Abstract
Background
This study sought to explore the value of the neutrophil to lymphocyte ratio (NLR) and interleukin-6 (IL-6) in predicting the prognosis of patients with non-metastatic colorectal cancer (CRC).
Methods
The data of 88 surgical CRC patients were retrospectively analyzed. A receiver operating characteristic (ROC) curve analysis was conducted to determine the patients’ thresholds for the NLR and IL-6. Kaplan-Meier curve and Cox regression models were used to assess the prognostic values.
Results
A ROC analysis was conducted to calculate the NLR cut-off value. The area under the curve (AUC) of the NLR was 0.739 [95% confidence interval (CI): 0.634 to 0.844] for overall survival (OS), and 0.799 (95% CI: 0.705 to 0.892) for disease-free survival (DFS). The AUC of IL-6 was 0.773 (95% CI: 0.670 to 0.876) for OS, and 0.817 (95% CI: 0.728 to 0.906) for DFS. The AUC of NLR + IL-6 was 0.805 (95% CI: 0.710 to 0.899) for OS and 0.853 (95% CI: 0.774 to 0.933) for DFS, which were higher than the NLR or IL-6 alone AUCs for OS and DFS. In addition, a high NLR and IL-6 value was significantly correlated with tumor differentiation and tumor-node-metastasis staging. The NLR was positively correlated with IL-6 level (r=0.481). The results of the Kaplan-Meier analysis showed that a high NLR + IL-6 value was correlated with worse OS and DFS.
Conclusions
A high NLR and IL-6 value is a better independent prognostic biomarker of CRC than the NLR or IL-6 level alone, and may be applied in clinical practice to identify high-risk patients.
Keywords: Colorectal cancer (CRC), neutrophil to lymphocyte ratio (NLR), interleukin-6, prognosis
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors of the digestive system, and is the fourth leading cause of cancer-related death in the world(1). The 5-year overall survival (OS) of patients with CRC has been significantly improved due to the rapid development of surgical technology and other therapies, such as chemotherapy, radiotherapy, targeted therapy and biotherapy (1). However, approximately 45% of patients undergoing colorectal resection cannot be cured, and these patients eventually develop recurrent cancer or die from tumor metastasis (2).
Inflammatory response plays an important role in the occurrence and development of CRC (3). The neutrophil to lymphocyte ratio (NLR) is a marker of the systemic inflammatory response, which has been confirmed to be associated with the prognosis of CRC patients (4,5). IL-6 is a pro-inflammatory factor that is associated with the development and prognosis of CRC (6). Recent research has explored the prognostic value of NLR and IL-6 in resectable stage II and III primary CRC. Thus, understanding the effect of the combined detection of NLR and IL6 on the prognosis of patients with CRC is of great clinical significance.
In this study, we first evaluated the prognostic value of the NLR or IL-6 level alone in CRC patients after surgery, and then further clarified their clinical significance by exploring the prognostic value of NLR + IL-6 combined in predicting the prognosis of patients with CRC.
We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-763).
Methods
Patients
We conducted a retrospective study of CRC patients who underwent surgery at The People’s Hospital of Leshan between January 2010 and February 2016. The CRC patients were pathologically confirmed to have stages II and III CRC, and no distant metastasis or local recurrence. The curative resections of all the CRC patients were performed by the same surgical team. The patients voluntarily received surgical treatment, and the final pathological diagnosis was CRC. The complete data of patient’s peripheral blood examination, including routine blood, biochemistry blood, and coagulation results, were obtained within 1 week before the operation.
Patients who had an infection or systemic inflammation, enterobrosis, hematological disease, intestinal obstruction, or history of other malignancies were excluded from the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of The People’s Hospital of Leshan (No. 20210701). All patients included in the study provided informed consent.
Data collection and laboratory methods
Data on patients’ clinical parameters were collected, including data on gender, age, tumor location, tumor size, operative adjuvant chemoradiotherapy, histological class, differentiation, tumor stage, and family histology. Tumor staging was performed according to tumor invasion and tumor-node-metastasis (TNM) classification (American Joint Committee on Cancer, 7th edition). Patients’ neutrophil and lymphocyte counts were measured within 3 days before the surgery. Blood was collected within 3 days before the surgery. The neutrophil and lymphocyte counts were detected in a clinical laboratory, and the NLR was then calculated. The NLR was defined as the absolute number of neutrophils divided by lymphocytes. Patients’ IL-6 levels were detected by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (CSB-E04638h, CUSABIO, Wuhan, China).
Survival and follow-up
All 103 patients were followed up with for 5 years. Twenty two patients were lost during the follow-up period for various reasons. The recurrence of CRC was confirmed by computed tomography, magnetic resonance imaging, and tumor markers, such as carcinoembryonic antigen, carbohydrate antigen 125, and finally confirmed by pathological examination. Disease-free survival (DFS) was measured from the date of surgery to the date of disease recurrence or the last follow-up examination without recurrence. OS was calculated from the date of diagnosis to the date of death or the last follow-up examination of survivors.
Statistical analysis
A receiver operating characteristic (ROC) curve analysis was conducted to identify the optimal preoperative NLR and IL-6 cut-off value. Based on these cut-off values, the CRC patients were divided into different groups according to their prognostic scores using the NLR, IL-6 level, and NLR + IL-6 value. The categorical and continuous variables are presented as number and mean ± standard deviation, respectively. The survival analysis was performed using the Kaplan-Meier survival curve. The Chi-square test was used to evaluate differences in patients’ clinicopathological characteristics. The Cox proportional-hazards regression model was used for the univariate and multivariate analyses of clinical variables to determine the independent prognostic factors. Differences were estimated by a log-rank test. P values <0.05 were considered statistically significant. All statistical analyses were performed with SPSS software version 22.0 (IBM Corporation, NY, USA).
Results
Clinical characteristics
A total of 88 patients (51.14% male and 48.86% female) with CRC were included in this study. The age of patients ranged from 25 to 81 years (46.59% of patients were aged <65, and 53.41% were aged ≥65), and patients had a mean age of 58.31±12.22 years. The mean pre-treatment NLR and IL-6 level were 2.94±0.76 and 219.20±38.25 pg/mL, respectively.
ROC curves of NLR and IL-6 for both OS and DFS
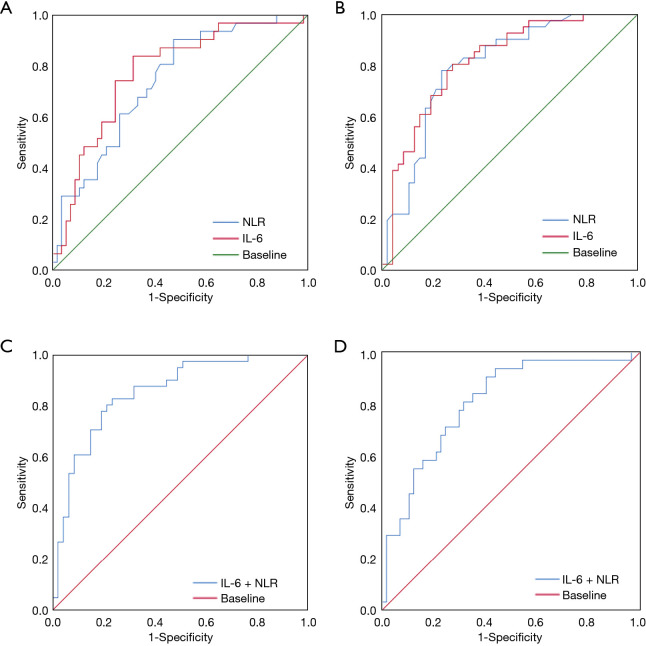
A ROC analysis was conducted to calculate the NLR cut-off value. The patients were divided into a high NLR (≥2.99) group and a low NLR (<2.99) group (see Figure 1). The area under the curve (AUC) was 0.739 [95% confidence interval (CI): 0.634 to 0.844] for OS (see Figure 1A), and 0.799 (95% CI: 0.705 to 0.892) for DFS (see Figure 1B). 2.99 was chosen as the optimal NLR value for evaluating OS and DFS. Similarly, based on the ROC analysis, the IL-6 cut-off value in our study was 213.83 pg/mL. The patients were divided into a high IL-6 (≥213.83) group and a low IL-6 (<213.83) group (see Figure 1). The AUC was 0.773 (95% CI: 0.670 to 0.876) for OS (see Figure 1A), and 0.817 (95% CI: 0.728 to 0.906) for DFS (see Figure 1B).
Figure 1.
ROC curve analysis of the NLR, IL-6 level and the NLR + IL-6 level in CRC patients. ROC curve analysis of the NLR and IL-6 level for OS (A) and DFS (B). ROC curve analysis of the NLR + IL-6 level for OS (C) and DFS (D). The prognostic scores of the NLR were assigned to scores of 1 (≥2.99) or scores of 0 (<2.99). The prognostic scores of IL-6 were assigned to scores of 1 (≥213.83 pg/mL) or scores of 0 (<213.83 pg/mL). The prognostic scores of the combination of the NLR and IL-6 level were scores of 2 (NLR ≥2.99 and ≥213.83 pg/mL), 1 (NLR ≥2.99 or ≥213.83 pg/mL), or 0 (neither NLR ≥2.43 nor ≥213.83 pg/mL). NLR, neutrophil to lymphocyte ratio; IL-6, interleukin-6; CRC, colorectal cancer; OS, overall survival; DFS, disease-free survival.
Correlations of NLR and IL-6 with clinicopathological factors
The correlations between NLR and IL-6 and various clinicopathological characteristics were analyzed. The results showed that a high NLR was significantly correlated with tumor differentiation and TNM staging. In addition, the high expression of IL-6 was correlated with tumor differentiation and TNM staging. However, there were no significant differences in the distribution of gender, age, tumor size, tumor location, and histologic class among patients (see Table 1). In addition, the results of the Spearman rank correlation analysis showed that the NLR was positively correlated with IL-6 (r=0.481).
Table 1. Characteristics of the 88 patients grouped by NLR and IL-6.
| Variables | Sum, n (%) | NLR | IL-6 | |||||
|---|---|---|---|---|---|---|---|---|
| ≥2.99 | <2.99 | P | ≥213.83 pg/mL | <213.83 pg/mL | P | |||
| Age (years) | ||||||||
| <65 | 41 (46.59) | 22 | 19 | 0.808 | 25 | 16 | 0.462 | |
| ≥65 | 47 (53.41) | 24 | 23 | 25 | 22 | |||
| Gender | ||||||||
| Female | 45 (51.14) | 21 | 24 | 0.673 | 20 | 25 | 0.286 | |
| Male | 43 (48.86) | 22 | 21 | 24 | 19 | |||
| Cancer site | ||||||||
| Rectum | 46 (52.27) | 25 | 21 | 0.393 | 28 | 18 | 0.212 | |
| Colon | 42 (47.73) | 19 | 23 | 20 | 22 | |||
| Histologic class | ||||||||
| Adenocarcinoma | 40 (45.45) | 22 | 18 | 0.793 | 27 | 15 | 0.414 | |
| Non-adenocarcinoma | 48 (54.55) | 25 | 23 | 26 | 22 | |||
| Differentiation | ||||||||
| Well/moderate | 68 (77.27) | 30 | 38 | 0.018 | 32 | 36 | 0.016 | |
| Poor | 30 (34.09) | 21 | 9 | 22 | 8 | |||
| Tumor stage | ||||||||
| II | 46 (52.27) | 17 | 29 | 0.005 | 19 | 27 | 0.05 | |
| III | 40 (45.45) | 27 | 13 | 25 | 15 | |||
| Tumor size (cm) | ||||||||
| <5 | 53 (60.23) | 27 | 25 | 0.458 | 24 | 28 | 0.823 | |
| ≥5 | 35 (39.77) | 21 | 14 | 17 | 18 | |||
NLR, neutrophil to lymphocyte ratio; IL-6, interleukin-6.
NLR + IL-6 is a superior prognostic biomarker
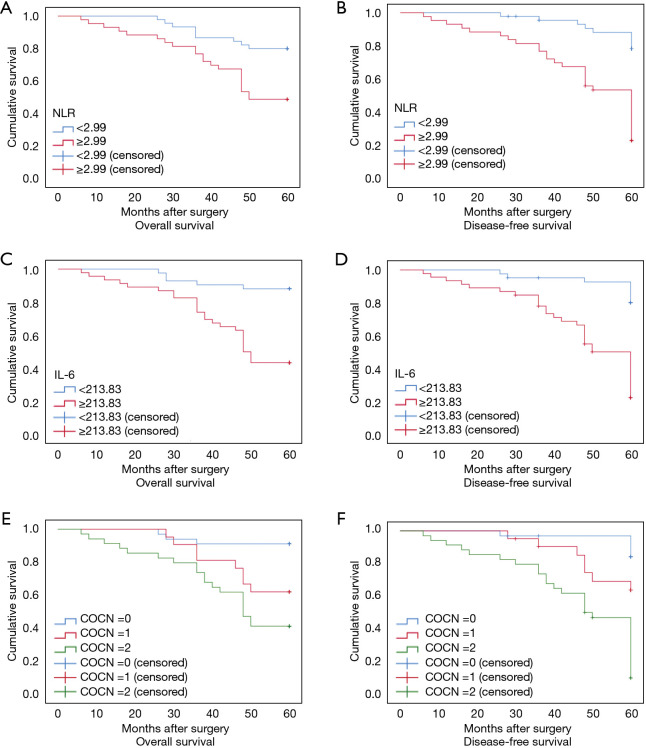
According to the results of this study and other studies (7-9), the NLR and IL-6 level are independent prognostic biomarkers for CRC. However, the question of whether the combined detection of NLR + IL-6 can also act as prognostic biomarkers for CRC had not been investigated. Thus, ROCs and the Kaplan-Meier method were used to assess the value of both NLR + IL-6 in predicting the prognosis of CRC patients. The results showed that the AUC of NLR + IL-6 was 0.805 (95% CI: 0.710 to 0.899) for OS (see Figure 1C) and 0.853 (95% CI: 0.774 to 0.933) for DFS (see Figure 1D), which were higher than the NLR or IL-6 alone AUCs for OS and DFS. In addition, the results of the Kaplan-Meier method showed that a high NLR + IL-6 value was correlated with worse OS and DFS (see Figure 2). These results suggest that the combined detection of NLR and IL-6 could be used as an important CRC prognostic biomarker, and the efficacy may be better than that of NLR or IL-6 alone.
Figure 2.
Kaplan-Meier survival curves for OS and DFS in CRC patients. (A) OS according to the NLR; (B) DFS according to the NLR; (C) OS according to IL-6 level; (D) DFS according to IL-6 level; (E) OS according to the NLR + IL-6 level; (F) DFS according to the NLR + IL-6 level. The prognostic scores of the NLR were assigned to scores of 1 (≥2.99) or scores of 0 (<2.99). The prognostic scores of IL-6 level were assigned to scores of 1 (≥213.83 pg/mL) or scores of 0 (<213.83 pg/mL). The prognostic scores of the combination of the NLR and IL-6 level (COCN) were scores of 2 (NLR ≥2.99 and IL-6 ≥213.83 pg/mL), 1 (NLR ≥2.99 or IL-6 ≥213.83 pg/mL), or 0 (neither NLR ≥2.43 nor IL-6 ≥213.83 pg/mL). NLR, neutrophil to lymphocyte ratio; IL-6, interleukin-6; CRC, colorectal cancer; OS, overall survival; DFS, disease-free survival.
Discussion
In this study, a high NLR + IL-6 value was correlated with worse OS and DFS, and was significantly correlated with tumor differentiation and TNM staging. In addition, the NLR was positively correlated with IL-6 level. Thus, these findings indicate that a high NLR + IL-6 value is a better independent prognostic biomarker of CRC than the NLR or IL-6 level alone, and may be applied in clinical practice to identify high-risk patients.
Recently, many studies have shown that inflammation response affects the occurrence and development of CRC (10). In the early immune response, inflammation may inhibit tumorigenesis; however, persistent inflammatory stimulation can promote tumor progression (11). Some inflammatory biomarkers have the ability to predict the prognosis of CRC (12,13). Neutrophils play a central role in inflammation within the tumor as they are attracted by CXCR2 ligands like CXCL1, CXCL2 and CXCL5, among others. Neutrophils release reactive oxygen species (ROS), reactive nitrogen (RNs) or proteases, and mediate angiogenesis, and also activate PI3K signaling, active promoter 2 (prok2) and MMP9, and finally promote tumorigenesis by inducing epithelial-mesenchymal transition (EMT) (14). The NLR has been proposed to be an indicator of cancer-related inflammation (15). Lymphocytes regulate the migration and proliferation of tumor cells and inhibit tumorigenesis (4,16). Conversely, neutrophils promote tumor angiogenesis and metastasis (17). The recruitment of neutrophils and lymphocytes plays an important role in the occurrence and development of CRC (18). An increased NLR in peripheral blood is associated with the poor prognosis of CRC (19). In our study, we demonstrated that a high NLR was significantly correlated with tumor differentiation and TNM staging. CRC patients with a high NLR had shorter OS and DFS than patients with a low NLR. This is consistent with other studies (7-9). These results suggest that high NLR values are positively correlated with the malignant degree of CRC and have prognostic significance in CRC.
Inflammation is closely related to the development of cancer. Gastrointestinal cancer appears to be particularly sensitive to inflammation (20,21). Inflammation can trigger and accelerate the development of cancer, leading to the formation of malignant tumors (22). IL-6 is a key regulator of CRC tumorigenesis, tumor growth, metastasis, and chemotherapy resistance, and it mainly activates the ERK, AKT, JAK2/STAT3 pathway and Twist initiating a vicious loop securing the EMT phenotype (23,24). Serum IL-6 levels have been shown to be associated with a poor prognosis, tumor burden, survival rate, and advanced stages of pancreatic cancer, lung cancer, esophageal cancer, breast cancer, ovarian cancer, and renal cancer (25-29). In addition, recent studies have shown that IL-6 may play a role in the occurrence and development of CRC. CRC patients with high IL-6 levels have a poor prognosis (30,31). In our study, the high expression of IL-6 was correlated with tumor differentiation and TNM staging. CRC patients with high IL-6 levels had shorter OS and DFS than patients with low IL-6 levels. These results are consistent with those of above studies. These results suggest that high IL-6 levels promote the malignant progression of CRC and have prognostic significance in CRC patients.
Many studies have confirmed the prognostic value of the NLR or IL-6 level alone in CRC patients (6,32). However, the prognostic value of the NLR combined with IL-6 level in CRC had not previously been clarified. NLR and IL-6 are significantly correlated with advanced-stage disease and reduced OS of esophageal squamous cell carcinoma patients (8). The NLR has been shown to be positively correlated with the expression of IL-6 in esophageal squamous cell carcinoma patients. Increased levels of IL-6 are associated with NLR values in laryngeal cancer (4). These results are in line with our results. In this study, we found that the NLR was positively correlated with IL-6 level in CRC. We found that high NLR + IL-6 values were correlated with worse OS and DFS, and were higher than those of NLR or IL-6 alone. These results imply that NLR + IL-6 can be used as an important prognostic biomarker, and the efficacy may be better than that of NLR or IL-6 alone. However, future studies are needed to validate the mechanism and prospectively validate the prognostic usefulness of NLR + IL-6 level in CRC patients, and to better guide the treatment of CRC. In addition, more patients should be added for future studies.
In our study, high NLR and IL-6 values were found to be positively correlated with the malignant degree of CRC and have prognostic significance in CRC patients. The combined detection of the NLR + IL-6 level could be used as an important prognostic biomarker, and the efficacy may be better than that of NLR or IL-6 alone. The NLR + IL-6 value may be applied in clinical practice to identify high-risk patients.
Acknowledgments
Funding: This study was supported by a first-class discipline construction project from the Colleges and Universities of Ningxia (NXYLXK2017A05).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of The People’s Hospital of Leshan (No. 20210701). All patients included in the study provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-763
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jgo-21-763
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-763). The authors have no conflicts of interest to declare.
References
- 1.Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet 2019;394:1467-80. 10.1016/S0140-6736(19)32319-0 [DOI] [PubMed] [Google Scholar]
- 2.Sada O, Ahmed K, Jeldo A, et al. Role of Anti-inflammatory Drugs in the Colorectal Cancer. Hosp Pharm 2020;55:168-80. 10.1177/0018578718823736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan Q, Zhao R, Xia L, et al. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: a meta-analysis of 26 observational studies. J Cancer Res Clin Oncol 2021;147:1077-87. 10.1007/s00432-020-03496-0 [DOI] [PubMed] [Google Scholar]
- 4.Du J, Liu J, Zhang X, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts survival in patients with laryngeal cancer. Oncol Lett 2018;15:1664-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva TH, Schilithz AOC, Peres WAF, et al. Neutrophil-lymphocyte ratio and nutritional status are clinically useful in predicting prognosis in colorectal cancer patients. Nutr Cancer 2020;72:1345-54. 10.1080/01635581.2019.1679198 [DOI] [PubMed] [Google Scholar]
- 6.Coşkun Ö, Öztopuz Ö, Özkan ÖF. Determination of IL-6, TNF-α and VEGF levels in the serums of patients with colorectal cancer. Cell Mol Biol (Noisy-le-grand) 2017;63:97-101. 10.14715/cmb/2017.63.5.18 [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Ramos M, Del Puerto-Nevado L, Zheng B, et al. Prognostic significance of neutrophil-to lymphocyte ratio and platelet-to lymphocyte ratio in older patients with metastatic colorectal cancer. J Geriatr Oncol 2019;10:742-8. 10.1016/j.jgo.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 8.Chen MF, Chen PT, Kuan FC, et al. The Predictive Value of Pretreatment Neutrophil-To-Lymphocyte Ratio in Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2019;26:190-9. 10.1245/s10434-018-6944-1 [DOI] [PubMed] [Google Scholar]
- 9.Sunakawa Y, Yang D, Cao S, et al. Immune-related Genes to Dominate Neutrophil-lymphocyte Ratio (NLR) Associated With Survival of Cetuximab Treatment in Metastatic Colorectal Cancer. Clin Colorectal Cancer 2018;17:e741-9. 10.1016/j.clcc.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Łaskowski P, Klim B, Ostrowski K, et al. Local inflammatory response in colorectal cancer. Pol J Pathol 2016;67:163-71. 10.5114/pjp.2016.61453 [DOI] [PubMed] [Google Scholar]
- 11.Ghuman S, Van Hemelrijck M, Garmo H, et al. Serum inflammatory markers and colorectal cancer risk and survival. Br J Cancer 2017;116:1358-65. 10.1038/bjc.2017.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe EK, Lee S, Kim SY, et al. Prognostic Effect of Inflammatory Genes on Stage I-III Colorectal Cancer-Integrative Analysis of TCGA Data. Cancers (Basel) 2021;13:751. 10.3390/cancers13040751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colloca GA, Venturino A, Guarneri D. Reduction of derived neutrophil-to-lymphocyte ratio after four weeks predicts the outcome of patients receiving second-line chemotherapy for metastatic colorectal cancer. Cancer Immunol Immunother 2021;70:1115-25. 10.1007/s00262-020-02761-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ocana A, Nieto-Jiménez C, Pandiella A, et al. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer 2017;16:137. 10.1186/s12943-017-0707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Zhang Y, Yv X, et al. Prognostic value of combined preoperative prognostic nutritional index and neutrophil to lymphocyte ratio in esophageal squamous cell carcinoma. Transl Cancer Res 2020;9:5117-27. 10.21037/tcr-19-2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krakowska M, Dębska-Szmich S, Czyżykowski R, et al. The prognostic impact of neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio in patients with advanced colorectal cancer treated with first-line chemotherapy. Prz Gastroenterol 2018;13:218-22. 10.5114/pg.2018.78287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda A, Yamada T, Matsumoto S, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio Predicts Survival After TAS-102 Treatment of Patients With Metastatic Colorectal Cancer. Anticancer Res 2019;39:4343-50. 10.21873/anticanres.13602 [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Jung HI, Kwon SH, et al. Preoperative neutrophil-lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis. Ann Surg Treat Res 2019;96:191-200. 10.4174/astr.2019.96.4.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dogan E, Bozkurt O, Sakalar T, et al. Impact of neutrophil-lymphocyte and platelet-lymphocyte ratio on antiEGFR and bevacizumab efficacy in metastatic colorectal cancer. J BUON 2019;24:1861-9. [PubMed] [Google Scholar]
- 20.Chung SS, Wu Y, Okobi Q, Adekoya D, Atefi M, Clarke O, et al. Proinflammatory Cytokines IL-6 and TNF-alpha Increased Telomerase Activity through NF-kappaB/STAT1/STAT3 Activation, and Withaferin A Inhibited the Signaling in Colorectal Cancer Cells. Mediators Inflamm. 2017;2017:5958429. 10.1155/2017/5958429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang WW, Wang QH, Peng P, et al. Effects of flurbiprofen axetil on postoperative serum IL-2 and IL-6 levels in patients with colorectal cancer. Genet Mol Res 2015;14:16469-75. 10.4238/2015.December.9.18 [DOI] [PubMed] [Google Scholar]
- 22.Kim B, Seo Y, Kwon JH, et al. IL-6 and IL-8, secreted by myofibroblasts in the tumor microenvironment, activate HES1 to expand the cancer stem cell population in early colorectal tumor. Mol Carcinog 2021;60:188-200. 10.1002/mc.23283 [DOI] [PubMed] [Google Scholar]
- 23.Heichler C, Scheibe K, Schmied A, et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut 2020;69:1269-82. 10.1136/gutjnl-2019-319200 [DOI] [PubMed] [Google Scholar]
- 24.Suarez-Carmona M, Lesage J, Cataldo D, et al. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol 2017;11:805-23. 10.1002/1878-0261.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumacher N, Rose-John S. ADAM17 Activity and IL-6 Trans-Signaling in Inflammation and Cancer. Cancers (Basel) 2019;11:1736. 10.3390/cancers11111736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol 2018;18:773-89. 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]
- 27.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 2018;15:234-48. 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browning L, Patel MR, Horvath EB, et al. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res 2018;10:6685-93. 10.2147/CMAR.S179189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumari N, Dwarakanath BS, Das A, et al. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol 2016;37:11553-72. 10.1007/s13277-016-5098-7 [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Ren G, Wang T, et al. Aberrantly expressed Fra-1 by IL-6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial-mesenchymal transition. Carcinogenesis 2015;36:459-68. 10.1093/carcin/bgv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu CC, Kuo HC, Wang FS, et al. Upregulation of TLRs and IL-6 as a marker in human colorectal cancer. Int J Mol Sci 2014;16:159-77. 10.3390/ijms16010159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo H, Murayama Y, Arita T, et al. The Prognostic Value of Preoperative Neutrophil-to-Lymphocyte Ratio in Colorectal Cancer. World J Surg 2016;40:2796-802. 10.1007/s00268-016-3595-x [DOI] [PubMed] [Google Scholar]