Abstract
Microsporidia are obligate intracellular parasites, recognized as causing chronic diarrhea and systemic disease in AIDS patients, organ transplant recipients, travelers, and malnourished children. Species of microsporidia that infect humans have been detected in drinking-water sources, and methods are needed to ascertain if these microsporidia are viable and capable of causing infections. In this study, Calcofluor White M2R and Sytox Green stains were used in combination to differentiate between live (freshly harvested) and dead (boiled) Encephalitozoon cuniculi spores. Calcofluor White M2R binds to chitin in the microsporidian spore wall. Dual-stained live spores appeared as turquoise-blue ovals, while dead spores appeared as white-yellow ovals at an excitation wavelength of 395 to 415 nm used for viewing the Calcofluor stain. Sytox Green, a nuclear stain, is excluded by live spores but penetrates compromised spore membranes. Dual-stained dead spores fluoresced bright yellow-green when viewed at an excitation wavelength of 470 to 490 nm, whereas live spores failed to stain with Sytox Green. After live and dead spores were mixed at various ratios, the number of viably stained spores detected in the dual-staining procedure correlated (P = 0.0025) with the expected numbers of viable spores. Spore mixtures were also assayed for infectivity in a focus-forming assay, and a correlation (P = 0.0002) was measured between the percentage of focus-forming microsporidia and the percentage of expected infectious spores in each mixture. By analysis of variance, no statistically significant differences were measured between the percentage of viably stained microsporidia and the percentage of infectious microsporidia (P = 0.964) in each mixture. These results suggest that Calcofluor White M2R and Sytox Green stains, when used together, may facilitate studies to identify viable microsporidia.
Microsporidia are obligate intracellular parasites that infect both vertebrate and invertebrate hosts and have caused significant economic losses in the fishery, silkworm, and honeybee industries. At least 15 species of microsporidia are known to infect mammals (6, 7, 10, 26). Microsporidiosis in humans is primarily an opportunistic infection in those with compromised immune systems, such as AIDS patients and individuals undergoing organ transplants, but recently the illness has also been recognized in malnourished children and travelers (5, 20, 21). Diarrhea is the most common symptom of microsporidiosis, but infected persons may also, or instead, develop keratitis, sinusitis, pneumonia, encephalitis, myositis, peritonitis, hepatitis, or nephritis (16, 26). Transmission is believed to be primarily by the fecal-oral or urinary-oral route (7, 16, 26). Increasing interest in the waterborne transmission of microsporidia has led to the inclusion of microsporidia on the Drinking Water Contaminant List and the Occurrence Priorities List of the U.S. Environmental Protection Agency (13). In addition to improving methods to detect the presence of microsporidian spores in water sources, it will be important to determine if these spores are viable and truly present a disease threat. The purpose of this study is to describe a staining method utilizing Calcofluor White M2R and Sytox Green that can distinguish between viable and dead microsporidian spores.
MATERIALS AND METHODS
Organisms.
Encephalitozoon cuniculi spores were collected from the supernatants of infected RK-13 cell cultures as previously described (8, 11). The spores were washed with Tris-buffered saline (TBS, pH 7.2), then with TBS containing 0.3% Tween 20, and finally with TBS, with each centrifugation performed at 400 × g for 15 min at 4°C. To remove host cell debris and immature stages of the microsporidia, the pellet was resuspended in TBS and mixed with an equal volume of 100% Percoll (final concentration, 50%; Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) followed by centrifugation at 500 × g for 30 min at 4°C. The pelleted spores were washed in TBS, counted on a hemocytometer, and diluted to give a final concentration of 108 spores per ml. Half of the spores were used fresh (live), and the other half were boiled for 10 min (dead). The live and dead spores were mixed in ratios of 0:100, 25:75, 50:50, 75:25, and 100:0, respectively, for use in the staining and infectivity studies.
Staining procedure.
Aliquots of 100 μl of each live-dead spore mixture were washed with TBS, resuspended in 100 μl of H2O containing 50 μM Sytox Green nucleic acid stain (Molecular Probes, Inc., Eugene, Oreg.), and incubated for 30 min at room temperature. The spores then were washed once in H2O, and 5 μl of each mixture was spotted onto slides. The slides were allowed to dry, quickly fixed in methanol, stained with 5 mg of Calcofluor White M2R (Sigma, St. Louis, Mo.)/ml of H2O for 5 min, rinsed in H2O, and allowed to dry. Slides were viewed under oil (without coverslipping) by using an Olympus AH-2 fluorescent microscope at a magnification of ×600. Dead spores were counted as yellow-green ovals through the 470- to 490-nm-excitation-wavelength filter used for viewing Sytox Green staining, and the total number of spores was counted as turquoise or white-yellow ovals through the 395- to 415-nm-excitation-wavelength filter used for viewing Calcofluor White M2R staining. At least 10 fields were counted per slide per spore mixture at a final ×600 magnification.
Infectivity assay.
A focus-forming assay was used to determine the percentage of infectious versus noninfectious microsporidia in each mixture of spores (19). Briefly, confluent monolayers of RK-13 cells in 24-well tissue culture plates (Costar Corp., Cambridge, Mass.) were seeded with 100-μl aliquots of each spore suspension mixture in triplicate. The medium was changed on day 3, and on day 7 the monolayers were fixed and Giemsa stained. Infected cells (foci) were counted by viewing the plates through a Bausch & Lomb inverted-light microscope at a magnification of ×200. The numbers of foci per 10 fields per well were averaged, and the standard deviations were determined. Percent values were calculated against the mean number of foci in wells inoculated with an equal number of freshly harvested microsporidia.
Statistical analyses.
Comparisons between the tested (i.e., measured) values of viable and infectious spores against expected numbers of viable and infectious spores in each mixture were measured by linear (Pearson) correlation using Graphpad Instat version 3.00 for Windows (Graphpad Software, San Diego, Calif. [www.graphpad.com]). Analysis of variance (ANOVA) was used to compare results for viably stained spores and infectious-focus-forming spores in each mixture.
RESULTS
Staining characteristics of live and dead E. cuniculi spores.
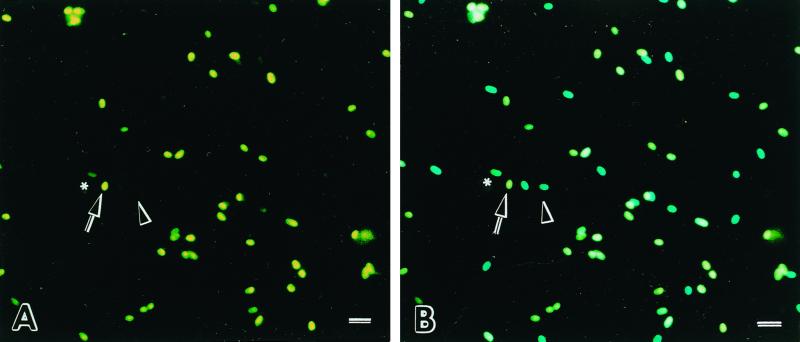
Freshly harvested live microsporidian spores that were double stained with Sytox Green and Calcofluor White M2R appeared as turquoise-blue ovals when seen through the 395- to 415-nm- excitation-wavelength filter used for viewing Calcofluor staining but were not visible or displayed a very pale green outline when observed through the 470- to 490-nm-excitation-wavelength filter used for viewing Sytox Green staining (Table 1). The dual-stained boiled (dead) microsporidia appeared as white-yellow ovals when viewed through the 395- to 415-nm- wavelength filter and as bright yellow-green ovals when viewed through the 470- to 490-nm-excitation-wavelength filter (Fig. 1).
TABLE 1.
Characteristics of live and dead E. cuniculi spores following dual staining with Calcofluor White M2R and Sytox Green
| Spore type | Appearance at excitation filter wavelength in nm (stain viewed) of:
|
|
|---|---|---|
| 395–415 (Calcofluor White M2R) | 470–490 (Sytox Green) | |
| Viable (live) | Turquoise-blue ovals | Not visible or barely visible green ovals |
| Boiled (dead) | White-yellowish ovals | Yellow-green ovals |
FIG. 1.
Freshly harvested (live) and boiled (dead) E. cuniculi spores stained with Sytox Green and Calcofluor White M2R. (A) When viewed through a blue filter (excitation wavelength, 470 to 490 nm), live spores are not visible or may appear as faint green oval cells (arrowhead), while dead spores fluoresce yellow-green (arrow). A few spores appeared semipermeant to the Sytox Green, suggesting dying spores (asterisk). (B) When the same area is viewed under a violet filter (excitation wavelength, 395 to 415 nm), dual-stained live spores appear as turquoise-blue ovals (arrowhead) and dead spores appear as bright white-yellow ovals (arrow). Bar = 10 μm.
Discrimination between live and dead E. cuniculi spores in mixtures.
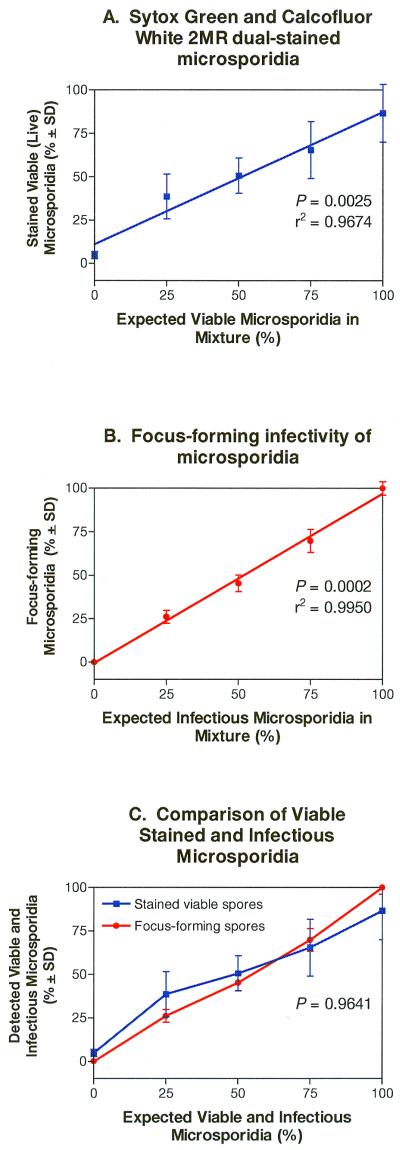
Freshly harvested (live) and boiled (dead) E. cuniculi spores were mixed in various ratios and stained with Sytox Green and Calcofluor. Dead and viable spores were counted in at least 10 fields per slide, and the mean percentage of viable spores in each mixture was plotted against the expected percentage of viable spores in each mixture (Fig. 2A). The results indicated that the mean percentage values of viably counted spores correlated with the expected percentage values of live spores in each mixture of spores (P = 0.0025; r2 = 0.9674).
FIG. 2.
Dual-staining procedure and infectivity assay of live and dead E. cuniculi spore mixtures. (A) Comparison of the percentage of viably stained and expected percentage of viable E. cuniculi spores in each mixture of live and dead spores. The number of spores stained by Sytox Green (dead) and Calcofluor White M2R (all spores) per field was counted. The comparison between the number of viably stained spores and expected number of spores was determined by linear (Pearson) correlation analysis (P = 0.0025). (B) Comparison of the detected percentage of focus-forming (infectious) organisms and expected percentage of viable E. cuniculi spores in the mixtures of live and dead spore suspensions. The comparison between the number of infectious-focus-forming spores and expected number of infectious spores in each mixture was determined by linear (Pearson) correlation analysis (P = 0.0002). (C) Comparison between viably stained and infectious E. cuniculi spores mixed in various ratios (i.e., composite of panels A and B). ANOVA was used for statistical comparison of viability determination and infectivity (P = 0.9641).
Infectivity of spores in the mixtures.
To correlate the infectivity of spores with viability staining characteristics, aliquots of each live-dead spore suspension were added to confluent monolayers of RK-13 host cells; 7 days later, the number of infected cells (foci) was counted. The mean percentage of infectious foci produced in each mixture was plotted against the expected percentage of infectious foci (based on the percentage of viable spores added to each mixture), and the results depicted in Fig. 2B show that there was a correlation between the measured and expected quantities of infectious foci generated from each spore suspension mixture (P = 0.0002, r2 = 0.9950).
Comparison of the dual-staining method with the infectivity assay for defining viability of spores.
ANOVA was used to compare the mean percentage of viably stained E. cuniculi spores in each mixture with the mean percentage of infectious-focus-forming spores in each mixture (Fig. 2C). No statistically significant differences were measured, suggesting that the dual-staining procedure can be used to measure viable and infectious microsporidia.
DISCUSSION
This study demonstrated a technique for combining the nucleic acid stain Sytox Green and the chitin stain Calcofluor M2R to assess the viability of E. cuniculi microsporidian spores. Staining procedures utilizing fluorescent brighteners, such as Calcofluor White M2R and Uvitex 2B (22–24), have proven to be sensitive for detecting microsporidia, particularly when corroborated with the modified trichrome blue staining procedure (9, 14, 15, 17, 18, 22, 25) or indirect immunofluorescence antibody staining (1–3, 12, 27, 28). Nucleic acid stains have been used previously as indicators of parasite cell viability, as applied by Belosevic and colleagues, who combined the use of the nucleic acid stain Sytox 59 with immunofluorescence staining to evaluate the viability of Cryptosporidium parvum oocysts (4). In this study, viable microsporidian spores from the species E. cuniculi were found to exclude the impermeant nucleic acid stain Sytox Green. In spore preparations stained singly with Sytox Green, dead cells fluoresced bright green, while viable spores did not take up this stain and could not be seen at 470 to 490 nm (not shown). In preparations stained with both Sytox Green and Calcofluor, dead spores, however, appeared as bright yellow-green ovals when viewed at 470 to 490 nm, which was likely due to the counterstaining with Calcofluor having shifted the Sytox Green staining from green towards yellow. Live and dead spores stained only with Calcofluor White M2R could not be differentiated when viewed through the 395- to 415-nm-wavelength-excitation filter, although variations in intensities of turquoise to white staining were seen. However, the dual-stained spores, when viewed through this filter, could readily be discerned as white-yellow dead spores and turquoise-blue live spores, suggesting that the Sytox Green counterstain caused a shift in color of the dead spores at the 395- to 415-nm wavelength. As a result, this viability staining procedure that combines the use of Calcofluor White M2R and Sytox Green can be used to count both viable and dead spores at the 395- to 415-nm filter wavelength. Alternatively, the total number of spores can be counted under bright-field microscopy, followed by counting dead spores using the 470- to 490-nm filter wavelength.
In the experiments presented here, the stained parasite smears were not coverslipped with mounting medium. Mounting medium was found to affect the appearance of these stained microsporidia by quenching the color or sharpness of the spores when viewed by fluorescence microscopy.
The results for viability of the spores determined by the combined use of the chitin stain Calcofluor M2R and the nucleic acid stain Sytox Green were not statistically significantly different from the results of the infectious-focus-forming assay. Furthermore, the dual-staining procedure presented here offers several advantages over infectivity assays used to detect viability. The combined staining procedure was quicker; it took less than 2 h, as opposed to the infectious-focus-forming assay, which required growing host cells, inoculation with spores, and incubation of the cultures for several days. Samples obtained from water sources or fecal samples can be stained for viability without concern for cell culture contamination, which would be problematic for performing an infectious focus-forming assay. Furthermore, species of microsporidia that presently cannot be grown in culture (e.g., Enterocytozoon bieneusi) would be precluded from testing for viability in a focus-forming assay but could be stained for viability using this procedure. This dual-staining procedure employing Sytox Green and Calcofluor White M2R thus opens the door to testing the viability of microsporidia recovered from water sources to evaluate potential health risks and also can be used to evaluate disinfection and drug treatment protocols.
ACKNOWLEDGMENTS
We thank Murphy Duwouis for photographic assistance. This work was supported by funding from the National Institutes of Health, Bethesda, Md. (RR00164, AI39968, and AI40323).
REFERENCES
- 1.Accoceberry I, Thellier M, Desportes-Livage I, Achbarou A, Biligui S, Danis M, Datry A. Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi. J Clin Microbiol. 1999;37:4107–4112. doi: 10.1128/jcm.37.12.4107-4112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldras A M, Orenstein J M, Kotler D P, Shadduck J A, Didier E S. Detection of microsporidia by indirect immunofluorescence antibody test using polyclonal and monoclonal antibodies. J Clin Microbiol. 1994;32:608–612. doi: 10.1128/jcm.32.3.608-612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckers P J A, Derks G J M, van Gool T, Rietveld F J R, Sauerwein R W. Encephalocytozoon intestinalis-specific monoclonal antibodies for laboratory diagnosis of microsporidiosis. J Clin Microbiol. 1996;34:282–285. doi: 10.1128/jcm.34.2.282-285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belosevic M R, Guy R A, Taghi-Kilani R, Neuman N F, Gyurek L L, Liyanage L R J, Millard P J, Finch G R. Nucleic acid stains as indicators of Cryptosporidium parvum oocyst viability. Int J Parasitol. 1997;27:787–798. doi: 10.1016/s0020-7519(97)00033-7. [DOI] [PubMed] [Google Scholar]
- 5.Bryan R T, Schwartz D A. Epidemiology of microsporidiosis. In: Wittner M, Weiss L, editors. The microsporidia and microsporidiosis. Washington, D.C.: American Society for Microbiology; 1999. pp. 502–516. [Google Scholar]
- 6.Canning E U, Lom J. The microsporidia of vertebrates. New York, N.Y: Academic Press; 1986. [Google Scholar]
- 7.Didier E S. Microsporidiosis. Clin Infect Dis. 1998;27:1–7. doi: 10.1086/514607. [DOI] [PubMed] [Google Scholar]
- 8.Didier E S, Didier P J, Friedberg D N, Stenson S M, Orenstein J M, Yee R W, Tio F O, Davis R M, Vossbrinck C, Millichamp N. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J Infect Dis. 1991;163:617–621. doi: 10.1093/infdis/163.3.617. [DOI] [PubMed] [Google Scholar]
- 9.Didier E S, Orenstein J M, Aldras A, Bertucci D, Rogers L B, Janney F A. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol. 1995;33:3138–3145. doi: 10.1128/jcm.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Didier E S, Snowden K F, Shadduck J A. Biology of microsporidian species infecting mammals. Adv Parasitol. 1998;40:283–320. doi: 10.1016/s0065-308x(08)60125-6. [DOI] [PubMed] [Google Scholar]
- 11.Didier E S, Vossbrinck C R, Baker M D, Rogers L B, Bertucci D B, Shadduck J A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111:411–421. doi: 10.1017/s0031182000065914. [DOI] [PubMed] [Google Scholar]
- 12.Enriquez F J, Ditrich O, Palting J D, Smith K. Simple diagnosis of Encephalitozoon sp. microsporidial infections by using a panspecific antiexospore monoclonal antibody. J Clin Microbiol. 1997;35:724–729. doi: 10.1128/jcm.35.3.724-729.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federal Register. Announcement of the drinking water contaminant list. Fed Regist. 1998;63:10273–10287. [Google Scholar]
- 14.Ignatius R, Henschel S, Liesenfeld O, Mansmann U, Schmidt W, Koppe S, Schneider T, Heise W, Futh U, Riecken E O, Hahn H, Ullrich R. Comparative evaluation of modified trichrome and Uvitex 2B stains for detection of low numbers of microsporidial spores in stool specimens. J Clin Microbiol. 1997;35:2266–2269. doi: 10.1128/jcm.35.9.2266-2269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokoskin E, Gyorkos T W, Camus A, Cedilotte L, Purtill T, Ward B. Modified technique for efficient detection of microsporidia. J Clin Microbiol. 1994;32:1074–1075. doi: 10.1128/jcm.32.4.1074-1075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotler D, Orenstein J M. Clinical syndromes associated with microsporidiosis. In: Wittner M, Weiss L, editors. The microsporidia and microsporidiosis. Washington, D.C.: American Society for Microbiology; 1999. pp. 258–292. [Google Scholar]
- 17.Rinder H, Janitschke K, Aspöck H, Da Silva A J, Deplazes P, Fedorko D P, Franzen C, Futh U, Hünger F, Lehmacher A, Meyer C G, Molina J-M, Sandfort J, Weber R, Löscher T The Diagnostic Multicenter Study Group on Microsporidia. Blinded, externally controlled multicenter evaluation of light microscopy and PCR for detection of microsporidia in stool specimens. J Clin Microbiol. 1998;36:1814–1818. doi: 10.1128/jcm.36.6.1814-1818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan N J, Sutherland G, Coughlan K, Globan M, Doultree J, Marshall J, Baird R W, Pedersen J, Dwyer B. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol. 1993;31:3264–3269. doi: 10.1128/jcm.31.12.3264-3269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt E C, Shadduck J A. Mechanisms of resistance to the intracellular protozoan Encephalitozoon cuniculi in mice. J Immunol. 1984;133:2712–2719. [PubMed] [Google Scholar]
- 20.Schwartz D A, Bryan R T. Microsporidia. In: Horsburgh R, editor. Pathology of emerging diseases. Washington, D.C.: American Society for Microbiology; 1997. pp. 61–94. [Google Scholar]
- 21.Schwartz D A, Bryan R T. The microsporidial infections: progress in epidemiology and prevention. In: Scheld W M, Craig W A, Hughes J M, editors. Emerging infections 3. Washington, D.C.: American Society for Microbiology; 1999. pp. 73–98. [Google Scholar]
- 22.van Gool T, Snijders F, Reiss P, Eeftinck J K, Schattenkerk, van den Bergh Weerman M A, Bartelsman J F, Bruins J J, Canning E U, Dankert J. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J Clin Pathol. 1993;46:694–699. doi: 10.1136/jcp.46.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vavra J, Dahbiova R, Hollister W S, Canning E U. Staining of microsporidian spores by optical brighteners with remarks on the use of brighteners for the diagnosis of AIDS associated human microsporidioses. Folia Parasitol (Prague) 1993;40:267–272. [PubMed] [Google Scholar]
- 24.Vavra J, Nohynkova E, Machala L, Spala J. An extremely rapid method for detection of microsporidia in biopsy materials from AIDS patients. Folia Parasitol (Prague) 1993;40:273–274. [PubMed] [Google Scholar]
- 25.Weber R, Bryan R T, Owen R L, Wilcox C M, Gorelkin L, Visvesvara G S. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. The Enteric Opportunistic Infections Working Group. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 26.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss L M, Cali A, Levee E, LaPlace D, Tanowitz H, Simon D, Wittner M. Diagnosis of Encephalitozoon cuniculi infection by western blot and the use of cross-reactive antigens for the possible detection of microsporidiosis in humans. Am J Trop Med Hyg. 1992;47:456–462. doi: 10.4269/ajtmh.1992.47.456. [DOI] [PubMed] [Google Scholar]
- 28.Zierdt C H, Gill V J, Zierdt W S. Detection of microsporidian spores in clinical samples by indirect fluorescent-antibody assay using whole-cell antisera to Encephalitozoon cuniculi and Encephalitozoon hellem. J Clin Microbiol. 1993;31:3071–3074. doi: 10.1128/jcm.31.11.3071-3074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]