups treated with these three drugs [117]. Nevertheless, death and sepsis were reported higher in the levilimab-treated group. A clinical trial is evaluating the efficacy and safety of levilimab in patients with severe COVID-19 (NCT04397562).
4.2. JAK-STAT inhibitors
Janus kinase (JAK)-1 and 2 and their downstream signaling pathway, signal transducer and activator of transcription (STAT), are downstream of the IL-6 pro-inflammatory pathway. The binding of IL-6 to membrane gp-130 induces the phosphorylation of JAK which phosphorylates and dimerizes the STAT-3 [118]. STAT-3 is a major intracellular protein that induces the expression of inflammatory transcription factors, such as NFκB. This finally leads to the secretion of multiple pro-inflammatory markers which induce the chemotaxis of the immune cells to the infection site [119]. Therefore, considering the role of JAK1 and JAK2 in the development of cytokine storm, inhibition of the JAK-STAT pathway can repress the inflammatory route induced by IL-6 in the hyperinflammatory condition in severe and critically ill patients [120]. A systematic review has analyzed the efficacy of JAK inhibitors in the improvement of COVID-19 symptoms and mortality. Based on this article, JAK inhibitors reduce ICU admission and mortality and also reduce the time-to-discharge in severe COVID-19 cases [120]. Multiple drugs have been developed as JAK-STAT inhibitors. Here, we discuss different JAK/STAT inhibitors and recent results/clinical trials of their administration in COVID-19 (Table 1).
4.2.1. Baricitinib (olumiant)
Baricitinib (olumiant) is a selective JAK1/2 inhibitor that was approved for anti-TNF-α-resistant RA patients [121], [122]. Recently, it has also been shown to hinder the endocytosis and assembly of the SARS-CoV-2 to the host cell by inhibiting AP2-associated protein kinase 1 (AAK1) [123], [124], [125]. Considering the inhibitory effects of baricitinib on the JAK-mediated inflammatory process and the entrance of SARS-CoV-2 to the host cell, it has been thought to be investigated in COVID-19 patients to improve the cytokine storm [126], [127], [128]. A study on 1033 COVID-19 patients intended to investigate the combination therapy of remdesivir and baricitinib on patient recovery and improvement of the clinical status. Patients receiving both drugs had a shorter time to recovery and up to 30% higher clinical improvement. Moreover, serious side effects were reported lower in patients receiving the combination of remdesivir and baricitinib [124]. The results of this study were published in the New England Journal of medicine after which FDA authorized the combination of remdesivir and baricitinib in children over two years of age and hospitalized adults who need respiratory support (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19). In a multicenter randomized phase III trial (COV-BARRIER), baricitinib did not alter the progression of the disease compared to the standard of care (including dexamethasone). However, the overall 28 and 60-day mortality rate was diminished up to 38.2 % in the group treated with both baricitinib and SOC [129], [130]. In another study, 12 moderately ill patients were treated with 4 mg/kg of oral baricitinib. Of the treated patients, none experienced any adverse events, none were transferred to the ICU, and all patients’ respiratory function improved after 2 weeks [131]. In a phase II study by Cantini et al., application of baricitinib successfully reduced the viral entry, decreased viral burden and mortality rate, reduced ICU-admission, improved the cytokine storm, and was not associated with significant side effects [132]. In conclusion, studies support using baricitinib, alone and in combination with remdesivir, in moderate to severe hospitalized COVID-19 patients. Several clinical trials are investigating the efficacy of treatment with baricitinib alone or in combination with dexamethasone or remdesivir (Table1). Future studies are still required to investigate the efficacy, dosing, and adverse effects of baricitinib.
4.2.2. Fedratinib (Inrebic)
Fedratinib (Inrebic) is a selective JAK-2 inhibitor approved for patients with intermediate or with a high risk of myelofibrosis in 2019 [133]. As discussed in the pathophysiology of COVID-19-induced cytokine storm, IL-6 stimulates naive CD4 T cells to Th17 cells. Th17 cells exert pro-inflammatory effects (through secreting IL-17) and anti-apoptotic effects that contribute to a severe inflammatory condition [134]. In a study, Fedratinib was reported to hinder the production of IL-17 by murine Th17 cells. Therefore, Fedratinib was suggested as a promising treatment of COVID-19 by targeting JAK-2 [134], [135]. No clinical trials have yet been registered to evaluate the efficacy of Fedratinib in COVID-19.
4.2.3. Tofacitinib
Tofacitinib is a pan-JAK inhibitor that specifically inhibits JAK3 and tyrosine kinase-2 and can therefore reduce the secretion of IL-6, IL-7, and IL-2 [135]. Tofacitinib has received FDA approval for RA and is also used as an anti-inflammatory drug for inflammatory bowel diseases (IBD) [135], [136]. In a large-scale study, 289 hospitalized COVID-19 patients were randomly divided into two groups. 89.3% of the patients received glucocorticoids. The treatment group received 10 mg of tofacitinib twice a day. Tofacitinib reduced the mortality rate and respiratory failure after 28 days [137]. Jacobs et al. reported a case of ulcerative colitis (UC) that was treated with 10 mg of tofacitinib twice a day. The patient was diagnosed with mild COVID-19 with a positive nasal swab. The patient was continued on tofacitinib twice a day. After a 5 day course, the patient’s clinical symptoms improved, and after two weeks, the patient did not have any symptoms of COVID-19 Jacobs et al., 2020;26 [138]:e64-e.. Although this case report demonstrated no progression in COVID infection despite continuing treatment with tofacitinib, it can not be concluded that tofacitinib had any positive effects on the improvement of the patient’s symptoms. The Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) is a database used for registration of COVID-19 outcomes in IBD patients. In a survey, Agrawal et al. investigated the outcome and characteristics of 37 IBD patients with COVID-19 who were treated with tofacitinib. The results of this analysis demonstrated that tofacitinib did not affect hospitalization, progression of the disease to severe infection, and requirement of ICU admission [139]. In another study, 62 patients with elevated CRP levels received tofacitinib. The tofacitinib-treated group experienced a significant reduction in inflammatory markers, ICU admission, and disease progression. The overall mortality was reduced which suggests the application of tofacitinib for COVID-19 [140]. Decision on the efficacy of tofacitinib in COVID-19 still has to be investigated and five clinical trials have been registered that aim to evaluate the efficacy of tofacitinib in reducing the COVID-19-associated cytokine storm (Table1).
4.2.4. Upadacitinib
Upadacitinib is a selective JAK1/3 inhibitor that has shown potent anti-inflammatory effects in ankylosing spondylitis (AS) and MTX-resistant moderate to severe RA patients [141], [142]. By blocking the JAK1/3, Upadacitinib inhibits the production of IL-6, GM-CSF, and IFN-γ [143]. Despite the anti-inflammatory potential of Upadacitinib, no clinical studies have yet reported the application of this drug in COVID-19.
4.2.5. Filgotinib
Filgotinib is another selective JAK1 inhibitor with a similar mechanism of action to Upadacitinib and has been used for the treatment of moderate to severe RA [142]. Similar to Upadacitinib, no clinical studies have yet reported the application of Filgotonib against COVID-19 infection.
4.2.6. Ruxolitinib
Ruxolitinib is a JAK1/2 inhibitor that has received approval for the treatment of myelofibrosis and is also used for treating steroid-resistant graft-versus hoist disease (GvHD) [144]. In a study on 105 COVID-19 patients, 14 patients received ruxolitinib for a median cumulative dose of 135 mg over a median of 9 days. 11/14 patients showed impressive improvement in clinical status on day 7 [144]. In another multicenter study, of the 43 patients, 22 received Ruxolitinib. Ruxolitinib-treated patients experienced a numerically faster improvement in clinical symptoms, superior improvement of chest CT findings, and favorable safety profile. None of the patients in the treatment group died after 28 days. Although there was no statistically significant difference between the control and treatment groups, the results of this study showed promising results in the improvement of COVID-19 patients after treatment with Ruxolitinib [145]. In another study, 18 COVID-19 patients with acute respiratory failure were treated with a high dose of Ruxolitinib (20 mg twice a day) for the first 48 h and 10 and 5 mg for two weeks. The results demonstrated that of the 18 patients that received high-dose ruxolitinib, 16 showed clinical improvement within the first 48 h and complete respiratory recovery after two weeks. Two patients did not respond to treatment and had progressive disease, and no significant side effects were observed [145]. Another paper reported clinical resolving of COVID-19 in a 53-year-old patient with hematopoietic stem cell transplant due to chronic myeloid leukemia (CML) who was treated with a high dose of ruxolitinib (10 mg twice daily) [146]. Similar results of the efficacy of ruxolitinib in critically ill COVID-19 patients have been reported [147], [148]. To conclude, the administration of ruxolitinib has shown significant improvement in the clinical status of COVID-19 patients with ARDS [149]. Despite promising results of ruxolitinib administration in critically ill patients, it can be also associated with hematologic and skin adverse effects [149] that need specific clinical considerations, which have been discussed in further sections.
4.2.7. Nezulcitinib (TD-0903)
Nezulcitinib is an inhaled pan-JAK inhibitor that has been recently thought to be investigated for respiratory damage in COVID-19 [150]. In a study, patients were treated with three different doses of nezulcitinib, 1 mg, 3 mg, and 10 mg daily for 7 days. Nezulcitinib improved the clinical status and reduced mortality in the treatment group [151]. This study revealed the efficacy and safety of inhaled nezulcitinib for respiratory damage of the COVID-19 and demonstrated that the inhaled form of JAK inhibitors could be an optimized route for drug application with minimal systemic side effects.
4.3. Soluble IL-6 inhibitors
IL-6 protein is present in the plasma in the form of soluble IL-6. Another reliable strategy for blocking the inflammatory cascade of IL-6 is using drugs that act by binding to and neutralizing soluble IL-6 protein. This leads to inhibition of its binding to IL-6R. These drugs are stratified as soluble IL-6 inhibitors and have previously shown favorable anti-inflammatory effects in different diseases [152]. This section aims on reviewing different soluble IL-6 inhibitors in suppressing COVID-19-associated cytokine storm.
4.3.1. Siltuximab
Siltuximab is a soluble IL-6 inhibitor that has been studied in COVID-19 associated hyperinflammation [150], [153]. Preliminary results of a pilot study have reported the potential efficacy of siltuximab in 21 COVID-19 patients with ARDS. In this study, administration of a median dose of 900 mg (11 mg/kg/day) siltuximab improved the clinical status of 33% of the patients [154]. Another cohort study reported the therapeutic potential of siltuximab in COVID-19 patients [155]. In a preliminary report of a prospective observational study, 30 patients who needed ventilatory support were included. The patients received 11 mg/kg of IV siltuximab and a second dose was repeated after 72 h. 18 patients also received corticosteroids in addition to the siltuximab. Of 30 patients, 16 experienced improved ventilatory status, four patients’ conditions were not changed or were deteriorated, and 10 patients died. Pentraxin-related protein (PTX3) is a member of the pentraxin family that has multiple functions on activation of TNFα induced protein 6 (TNFAIP6) and complement C1q [156]. PTX-3 induction results in the activation of innate immune effector cells, such as macrophages and DCs, and finally, contribute to alveolar damage and ARDS. In this study, improved ventilatory status was associated with reduced serum levels of IL-8, a major cytokine of neutrophil chemotaxis, and PTX-3. The authors concluded that treatment with siltuximab improved the ventilatory status and respiratory function by inhibiting the complement system and the pro-inflammatory effects of IL-8, independent of IL-6. Moreover, no serious or unexpected side effects were observed in patients receiving siltuximab [157]. These data have brought the context of using siltuximab as a treatment option for COVID-19-induced ARDS (Table1).
4.3.2. Olokizumab
Olokizumab is a humanized monoclonal antibody that represses the IL-6 pathway by attaching to the 3rd attachment site of IL-6 and blocking its interaction with gp130 [158]. It has been previously used as an anti-inflammatory agent for the treatment of RA patients [159] that did not show adequate response to TNF- inhibitors [54], [160]. Its efficacy is as much as TCZ in multiple doses but its adverse effects are mild to moderate and have better effects on CRP levels than placebo. Moreover, olokizumab can repress the inflammatory process by inhibiting the activation of macrophages [161]. Antonov et. al used Olokizumab on 610 COVID-19 patients with the 160 mg/ml subcutaneous dose. They showed that Olokizumab improved general condition from the first day, decreased the temperature to normal, and prevented the progression of the disease to severe mode [162]. In an ongoing clinical trial NCT04452474, they are using single-dose 64 mg of Olokizumab at day 29 in patients with severe COVID-19. They included 100 patients, 50 as case and 50 as control (placebo group). The patients will be followed up for 60 days after the injection. In another completed clinical trial (NCT04380519), they recruited 189 randomized COVID-19 patients on the 15th day of hospitalization and administered 64 mg of Olokizumab to one-third of them, 80 mg of RPH-104 to other one-third, and normal saline as a placebo to the remaining patients. This study is still in its ongoing phase.
4.3.3. Clazakizumab
Clazakizumab is a humanized monoclonal antibody that directly inhibits IL-6 and has been used for the treatment of RA [163]. In RA patients, Clazakizumab has been shown to have 3 to 4 times more adverse effects than MTX [164]. In a case report by Vaidya and et al., 25 mg of Clazakizumab was used for the treatment of a transplanted 61-year-old severe COVID-19 patient in a single dose. At the end of the 11th day of admission, the patient was good enough to be discharged [165]. The clinical trials of Clazakizumab are listed in Table 1.
4.3.4. Vobarilizumab (ALX-0061)
Vobarilizumab (ALX-0061) is a bispecific nanobody against albumin and IL-6R and has been used for the treatment of moderate to severe RA in a phase 2b clinical trial [166] and further evaluation of its efficacy against systemic lupus erythematosus (SLE) is under investigation [167], [168], [169], [170]. It has 10 times more than TCZ and has 2000 times more affinity than TCZ to the receptor. However, its affinity to mIL-6r is 45 times less than TCZ [171]. ALX0061 is expected to have more efficacy than TCZ by inhibiting the trans-signaling pathway and fewer side effects [172]. There are no reported studies of vobarilizumab in the treatment of COVID-19.
4.4. Non-specific anti-inflammatory strategies
As mentioned earlier, anti-inflammatory treatment is recommended for suppressing the ARDS and cytokine storm in the severe phase of the disease [173]. Other anti-inflammatory strategies, such as corticosteroids, intravenous immunoglobulin (IVIG), convalescent serum therapy, can also be used in addition to the IL-6 receptor, JAK, and soluble IL-6 inhibitors. Here, we aim to discuss the anti-inflammatory effects of corticosteroid therapy and convalescent plasma in COVID-19.
4.4.1. Corticosteroids
Corticosteroids activate glucocorticoid receptors (GRs) in the cell cytoplasm. Activated GRs attach to glucocorticoid response elements (GREs). GREs are located on the promoter region of steroid-sensitive genes that encode anti-inflammatory factors, such as annexin1, SLP-1, MKP-1, IKAPAB, ALFA, and GILZ [174], [175]. Moreover, IkBα acts as a negative regulator of NF-kB which is degraded by IkB kinase (IKK) in the process of NF-kB activation. Dissociation of NF-kB from IkBα activates NF-kB. Activated NF-kB translocates into the nucleus environment where it binds to NF-kB responsive genes promoter. Corticosteroids interact with these molecules to stop the inflammation process by inhibiting the NF-kB pathway and thus inhibiting the secretion of pro-inflammatory cytokines such as IL-6 [176], [177]. Then, glucocorticoids limit vascular permeability by inhibiting antigen-presenting cells (APC) of secreting histamine and lipid mediators such as PGE2 and LTB4. Moreover, corticosteroids inhibit neutrophil chemotaxis by decreasing E-selectin expression, inducing neutrophil apoptosis, and enhancing macrophage phagocytosis by enhancing Annexin-1 expression [178]. The suppression of inflammation is also induced by glucocorticosteroids by increasing the expression of TGF-β and IL-10 [179]. The anti-inflammatory properties of corticosteroids have led these drugs to be considered for suppressing the inflammatory process in severe COVID-19 [180], [181].
The RECOVERY trial is the most copious study that has been conducted to evaluate the therapeutic efficacy of corticosteroids in COVID-19. In this study, Horby et al. used 6 mg/day dexamethasone for 5–7 days in 6425 COVID-19 patients. They showed that the mortality of mechanically ventilated patients was reduced to one-third (from 40.7% to 29%) and to 20% in patients who needed non-invasive oxygen supplementation (from 25% to 21.5%). Nevertheless, corticosteroid treatment did not affect mortality in patients who did not require oxygen supplementation. Moreover, ICU admission length was shortened, radiologic features were improved, and the need for oxygen therapy was reduced in severe patients. They showed that the mechanisms of glucocorticoids action were inhibiting cytokine storm, production of anti-inflammatory cytokines, inhibiting phagocytes, decreasing neutrophils recall, and decreasing alveolar edema and vascular leakage [182], [183]. Other studies have also reported the beneficial effect of corticosteroids in the treatment of severe COVID-19 [184], [185], [186].
On the contrary, some studies, such as the COVIDOSE study, have shown that treating COVID-19 pneumonia with corticosteroids has no advantages to be considered as the reason to use them in the therapeutic protocol for COVID-19 [187], [188], [189]. There are clinical trials that are investigating the pros of corticosteroids use in COVID-19 patients. Qin et al. (ChiCTR2000029386) are conducting a clinical trial of using 1–2 mg/Kg/day for 3 days of methylprednisolone on 48 COVID-19 patients to assess the efficacy of corticosteroid therapy in COVID-19 patients. Using corticosteroids alone or in combination with other IL-6-inhibitors, such as TCZ, could be accompanied by some adverse effects and challenges, which are discussed in further sections.
4.4.2. Convalescent plasma
Serum therapy was used in the H1N1 influenza pandemic in 2009. Whereas lots of cytokines are released to the bloodstream in the COVID-19 cytokine storm, using multiple and consecutive drugs could be more tolerated by therapeutic plasma exchange (TPE) [190], [191], [192]. TPE is used for eliminating plasma antibodies, toxins, and abnormal proteins that have been produced due to COVID-19. It also is considered as a replacement for decreased plasma volume and its constituents due to infection [193]. The volume of TPE should be precisely supervised because it can exert volume overload in patients. To prevent this complication, a specific amount of plasma should be drained from the body and be replaced by frozen albumin, plasma, and TPE [194]. The effectiveness of TPE is related to the proportion of extracted plasma volume to total patient’s plasma volume, distribution of pathogenic particles, and their production-elimination rate. One time TPE purifies plasma up to 65% and it will rise to 85% by the second administration of TPE [195], [196].
In a study by Khamis et al., they recruited 31 severe COVID-19 patients admitted to ICU with ARDS for TPE treatment. They showed less intubation, reduced mortality rate, better laboratory parameters amendment, and faster respiratory recovery in patients treated with TPE compared to conventional therapy [197]. Other studies have shown that treatment with convalescent plasma can reduce the severity of COVID-19 infection, viral load, inflammatory markers, and blood viscosity [198], [199], [200], [201], [202]. In conclusion, treatment with convalescent plasma is an effective strategy for suppressing the hyperinflammatory condition of the severe COVID-19 through neutralization of viral particles and inflammatory cytokines, including IL-6 [203].
4.4.3. Intravenous immunoglobulins (IVIG)
Intravenous immunoglobulins (IVIG) is an IgG product derived from the plasma of thousands of donors and is used for the treatment of infectious and inflammatory conditions [203]. IVIG can repress the inflammation through several mechanisms. Inhibiting the FcγR of the innate immune cells, inhibition of the complement system, and inhibition of lymphocyte activation are some examples of inflammation suppression by IVIG. Studies have investigated the efficacy of IVIG in the treatment of COVID-19 pneumonia [204], [205] and multiple clinical trials are investigating this (Table 1).
4.4.4. Hyperbaric oxygen therapy (HBOT) and ozone therapy
Hyperbaric oxygen therapy (HBOT) is a method for delivering high-pressure oxygen (more than 1 ATA) to patients. In addition to its effect in overcoming the air-blood barrier of the lungs, HBOT has beneficial effects on reducing inflammation has therefore been considered as a potential treatment option in COVID-19 [206], [207], [208]. A study analyzed treatment with hyperbaric oxygen at 2 atmospheres for 90 min in 20 COVID-19 cases. This study concluded that this treatment approach was safe but did not show specific efficacy in improving respiratory distress [209]. However, another study evaluated the therapeutic efficacy of HBOT in 5 COVID-19 cases with hypoxemia and tachypnea. All patients experienced improved respiratory conditions with elevated O2 saturation and did not require mechanical ventilation [210]. A systematic review aimed to assess the efficacy of hyperbaric oxygen therapy on severe COVID-19 by including eight articles. This study concluded that hyperbaric oxygen therapy is a safe and effective treatment method for severe COVID-19 [211].
Ozone (O3) is an allotrope of oxygen with potent virucidal and anti-inflammatory effects [212]. Therefore, the application of ozone (O3) therapy has been considered to suppress the severe inflammatory process in COVID-19 [213]. A study evaluated rectal ozone for the treatment of 14 patients with severe pulmonary COVID-19 infection. The results of this study indicated that ozone-treated patients had reduced inflammation and improved oxygenation. Mortality was lower in the ozone-treated group but was not statistically different [214].
5. Challenges of IL-6 inhibition in COVID-19
Despite promising results, management of COVID-19-associated CRS through IL-6 pathway suppression is accompanied by some challenges. Understanding these challenges and developing novel strategies to overcome them can improve the therapeutic outcome of severe COVID-19 treated with IL-6 inhibitors. The wide range efficacy of TCZ is the most important dilemma. Although Tocilizumab treatment has been recommended in many studies, its efficacy has been reported to vary according to studies. Moreover, some patients are refractory and do not respond to TCZ [75]. The variety of efficacy can be attributed to some reasons. Timing for initiating the corticosteroid and Tocilizumab is one of the most significant factors that determine the efficacy of the drug. Too early usage has less efficacy due to lower serum levels of IL-6 and too late usage also has less efficacy due to the pulmonary damage that occurred before treatment [200], [215]. In a study, late administration of corticosteroids did not improve the outcome and respiratory failure [200]. Severe patients have a higher level of inflammatory cytokines such as IL-6 so it has been shown that TCZ is more effective in severe patients than in moderate/mild patients [216]. Another factor that can affect the efficacy of TCZ is the age and comorbidities of the patients. Most of the published studies show that the use of the TCZ was preferred for younger patients with fewer comorbidities such as diabetes, chronic heart diseases, etc. So this is why the available data cannot be generalized to all COVID-19 patients and the rate of the Tocilizumab efficacy should be assessed in a wider range of age [188]. The recommended dose for TCZ in COVID-19 is 400–800 mg but in a study by Strohbehn et al., they used 40–200 mg of TCZ instead of a routine dose. They concluded that a lower dose of the TCZ has a faster and better effect on diminishing the inflammation induced by severe COVID-19 [189]. In TCZ-refractory cases that do not respond to treatment, adjuvant treatment with an IL-1 inhibitor can improve the condition. In a study, mechanical ventilation and fatality were reduced after injection of anakinra, an IL-1 inhibitor, in COVID-19-associated ARDS patients that had not responded to standard-of-care regimen and TCZ [217].
Similar to other systemic drugs, the application of TCZ is associated with side effects. Since TCZ blocks the signaling of IL-6 and its downstream pathway, which has a substantial role in the immune response against exogenic pathogens, treatment with TCZ could result in bacterial and fungal superinfections [78]. Constitutional symptoms such as fatigue, malaise, anorexia fever, dry cough, nausea, headache, hypertension, pruritis, and even anaphylaxis occur in response to TCZ injection [87], [98]. Treatment with TCZ can also lead to an increase in liver enzymes, including alanine transaminase (ALT), aspartate transaminase (AST), bilirubin, and an increase in serum triglyceride (TG), and LDL [218]. Despite the increase in hepatic enzymes, the function of the liver is maintained at a normal level [219]. Thrombocytopenia and mild neutropenia have also been reported as side effects of TCZ therapy in COVID-19 patients [84].
Although TCZ has FDA approval for RA treatment, the long-term effects of this drug still need to be studied, especially in COVID-19. Application of TCZ could be associated with mild to severe complications that could even be lethal in some cases. Radbel et al. [220] reported two cases of diagnosed COVID-19 patients with CRS to develop viral myocarditis and Hemophagocytic Lymphohistiocytosis (sHLH) after receiving two doses of TCZ. The suppression of the immune response after receiving TCZ could have been the underlying mechanism of sHLH and myocarditis of these patients.
Since transplanted patients are recommended to use immunosuppressive drugs for many years to avoid rejection, another challenge in the injection of TCZ is whether or not to use it in solid organ transplanted patients. A study by Perez-Saez and et al. used Tocilizumab in 80 kidney transplanted COVID-19 patients. They showed that TCZ reduced the mortality rate by 38% in severely ill patients compared to those that have not been treated with Tocilizumab [221]. In contrast, another study showed that using Tocilizumab in solid organ transplanted COVID-19 patients increased the risk of mortality, mechanical ventilation need, ICU admission, and renal replacement therapy. They concluded that the hazardous effects of the TCZ were due to the patient's differences in their study groups. Patients who had been treated with Tocilizumab were in more severe condition and therefore had a worse outcome than the non-TCZ treated patients [221].
The combination of TCZ with other immune-suppressing agents is still a question. Some studies show that a combination of corticosteroids (CS) and TCZ has more efficacy than TCZ or CS alone therapy [184], [188], [222]. Rubio-Rivas and et al. showed that CS, especially dexamethasone, is recommended in all severe COVID-19 patients and they also showed that a combination of TCZ with CS due to its anti-inflammatory and inhibitory effects on IL-6 is beneficial in severely ill patients [184]. Medrano et al. recommend that in patients older than 65-year-old with severe COVID-19, treatment with TCZ in combination with CS has superior efficacy compared with CS alone [188]. Balena and et al. recruited 206 COVID-19 patients and treated 16 of them with TCZ. They concluded that a combination of TCZ and CS is beneficial in older and more severe patients despite a trend to secondary infection after treatment with TCZ in severe COVID-19 patients [185]. Luis et al. showed that early aggressive immunomodulatory treatment with TCZ and CS had prominent efficacy in severe COVID-19 infection [186].
Tocilizumab can be combined with JAK inhibitors to achieve a better outcome in COVID-19. Although this strategy has shown beneficial effects but also increases the incidence of superinfection [223], [224]. There is not any clear explanation for the mechanism of this complication but it mostly seems to be related to the inflammatory cytokine-blocking side effects of these drugs besides their main mechanism of action. The best way to control and early treatment of probable superinfection induced by these drugs is using them just in hospitalized patients under direct surveillance [225], [226].
JAK inhibitors, including upadacitinib and baricitinib, can have some unique but controllable side effects, of which the most important are an increase in the risk of co-infections and liver injury. Upadacitinib increases the rate of cholestasis. Therefore, patients who are treated with this drug should be assessed periodically by trans abdominal ultrasonography for early diagnosis of cholestasis. Therefore, it is highly recommended to check liver function tests at certain times after JAK inhibitors treatment, especially in women treated with JAK inhibitors [227]. In addition, baricitinib has been reported to induce neutropenia and lymphocytopenia and therefore increases the risk of co-infections [228]. Moreover, an important challenge is that baricitinib increases the risk of thromboembolic events. Due to the hyperinflammatory condition of the COVID-19, patients receiving baricitinib are at a higher risk of thromboembolic events [229]. Therefore, coagulation factors must be routinely monitored in patients receiving baricitinib [230], [231]. Ruxolitinib is another JAK inhibitor that has been associated with side effects. In a case report, two COVID-19 patients who received ruxolitinib were reported to present with a reduction in platelet count, decreased hemoglobin and hematocrit, skin purpuric rashes, secondary labial herpes, and deep tissue infection. These side effects were relieved by the administration of antibiotics and antiviral treatments [232].
Since the start of the pandemic, there have been huge efforts to control the infection, of which the development of the SARS-CoV-2 vaccine is the most important one. Despite the immune-stimulating properties, vaccine injection must be conducted with specific considerations in vulnerable groups, especially in patients receiving immunocompromising drugs. Corticosteroids, JAK inhibitors, and TCZ are the most important group of drugs that can affect the immune response to the vaccine. Based on initial reports from MAJIK-SFR Registry (NCT04602091), the overall response to SARS-CoV-2 vaccines remained high in patients on JAK inhibitors. Nevertheless, based on the lack of antibody production, some patients were considered non-responders. Compared to other JAK inhibitors, upadacitinib was associated with a higher non-response rate [233]. Based on another report, the humoral immune response to the BNT162b2 mRNA (Pfizer) vaccine was not impaired in MF patients receiving ruxolitinib [234]. The effects of immunomodulatory drugs on the cellular immune response to the SARS-CoV-2 vaccine are not elucidated yet and need to be considered in future studies.
6. Clinical considerations and future prospectives
Diverse results have been observed in different studies that have evaluated the therapeutic efficacy of IL-6-inhibitors and JAK inhibitors in COVID-19 [235], [236]. Here, we aim to review the most important clinical keys to achieve optimal clinical results of treatment with IL-6 and JAK inhibitors. Personalized therapy, giving the right drug to the right patient at the right time, has gained high attraction during the last years. To achieve optimal efficacy, choosing the right patient for TCZ injection and infusion of TCZ at the right time are two main factors. Some factors have been shown to reduce the response to treatment with TCZ. Starting TCZ in late stages of the disease, in patients under mechanical ventilatory support [237], in patients whose serum level of CRP does not change after TCZ injection, in decreased serum levels of miR-146a [238], and hyperglycemic patients, are some factors that minimize the response probability to TCZ [239].
Based on previous studies, the onset of respiratory involvement is associated with the activation of the IL-6-mediated hyperinflammatory condition and the production of pro-inflammatory markers, such as CRP [240]. Studies have tried to determine the best period for injection of TCZ. In a phase II trial (NCT04317092), TCZ reduced the 30-day mortality rate of the patients and this reduction was detected to be more obvious in patients who received TCZ before they needed mechanical ventilatory support [241]. It was proposed by a study that treatment should be started as soon as possible and before the progression of the disease to the severe phase [242]. Hernandez et al. showed that administration of TCZ before deterioration of respiratory condition (in patients requiring Fio2 < 0.5) is more efficacious in improving survival. Nevertheless, administration of TCZ in critical cases who needed ventilatory support for maintaining saturation >93% (patients requiring Fio2 > 0.5) did not show clinical efficacy. The authors concluded that the timing of injection is a major determinant of TCZ efficacy and late administration of TCZ in the respiratory failure phase can not improve the respiratory condition [243], [244]. A study investigated the therapeutic efficacy of TCZ in patients who required more respiratory support (Fio2 > 45%) with those requiring less respiratory support (Fio2 < 45%). This study demonstrated that early treatment with TCZ in patients who required Fio2 < 45% was more effective to reduce the need for mechanical ventilation and mortality [245]. In another study, the patients who were primarily deteriorated and were under mechanical ventilation showed a lower response to the intravenous injection of TCZ [246], which confirms that administration of TCZ at the right time, especially before starting the mechanical ventilatory support [242], [247], [248]. A better outcome was also achieved in patients who had increased inflammatory markers, such as CRP [249]. It was shown that TCZ can show its optimal efficacy when used in patients with progressive COVID-19 who are under respiratory support but are not too ill to need mechanical ventilatory support. These patients are determined as patients with higher platelet count and high inflammatory markers, such as D-dimer, CRP, and prolactin [250], [251]. To obtain optimal efficacy, TCZ may be needed to be repeated for at least two injections. Luo et al. showed that two doses of TCZ had higher efficacy in reducing CRP and suppressing the hyperinflammatory status compared to a single dose of TCZ [252].
Hyperglycemic patients show a poor response to treatment with TCZ. In a retrospective study by Marfella et al., it was shown that treatment of hyperglycemic patients with TCZ was less effective to reduce the risk of severe COVID-19 compared to normoglycemic patients. This could be attributed to the higher serum levels of IL-6 or higher IL-6R expression in hyperglycemic status [253], [254]. Therefore, to achieve optimal response, appropriate blood glucose control is mandated in patients receiving TCZ. Anti-IL-6 drug dosage and route of administration is another issue that has a noticeable impact on treatment outcomes. Most studies have used intravenous TCZ with a dosage of 4–8 mg/kg in one or two doses (up to 600 mg) [252], [255], [256]. Another route of TCZ administration is the subcutaneous injection with 162 mg twice a day (up to 324 mg total dose daily) [99], [257]. The route of administration does not seem to impact the outcome [258]; however, dosage still needs to be evaluated in further studies [98].
In some cases, patients who have partial or no response to one drug could benefit from another drug from the same family. Cingolani et al. reported improvement of respiratory status after administration of oral baricitinib in a 71-year-old COVID-19-associated respiratory failure who had a partial response to sarilumab [259]. Aldolase-reductase inhibitors are glucose-lowering drugs that are conventionally used for DM. Due to their anti-inflammatory properties, it was hypothesized that combined treatment of TCZ with these drugs can improve the hyperinflammatory status in COVID-19. In a case report of COVID-19 myocarditis, the patient was treated by TCZ plus CS in combination with an aldose reductase inhibitor (AT-001). They concluded that a combination of TCZ and AT-001 with routine CS therapy can be a potential alternative for CS alone treatment in patients who are severely ill and do not respond to CS [225].
Corticosteroids are drugs with multiple adverse effects, such as bacterial super-infection, cardiovascular complications, and hyperglycemia risk, and should be used cautiously in severe patients, hypoxic patients, and patients with a history of glucocorticoids use [260]. In the term of methylprednisolone, it has been shown that it should be used in a dosag less than <1 mg/kg/day and not be used for more than one week [181] and IV dexamethasone must be used with a dosage of 6 mg/day in COVID-19 patients [261]. Another severe complication of corticosteroid use is intestinal perforation which is related to the high dose of corticosteroids. A study reported a 66-year old man with metabolic syndrome and COVID-19-associated acute respiratory failure under treatment with high dose methylprednisolone and TCZ to be diagnosed with acute intestinal perforation [261]. Since other studies had previously reported the same complication in RA patients treated with TCZ/corticosteroids [262], [263], intestinal perforation must be considered as a lethal complication of treatment with corticosteroids and TCZ in COVID-19 patients.
In some severe COVID-19 patients, monotherapy with IL-6 inhibitors, corticosteroids, or JAK inhibitors can not improve respiratory failure and ARDS. Therefore, combination therapy has been considered in some studies (Table 1) [264]. Concomitant inhibition of IL-6 and IL-6R can act synergistically to inhibit the IL-6 inflammatory cascade. In a study, a combination of TCZ and siltuximab reduced serum IL-6/IL-6R ratio and effectively suppressed the hyperinflammatory status compared to monotherapy with each drug [265]. Anti-inflammatory drugs and TCZ could act synergistically to improve the outcome in patients with severe infection. In a study, TCZ and methylprednisolone were administered alone or in combination in non-intubated patients. The results demonstrated that early anti-inflammatory treatment with TCZ, methylprednisolone, or both, can improve the clinical outcome [266]. Another study investigated the effect of a 5-day treatment with high-dose methylprednisolone (250 mg on day 1 followed by 80 mg on following days) followed by a single dose infusion of 8 mg/kg TCZ in 43% of the patients. The results indicated that treated patients had 65% less mortality, 71% less mechanical ventilation, and 79% better primary outcome [266]. The authors concluded that treatment with glucocorticoids, followed by adjunctive injection of TCZ, can substantially promote the outcome in deteriorating COVID-19 patients. Combination therapy with corticosteroids and TCZ can also be used for the management of COVID-19-associated side effects, such as myocarditis and cerebral edema. In a study by Coyle et al, a 57-year-old male COVID-19 patient was diagnosed with acute myocarditis with extreme elevation in troponin. The patient was treated with high-dose 500 mg IV methylprednisolone for 4 days. Since the inflammatory markers were increased and the patient’s hemodynamic status became unstable, the patient was started on a single infusion of 4 mg/kg TCZ and an AT-001 aldolase-reductase inhibitor (ARI). After combination therapy with these drugs, the patient’s clinical status and inflammatory markers were significantly improved and he was fully recovered and discharged on day 19 [225].
The complement system is one of the directors of the innate immune system and contributes to the cytokine storm in COVID-19 [170], [267]. Therefore, another potent combination therapy is the combination of complement inhibitors with IL-6R or JAK inhibitors. In a study, eculizumab, a monoclonal antibody against C5a, was used to treat ARDS in COVID-19 patients. The results indicated that the patients experienced significant improvement in serum D-dimer levels, respiratory status, and radiologic findings. The authors concluded that combination therapy using a complement inhibitor and a JAK inhibitor can treat the ARDS by simultaneous inhibition of adaptive and innate hyper-immune responses [268]. Other than IL-6, several cytokines and interleukins participate in the progression of ARDS in COVID-19. In some severe cases that do not respond to treatment, TCZ can also be combined with immunomodulatory drugs of other classes, such as IL-1 inhibitors. Aomar-Millán and et al. showed that Anakinra (an IL-1 inhibitor) can be used with TCZ and CS in treating severe COVID-19 patients that had not responded to TCZ plus CS therapy. The reason for the synergetic effect of Anakinra and TCZ is that IL-6 and IL-1 both are main inflammatory agents in COVID-19 and thus the combination use of them has a synergetic effect in severely ill COVID-19 patients [269]. Moreover, a combination of anakinra and methylprednisolone was reported to decrease the mortality in COVID-19 patients with hyperinflammation and respiratory failure in an observational retrospective study on 120 patients [270].
7. Conclusion
Cytokine storm-induced ARDS is the major cause of respiratory damage and lung damage in severe COVID-19. IL-6 has a key role in this hyperinflammatory process and corresponding ARDS. Thus, inhibition of the IL-6 pathway is an appropriate method for reversing pulmonary failure and reducing mortality in COVID-19 (271). Specific IL-6 inhibitors, including tocilizumab, sarilumab, clazakizumab, levilimab, non-specific anti-inflammatory drugs such as corticosteroids, and JAK-inhibitors such as tofacitinib, baricitinib, etc, are the latest developed agents for inhibition of IL-6 pathway that have been studied and have shown promising effects against severe COVID-19. Among IL-6 pathway inhibitors, tocilizumab and baricitinib have shown promising results for severe COVID-19 treatment; however, data either do not support other drugs for severe COVID-19 treatment, or future studies are still mandated to determine their efficacy. Despite promising results, the application of IL-6 pathway antagonists can be associated with side effects, such as the risk of superinfection and hepatic damage. Moreover, to reach optimal efficacy by using these drugs, specific clinical considerations such as the appropriate timing and dosage must be considered. In some severe cases that do not respond to monotherapy, a combination of two or more drugs from the same class or different classes can be helpful. Further studies are indicated to investigate the treatment options, efficacy, possible unexpected adverse effects, and future approaches to achieve improved outcomes in COVID-19 by blocking the IL-6 signaling pathway.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to deeply appreciate healthcare workers in the field of COVID-19 and wish all scholars be safe. We wish that proper control of the pandemic be available by global vaccination. We also acknowledge Mohammad Javad Yavari, M.D., for his constructive comments on revising the manuscript.
Author Contribution.
R.E., P.K., and A.H. contributed to data gathering, writing the primary manuscript, and designing the tables. R.E. and P.K. contributed to designing the figure. A.E. contributed to the hypothesis, correspondence, and revising of the final manuscript before submission. All the authors viewed and confirmed the final manuscript before submission.
Availability of data and material
Data are immediately provided upon request by the reviewers.
References
- 1.Esmaeilzadeh A., Elahi R. Immunobiology and immunotherapy of COVID-19: A clinically updated overview. J. Cell. Physiol. 2021;236(4):2519–2543. doi: 10.1002/jcp.30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tahmasebi S., Khosh E., Esmaeilzadeh A. The outlook for diagnostic purposes of the 2019-novel coronavirus disease. J. Cell. Physiol. 2020;235(12):9211–9229. doi: 10.1002/jcp.29804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng Z., Xiang M., Guan H., Liu Y., Zhang H., Xia L., et al. Early fibroproliferative signs on high-resolution CT are associated with mortality in COVID-19 pneumonia patients with ARDS: a retrospective study. Therapeut. Adv. Chronic Dis. 2021;12 doi: 10.1177/2040622320982171. 2040622320982171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baratella E., Ruaro B., Marrocchio C., Starvaggi N., Salton F., Giudici F., et al. Interstitial lung disease at high resolution CT after SARS-CoV-2-related acute respiratory distress syndrome according to pulmonary segmental anatomy. J. o Clin. Med. 2021;10(17):3985. doi: 10.3390/jcm10173985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali J., Ali Q., Hafeez M., Malik A. Clinical features, diagnosis and treatment of COVID-19. Biol. Clin. Sci. Res. J. 2020;2020(1) [Google Scholar]
- 7.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Rostami M.R., Leopold P.L., Mezey J.G., O’Beirne S.L., Strulovici-Barel Y., et al. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am. J. Respir. Crit. Care Med. 2020;202(2):219–229. doi: 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruaro B., Salton F., Braga L., Wade B., Confalonieri P., Volpe M.C., et al. The history and mystery of alveolar epithelial type II cells: Focus on their physiologic and pathologic role in lung. Int. J. Mol. Sci. 2021;22(5):2566. doi: 10.3390/ijms22052566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan S., Jiang S.-C., Zhang Z.-W., Fu Y.-F., Hu J., Li Z.-L. The role of alveolar edema in COVID-19. Cells. 2021;10(8):1897. doi: 10.3390/cells10081897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kommoss F.K., Schwab C., Tavernar L., Schreck J., Wagner W.L., Merle U., et al. The Pathology of Severe COVID-19-Related Lung Damage: Mechanistic and Therapeutic Implications. Deutsches Ärzteblatt Int. 2020;117(29–30):500. doi: 10.3238/arztebl.2020.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song C.-Y., Xu J., He J.-Q., Lu Y.-Q. Immune dysfunction following COVID-19, especially in severe patients. Sci. Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-72718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. 2020;55(6) doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hariharan A., Hakeem A.R., Radhakrishnan S., Reddy M.S., Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2021;29(1):91–100. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gil-Etayo F.J., Suàrez-Fernández P., Cabrera-Marante O., Arroyo D., Garcinuño S., Naranjo L., et al. T-helper cell subset response is a determining factor in COVID-19 progression. Front. Cell. Infect. Microbiol. 2021;11:79. doi: 10.3389/fcimb.2021.624483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhall A., Patiyal S., Sharma N., Usmani S.S., Raghava G.P. Computer-aided prediction and design of IL-6 inducing peptides: IL-6 plays a crucial role in COVID-19. Briefings Bioinf. 2021;22(2):936–945. doi: 10.1093/bib/bbaa259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garbers C., Aparicio-Siegmund S., Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr. Opin. Immunol. 2015;34:75–82. doi: 10.1016/j.coi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Prakash S., Sen R.K., Tripathy S.K., Sen I.M., Sharma R., Sharma S. Role of interleukin-6 as an early marker of fat embolism syndrome: a clinical study. Clin. Orthopaedics Related Res. 2013;471(7):2340–2346. doi: 10.1007/s11999-013-2869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galván-Román J.M., Rodríguez-García S.C., Roy-Vallejo E., Marcos-Jiménez A., Sánchez-Alonso S., Fernández-Díaz C., et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J. Allergy Clin. Immunol. 2021;147(1) doi: 10.1016/j.jaci.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen B.K., Steensberg A., Fischer C., Keller C., Keller P., Plomgaard P., et al. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proc. Nutr. Soc. 2004;63(2):263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- 22.Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012;122(4):143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 23.Kamimura D., Ishihara K., Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 2003:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 24.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018;18(12):773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh C.-Y., Chen C.-A., Huang C.-Y., Chang M.-C., Lee C.-N., Su Y.-N., et al. IL-6-encoding tumor antigen generates potent cancer immunotherapy through antigen processing and anti-apoptotic pathways. Mol. Ther. 2007;15(10):1890–1897. doi: 10.1038/sj.mt.6300243. [DOI] [PubMed] [Google Scholar]
- 26.Galun E., Zeira E., Pappo O., Peters M., Rose-John S. Liver regeneration induced by a designer human IL-6/sIL-6R fusion protein reverses severe hepatocellular injury. FASEB J. 2000;14(13):1979–1987. doi: 10.1096/fj.99-0913com. [DOI] [PubMed] [Google Scholar]
- 27.Fielding C.A., McLoughlin R.M., McLeod L., Colmont C.S., Najdovska M., Grail D., et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 2008;181(3):2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 28.Rose-John S., Waetzig G.H., Scheller J., Grötzinger J., Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opinion Therapeutic Targets. 2007;11(5):613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 29.Mitsuyama K., Matsumoto S., Rose-John S., Suzuki A., Hara T., Tomiyasu N., et al. STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut. 2006;55(9):1263–1269. doi: 10.1136/gut.2005.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atal S., Fatima Z. IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharmaceut. Med. 2020;34(4):223–231. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.März P., Herget T., Lang E., Otter U., Rose-John S. Activation of gp 130 by IL-6/soluble IL-6 receptor induces neuronal differentiation. Eur. J. Neurosci. 1997;9(12):2765–2773. doi: 10.1111/j.1460-9568.1997.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 32.Esmaeilzadeh A., Rostami S., Yeganeh P.M., Tahmasebi S., Ahmadi M. Recent advances in antibody-based immunotherapy strategies for COVID-19. J. Cell. Biochem. 2021;122(10):1389–1412. doi: 10.1002/jcb.30017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Zhao B., Qu Y. Detectable serum SARS-CoV-2 viral load is closely associated with drastically elevated Interleukin-6 (IL-6) levels in critically ill COVID 19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146(1) doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedrick T.L., Murray B.P., Hagan R.S., Mock J.R. COVID-19: clean up on IL-6. Am. J. Respir. Cell Mol. Biol. 2020;63(4):541–543. doi: 10.1165/rcmb.2020-0277LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ascierto P.A., Fu B., Wei H. IL-6 modulation for COVID-19: the right patients at the right time? Journal for ImmunoTherapy of. Cancer. 2021;9(4) doi: 10.1136/jitc-2020-002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.P.A. Ascierto, B. Fu, H. Wei, JJfIoC. IL-6 modulation for COVID-19: the right patients at the right time? 9(4) (2021). [DOI] [PMC free article] [PubMed]
- 38.Yin X.-X., Zheng X.-R., Peng W., Wu M.-L., Mao X.-Y. Vascular endothelial growth factor (VEGF) as a vital target for brain inflammation during the COVID-19 outbreak. ACS Chem. Neurosci. 2020;11(12):1704–1705. doi: 10.1021/acschemneuro.0c00294. [DOI] [PubMed] [Google Scholar]
- 39.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19(7) doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samavati L., Uhal B.D. ACE2, much more than just a receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020;10:317. doi: 10.3389/fcimb.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y., Zhou W., Yang L., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.S. Diehl, M. Rincón, JMi. The two faces of IL-6 on Th1/Th2 differentiation, 39(9) (2002) 531–536. [DOI] [PubMed]
- 45.Diehl S., Anguita J., Hoffmeyer A., Zapton T., Ihle J.N., Fikrig E., et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13(6):805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 46.Sanceau J., Wijdenes J., Revel M., Wietzerbin J. IL-6 and IL-6 receptor modulation by IFN-gamma and tumor necrosis factor-alpha in human monocytic cell line (THP-1). Priming effect of IFN-gamma. J. Immunol. 1991;147(8):2630–2637. [PubMed] [Google Scholar]
- 47.Orange J.S., Biron C.A. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 1996;156(12):4746–4756. [PubMed] [Google Scholar]
- 48.Zhang W., Liu Y., Yan Z., Yang H., Sun W., Yao Y., et al. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J. ImmunoTher. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang N., Zeng Y., Du W., Zhu J., Shen D., Liu Z., et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int. J. Oncol. 2016;49(4):1360–1368. doi: 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- 50.Passos S.T., Silver J.S., O’Hara A.C., Sehy D., Stumhofer J.S., Hunter C.A. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J. Immunol. 2010;184(4):1776–1783. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishihara M., Ogura H., Ueda N., Tsuruoka M., Kitabayashi C., Tsuji F., et al. IL-6–gp130–STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int. Immunol. 2007;19(6):695–702. doi: 10.1093/intimm/dxm045. [DOI] [PubMed] [Google Scholar]
- 52.Rochman I., Paul W.E., Ben-Sasson S. IL-6 increases primed cell expansion and survival. J. Immunol. 2005;174(8):4761–4767. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]
- 53.Kuppalli K., Rasmussen A.L. A glimpse into the eye of the COVID-19 cytokine storm. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darif D., Hammi I., Kihel A., Saik I.E.I., Guessous F., Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021;104799 doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohamadian M., Chiti H., Shoghli A., Biglari S., Parsamanesh N., Esmaeilzadeh A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene Med. 2021;23(2) doi: 10.1002/jgm.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moradian N., Gouravani M., Salehi M.A., Heidari A., Shafeghat M., Hamblin M.R., et al. Cytokine release syndrome: inhibition of pro-inflammatory cytokines as a solution for reducing COVID-19 mortality. Eur. Cytokine Netw. 2020;31(3):81–93. doi: 10.1684/ecn.2020.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo W., Li Y.-X., Jiang L.-J., Chen Q., Wang T., Ye D.-W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 2020;41(8):531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lagunas-Rangel F.A., Chávez-Valencia V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pomponio G., Ferrarini A., Bonifazi M., Moretti M., Salvi A., Giacometti A., et al. Tocilizumab in COVID-19 interstitial pneumonia. J. Intern. Med. 2021;289(5):738–746. doi: 10.1111/joim.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samaee H., Mohsenzadegan M., Ala S., Maroufi S.S., Moradimajd P. Tocilizumab for treatment patients with COVID-19: recommended medication for novel disease. Int. Immunopharmacol. 2020;107018 doi: 10.1016/j.intimp.2020.107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nissen C.B., Sciascia S., de Andrade D., Atsumi T., Bruce I.N., Cron R.Q., et al. The role of antirheumatics in patients with COVID-19. The Lancet. Rheumatology. 2021 doi: 10.1016/S2665-9913(21)00062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tahmasebi S., Elahi R., Khosh E., Esmaeilzadeh A. Programmable and multi-targeted CARs: a new breakthrough in cancer CAR-T cell therapy. Clin. Transl. Oncol. 2021;23(6):1003–1019. doi: 10.1007/s12094-020-02490-9. [DOI] [PubMed] [Google Scholar]
- 65.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Price C.C., Altice F.L., Shyr Y., Koff A., Pischel L., Goshua G., et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: survival and clinical outcomes. Chest. 2020;158(4):1397–1408. doi: 10.1016/j.chest.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassoun A., Thottacherry E.D., Muklewicz J. Utilizing tocilizumab for the treatment of cytokine release syndrome in COVID-19. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fomina D.S., Mar'yana A.L., Beloglazova I.P., Mutovina Z.Y., Poteshkina N.G., Samsonova I.V., et al. Temporal clinical and laboratory response to interleukin-6 receptor blockade with Tocilizumab in 89 hospitalized patients with COVID-19 pneumonia. Pathogens Immun. 2020;5(1):327. doi: 10.20411/pai.v5i1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Selvaraj V., Khan M.S., Bavishi C., Dapaah-Afriyie K., Finn A., Lal A., et al. Tocilizumab in hospitalized COVID-19 patients: A meta-analysis of randomized controlled trials. medRxiv. 2021 doi: 10.1007/s00408-021-00451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kewan T., Covut F., Al-Jaghbeer M.J., Rose L., Gopalakrishna K., Akbik B. Tocilizumab for treatment of patients with severe COVID–19: A retrospective cohort study. EClinicalMedicine. 2020;24:100418. doi: 10.1016/j.eclinm.2020.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ignatius E.H., Wang K., Karaba A., Robinson M., Avery R.K., Blair P., editors. Tocilizumab for the treatment of COVID-19 among hospitalized patients: a matched retrospective cohort analysis. Open Forum Infectious Diseases. Oxford University Press US; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canziani L.M., Trovati S., Brunetta E., Testa A., De Santis M., Bombardieri E., et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J. Autoimmun. 2020;114 doi: 10.1016/j.jaut.2020.102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaye A.G., Siegel R. The efficacy of IL-6 inhibitor Tocilizumab in reducing severe COVID-19 mortality: a systematic review. PeerJ. 2020;8 doi: 10.7717/peerj.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aziz M., Haghbin H., Abu Sitta E., Nawras Y., Fatima R., Sharma S., et al. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J. Med. Virol. 2021;93(3):1620–1630. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 77.Ramaswamy M., Mannam P., Comer R., Sinclair E., McQuaid D.B., Schmidt M.L. Off-label real world experience using tocilizumab for patients hospitalized with COVID-19 disease in a regional community health system: a case-control study. medrxiv. 2020 [Google Scholar]
- 78.Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2021;73(2):e445–e454. doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M.P., et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Internal Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wadud N., Ahmed N., Shergil M.M., Khan M., Krishna M.G., Gilani A., et al. Improved survival outcome in SARs-CoV-2 (COVID-19) acute respiratory distress syndrome patients with tocilizumab administration. medRxiv. 2020 [Google Scholar]
- 81.Klopfenstein T., Zayet S., Lohse A., Balblanc J.-C., Badie J., Royer P.-Y., et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Medecine et maladies infectieuses. 2020;50(5):397–400. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossotti R., Travi G., Ughi N., Corradin M., Baiguera C., Fumagalli R., et al. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. J. Infect. 2020;81(4):e11–e17. doi: 10.1016/j.jinf.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S., et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morena V., Milazzo L., Oreni L., Bestetti G., Fossali T., Bassoli C., et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur. J. Int. Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eimer J., Vesterbacka J., Svensson A.K., Stojanovic B., Wagrell C., Sönnerborg A., et al. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. J. Intern. Med. 2020 doi: 10.1111/joim.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rojas-Marte G., Khalid M., Mukhtar O., Hashmi A.T., Waheed M.A., Ehrlich S., et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case–controlled study. QJM: Int. J. Med. 2020;113(8):546–550. doi: 10.1093/qjmed/hcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strohbehn G.W., Heiss B.L., Rouhani S.J., Trujillo J.A., Yu J., Kacew A.J., et al. COVIDOSE: Low-dose tocilizumab in the treatment of Covid-19. medRxiv. 2020 doi: 10.1002/cpt.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alattar R., Ibrahim T.B., Shaar S.H., Abdalla S., Shukri K., Daghfal J.N., et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 2020;92(10):2042–2049. doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borku Uysal B., Ikitimur H., Yavuzer S., Ikitimur B., Uysal H., Islamoglu M.S., et al. Tocilizumab challenge: A series of cytokine storm therapy experiences in hospitalized COVID-19 pneumonia patients. J. Med. Virol. 2020;92(11):2648–2656. doi: 10.1002/jmv.26111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Issa N., Dumery M., Guisset O., Mourissoux G., Bonnet F., Camou F. Feasibility of tocilizumab in ICU patients with COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin. Exp. Rheumatol. 2020;38(3):529–532. [PubMed] [Google Scholar]
- 92.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N. Engl. J. Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F., et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8(5):695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Int. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carvalho V., Turon R., Goncalves B., Ceotto V., Kurtz P., Righy C. Effects of tocilizumab in critically ill patients with COVID-19: a quasi-experimental study. medRxiv. 2020 [Google Scholar]
- 97.Lan S.-H., Lai C.-C., Huang H.-T., Chang S.-P., Lu L.-C., Hsueh P.-R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mastroianni A., Greco S., Apuzzo G., De Santis S., Oriolo C., Zanolini A., et al. Subcutaneous tocilizumab treatment in patients with severe COVID-19–related cytokine release syndrome: an observational cohort study. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kimmig L.M., Wu D., Gold M., Pettit N.N., Pitrak D., Mueller J., et al. Il-6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Front. Med. 2020;7:689. doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benucci M., Giannasi G., Cecchini P., Gobbi F.L., Damiani A., Grossi V., et al. COVID-19 pneumonia treated with sarilumab: a clinical series of eight patients. J. Med. Virol. 2020 doi: 10.1002/jmv.26062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khiali S., Rezagholizadeh A., Entezari-Maleki T. A comprehensive review on sarilumab in COVID-19. Expert Opin. Biol. Ther. 2021;21(5):615–626. doi: 10.1080/14712598.2021.1847269. [DOI] [PubMed] [Google Scholar]
- 103.Della-Torre E., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S., et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann. Rheum. Dis. 2020;79(10):1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gremese E., Cingolani A., Bosello S.L., Alivernini S., Tolusso B., Perniola S., et al. Sarilumab use in severe SARS-CoV-2 pneumonia. EClinicalMedicine. 2020;27 doi: 10.1016/j.eclinm.2020.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montesarchio V., Parrella R., Iommelli C., Bianco A., Manzillo E., Fraganza F., et al. Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. J. ImmunoTher. Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lescure F.-X., Honda H., Fowler R.A., Lazar J.S., Shi G., Wung P., et al. Sarilumab treatment of hospitalised patients with severe or critical COVID-19: a multinational, randomised, adaptive, phase 3, double-blind, placebo-controlled trial. medRxiv. 2021 [Google Scholar]
- 107.Lescure F.-X., Honda H., Fowler R.A., Lazar J.S., Shi G., Wung P., et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respirat. Med. 2021;9(5):522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takeuchi T., Thorne C., Karpouzas G., Sheng S., Xu W., Rao R., et al. Sirukumab for rheumatoid arthritis: the phase III SIRROUND-D study. Ann. Rheum. Dis. 2017;76(12):2001–2008. doi: 10.1136/annrheumdis-2017-211328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takeuchi T., Yamanaka H., Harigai M., Tamamura R., Kato Y., Ukyo Y., et al. Sirukumab in rheumatoid arthritis refractory to sulfasalazine or methotrexate: a randomized phase 3 safety and efficacy study in Japanese patients. Arthritis Res. Therapy. 2018;20(1):1–11. doi: 10.1186/s13075-018-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Convertino I., Tuccori M., Ferraro S., Valdiserra G., Cappello E., Focosi D., et al. Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients. Crit. Care. 2020;24(1):1–6. doi: 10.1186/s13054-020-03020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garbers C., Rose-John S. Genetic IL-6R variants and therapeutic inhibition of IL-6 receptor signalling in COVID-19. Lancet Rheumatol. 2021;3(2):e96–e97. doi: 10.1016/S2665-9913(20)30416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schreiber S., Aden K., Bernardes J.P., Conrad C., Tran F., Höper H., et al. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients With Active Inflammatory Bowel Disease. Gastroenterology. 2021;160(7) doi: 10.1053/j.gastro.2021.02.062. [DOI] [PubMed] [Google Scholar]
- 113.Chen B., Zhang S., Wang B., Chen H., Li Y., Cao Q., et al. 775b Olamkicept, an IL-6 Trans-Signaling Inhibitor, is Effective for Induction of Response and Remission in A Randomized, Placebo-Controlled Trial in Moderate to Severe Ulcerative Colitis. Gastroenterology. 2021;161(2):e28–e29. [Google Scholar]
- 114.Mazurov V., Korolev M., Kundzer A., Soroka N., Kastanayan A., Povarova T., et al. BMJ Publishing Group Ltd; 2021. POS0624 efficacy and safety of levilimab in combination with methotrexate in subjects with active rheumatoid arthritis: Phase III, double-blind, placebo-controlled, randomized trial. [DOI] [PubMed] [Google Scholar]
- 115.Lomakin N.V., Bakirov B.A., Protsenko D.N., Mazurov V.I., Musaev G.H., Moiseeva O.M., et al. The efficacy and safety of levilimab in severely ill COVID-19 patients not requiring mechanical ventilation: results of a multicenter randomized double-blind placebo-controlled phase III CORONA clinical study. Inflamm. Res. 2021;1–14 doi: 10.1007/s00011-021-01507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lomakin N., Bakirov B., Musaev G., Protsenko D., Moiseeva O., Pasechnik E., et al. BMJ Publishing Group Ltd; 2021. POS1214 the dynamics of inflammatory markers in COVID-19 patients treated with levilimab. [Google Scholar]
- 117.Bobkova S., Zhukov A., Protsenko D., Samoylenko V., Tyurin I. Comparative study of monoclonal anti-IL6 antibodies in severe new coronavirus disease COVID-19 patients. Retrospective Cohort Study City. 2021;22 [Google Scholar]
- 118.Luo J., Lu S., Yu M., Zhu L., Zhu C., Li C., et al. The potential involvement of JAK-STAT signaling pathway in the COVID-19 infection assisted by ACE2. Gene. 2021;768 doi: 10.1016/j.gene.2020.145325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Favoino E., Prete M., Catacchio G., Ruscitti P., Navarini L., Giacomelli R., et al. Working and safety profiles of JAK/STAT signaling inhibitors. Are these small molecules also smart? Autoimmun. Rev. 2021;102750 doi: 10.1016/j.autrev.2021.102750. [DOI] [PubMed] [Google Scholar]
- 120.Walz L., Cohen A.J., Rebaza A.P., Vanchieri J., Slade M.D., Cruz C.S.D., et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect. Dis. 2021;21(1):1–10. doi: 10.1186/s12879-020-05730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kubo S., Nakayamada S., Tanaka Y. Baricitinib for the treatment of rheumatoid arthritis and systemic lupus erythematosus: a 2019 update. Expert Rev. Clin. Immunol. 2019;15(7):693–700. doi: 10.1080/1744666X.2019.1608821. [DOI] [PubMed] [Google Scholar]
- 122.Praveen D., Puvvada R.C., Aanandhi V. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Favalli E.G., Biggioggero M., Maioli G., Caporali R. Baricitinib for COVID-19: a suitable treatment? Lancet. Infect. Dis. 2020;20(9):1012–1013. doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet(London, England) 2020;395(10223) doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saber-Ayad M., Hammoudeh S., Abu-Gharbieh E., Hamoudi R., Tarazi H., Al-Tel T.H., et al. Current Status of Baricitinib as a Repurposed Therapy for COVID-19. Pharmaceuticals. 2021;14(7):680. doi: 10.3390/ph14070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M., et al. JAK inhibition as a new treatment strategy for patients with COVID-19. Int. Arch. Allergy Immunol. 2020;181(6):467–475. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stebbing J., Krishnan V., de Bono S., Ottaviani S., Casalini G., Richardson P.J., et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020;12(8) doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Petrone L., Petruccioli E., Alonzi T., Vanini V., Cuzzi G., Fard S.N., et al. In-vitro evaluation of the immunomodulatory effects of Baricitinib: Implication for COVID-19 therapy. J. Infect. 2021;82(4):58–66. doi: 10.1016/j.jinf.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marconi V.C., Ramanan A.V., de Bono S., Kartman C.E., Krishnan V., Liao R., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. The Lancet. Respir. Med. 2021 doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kalil A.C., Stebbing J. Baricitinib: the first immunomodulatory treatment to reduce COVID-19 mortality in a placebo-controlled trial. The Lancet. Respir. Med. 2021 doi: 10.1016/S2213-2600(21)00358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 2020;81(2):318. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cantini F., Niccoli L., Nannini C., Matarrese D., Di Natale M.E., Lotti P., et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mullally A., Hood J., Harrison C., Mesa R. Fedratinib in myelofibrosis. Blood Adv. 2020;4(8):1792–1800. doi: 10.1182/bloodadvances.2019000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guglani L., Khader S.A. Th17 cytokines in mucosal immunity and inflammation. Curr. Opin. HIV AIDS. 2010;5(2):120. doi: 10.1097/COH.0b013e328335c2f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Casillo G.M., Mansour A.A., Raucci F., Saviano A., Mascolo N., Iqbal A.J., et al. Could IL-17 represent a new therapeutic target for the treatment and/or management of COVID-19-related respiratory syndrome? Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zerbini C.A., Lomonte A.B.V. Tofacitinib for the treatment of rheumatoid arthritis. Expert Rev. Clin. Immunol. 2012;8(4):319–331. doi: 10.1586/eci.12.19. [DOI] [PubMed] [Google Scholar]
- 137.Guimarães P.O., Quirk D., Furtado R.H., Maia L.N., Saraiva J.F., Antunes M.O., et al. Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jacobs J., Clark-Snustad K., Lee S. Case report of a SARS-CoV-2 infection in a patient with ulcerative colitis on tofacitinib. Inflamm. Bowel Dis. 2020;26(7) doi: 10.1093/ibd/izaa093. e64-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Agrawal M., Brenner E.J., Zhang X., Modesto I., Woolcott J., Ungaro R.C., et al. Characteristics and Outcomes of IBD Patients with COVID-19 on Tofacitinib Therapy in the SECURE-IBD Registry. Inflamm. Bowel Dis. 2020 doi: 10.1093/ibd/izaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maslennikov R., Ivashkin V., Vasilieva E., Chipurik M., Semikova P., Semenets V., et al. Tofacitinib reduces mortality in coronavirus disease 2019 Tofacitinib in COVID-19. Pulm. Pharmacol. Ther. 2021;69 doi: 10.1016/j.pupt.2021.102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fleischmann R., Pangan A.L., Song I.H., Mysler E., Bessette L., Peterfy C., et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 142.Biggioggero M., Becciolini A., Crotti C., Agape E., Favalli E.G. Upadacitinib and filgotinib: the role of JAK1 selective inhibition in the treatment of rheumatoid arthritis. Drugs Context. 2019;8 doi: 10.7573/dic.212595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peterson D., Damsky W., King B. Reply: Calm before the storm: understanding the role of Janus kinase inhibitors in COVID-19. J. Am. Acad. Dermatol. 2020;83(1):e67–e68. doi: 10.1016/j.jaad.2020.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yeleswaram S., Smith P., Burn T., Covington M., Juvekar A., Li Y., et al. Inhibition of cytokine signaling by ruxolitinib and implications for COVID-19 treatment. Clin. Immunol. 2020;218 doi: 10.1016/j.clim.2020.108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146(1) doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Innes A.J., Cook L.B., Marks S., Bataillard E., Crossette-Thambiah C., Sivasubramaniam G., et al. Ruxolitinib for tocilizumab-refractory severe COVID-19 infection. Br. J. Haematol. 2020 doi: 10.1111/bjh.16979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaplanski G., Bontemps D., Esnault P., Blasco V., Carvelli J., Delarbre D., et al. Combined Anakinra and Ruxolitinib treatment to rescue extremely ill COVID-19 patients: A pilot study. Autoimmun. Rev. 2021;20(2) doi: 10.1016/j.autrev.2020.102726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bagca B.G., Avci C.B. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020;54:51–61. doi: 10.1016/j.cytogfr.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.G. Caocci, G. La Nasa, Could ruxolitinib be effective in patients with COVID-19 infection at risk of acute respiratory distress syndrome (ARDS)? 2020. [DOI] [PMC free article] [PubMed]
- 150.Pfeifer N.D., Lo A., Bourdet D.L., Colley K., Singh D. Phase I study in healthy participants to evaluate safety, tolerability, and pharmacokinetics of inhaled nezulcitinib, a potential treatment for COVID-19. Clin. Transl. Sci. 2021 doi: 10.1111/cts.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh D., Bogus M., Moskalenko V., Lord R., Moran E.J., Crater G.D., et al. A phase 2 multiple ascending dose study of the inhaled pan-JAK inhibitor nezulcitinib (TD-0903) in severe COVID-19. Eur. Respir. J. 2021;58(4) doi: 10.1183/13993003.00673-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Doyle E., Bentley D., Dodds M. COVID-19: Simulation Study of Tocilizumab and Siltuximab Interventions in the Context of Acute Respiratory Distress Syndrome. Authorea Preprints. 2020 [Google Scholar]
- 153.Palanques-Pastor T., López-Briz E., Andrés J.L.P. Involvement of interleukin 6 in SARS-CoV-2 infection: siltuximab as a therapeutic option against COVID-19. Eur. J. Hospital Pharm. 2020;27(5):297–298. doi: 10.1136/ejhpharm-2020-002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gritti G., Raimondi F., Ripamonti D., Riva I., Landi F., Alborghetti L., et al. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. medRxiv. 2020 [Google Scholar]
- 155.Gritti G., Raimondi F., Ripamonti D., Riva I., Landi F., Alborghetti L., et al. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. medRxiv. 2020 [Google Scholar]
- 156.Inoue K., Kodama T., Daida H. Pentraxin 3: a novel biomarker for inflammatory cardiovascular disease. Int. J. Vasc. Med. 2012;2012 doi: 10.1155/2012/657025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gritti G., Raimondi F., Bottazzi B., Ripamonti D., Riva I., Landi F., et al. Siltuximab downregulates interleukin-8 and pentraxin 3 to improve ventilatory status and survival in severe COVID-19. Leukemia. 2021;1–5 doi: 10.1038/s41375-021-01299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shaw S., Bourne T., Meier C., Carrington B., Gelinas R., Henry A., editors. Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs. Taylor & Francis; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Genovese M.C., Fleischmann R., Furst D., Janssen N., Carter J., Dasgupta B., et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann. Rheum. Dis. 2014;73(9):1607–1615. doi: 10.1136/annrheumdis-2013-204760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang L., Liu W., Yu X., Wu M., Reichert J.M., Ho M. COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antibody Therapeutics. 2020;3(3):205–212. doi: 10.1093/abt/tbaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tsvetov V., Matveev A., Sychev D. Rationality of routine clinical use of olokizumab in COVID-19. Kachestvennaya Klinicheskaya Praktika= Good Clinical Practice. 2020;4S:68–70. [Google Scholar]
- 162.Antonov V., Ignatova G., Pribytkova O., Sleptsova S., Strebkova E., Khudyakova E., et al. Experience of olokizumab use in COVID-19 patients. Ter. Arkh. 2020;92(12):148–154. doi: 10.26442/00403660.2020.12.200522. [DOI] [PubMed] [Google Scholar]
- 163.Gracia-Hernandez M., Sotomayor E.M., Villagra A. Targeting macrophages as a therapeutic option in COVID-19. Front. Pharmacol. 2020;11:1659. doi: 10.3389/fphar.2020.577571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen P.-L., Lee N.-Y., Cia C.-T., Ko W.-C., Hsueh P.-R. A review of treatment of coronavirus disease 2019 (COVID-19): therapeutic repurposing and unmet clinical needs. Front. Pharmacol. 2020:11. doi: 10.3389/fphar.2020.584956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vaidya G., Czer L.S., Kobashigawa J., Kittleson M., Patel J., Chang D., editors. Successful treatment of severe COVID-19 pneumonia with clazakizumab in a heart transplant recipient: a case report. Transplantation proceedings. Elsevier; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weinblatt M., Dorner T., Zeldin R., editors. Results of a phase IIb study of vobarilizumab, an anti-interleukin 6 receptor nanobody, in patients with moderate-to-severe rheumatoid arthritis despite treatment with methotrexate. Journal of rheumatology Conference: 72nd annual meeting of the canadian rheumatology association. CRA; 2017. [Google Scholar]
- 167.Ezzikouri S., Nourlil J., Tsukiyama-Kohara K., Kohara M., El Ossmani H., Windisch M.P., et al. Nanobodies: an unexplored opportunity to combat COVID-19. J. Biomol. Struct. Dyn. 2020;1–3 doi: 10.1080/07391102.2020.1845801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Han Q., Guo M., Zheng Y., Zhang Y., De Y., Xu C., et al. Current evidence of interleukin-6 signaling inhibitors in patients with COVID-19: a systematic review and meta-analysis. Front. Pharmacol. 2020;11:2119. doi: 10.3389/fphar.2020.615972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bonek K., Roszkowski L., Massalska M., Maslinski W., Ciechomska M. Biologic Drugs for Rheumatoid Arthritis in the Context of Biosimilars, Genetics, Epigenetics and COVID-19 Treatment. Cells. 2021;10(2):323. doi: 10.3390/cells10020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hannon C.W., McCourt C., Lima H.C., Chen S., Bennett C. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst. Rev. 2021;3 doi: 10.1002/14651858.CD007478.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Van Roy M., Van de Sompel A., De Smet K., Jacobs J., Denayer T., Ulrichts H., et al. FRI0021 Alx-0061, an anti-IL-6r nanobody® for therapeutic use in rheumatoid arthritis, demonstrates in vitro a differential biological activity profile as compared to tocilizumab. Ann. Rheumatic Dis. 2013;72(Suppl 3) A375-A. [Google Scholar]
- 172.Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sharun K., Tiwari R., Dhama J., Dhama K. Dexamethasone to combat cytokine storm in COVID-19: Clinical trials and preliminary evidence. Int. J. Surg. (London, England). 2020;82:179. doi: 10.1016/j.ijsu.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stahn C., Löwenberg M., Hommes D.W., Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol. Cell. Endocrinol. 2007;275(1–2):71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 175.Salton F., Confalonieri P., Meduri G.U., Santus P., Harari S., Scala R., editors. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open forum infectious diseases. Oxford University Press US; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Unlap T., Jope R.S. Inhibition of NFkB DNA binding activity by glucocorticoids in rat brain. Neurosci. Lett. 1995;198(1):41–44. doi: 10.1016/0304-3940(95)11963-w. [DOI] [PubMed] [Google Scholar]
- 177.Meduri G.U., Bridges L., Shih M.-C., Marik P.E., Siemieniuk R.A., Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42(5):829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 178.Schleimer R.P., Freeland H., Peters S., Brown K., Derse C. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J. Pharmacol. Exp. Ther. 1989;250(2):598–605. [PubMed] [Google Scholar]
- 179.Centrella M., McCARTHY T.L., Canalis E. Glucocorticoid regulation of transforming growth factor beta 1 activity and binding in osteoblast-enriched cultures from fetal rat bone. Mol. Cell. Biol. 1991;11(9):4490–4496. doi: 10.1128/mcb.11.9.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mattos-Silva P., Felix N.S., Silva P.L., Robba C., Battaglini D., Pelosi P., et al. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir. Physiol. Neurobiol. 2020;280 doi: 10.1016/j.resp.2020.103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Prescott H.C., Rice T.W. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA. 2020;324(13):1292–1295. doi: 10.1001/jama.2020.16747. [DOI] [PubMed] [Google Scholar]
- 182.Group TRC Dexamethasone in hospitalized patients with Covid-19—preliminary report. New Engl. J. Med. 2020 [Google Scholar]
- 183.Mahase E. Covid-19: demand for dexamethasone surges as RECOVERY trial publishes preprint. BMJ: Brit. Med. J. (Online) 2020;369 doi: 10.1136/bmj.m2512. [DOI] [PubMed] [Google Scholar]
- 184.Rubio-Rivas M., Ronda M., Padulles A., Mitjavila F., Riera-Mestre A., García-Forero C., et al. Beneficial effect of corticosteroids in preventing mortality in patients receiving tocilizumab to treat severe COVID-19 illness. Int. J. Infectious Dis. 2020;101:290–297. doi: 10.1016/j.ijid.2020.09.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Balena F., Bavaro D.F., Fabrizio C., Bottalico I.F., Calamo A., Santoro C.R., et al. Tocilizumab and corticosteroids for COVID-19 treatment in elderly patients. J. Gerontol. Geriatr. 2020;68:197–203. [Google Scholar]
- 186.Luis B.-M., Miguel M.-B., Pedro D.-L., David I.-P., Itziar A., Ana G.-H., et al. Benefits of early aggressive immunomodulatory therapy (tocilizumab and methylprednisolone) in COVID-19: single center cohort study of 685 patients. J. Translat. Autoimmun. 2021:100086. doi: 10.1016/j.jtauto.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li L., Li R., Wu Z., Yang X., Zhao M., Liu J., et al. Therapeutic strategies for critically ill patients with COVID-19. Ann. Intensive Care. 2020;10(1):1–9. doi: 10.1186/s13613-020-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.López-Medrano F., Asín M.-A.-P.-J., Fernández-Ruiz M., Carretero O., Lalueza A., de la Calle G.M., et al. Combination therapy with tocilizumab and corticosteroids for aged patients with severe COVID-19 pneumonia: a single-center retrospective study. Int. J. Infectious Dis. 2021;105:487–494. doi: 10.1016/j.ijid.2021.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Strohbehn G.W., Heiss B.L., Rouhani S.J., Trujillo J.A., Yu J., Kacew A.J., et al. COVIDOSE: A Phase II Clinical Trial of Low-Dose Tocilizumab in the Treatment of Noncritical COVID-19 Pneumonia. Clin. Pharmacol. Ther. 2021;109(3):688–696. doi: 10.1002/cpt.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang S., Peng Y., Wang R., Jiao S., Wang M., Huang W., et al. An antibody-dependent enhancement (ADE) activity eliminated neutralizing antibody with potent prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. bioRxiv. 2020 doi: 10.1038/s41467-020-19568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Focosi D., Anderson A.O., Tang J.W., Tuccori M. Convalescent plasma therapy for COVID-19: state of the art. Clin. Microbiol. Rev. 2020;33(4):e00072–e120. doi: 10.1128/CMR.00072-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Faisal S., Aman K., Rahman A.u. Innate immune-mediated antiviral response to SARS-CoV-2 and convalescent sera a potential prophylactic and therapeutic agent to tackle COVID-19. Antibody Therapeut. 2020;3(3):212–220. doi: 10.1093/abt/tbaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Investig. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;323(16):1561–1562. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 195.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tiberghien P., de Lamballerie X., Morel P., Gallian P., Lacombe K., Yazdanpanah Y. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how? Vox Sang. 2020;115(6):488–494. doi: 10.1111/vox.12926. [DOI] [PubMed] [Google Scholar]
- 197.Khamis F., Al-Zakwani I., Al Hashmi S., Al Dowaiki S., Al Bahrani M., Pandak N., et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int. J. Infectious Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zeng H., Wang D., Nie J., Liang H., Gu J., Zhao A., et al. The efficacy assessment of convalescent plasma therapy for COVID-19 patients: a multi-center case series. Signal Transduct. Targeted Therapy. 2020;5(1):1–12. doi: 10.1038/s41392-020-00329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Roshandel E., Sankanian G., Salimi M., Jalili A., Salari S., Sadeghi A., et al. Plasma exchange followed by convalescent plasma transfusion in COVID-19 patients. Transfus. Apheres. Sci. 2021;103141 doi: 10.1016/j.transci.2021.103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mongardon N., Piagnerelli M., Grimaldi D., Perrot B., Lascarrou J.-B. Impact of late administration of corticosteroids in COVID-19 ARDS. Intensive Care Med. 2021;47(1):110–112. doi: 10.1007/s00134-020-06311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 2020;35(14) doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tamburello A., Marando M. Immunoglobulins or convalescent plasma to tackle COVID-19: buying time to save lives–current situation and perspectives. Swiss Med. Weekly. 2020;150(1718) doi: 10.4414/smw.2020.20264. [DOI] [PubMed] [Google Scholar]
- 203.Kesici S., Yavuz S., Bayrakci B. Get rid of the bad first: therapeutic plasma exchange with convalescent plasma for severe COVID-19. Proc. Natl. Acad. Sci. 2020;117(23):12526–12527. doi: 10.1073/pnas.2006691117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int. J. Mol. Sci. 2020;21(7):2272. doi: 10.3390/ijms21072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sakoulas G., Geriak M., Kullar R., Greenwood K., Habib M., Vyas A., et al. Intravenous Immunoglobulin (IVIG) significantly reduces respiratory morbidity in COVID-19 pneumonia: a prospective randomized trial. medRxiv. 2020 [Google Scholar]
- 206.Senniappan K., Jeyabalan S., Rangappa P., Kanchi M. Hyperbaric oxygen therapy: Can it be a novel supportive therapy in COVID-19? Indian J. Anaesthesia. 2020;64(10):835. doi: 10.4103/ija.IJA_613_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Guo D., Pan S., Wang M., Guo Y. Hyperbaric oxygen therapy may be effective to improve hypoxemia in patients with severe COVID-2019 pneumonia: two case reports. Undersea Hyperb. Med. 2020;47(2):181–187. [PubMed] [Google Scholar]
- 208.Arıcıgil M., Dündar M.A., Yücel A., Arbağ H., Arslan A., Aktan M., et al. Anti-inflammatory effects of hyperbaric oxygen on irradiated laryngeal tissues☆. Brazil. J. Otorhinolaryngol. 2018;84:206–211. doi: 10.1016/j.bjorl.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gorenstein S.A., Castellano M.L., Slone E.S., Gillette B., Liu H., Alsamarraie C., et al. Hyperbaric oxygen therapy for COVID-19 patients with respiratory distress: treated cases versus propensity-matched controls. Undersea Hyperb. Med. 2020;405–13 [PubMed] [Google Scholar]
- 210.Thibodeaux K., Speyrer M., Raza A., Yaakov R., Serena T.E. Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: a retrospective case series. J. Wound Care. 2020;29(Sup5a):S4–S8. doi: 10.12968/jowc.2020.29.Sup5a.S4. [DOI] [PubMed] [Google Scholar]
- 211.Oliaei S., SeyedAlinaghi S., Mehrtak M., Karimi A., Noori T., Mirzapour P., et al. The effects of hyperbaric oxygen therapy (HBOT) on coronavirus disease-2019 (COVID-19): a systematic review. Eur. J. Med. Res. 2021;26(1):1–12. doi: 10.1186/s40001-021-00570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Simonetti V., Quagliariello V., Franzini M., Iaffaioli R., Maurea N., Valdenassi L. Ozone exerts cytoprotective and anti-inflammatory effects in cardiomyocytes and skin fibroblasts after incubation with doxorubicin. Evidence-Based Complement. Alternat. Med. 2019;2019 doi: 10.1155/2019/2169103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fernández-Cuadros M.E., Albaladejo-Florín M.J., Peña-Lora D., Álava-Rabasa S., Pérez-Moro O.S. Ozone (O3) and SARS-CoV-2: physiological bases and their therapeutic possibilities according to COVID-19 evolutionary stage. SN Comprehensive. Clin. Med. 2020:1–9. doi: 10.1007/s42399-020-00328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fernández-Cuadros M.E., Albaladejo-Florín M.J., Álava-Rabasa S., Gallego-Galiana J., Pérez-Cruz G.F., Usandizaga-Elio I., et al. Compassionate Use of Rectal Ozone (O 3) in Severe COVID-19 Pneumonia: a Case-Control Study. SN Comprehen. Clin. Med. 2021;3(5):1185–1199. doi: 10.1007/s42399-021-00849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lapidus N., Zhou X., Carrat F., Riou B., Zhao Y., Hejblum G. Biased and unbiased estimation of the average length of stay in intensive care units in the Covid-19 pandemic. Ann. Intensive Care. 2020;10(1):1–9. doi: 10.1186/s13613-020-00749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.de Cáceres C., Martínez R., Bachiller P., Marín L., García J.M. The effect of tocilizumab on cytokine release syndrome in COVID-19 patients. Pharmacol. Rep. 2020;72(6):1529–1537. doi: 10.1007/s43440-020-00186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.J. Alijotas-Reig, E. Esteve-Valverde, D. Ruiz-Hidalgo, G. Lopez-Sanchez, F. Martinez-Valle, J. Trape-Pujol, et al.. Anakinra as rescue therapy to treat patients with severe COVID-19 refractory to tocilizumab, 2020.
- 218.Michot J.-M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F., et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann. Oncol. 2020;31(7):961. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Serviddio G., Villani R., Stallone G., Scioscia G., Foschino-Barbaro M.P., Lacedonia D. COVID19-Tocilizumab and liver injury in patients with COVID-19. Therapeut. Adv. Gastroenterol. 2020;13 doi: 10.1177/1756284820959183. 1756284820959183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158(1):e15–e19. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pérez-Sáez M.J., Blasco M., Redondo-Pachón D., Ventura-Aguiar P., Bada-Bosch T., Pérez-Flores I., et al. Use of tocilizumab in kidney transplant recipients with COVID-19. Am. J. Transplant. 2020;20(11):3182–3190. doi: 10.1111/ajt.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Perrone F., Piccirillo M.C., Ascierto P.A., Salvarani C., Parrella R., Marata A.M., et al. Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial. J. Translat. Med. 2020;18(1):1–11. doi: 10.1186/s12967-020-02573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Alijotas-Reig J., Esteve-Valverde E., Belizna C., Selva-O'Callaghan A., Pardos-Gea J., Quintana A., et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmu. Rev. 2020;19(7):102569. doi: 10.1016/j.autrev.2020.102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tanner T., Wahezi D.M. Hyperinflammation and the utility of immunomodulatory medications in children with COVID-19. Paediatr. Respir. Rev. 2020 doi: 10.1016/j.prrv.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Coyle J., Igbinomwanhia E., Sanchez-Nadales A., Danciu S., Chu C., Shah N. A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001. Case Rep. 2020;2(9):1331–1336. doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.E. Raschi, P. Caraceni, E. Poluzzi, F. De Ponti, JEOoDS. Baricitinib, JAK inhibitors and liver injury: a cause for concern in COVID-19? 19(10) (2020) 1367–1369. [DOI] [PubMed]
- 228.D. Praveen, R.C. Puvvada, V. Aanandhi, JIjoaa. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19 55(5) (2020) 105967. [DOI] [PMC free article] [PubMed]
- 229.P.P. Dobesh, T.C. Trujillo, JPTJoHP, Therapy D. Coagulopathy, Venous Thromboembolism, and Anticoagulation in Patients with COVID‐19 40(11) (2020) 1130–1151. [DOI] [PMC free article] [PubMed]
- 230.S.C. Jorgensen, C.L. Tse, L. Burry, L.D. Dresser, JPTJoHP, Therapy D. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID‐19 40(8) (2020) 843-856. [DOI] [PMC free article] [PubMed]
- 231.T. Giménez Poderós, S. Gallardo Borge, P. Vazquez‐Ferreiro, JPTJoHP, Therapy D. Risk of Venous Thromboembolism Associated with Tofacitinib and Baricitinib: A Systematic Review and indirect Meta‐Analysis 40(12) (2020) 1248-1264. [DOI] [PubMed]
- 232.V. Gaspari, C. Zengarini, S. Greco, V. Vangeli, A. Mastroianni, JIjoaa. Side effects of ruxolitinib in patients with SARS-CoV-2 infection: two case reports 56(2) (2020) 106023. [DOI] [PMC free article] [PubMed]
- 233.R. Seror, M. Camus, J.-H. Salmon, C. Roux, E. Dernis, A. Basch, et al., Do JAK inhibitors affect immune response to COVID-19 vaccination? Data from the MAJIK-SFR Registry 2021. [DOI] [PMC free article] [PubMed]
- 234.G. Caocci, O. Mulas, D. Mantovani, A. Costa, A. Galizia, L. Barabino, et al., Ruxolitinib does not impair humoral immune response to COVID-19 vaccination with BNT162b2 mRNA COVID-19 vaccine in patients with myelofibrosis (2021) 1–3. [DOI] [PMC free article] [PubMed]
- 235.J. Radbel, N. Narayanan, P.J.J.C. Bhatt, Use of tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report 158(1) (2020) e15–e19. [DOI] [PMC free article] [PubMed]
- 236.S.K. Alzghari, V.S. Acuña, JJoCV. Supportive treatment with tocilizumab for COVID-19: a systematic review 127 (2020) 104380. [DOI] [PMC free article] [PubMed]
- 237.M. Mikulska, L.A. Nicolini, A. Signori, A. Di Biagio, C. Sepulcri, C. Russo, et al., Tocilizumab and steroid treatment in patients with COVID-19 pneumonia 15(8) (2020) e0237831. [DOI] [PMC free article] [PubMed]
- 238.J. Sabbatinelli, A. Giuliani, G. Matacchione, S. Latini, N. Laprovitera, G. Pomponio, et al.. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients 193 (2021) 111413. [DOI] [PMC free article] [PubMed]
- 239.O. Moreno-Pérez, M. Andres, J.-M. Leon-Ramirez, J. Sánchez-Payá, J.C. Rodríguez, R. Sánchez, et al., Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: A retrospective cohort study 114 (2020) 102523. [DOI] [PMC free article] [PubMed]
- 240.A. Sanchez-Montalva, J. Selares-Nadal, J. Espinosa-Pereiro, N. Fernandez-Hidalgo, S. Perez-Hoyos, F. Salvador, et al., Early outcomes of tocilizumab in adults hospitalized with severe COVID19. An initial report from the Vall dHebron COVID19 prospective cohort study, 2020.
- 241.F. Perrone, M.C. Piccirillo, P.A. Ascierto, C. Salvarani, R. Parrella, A.M. Marata, et al., Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 phase 2 trial 2020.
- 242.R.M. Petrak, N.C. Skorodin, N.W. Van Hise, R.M. Fliegelman, J. Pinsky, V. Didwania, et al., Tocilizumab as a therapeutic agent for critically ill patients infected with sARS‐CoV‐2, 2020. [DOI] [PMC free article] [PubMed]
- 243.M.G. Hernández-Mora, A.C. Úbeda, L. Prieto-Pérez, F.V. Álvarez, B.Á. Álvarez, M.J.R. Nieto, et al., Compassionate use of tocilizumab in severe SARS-CoV2 pneumonia. 102 (2021) 303–309. [DOI] [PMC free article] [PubMed]
- 244.M. Gorgolas, A. Cabello, L.P. Perez, F.V. Alvarez, B.A. Alvarez, M.J.R. Nieto, et al., Compassionate Use of Tocilizumab in Severe SARS-CoV2 Pneumonia. When late administration is too late, 2020.
- 245.P. Sinha, A. Mostaghim, C.G. Bielick, A. McLaughlin, D.H. Hamer, L.M. Wetzler, et al,. Early administration of Interleukin-6 inhibitors for patients with severe Covid-19 disease is associated with decreased intubation, reduced mortality, and increased discharge. 99 (2020) 28–33. [DOI] [PMC free article] [PubMed]
- 246.L. Campins, R. Boixeda, L. Perez-Cordon, R. Aranega, C. Lopera, L.J.C. Force, et al., Early tocilizumab treatment could improve survival among COVID-19 patients 38(3) (2020) 578. [PubMed]
- 247.G. Rojas-Marte, M. Khalid, O. Mukhta,r A.T. Hashmi, M.A. Waheed, S. Ehrlich, et al., Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case–controlled study 113(8) (2020) 546–550. [DOI] [PMC free article] [PubMed]
- 248.J. Martínez-Sanz, A. Muriel, R. Ron, S. Herrera, J.A. Pérez-Molina, S. Moreno, et al., Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicentre cohort study. 27(2) (2021) 238–243. [DOI] [PMC free article] [PubMed]
- 249.Ş. Keske, S. Tekin, B. Sait, P. İrkören, M. Kapmaz, C. Çimen, et al., Appropriate use of tocilizumab in COVID-19 infection 99 (2020) 338–343. [DOI] [PMC free article] [PubMed]
- 250.I.M. Tleyjeh, Z. Kashour, M. Damlaj, M. Riaz, H. Tlayjeh, M. Altannir, et al., Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis, 2020. [DOI] [PMC free article] [PubMed]
- 251.P. Luo, Y. Liu, L. Qiu, X. Liu, D. Liu, J.J. Li, Jomv. Tocilizumab treatment in COVID‐19: a single center experience 92(7) (2020) 814-818. [DOI] [PMC free article] [PubMed]
- 252.Marfella R., Paolisso P., Sardu C., Bergamaschi L., D’Angelo E.C., Barbieri M., et al. Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diabetes Metab. 2020;46(5):403–405. doi: 10.1016/j.diabet.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ghaebi M., Tahmasebi S., Jozghorbani M., Sadeghi A., Thangavelu L., Zekiy A.O., et al. Risk factors for adverse outcomes of COVID-19 patients: Possible basis for diverse responses to the novel coronavirus SARS-CoV-2. Life Sci. 2021;119503 doi: 10.1016/j.lfs.2021.119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.L.M. Canziani, S. Trovati, E. Brunetta, A. Testa, M. De Santis, E. Bombardieri, et al., Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients 114 (2020) 102511. [DOI] [PMC free article] [PubMed]
- 255.X. Xu, M. Han, T. Li, W. Sun, D. Wang, B. Fu, et al., Effective treatment of severe COVID-19 patients with tocilizumab. 117(20) (2020) 10970–10975. [DOI] [PMC free article] [PubMed]
- 256.Mazzitelli M., Arrighi E., Serapide F., Pelle M.C., Tassone B., Lionello R., et al. Use of subcutaneous tocilizumab in patients with COVID-19 pneumonia. J. Med. Virol. 2020 doi: 10.1002/jmv.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kaminski M.A., Sunny S., Balabayova K., Kaur A., Gupta A., Abdallah M., et al. Tocilizumab therapy for COVID-19: A comparison of subcutaneous and intravenous therapies. Int. J. Infectious Dis. 2020;101:59–64. doi: 10.1016/j.ijid.2020.09.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cingolani A., Tummolo A., Montemurro G., Gremese E., Larosa L., Cipriani M., et al. Baricitinib as rescue therapy in a patient with COVID-19 with no complete response to sarilumab. Infection. 2020;48(5):767–771. doi: 10.1007/s15010-020-01476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cano E.J., Fuentes X.F., Campioli C.C., O’Horo J.C., Saleh O.A., Odeyemi Y., et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest. 2021;159(3):1019–1040. doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Guardiola P.G., Ares J.Á.D., Tomás N.P., Tomás J.C.S., Martínez S.N. Intestinal perforation in patient with COVID-19 infection treated with tocilizumab and corticosteroids. Report of a clinical case. Cirugia Espan∼ ola. 2020 doi: 10.1016/j.ciresp.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Notaro S., Sorrentino M., Ruocco A., Notaro A., Corcione A., Murino P., et al. Combined use of high doses of vasopressin and corticosteroids in a patient with Crohn’s disease with refractory septic shock after intestinal perforation: a case report. J. Med. Case Rep. 2017;11(1):1–4. doi: 10.1186/s13256-017-1456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Emery P., Keystone E., Tony H., Cantagrel A., Van Vollenhoven R., Sanchez A., et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 2008;67(11):1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bermejo A.F.C., Ruiz-Antorán B., Cruz A.F., Sempere E.D., Díaz A.C., Rubio E.M., et al. Sarilumab versus standard of care for the early treatment of COVID-19 pneumonia in hospitalized patients: SARTRE: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):1–3. doi: 10.1186/s13063-020-04633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Doyle E.B., Bentley D., Dodds M.G. COVID-19 acute respiratory distress syndrome: A simulation study of the effects of combination therapy with tocilizumab and siltuximab. Br. J. Clin. Pharmacol. 2021 doi: 10.1111/bcp.15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mikulska M., Nicolini L.A., Signori A., Di Biagio A., Sepulcri C., Russo C., et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS ONE. 2020;15(8) doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Benmansour N.C., Carvelli J., Vivier E. Complement cascade in severe forms of COVID-19: Recent advances in therapy. Eur. J. Immunol. 2021;1–8 doi: 10.1002/eji.202048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Giudice V., Pagliano P., Vatrella A., Masullo A., Poto S., Polverino B.M., et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front. Pharmacol. 2020;11:857. doi: 10.3389/fphar.2020.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Aomar-Millán I.F., Salvatierra J., Torres-Parejo Ú., Faro-Miguez N., Callejas-Rubio J.L., Ceballos-Torres Á., et al. Anakinra after treatment with corticosteroids alone or with tocilizumab in patients with severe COVID-19 pneumonia and moderate hyperinflammation. A retrospective cohort study. Intern. Emerg. Med. 2021;16(4):843–852. doi: 10.1007/s11739-020-02600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bozzi G., Mangioni D., Minoia F., Aliberti S., Grasselli G., Barbetta L., et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: An observational cohort study. J. Allergy Clin. Immunol. 2021;147(2) doi: 10.1016/j.jaci.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.S. Atal, Z. Fatima, JPm. IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? 34(4) (2020) 223-231. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are immediately provided upon request by the reviewers.