Abstract
Cardiac fibroma is a rare primary benign cardiac tumor, especially in adults. It often occurs in the interventricular septum and free wall of the left ventricle and is solitary and space-occupying with clear boundaries. Here we report a 27-year-old male with a cardiac fibroma in the right ventricle, with extensive infiltrative growth. He was admitted to hospital with the complaint of exertional chest tightness, shortness of breath, hemoptysis, and edema of lower extremities. Ultrasound showed a large right ventricular mass blocking the outflow tract. The patient underwent palliative resection. Pathologic examination and Masson staining showed that collagen tissue proliferated and infiltrated myocardial fibers. The final diagnosis was cardiac fibroma.
Keywords: Adult, heart, right ventricle, cardiac fibroma, infiltrative
Introduction
Cardiac fibroma is a rare primary cardiac tumor, mostly occurring in infants and children, but rarely in adults. It often occurs in the free wall and ventricular septum of the left ventricle, and by imaging, most of them are isolated space-occupying lesions with clear boundaries. Here we report a 27-year-old male with a cardiac fibroma in the right ventricle. The tumor showed extensive infiltrative growth and could not be completely removed by operation. The clinical and histopathologic features are reported and the relevant literature is reviewed in order to improve the diagnosis of fibroma by clinicians and pathologists, and improve outcome.
Case report
A male patient, 27 years old, had exertional chest tightness and shortness of breath for 1 year, aggravated for 1 month, and hemoptysis with edema of lower extremities for 2 days. He was admitted to the department of cardiac surgery in our hospital on February 6, 2020. Imaging examination: Cardiac color doppler examination showed that a mixed echogenic mass in the right ventricle apex, right ventricular outflow tract, and pulmonary valve. The range of the apical masses was about 35.4×18.5 mm. The range of the right ventricular outflow tract masses was about 34.4×19.2 mm and the masses involved the tricuspid valve and pulmonary valve, with a wide base. The tricuspid valve was limited in opening and in closing, and a small amount of reflux signal could be detected. The right ventricular outflow tract was narrow, about 7 mm wide, and the right atrium was enlarged, in which a cloud-like echo could be detected (Figure 1A, 1B).
Figure 1.
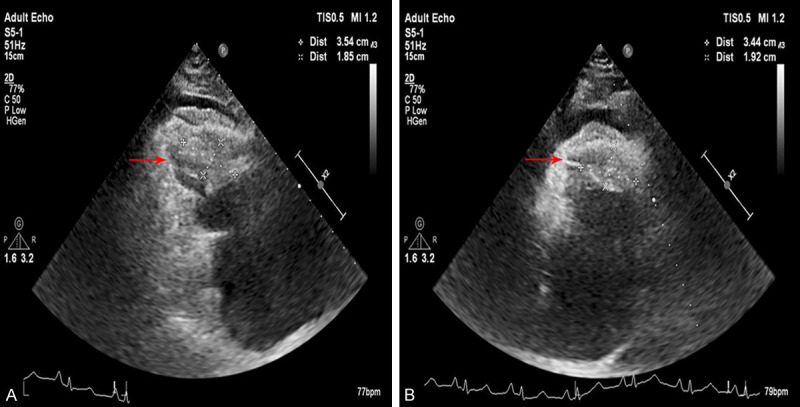
Cardiac color doppler examination. A. The range of the apical masses was about 35.4×18.5 mm. B. The range of the right ventricular outflow tract masses was about 34.4×19.2 mm.
Operation
The patient underwent palliative resection of the cardiac mass and mechanical tricuspid valve replacement on February 10, 2020. During the operation, there was about 100 ml of pale yellow pericardial effusion in the pericardium, and the right atrium was enlarged. A large tumor could be seen in the right ventricle and right ventricular outflow tract, which was firm and extensively invaded the right ventricular wall, interventricular septum, apex, right ventricular outflow tract, and the root of pulmonary valve. A small gap could be seen between the tumor, the right ventricular wall, and the right ventricular outflow tract, resulting in stenosis of the outflow tract. The narrowest part was about 0.7 cm. The tumor extensively and compactly invaded the apex, tricuspid valve, chordae tendineae, and papillary muscle, with fusion of subvalvular apparatus, leading to severe stenosis of the tricuspid valve. The opening of the tricuspid valve was about 0.5 cm. During the operation, considering that the tumor infiltrated the myocardium, the tumor could not be completely removed, so palliative tumor resection was used. Due to the severe stenosis of the tricuspid valve and the poor repair effect, it was replaced with a mechanical valve.
Pathologic examination
(1) Gross specimen: The operative specimens were a pile of gray-white and gray-yellow non-plastic tissues, about 4.5×3.0×1.5 cm. Gray-white tissue was tough, gray-yellow tissue was myocardium, and the two grew interspersed (Figure 2A). (2) H&E staining: Microscopically, there was significant proliferation of fibrous and collagen tissue, where the tumor cells were arranged in bundles, and some infiltrated myocardial fibers. The cell density was variable, most of which was sparse, and the nuclei were spindle-shaped without mitosis or necrosis. Collagen fibers were abundant, with scattered foci of lymphocytic infiltration (Figure 2B). (3) Immunohistochemical staining: Tumor cells expressed vimentin (Figure 2C), and had scattered expression of SMA (Figure 2D). There was cytoplasmic expression of β-catenin (Figure 2E), negative for desmin and CD34. Ki-67 labeling index was less than 1%. (4) Special staining: Masson staining showed that blue-stained collagen fibers proliferated and infiltrated the striated muscle tissue (red) (Figure 2F). The diagnosis was cardiac fibroma. The patient recovered well after operation, and the cardiac ultrasound was reexamined on the 4th day after operation. The results showed that the right ventricular inflow and outflow tract were unobstructed, the apex was fair, the tricuspid valve was an artificial valve, and no obvious reflux was detected (Figure 3). The patient was discharged on the 14th day after operation. On the 30th day after operation, the patient was followed up by telephone, and cardiac function was good, without evidence of recurrence.
Figure 2.
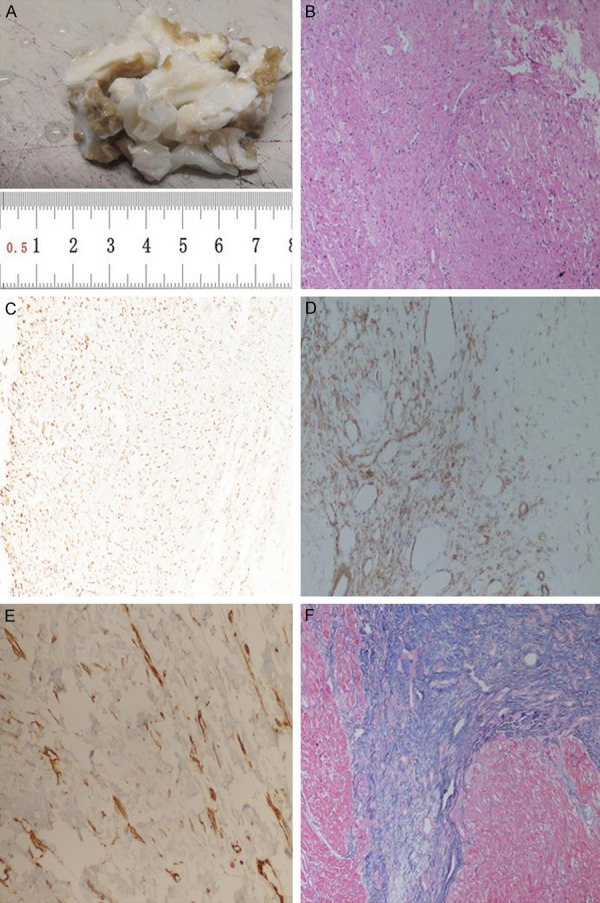
A. Gross specimen observation revealed a pile of gray-white and gray-yellow non-plastic tissues, about 4.5×3.0×1.5 cm. B. Microscopically, there was significant proliferation of fibrous and collagen tissue, which infiltrated cardiac muscle fibers (200×). C. Immunohistochemical staining: tumor cells had scattered expression of vimentin (200×). D. Immunohistochemical staining: tumor cells focally expressed SMA (200×). E. Immunohistochemical staining: tumor cells focally expressed β-catenin (200×). F. Masson staining showed that blue-stained collagen fibers proliferated and infiltrated the striated muscle tissue (red) (200×).
Figure 3.
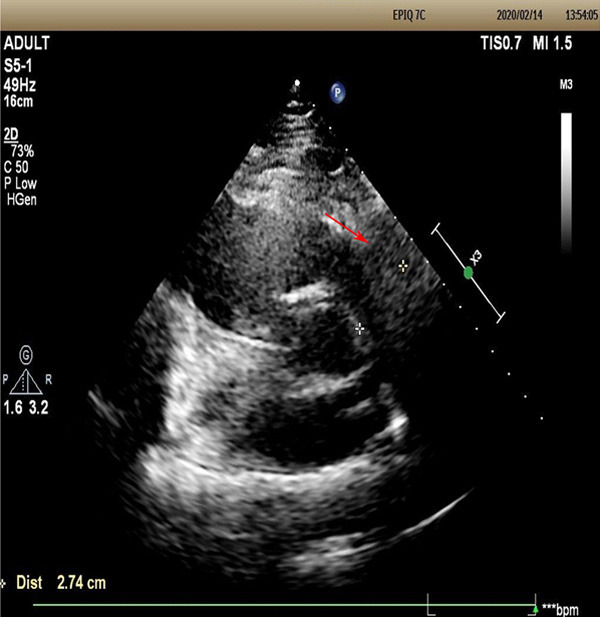
Posoperative cardiac ultrasound showed that the right ventricular inflow and outflow tract were unobstructed, the apex was fair, the tricuspid valve was an artificial valve, and no obvious reflux was detected.
Discussion
Cardiac fibroma is a benign congenital mesenchymal tumor, that consists of fibroblasts and matrix containing collagen fibers. Cardiac fibroma mostly occurs in infants or fetuses, accounting for 6%-25% of primary cardiac tumors in infancy, and it is the second-most common tumor after rhabdomyoma [1]. Clinical symptoms include arrhythmia, conduction disturbance, chest pain, dyspnea, fatigue or fainting, and even sudden death. Imaging examination mainly includes cardiac echocardiography, computed tomography (CT) and magnetic resonance imaging (MRI). Cardiac myxoma is the most common primary cardiac tumor in adults, accounting for about 40% of benign tumors [2]. Cardiac fibroma is extremely rare in adults [3]. Patients with Gorlin syndrome (nevoid basal cell carcinoma syndrome) are prone to develop cardiac fibroma, and the etiology of sporadic cardiac fibroma is not clear. Most tumors occur in the left ventricle (about 60%), followed by the right ventricle (about 30%) [4]. The tumor should be detected and resected completely as early as possible to prevent invasive growth.
This case was a 27-year-old male. Cardiac color doppler examination showed an irregular mass in the right ventricle, with a wide base, involving tricuspid valve and pulmonary valve. During the operation, it was found that the tumor extensively invaded the right ventricular wall, interventricular septum, apex, and right ventricular outflow tract, and the root of pulmonary valve. It also extensively and compactly invaded the apex tricuspid valve, chordae tendineae, and papillary muscle. The tumor involved a wide range (ventricle, interventricular septum, apex, pulmonary valve and tricuspid valve) and grew interspersed with myocardial tissue.
Cardiac fibromas are mostly isolated lesions with clear boundaries and no envelope, with a diameter of about 1-10 cm. The cut surface is braided, and the tumor margin and myocardial tissue grow alternately, often protruding into the ventricular cavity. In this case, the tumor infiltrated the myocardium significantly. Tumors in children are rich in fibroblasts, while collagen and fibers are rare. In adult patients, tumors are mainly composed of collagen and fibers, and the density of fibroblasts is low. Calcification occurs in 25%-50% of cases. Necrosis and cystic change are occasionally seen. Lymphocyte and monocyte infiltration can be seen around the blood vessels and at the junction of the lesion and myocardium [5]. Tumor cells show myofibroblast differentiation, positive for vimentin and SMA. The histologicappearance of this case is consistent with the above, and the diagnosis is clear. In cardiac fibroma, the content of collagen fibers is related to the age of the patient. The older the patient is, the richer in collagen fibers. The intersection of tumor tissue and myocardial fibers is the pathologic basis of ventricular tachycardia and sudden cardiac arrest, and the cause of arrhythmia in patients with partial tumor resection [6].
Cardiac fibroma needs to be distinguished from: (1) Cardiac rhabdomyoma: vesicular striated muscle cells containing a large amount of glycogen can be seen, with striations in the cytoplasm. Immunohistochemistry: desmin (+). (2) Papillary elastofibroma: this is a papillary growth, consisting of mucoid stroma and elastic fibers. The papillary surface is lined with a layer of endothelial cells. VG staining of elastic fibers is positive. (3) Deep invasive fibromatosis is a fusiform fibroblast/myofibroblast proliferation, rich in collagen, that is similar to cardiac fibroma, but the former usually occurs in the abdominal wall or retroperitoneum, and the cells are often arranged in bundles. Positive immunohistochemistry for β-Catenin in the nucleus can help to distinguish the two.
Cardiac fibroma should be surgically resected first. If the tumor cannot be completely removed due to the invasive growth of the tumor, implantation of a defibrillator may be considered. Partial resection of the tumor may relieve obstruction and compression to a certain extent, which helps the recovery of patients. For patients with larger and unresectable tumors, heart transplantation may be considered [5]. Interventricular septum involvement in patients with cardiac fibroma may cause conduction dysfunction, which is one of the factors of poor prognosis. Cardiac fibroma in adults is rare, and its prognosis is better than that of children [7]. Complete resection of tumor may prolong asymptomatic survival, and the relapse rate is low.
Acknowledgements
This project was supported by: Molecular mechanism of immunoglobulin ILT4 regulating hematogenous metastasis of NSCLC through VEGFA (No. 2019YD028); Immunoglobulin ILT4 participates in angiogenesis and signal pathway of NSCLC by regulating VEGFA (No. 2018WS024); Upregulation of GTP cyclohydrolase-1 expression in non-small cell lung cancer by PDGF-BB and induction of tumor lymphangiogenesis (No. 2020YD011).
Disclosure of conflict of interest
None.
References
- 1.Dominguez C, Perkins A, Duque A, Bravo V. Primary cardiac tumors in infancy: a case report and literature review. Acad Forensic Pathol. 2017;7:112–118. doi: 10.23907/2017.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uzun O, Wilson DG, Vujanic GM, Parsons JM, De Giovanni JV. Cardiac tumours in children. Orphanet J Rare Dis. 2007;2:11. doi: 10.1186/1750-1172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho SH, Fritz T, Cronin LJ, Cohle SD. Primary cardiac fibroma in an adult. Case Rep Cardiol. 2015;2015:713702. doi: 10.1155/2015/713702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidari A, Sabzi F, Faraji R. Right atrial fibroma in an adult patient. Ann Card Anaesth. 2018;21:65–67. doi: 10.4103/aca.ACA_121_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nwachukwu H, Li A, Nair V, Nguyen E, David TE, Butany J. Cardiac fibroma in adults. Cardiovasc Pathol. 2011;20:e146–152. doi: 10.1016/j.carpath.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Carreon CK, Sanders SP, Perez-Atayde AR, Del Nido PJ, Walsh EP, Geva T, Alexander ME. Interdigitating myocardial tongues in pediatric cardiac fibromas: plausible substrate for ventricular tachycardia and cardiac arrest. JACC Clin Electrophysiol. 2019;5:563–575. doi: 10.1016/j.jacep.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Piazza N, Chughtai T, Toledano K, Sampalis J, Liao C, Morin JF. Primary cardiac tumours: eighteen years of surgical experience on 21 patients. Can J Cardiol. 2004;20:1443–1448. [PubMed] [Google Scholar]


