Abstract
Objectives
Older nursing home residents make up the population at greatest risk of morbidity and mortality from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. No studies have examined the determinants of long-term antibody responses post vaccination in this group.
Design
Longitudinal cohort study.
Setting and Participants
Residents from 5 nursing homes assessed before vaccination, and 5 weeks and 6 months post vaccination, with the BNT162b2 messenger RNA SARS-CoV-2 vaccine.
Methods
Comprehensive clinical assessment was performed, including assessment for comorbidity, frailty, and SARS-CoV-2 infection history. Serum nucleocapsid and anti-spike receptor binding domain (RBD) antibodies were analyzed at all timepoints. An in vitro angiotensin-converting enzyme (ACE2) receptor-spike RBD neutralization assay assessed serum neutralization capacity.
Results
Of 86 participants (81.1 ± 10.8 years; 65% female), just under half (45.4%; 39 of 86) had evidence of previous SARS-CoV-2 infection. All participants demonstrated a significant antibody response to vaccination at 5 weeks and a significant decline in this response by 6 months. SARS-CoV-2 infection history was the strongest predictor of antibody titer (log-transformed) at both 5 weeks [β: 3.00; 95% confidence interval (CI): 2.32–3.70; P < .001] and 6 months (β: 3.59; 95% CI: 2.89–4.28; P < .001). Independent of SARS-CoV-2 infection history, both age in years (β: −0.05; 95% CI: −0.08 to −0.02; P < .001) and frailty (β: −0.22; 95% CI: −0.33 to −0.11; P < .001) were associated with a significantly lower antibody titer at 6 months. Anti-spike antibody titers at both 5 weeks and 6 months significantly correlated with in vitro neutralization capacity.
Conclusions and Implications
In older nursing home residents, SARS-CoV-2 infection history was the strongest predictor of anti-spike antibody titers at 6 months, whereas age and frailty were independently associated with lower titers at 6 months. Antibody titers significantly correlated with in vitro neutralization capacity. Although older SARS-CoV-2 naïve nursing home residents may be particularly vulnerable to breakthrough SARS-CoV-2 infection, the relationship between antibody titers, SARS-CoV-2 infection, and clinical outcomes remains to be fully elucidated in this vulnerable population.
Keywords: Nursing homes, long-term care, vaccine, SARS-CoV-2.0, COVID-19, antibody
Older adults and those living with frailty are at greatest risk from infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), exemplified in older adults resident in nursing homes.1, 2, 3 In the United States, nursing home deaths have represented nearly one-third of all deaths from SARS-CoV-2 infection, which has been mirrored internationally.4, 5, 6, 7 Nursing home residents are typically older, with higher levels of frailty and medical comorbidity and vaccinations are typically less efficacious in this group than in their community-dwelling counterparts.8, 9, 10 Recent studies have supported the clinical efficacy of SARS-CoV-2 vaccination in older nursing home residents.11 , 12
Of note, early studies demonstrating the persistence of antibodies against the receptor binding domain (RBD) of the SARS-CoV-2 Spike protein at 6 months after vaccination did not include older adults with frailty or those resident in nursing homes.13 , 14 Although a small number of studies have supported the immunogenicity of the BNT162b2 vaccine to induce an initial antibody response after vaccination in nursing home residents, this response is significantly lower than community-dwelling younger adults, particularly in SARS-CoV-2-naïve residents.15, 16, 17, 18, 19 In addition, longer-term data have recently suggested that nursing home residents experience a faster decline in humoral response after vaccine than younger community-dwelling adults.20 , 21 Importantly, emerging evidence also suggests that anti-Spike immunoglobulin (Ig)G levels correlate with vaccine-induced protection in nursing homes experiencing SARS-CoV-2 outbreaks.22 Despite this, no studies have examined the determinants of long-term vaccine-indued humoral immune responses in nursing home residents. This is particularly important in the context of breakthrough infections and the current vaccine booster programs that have demonstrated clinical efficacy in older adults.23 , 24
Methods
Study Design and Setting
COVID-19 in Nursing Home Residents: Predicting Disease Severity, Outcomes and Anti-Viral Immune Responses (NH-COVAIR) recruited participants from 5 nursing homes in Dublin, Ireland. Full ethical approval was granted from the local ethics committee (Reference: 20-NREC-COV-049). In accordance with the Declaration of Helsinki, all participants provided fully informed consent, or assent following discussion with family members/carers if fully informed direct consent was unobtainable because of cognitive impairment. Individuals resident in nursing homes, not currently receiving systemic immunosuppressive therapy/chemotherapy, without systemic autoimmune disease, acute infections, or with a known active malignancy were included. Participants were enrolled before receiving the BNT162b2 vaccine and were assessed at baseline, at 5 weeks, and at 6 months after completed vaccination (second dose).
Clinical Assessment
Comprehensive clinical assessment included demographics (age, sex, body mass index), detailed medical history (number/detail of medical conditions and regular medications), and frailty assessment. Frailty status was assessed using the FRAIL-NH scale, a tool specifically designed and validated for nursing home residents.25 Those with sarcopenia at risk of adverse outcomes were identified using the SARC-F tool.26 Grip strength (measured in kg) was measured in both hands and the arithmetic mean computed in kg. The Barthel Index was used to assess activities of daily living.27
SARS-CoV-2 Infection History
As part of a national screening program, all residents underwent weekly real-time polymerase chain reaction of nasopharyngeal swabs for SARS-CoV-2. The results of these, and previous SARS-CoV-2 infection history, were collected. Individuals were classified as having previous SARS-CoV-2 if they had a positive nasopharyngeal swab or a positive reading on the qualitative nucleocapsid serological assay at any timepoint during the study (see later in this article).
Measurement of SARS-CoV-2 Antibodies
Serum samples at all timepoints were analyzed using the Elecsys anti-SARS-CoV-2 assay from Roche Diagnostics, a validated electrochemiluminescence assay. Samples were analyzed using (1) a qualitative assay against the nucleocapsid antigen of SARS-CoV-2 reporting results as reactive (<1.0) or nonreactive (>1.0) to examine for SARS-CoV2 exposure, and (2) a quantitative assay (in U/mL) against the Spike RBD of SARS-CoV2 to assess antibody titer after vaccination in nursing home residents. Both assays underwent full validation previously published.28
Neutralization Assays
A previously validated in-house neutralization assay was used to assess the ability of serum antibodies to neutralize the angiotensin-converting enzyme (ACE2) receptor-Spike RBD.29 Plates were coated with recombinant RBD (aa319–541; Peak Proteins) in phosphate-buffered saline (PBS) and blocked with 3% milk powder in PBS-0.1%Tween (PBST). Serum samples were diluted 1:50 in 1% milk in PBST. A commercial IgG1 anti-spike antibody containing 2% normal human serum was used as a positive control on each plate (ThermoFisher Scientific). Plates were washed 3 times with PBST and 50 μL per well of 10 μg/mL biotinylated ACE2 added to the plate and left for 1 hour. After 3 further wash steps, 100 μL per well streptavidin horseradish peroxidase (HRP) (diluted 1:40 in PBS) was added for 20 minutes. The streptavidin HRP was removed and plates were washed 3 times. After washing, 100 μL SigmaFast OPD was added to each well for 10 minutes and the reaction stopped using 3M hydrochloric acid and optical density measured using a plate reader at 492 nm. Results are presented as % neutralization in comparison with a pool of 6 pre-pandemic controls, with a lower percentage indicating greater neutralization capacity.29
Statistical Analysis
Data were analyzed using STATA v17.0 (StataCorp). Graphs were prepared using GraphPad Prism V9.2 (GraphPad Software). Descriptive statistics were reported as means (with SD), medians [with interquartile range (IQR)], and proportions (with percentages) as appropriate. Antibody titers were analyzed using nonparametric statistics given their non-normal distribution (Wilcoxon rank-sum and sign-rank tests). Exploratory analysis was conducted to examine potential factors associated with vaccine response at 5 weeks and durability of this response at 6 months. Stepwise linear regression used log-transformed antibody levels as the dependent variable. Regression results are reported as beta (β) coefficients with corresponding 95% CI. We included individual predictors with a P value of <.10 on bivariate analysis for inclusion in a multivariate model containing all significant predictors. Spearman's rho was used to assess the correlation between antibody titer and neutralization capacity.
Results
Study Population and Characteristics
Eighty-six (81.1 ± 10.8 years; 65% female) participants were recruited. Just fewer than half (45.4%; 39/86) had evidence of previous SARS-CoV-2 (n = 32 with previous positive nasopharyngeal swab, n = 7 with negative swabs but a reactive nucleocapsid antibody result at baseline). All underwent clinical assessment at baseline and had repeat blood samples at 5 weeks (median 34 days; IQR: 27–35 days) and 6 months (median 182 days; IQR: 180–184 days). No SARS-CoV-2–naïve individuals at baseline subsequently had a reactive nucleocapsid result or positive swab at either the 5-week or 6-month follow-up. Over the 6-month follow-up, 8 residents died (age 84 ± 10 years; 2 of 8 were women), 2 of whom had previous COVID-19. No nursing homes reported incident outbreaks, and incident deaths were not caused by acute COVID-19 illness. Clinical characteristics are provided in Table 1 .
Table 1.
Descriptive Characteristics and Antibody Titers of NH-COVAIR Study Participants
| Characteristic | Previous SARS-CoV-2 Infection (n = 39) | SARS-CoV-2 Naive (n = 47) |
|---|---|---|
| Age, y (SD) | 80.8 (10.1) | 81.4 (11.5) |
| Gender, female (%) | 23 (59.0) | 33 (70.2) |
| Body Mass Index, m/kg2 (SD) | 27.8 (5.8) | 26.1 (6.9) |
| Cognitive Status | ||
| Mild cognitive impairment (%) | 9 (23.1) | 14 (29.8) |
| Dementia (%) | 7 (17.95) | 13 (27.7) |
| Medical history | ||
| Comorbidity count (IQR) | 5 (4–7) | 4 (3–6) |
| Diabetes (%) | 7 (17.8) | 11 (23.4) |
| Neurodegenerative disorder (%) | 6 (15.4) | 4 (8.5) |
| Solid tumor (%) | 5 (15.4) | 5 (10.6) |
| Chronic obstructive pulmonary disease (%) | 7 (18.0) | 8 (17.0) |
| Stroke (%) | 10 (25.6) | 9 (19.2) |
| Congestive cardiac failure (%) | 8 (20.5) | 3 (6.8) |
| Myocardial Infarction (%) | 4 (10.3) | 4 (8.5) |
| Medication history | ||
| Number of regular medications (IQR) | 13 (10–18) | 13 (11–16) |
| Regular inhaled steroid (%) | 5 (12.8) | 4 (8.5) |
| Regular oral steroid (%) | 2 (5.1) | 2 (4.3) |
| Regular NSAID (%) | 11 (28.2) | 16 (34.0) |
| Frailty assessment | ||
| FRAIL-NH | ||
| Nonfrail | 20 (51.3) | 24 (51.1) |
| Pre-frail | 13 (13.3) | 18 (38.3) |
| Frail | 6 (15.38) | 5 (10.6) |
| SARC-F >3 (%) | 30 (76.9) | 38 (80.1) |
| Grip strength (IQR) (kg) | 11 (7–17) | 14 (7.5–18.5) |
| Barthel index (IQR) | 10 (4–14) | 9 (4–14) |
| Anti-Spike RBD antibody titer, U/mL (SD) | ||
| Baseline (pre-vaccination) | 160 (80–766) | 4 (0) |
| 5 wk after 2nd dose | 22,451 (14,021–25,000) | 633 (170–1848) |
| 6 mo after 2nd dose | 6332 (3372–9667) | 133 (54–337) |
NSAID, nonsteroidal anti-inflammatory drug.
Data are presented as means with SDs or medians with IQRs.
All participants received 2 doses of the Pfizer BioNTech BNT162b2 messenger RNA (mRNA) vaccine. Side effects after either dose, reported by half of participants (50%; 43 of 86), were limited to local injection site reactions, muscle pain, and tiredness.
Post-Vaccine Antibody Response in Nursing Home Residents
All participants mounted a significant anti-Spike RBD antibody response at 5 weeks after vaccination (median: 3258 U/mL; IQR: 455–22,755 U/mL) (z = −7.6, P < .001). This response was much greater in individuals with previous SARS-CoV-2 (median: 22,451; IQR: 14,021–25,000 U/mL) (z = −6.5, P < .001) than in SARS-CoV-2–naïve participants (median: 633 U/mL; IQR: 170–1848 U/mL).
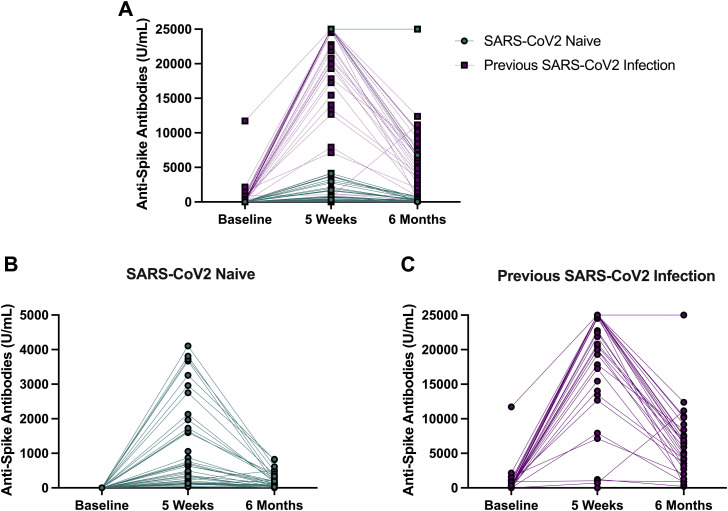
By 6 months, all participants experienced a significant decline in SARS-CoV-2 anti-Spike antibodies (from 3258 U/mL; 455–22,755 U/mL at 5 weeks to 560 U/mL; 114–6345 U/mL at 6 months). This was seen in both previously infected (22,451 U/mL; IQR: 14,021–25,000 U/mL at 5 weeks to 6332 U/mL; IQR: 3372–9667 U/mL at 6 months; z = 4.7, P < .001) and uninfected (632.5 U/mL; IQR: 170–1848 U/mL at 5 weeks to 133; IQR: 54–337 U/mL at 6 months; z = 5.0, P < .001) individuals. Data are presented graphically in Figure 1 .
Fig. 1.
Antibody response to SARS-CoV-2 mRNA vaccine in older nursing home residents. (A) Antibody responses in all participants (N = 86) at baseline, 5 weeks, and 6 months after vaccination (top). (B) Antibody responses in SARS-CoV-2–naïve individuals (bottom left). (C) Antibody responses in participants with previous SARS-CoV-2 infection (bottom right).
On exploratory bivariate analysis, previous SARS-CoV-2 was strongly associated with log-transformed antibody titer at 5 weeks (β: 3.00; 95% CI: 2.32–3.70; P < .001) and 6 months (β: 3.59; 95% CI: 2.89–4.28; P < .001). In addition, both age (β: −0.08; 95% CI: −0.13 to −0.02; P = .004) and frailty (β: −0.20; 95% CI: −0.40 to −0.01; P = .004) were associated with lower antibody levels at 6 months. No other predictors were associated with antibody response at 5 weeks or 6 months on bivariate analysis. On multivariate analysis, previous SARS-CoV-2 (β: 3.43; 95% CI: 2.83–4.04; P < .001), increasing age (β: −0.05; 95% CI: −0.08 to −0.02; P < .001) and frailty (β: −0.22; −0.33 to −0.11; P < .001) all remained significantly associated with antibody titer at 6 months. Comorbidity count, number of regular medications, use of steroid medication or nonsteroidal anti-inflammatory drugs, SARC-F score, grip strength, and Barthel Index were not associated with antibody titers at either follow-up timepoint.
In Vitro Neutralization Capacity
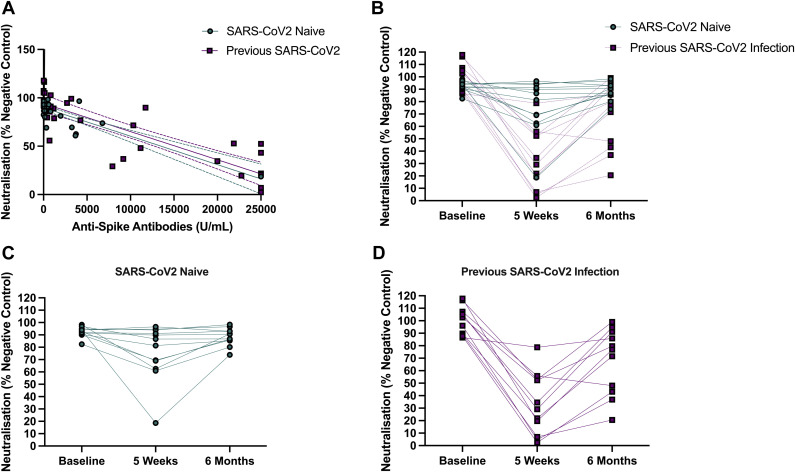
To assess the in vitro neutralization capacity of the vaccine response in nursing home residents, an in vitro ACE2 receptor-Spike RBD neutralization assay was used. Serum from a subsample of participants representative of the overall study population (n = 24; n = 12 with previous SARS-CoV-2 infection and n = 12 SARS-CoV-2–naïve participants) were analyzed. There were significant correlations between neutralization capacity at 5 weeks (Spearman's rho: −0.85, P < .001) and 6 months (Spearman's rho: −0.55, P = .006). Results of neutralization assays are presented visually in Figure 2 .
Fig. 2.
Neutralization capacity of the antibody response to SARS-CoV-2 vaccination. (A) Correlation between neutralization capacity and anti-Spike antibody titer (n = 24) (top left). (B) Neutralization capacity at baseline, 5 weeks, and 6 months post vaccination with a SARS-CoV-2 mRNA vaccine (top right). (C) Neutralization capacity over time in SARS-CoV-2–naïve participants. (D) Neutralization capacity over time in participants previously infected with SARS-CoV-2. Note: Neutralization values are presented as a percentage of a pool of 6 pre-pandemic controls, with greater. Note: Neutralization values are presented as a percentage of a pool of 6 pre-pandemic controls, with greater values indicating poorer neutralization capacity.
Discussion
In the current study, we found that past infection was the strongest determinant of 6-month vaccine-induced anti-Spike antibody titer in older nursing home residents. Further, both age and frailty were independently associated with significantly lower antibody titers at 6 months. Although our data may suggest that older SARS-CoV-2–naïve residents are at greater risk of breakthrough infection, the complex relationship among antibody levels, breakthrough infection, and clinical outcomes is yet to be fully elucidated.
The most striking finding is the large difference in 6-month antibody titer between SARS-CoV-2–naïve and previously infected individuals. There may be several reasons for this. In the first instance, this may be because of more potent immunological memory during SARS-CoV-2 infection than post vaccination.21 Further, infection with SARS-CoV-2 may result in a greater number of polyfunctional antibodies against viral antigens than vaccination against the Spike protein alone. Future research should aim to elucidate whether vaccination with the same antigen (Spike) or alternative antigens may result in a stronger long-term induction of antibodies, particularly with the commencement of booster programs. Further, we demonstrated that antibody titers were significantly correlated with in vitro neutralization capacity, hinting that the more robust antibody response in those previously infected may have more neutralization capacity against SARS-CoV-2 in vivo.
Our findings are particularly relevant in the context of ongoing booster programs commenced in several jurisdictions.24 , 30 Given the significantly higher antibody levels in individuals with past SARS-CoV-2 infection, our data suggest that SARS-CoV-2–naïve nursing home residents may be capable of inducing higher antibody titers with additional exposure to viral antigen. Of note, early clinical data suggest that a third dose of BNT162b2 vaccine is well tolerated in older adults.30 Taken together, the current data support these booster programs by demonstrating that additional doses could be beneficial if they are to boost antibody responses in this vulnerable population, particularly in those with no history of SARS-CoV-2 infection.
Importantly, our study only measured the humoral immune response to SARS-CoV-2 Spike protein and by design did not assess the cellular immune responses to SARS-CoV-2. Determining the differences in T-cell response between those previously infected and SARS-CoV-2–naïve nursing home residents is a crucial area for future research. Although our findings demonstrate a sharp decline in the humoral response at 6 months, the clinical correlates of this in terms of breakthrough SARS-CoV-2 infection, infection severity, and mortality are yet to be determined. A further limitation of the current study is the relatively small sample size; however, we were still able to demonstrate the striking effects of past COVID-19 illness on 6-month vaccine-induced antibody titers.
Conclusions and Implications
We demonstrated a significant association between SARS-CoV-2 infection history, age, frailty, and antibody responses in older nursing home residents at 6 months post vaccination. Our findings suggest that SARS-CoV-2–naïve nursing home residents may benefit from ongoing vaccine booster programs. Our findings call for further research into the complex relationship among SARS-CoV-2 antibody titers, breakthrough SARS-CoV-2 infection, and clinical outcomes in older nursing home residents.
Acknowledgments
We acknowledge the Meath Foundation, Clinical Chemistry and Laboratory Medicine Department in Tallaght University Hospital, local Nursing Homes in the Tallaght University Hospital catchment area, all study participants, their families, and carers.
Footnotes
N.M.B. and S.P.K. contributed equally to this work.
The NH-COVAIR Study was funded by a grant from the Meath Foundation, Tallaght University Hospital. A.H.D. has been awarded the Irish Clinical Academic Training (ICAT) Programme, supported by the Wellcome Trust and the Health Research Board (Grant Number 203930/B/16/Z), the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division, Northern Ireland. N.B. is funded under the Science Foundation Ireland Phase 2 COVID-19 Rapid Response Call (20/COV/8487) and the Health Research Board COVID-19 Rapid Response Call (COV19–2020–053).
The authors declare no conflicts of interest.
References
- 1.Geriatric Medicine Research Collaborative, Covid Collaborative, Welch C., et al. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021;50:517–630. doi: 10.1093/ageing/afab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fallon A., Dukelow T., Kennelly S.P., O'Neill D. COVID-19 in nursing homes. QJM. 2020;113:391–392. doi: 10.1093/qjmed/hcaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennelly S.P., Dyer A.H., Noonan C., et al. Asymptomatic carriage rates and case fatality of SARS-CoV-2 infection in residents and staff in Irish nursing homes. Age Ageing. 2021;50:49–54. doi: 10.1093/ageing/afaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Medical Directors Association Interim recommendations for communal activities and visitation in post-acute and long-term care facilities. https://paltc.org/sites/default/files/Interim Guidance for Communial Activities and Visitation 25FEB2021 - FINAL.pdf
- 5.Centers for Disease Control and Prevention COVID-19 vaccine-breakthrough case investigation and reporting. https://www.cdc.gov/vaccines/covid-19/health-departments/breathrough-cases.html
- 6.Sciensano Surveillance in residential care centres. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Surveillance_WZC.pdf
- 7.Morciano M., Stokes J., Kontopantelis E., Hall I., Turner A.J. Excess mortality for care home residents during the first 23 weeks of the COVID-19 pandemic in England: a national cohort study. BMC Med. 2021;19:71. doi: 10.1186/s12916-021-01945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briggs R., Coughlan T., Collins R., et al. Nursing home residents attending the emergency department: clinical characteristics and outcomes. QJM. 2013;106:803–808. doi: 10.1093/qjmed/hct136. [DOI] [PubMed] [Google Scholar]
- 9.Falconer M., O'Neill D. Profiling disability within nursing homes: a census-based approach. Age Ageing. 2007;36:209–213. doi: 10.1093/ageing/afl185. [DOI] [PubMed] [Google Scholar]
- 10.Rolland Y., Cesari M., Morley J.E., et al. Editorial: COVID-19 vaccination in frail people: lots of hope and some questions. J Nutr Health Aging. 2021;25:146–147. doi: 10.1007/s12603-021-1591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shratri M., Krutikov M., Palmer T., et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;21:1529–1538. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabezas C., Coma E., Mora-Fernandez N., et al. Associations of BNT162b2 vaccination with SARS-CoV-2 vaccination with SARS-CoV-2 infection and hospital admission and death with covid-19 in nursing homes and healthcare workers in Catalonia: prospective cohort study. BMJ. 2021;374:n1868. doi: 10.1136/bmj.n1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doria-Rose N., Suthar M.S., Makowski M., et al. 2021 antibody persistent through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N Eng J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naaber P., Tserel L., Kangro K., et al. Dynamics of antibody response to BNT162b2 vaccine after 6 months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blain H., Tuaillon E., Gamon L., et al. Spike antibody levels of nursing home residents with or without prior COVID-19 3 weeks after a single BNT162b2 vaccine dose. JAMA. 2021;325:18. doi: 10.1001/jama.2021.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Praet J.T.V., Vandecasteele S., De Roo A., et al. Humoral and cellular immunogenicity of the BNT162b2 messenger RNA coronavirus disease. 2019 Vaccine in Nursing Home Residents. Clin Infect Dis. 2021;73:2145–2147. doi: 10.1093/cid/ciab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmeron Rios S., Romero M., Zamora E.B.C., et al. Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study. J Am Geriatr Soc. 2021;69:1441–1447. doi: 10.1111/jgs.17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nace D.A., Kip K.E., Mellors J.Q., et al. Angitbody responses after mRNA-based COVID-19 vaccination in residential older adults: implications for reopening. J Am Med Dir Assoc. 2021;22:1593–1598. doi: 10.1016/j.jamda.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canaday D.H., Carias L., Oyebanji O.A., et al. Reduced BNT162b2 messenger RNA vaccine response in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-naïve nursing home residents. Clin Infect Dis. 2021;73:2112–2115. doi: 10.1093/cid/ciab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breznik J.A., Zhang A., Huynh A., et al. Antibody responses 3–5 months post-vaccination with mRNA-1273 or BNT163b2 in nursing home residents. J Am Med Dir Assoc. 2021;22:2512–2514. doi: 10.1016/j.jamda.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miguez H.M., Gomez M.O., Monte sGarcia M., et al. Cellular and humoral response, six months after two doses of BNT162b2 mRNA COVID 19 vaccination, in residents and staff of nursing homes for the elderly. J Infect Dis. 2022;225:355–357. doi: 10.1093/infdis/jiab546. [DOI] [PubMed] [Google Scholar]
- 22.Blain H, Tuaillon E, Gamon L, et al. Receptor binding domain-IgG levels correlate with proection in residents facing SARS-CoV-2 B.1.1.7 outbreaks. Allergy. Published online October 14, 2021. https://doi.org/10.1111/all.15142. [DOI] [PMC free article] [PubMed]
- 23.Cavanaugh A.M., Fortier S., Lewis P., et al. COVID-19 outbreak associated with a SARS-CoV-2R.1 lineage variant in a skilled nursing facility after vaccination program – Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:639–643. doi: 10.15585/mmwr.mm7017e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barda N., Dagan N., Cohen C., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaehr E., Visvanathan R., Malmstrom T., Morley J.E. Frailty in nursing homes: the FRAIL-NH scale. J Am Med Dir Assoc. 2015;16:87–89. doi: 10.1016/j.jamda.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Malmstrom T.K., Morely J.E. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531–532. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel Index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 28.Leonard A., Prior A.R., Reilly P., et al. High anti-SARS-CoV-2 antibody seroprevalence in healthcare workers in an Irish university teaching hospital. Ir J Med Sci. 2021;30:1–6. doi: 10.1007/s11845-021-02690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phelan T., Dunne J., Conlon C., et al. Dynamic assay for profiling anti-SARS-CoV-2 antibodies and their ACE2/Spike RBD neutralization capacity. Viruses. 2021;13:1371. doi: 10.3390/v13071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David S., Shamir-Stein N.S., Gez S.B., Lerner U., Rahamim-Cohen D., Zohar A.E. Reactogenicity of a third BNT162b2 mRNA COVID-19 vaccine among immunocompromised individuals and seniors—A nationwide survey. Clin Immunol. 2021;232:108860. doi: 10.1016/j.clim.2021.108860. [DOI] [PMC free article] [PubMed] [Google Scholar]