Abstract
Objective: To determine the efficacy and safety of bevacizumab (Bz) combined with temozolomide (TMZ) in the treatment of recurrent malignant gliomas and its influence on serum tumor markers (STMs). Methods: The clinical data of 73 patients with recurrent malignant gliomas admitted to the First People’s Hospital of Shuangliu District from April 2016 to June 2018 were analyzed retrospectively. Patients were divided into two groups according to different therapies: the control group (n=33) treated with TMZ, and the research group (n=40) treated with Bz combined with TMZ (Bz+TMZ). The overall response rate (ORR), disease control rate (DCR) and incidence of adverse reactions (ARs) were observed after 4 courses of treatment. The levels of STMs were detected. Additionally, the Karnofsky Performance Scale (KPS) score and quality of life (QoL) before and after treatment were compared between the two groups. The 1-year and 2-year survival rates as well as median survival time (MST) were also compared after 2-year follow-up. Treatment satisfaction was recorded and compared. Results: After treatment, the research group exhibited better ORR and DCR than the control group; The incidence of ARs differed insignificantly between the two arms; The serum levels of Vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and transforming growth factor β (TGF-β) in the research group were statistically lower than those in the control group; The KPS score and QoL score increased significantly in both arms, and were higher in the research group compared with the control group; the research group was also superior to the control group in treatment satisfaction. The research group showed higher 1-year and 2-year survival rates than the control group. Conclusions: BZ+TMZ is effective in treating recurrent malignant gliomas, which can improve the QoL and survival of patients.
Keywords: Bevacizumab, temozolomide, recurrent glioma, serum tumor markers, survival rate
Introduction
Malignant glioma is a common malignancy of the central nervous system, with high prevalence, rapid progression, and increased risk of death in patients [1]. With the population aging, the incidence of malignant gliomas is increasing, especially glioblastoma, which accounts for 46.1% of primary malignant central nervous system tumors, with the highest incidence found in the elderly [2]. The main pathological types of the disease include glioblastoma, anaplastic astrocytoma, anaplastic oligodendrocytoma, and anaplastic oligodendrocytoma [3]. The major clinical manifestations are cranial hypertension symptoms (headache, nausea and vomiting, visual impairment, etc.) that occur in 90% of patients, as well as other presentations such as mental changes, seizures, and focal neurological symptoms. The severity of symptoms is related to the size and location of the tumor, as well as the age of the patient [4].
Malignant gliomas are mostly located in the cerebral hemisphere, with rapid tumor growth and obvious peritumoral space-occupying effect [5]. Currently, magnetic resonance imaging (MRI) is most widely used to assist the diagnosis of the disease in clinic, and the MRI lesion site is characterized by a mass with mixed density and uneven ring enhancement with edema zone surrounding it [6]. At present, the primary clinical treatment scheme is to maximize the surgical resection on the premise of guaranteeing the neurological function, combined with drug therapy after surgery. Although this scheme can prolong the survival of patients, it is difficult to completely remove the tumor due to high degree of malignancy, strong invasiveness and other biological characteristics, as well as the susceptibility to relapse induced by the residual tumor cells [7,8]. The prognosis of recurrent malignant gliomas is poor and there is currently no recognized optimal treatment plan [9].
Dose-dense regimen of temozolomide (TMZ) is the most extensively used clinical treatment, but the effect is not ideal [10,11]. Bevacizumab (Bz) is a human vascular endothelial growth factor receptor (VEGFR) monoclonal antibody, which can specifically bind to vascular endothelial growth factor (VEGF), block the downstream conduction pathway, reduce tumor angiogenesis, and inhibit tumor growth [12,13]. Malignant gliomas are rich in tumor blood vessels. Therefore, anti-angiogenesis therapy has become a vital strategy for the treatment of malignant gliomas [14]. VEGF, epidermal growth factor (EGF) and transforming growth factor β (TGF-β) are important serological indicators and common monitoring factors in the progression of glioma [15]. However, the employment of Bz combined with TMZ (Bz+TMZ) on recurrent malignant gliomas is rarely reported [16].
In this study, patients with recurrent malignant gliomas were treated with Bz+TMZ to explore the efficacy and safety of this combination therapy and its influence on serum tumor markers (STMs). The combined antivascular targeted therapy and chemotherapy may help with exploration of the mechanism of tumor recurrence, and thus to provide more monitoring indicators for clinical evaluation and treatment effect of malignant gliomas.
Materials and methods
Patient data
In this retrospective study, 73 patients with recurrent malignant gliomas admitted to the First People’s Hospital of Shuangliu District during April 2016 and June 2018 were enrolled and divided into two groups: the control group (n=33) treated with TMZ monotherapy, and the research group (n=40) treated by Bz+TMZ. In the control group, there were 19 males and 14 females with an average age of (54.83±9.12) years old (range: 30-72). In the research group, the male to female ratio was 25:15 females, and the mean age was (55.06±9.74) years old (range: 28-70). This study was approved by the Ethics Committee of our hospital, and the subjects and their families were informed and signed a fully informed consent.
Inclusion and exclusion criteria
Inclusion criteria
(1) Meeting the diagnostic criteria of malignant gliomas [17]; (2) World Health Organization (WHO) classification III-IV; (3) First relapse confirmed by MRI; (4) Karnofsky Performance Scale (KPS) score ≥60; (5) Presence of measurable or evaluable lesions; (6) No contraindications of chemotherapy, and high compliance with treatment and follow-up.
Exclusion criteria
(1) Prior Bz treatment; (2) Severe primary heart, liver, kidney and other organ diseases, or coagulation dysfunction; (3) Bleeding tendency, history of cerebral hemorrhage/cerebral embolism/severe hypertension, or severe intracranial infection; (4) Cognitive impairment, or central nervous system and severe peripheral nerve diseases; (5) Incomplete clinical data.
Treatment methods
This study was a retrospective study, and patients were divided into a control group and a research group according to different treatment regimens. The control group was treated with TMZ monotherapy, while the research group was treated with the combination therapy of Bz+TMZ. Both groups were given 100-120 mg/m2 TMZ capsules (dose-dense regimen) (Tasly, Jiangsu, China, H20040637) on an empty stomach at an interval of 1 week after continuous treatment for 7 days, and continued to take the drug for 7 days at an interval of 1 week, with 28 days as a course of treatment. In the subsequent chemotherapy, the dose of TMZ could be reduced by 80 mg/m2 if the bone marrow was suppressed and the neutrophil count was >1.5 × 109/L. On this basis, the research group received 5 mg/m2 Bz injection (Qilu Pharmaceutical, Jinan, China, S20190040) diluted in 100-250 mL 0.9% sodium chloride solution, for intravenous drip once every two weeks for more than 90 min. If there were no adverse reactions (ARs), the infusion time could be reduced to 60 min. Patients in both arms were evaluated once after 2 courses of chemotherapy. Chemotherapy was discontinued for patients with progressive disease (PD). Those with stable disease (SD) or partial response (PR) continued the original chemotherapy after another two courses of chemotherapy, with a total of 4 courses, and the treatment efficacy was evaluated until the completion of chemotherapy.
Outcome measures
Primary outcome measures
(1) Evaluation of overall response rate (ORR): Complete response (CR): complete disappearance of the lesion, lasting for over 4 weeks; PR: a ≥30% reduction in the lesion size for at least 4 weeks, with no new lesions; SD: a condition between PR and PD; PD: a >20% increase in the two-diameter product of the lesion, or the appearance of new lesions. ORR = (CR cases + PR cases)/total cases × 100%, disease control rate (DCR) = (CR cases + PR cases + SD cases)/total cases × 100%. (2) Incidence of adverse reactions (ARs): During treatment, ARs such as nausea, vomiting, fever, myelosuppression and skin ulceration were observed. (3) STMs: Venous blood was collected on an empty stomach before and 3 months after treatment. The levels of VEGF, EGF and TGF-β were detected by ELISA in strict accordance with the kit instructions of human VEGF ELISA, human EGF ELISA and human TGF-β ELISA (Jingkang Bioengineer, Shanghai, China, Cat. Nos. JK-ELISA-00407, JK JK-ELISA-01184 and JKSW70015).
Secondary outcome measures
(1) KPS score: The functional state of patients was assessed by the KPS [18]. The higher the score, the better the patient’s health and tolerance to the side effects brought by the treatment. It is generally considered that a KPS score above 80 is independent, that is, self-care level; 50-70 is divided into semi-independent, that is, living semi-self-care, and <50 means dependent, that is, living with considerable assistance from others. Patients with more than 80 points had better postoperative condition and longer survival. (2) Quality of life (QoL) score: Referring to the QoL standards of cancer patients [19], patients’ QoL was evaluated from 12 dimensions of appetite, spirit, sleep, fatigue, pain, family’s understanding and cooperation, colleagues’ understanding and cooperation, self-understanding of cancer, attitude towards treatment, daily life, side effects of treatment, and facial expression, with a total score of 60 points. Evaluation criteria: <20 points, extremely poor QoL; 21-30 points, poor QoL; 31-40 points, average QoL; 41-50 points, fair QoL; 51-60 points, good QoL. (3) Long-term survival rate: The 1-year and 2-year survival rates of patients were followed up and the median survival time (MST) was calculated. (4) Treatment satisfaction: The self-made treatment satisfaction questionnaire was used to evaluate the treatment satisfaction of patients after treatment. There are 20 questions in total, with 5 points for each question. A total score <70 was considered as dissatisfied, 70-89 as satisfied, and ≥90 as very satisfied. Satisfaction = (very satisfied cases + satisfied cases)/total cases × 100%.
Statistical methods
SPSS24.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis and GraphPad Prism 7 was used for image rendering of the data. Categorical variables, described as [n(%)], were compared by the Chi-square test. Continuous variables were expressed as Mean ± standard deviation (x̅±SD); The independent samples t-test was used for inter-group comparisons and paired t-test was adopted for intra-group comparisons before and after treatment. P<0.05 was considered statistically significant.
Results
General data
There were no significant differences between the two groups in general data such as gender, age, body mass index (BMI), pathological grade, time to recurrence, marital status, residence, educational background, and smoking/hypertension/diabetes history (all P>0.05) Table 1.
Table 1.
Comparison of general information between the two groups [n (%)] (x̅±SD)
| Classification | Research group (n=40) | Control group (n=33) | t/χ2 value | P value |
|---|---|---|---|---|
| Gender | 0.183 | 0.668 | ||
| Male | 25 (62.50) | 19 (57.58) | ||
| Female | 15 (37.50) | 14 (42.42) | ||
| Age (years old) | 55.06±9.74 | 54.83±9.12 | 0.103 | 0.918 |
| BMI (kg/m2) | 23.18±2.57 | 23.25±2.65 | 0.114 | 0.909 |
| Pathological grading | 0.065 | 0.797 | ||
| III | 28 (70.00) | 24 (72.73) | ||
| IV | 12 (30.00) | 9 (27.27) | ||
| Time to recurrence (months) | 15.02±4.81 | 15.25±4.74 | 0.204 | 0.838 |
| Marital status | 0.206 | 0.649 | ||
| Married | 31 (77.50) | 27 (81.82) | ||
| Single | 9 (22.50) | 6 (18.18) | ||
| Residence | 0.072 | 0.788 | ||
| Urban | 23 (57.50) | 20 (60.61) | ||
| Rural | 17 (42.50) | 13 (39.39) | ||
| Educational background | 0.612 | 0.433 | ||
| ≥High school | 10 (25.00) | 11 (33.33) | ||
| <High school | 30 (75.00) | 22 (66.67) | ||
| History of smoking | 0.557 | 0.455 | ||
| Yes | 18 (45.00) | 12 (36.36) | ||
| No | 22 (55.00) | 21 (63.64) | ||
| History of hypertension | 0.069 | 0.792 | ||
| Yes | 11 (27.50) | 10 (30.30) | ||
| No | 29 (72.50) | 23 (69.70) | ||
| History of diabetes | 0.017 | 0.894 | ||
| Yes | 9 (22.50) | 7 (21.21) | ||
| No | 31 (77.50) | 26 (78.79) |
Note: BMI, body mass index.
ORR
After treatment, the ORR was 57.50% in the research group and 30.30% in the control group, and the DCR was 87.50% in the research group and 63.64% in the control group. The data revealed evidently higher ORR and DCR in the research group compared with the control group (P<0.05) Table 2.
Table 2.
Comparison of the total effective rate between the two groups after treatment [n (%)]
| Groups | Complete response | Partial response | Stable disease | Progressive disease | Overall response rate | Disease control rate |
|---|---|---|---|---|---|---|
| Research group (n=40) | 5 (12.50) | 18 (45.00) | 12 (30.00) | 5 (12.50) | 23 (57.50) | 35 (87.50) |
| Control group (n=33) | 1 (3.03) | 9 (27.27) | 11 (33.33) | 12 (36.36) | 10 (30.30) | 21 (63.64) |
| χ2 | - | - | - | - | 5.400 | 5.764 |
| P | - | - | - | - | 0.020 | 0.016 |
Incidence of ARs
After treatment, the incidence of ARs was 20.00% in the research group and 39.39% in the control group, with no significant difference between the two groups (P>0.05) Table 3.
Table 3.
Comparison of the incidence of adverse reactions between two groups after treatment [n (%)]
| Groups | Myelosuppression | Fever | Hypertension | Nausea and vomiting | Skin ulceration | Total incidence |
|---|---|---|---|---|---|---|
| Research group (n=40) | 2 (5.00) | 1 (2.50) | 2 (5.00) | 2 (5.00) | 1 (2.50) | 8 (20.00) |
| Control group (n=33) | 3 (9.09) | 2 (6.06) | 2 (6.06) | 3 (9.09) | 3 (9.09) | 13 (39.39) |
| χ2 | - | - | - | - | - | 3.319 |
| P | - | - | - | - | - | 0.068 |
KPS score
The KPS score, differed insignificantly between the two arms before therapy, elevated remarkably in the research group and the control group after treatment, with higher score in the research group (P<0.05) Figure 1.
Figure 1.
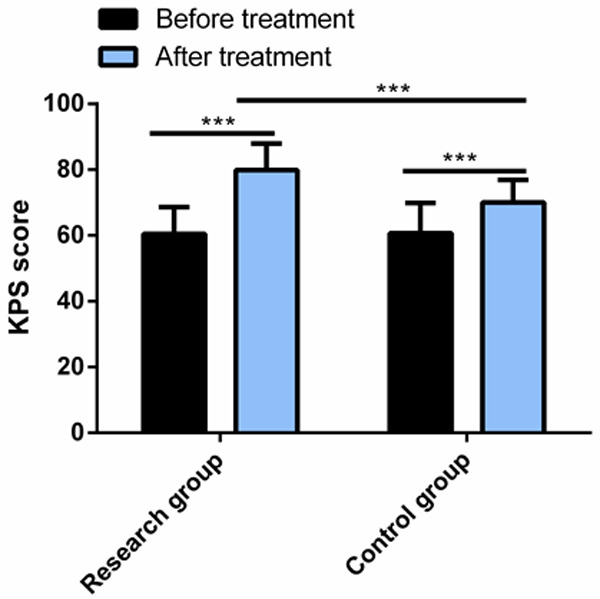
Comparison of KPS score between the two groups. After treatment, the KPS score of both groups increased significantly, and the KPS score of the research group was significantly higher than that of the control group. Note: ***P<0.001; KPS, Karnofsky Performance Scale.
QoL score
The QoL score, which showed no evident difference between the control group and the research group before therapy, increased significantly in both arms after treatment, especially in the research group (P<0.05) Figure 2.
Figure 2.
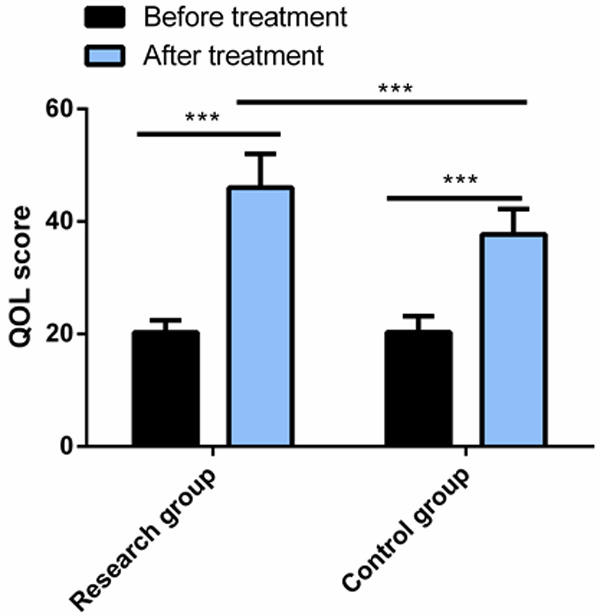
Comparison of QoL score between the two groups. After treatment, the QoL score increased significantly in both groups, and the score in the research group was significantly higher than that in the control group. Note: ***P<0.001; QoL, quality of life.
STMs
VEGF, EGF and TGF-β levels, which were not statistically different between the control group and the research group before therapy, decreased significantly in both arms after treatment, with lower levels in the research group (all P<0.05) Figure 3.
Figure 3.
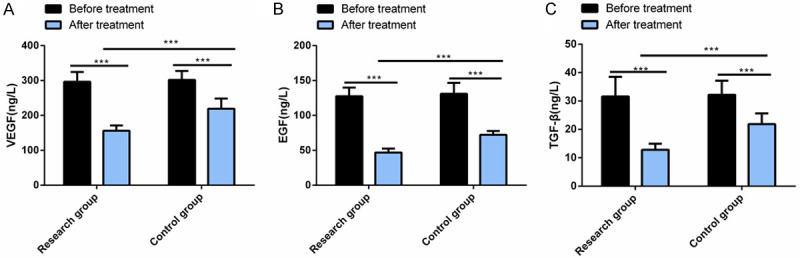
Comparison of serum tumor markers between the two groups. After treatment, the levels of VEGF (A), EGF (B) and TGF-β (C) decreased significantly in the two groups, and their levels in the research group were significantly lower than those in the control group. Note: ***P<0.001; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; TGF-β, transforming growth factor β.
Long term survival rate
The 1-year and 2-year survival rates as well as the MST were significantly higher in the research group than in the control group (P<0.05) Table 4.
Table 4.
Comparison of long-term survival between the two groups after treatment [n (%)]
| Groups | 1-year survival rate | 2-year survival rate | Median survival time (months) |
|---|---|---|---|
| Research group (n=40) | 31 (77.50) | 28 (70.00) | 23.62±3.44 |
| Control group (n=33) | 15 (45.45) | 12 (36.36) | 14.87±1.21 |
| t/χ2 | 7.967 | 8.259 | 13.910 |
| P | 0.004 | 0.004 | <0.001 |
Treatment satisfaction
After treatment, the treatment satisfaction of the research group was 92.50%, a rate notably higher than that of 69.70% in the control group (P<0.05) Table 5.
Table 5.
Comparison of treatment satisfaction between two groups [n (%)]
| Classification | Research group (n=40) | Control group (n=33) | χ2 value | P value |
|---|---|---|---|---|
| Very satisfied | 27 (67.50) | 10 (30.30) | - | - |
| Satisfied | 10 (25.00) | 13 (39.39) | - | - |
| Dissatisfied | 3 (7.50) | 10 (30.30) | - | - |
| Treatment satisfaction | 37 (92.50) | 23 (69.70) | 6.424 | 0.011 |
Discussion
Malignant glioma is highly malignant and often infiltrated in the brain tissue, so it is difficult to completely remove the tumor tissue by surgery. The faster the growth of intracranial mass, the higher the risk of recurrence of the disease after treatment; And those with recurrence have a very poor prognosis and greatly shortened survival time [20,21]. At present, there is no standard or effective treatment scheme for malignant gliomas in clinic [22]. While for recurrent malignant gliomas, the clinical treatment methods include reoperation, concurrent chemotherapy, radiotherapy, molecular targeted drugs and other comprehensive treatment methods, which can effectively control the growth of postoperative residual tumor tissue and prolong the survival of patients [23,24]. Herein, we used Bz+TMZ for patients with recurrent malignant gliomas, hoping to provide useful value for clinical practice.
Xu T et al. [25] found that Bz combined with irinotecan can improve the prognosis of patients with recurrent malignant gliomas, improve the treatment efficiency and prolong the survival time. In the study of Franceschi E et al. [26], it was found that TMZ combined with radiotherapy for patients with recurrent malignant gliomas can prolong the disease progression period and reduce mortality. In the present study, we found that patients in the research group had higher ORR and DCR, with no significant difference in the incidence of ARs compared with the control group. It suggests that Bz+TMZ can significantly improve the treatment efficacy and DCR of patients, similar to the research results of Xu T et al. and Franceschi E et al. (please add references here). Besides, notably higher KPS score and QoL score were observed in the research group after treatment, indicating that Bz+TMZ can greatly improve the QoL and prognosis of patients. Rades D et al. [27] reported that patients with recurrent glioblastoma multiforme treated with radiotherapy showed statistically increased KPS scores and improved QoL, which accorded with our research findings. Maitre P et al. [28] pointed out that high-dose salvage re-irradiation for patients with recurrent gliomas can validly improve their QoL and activities of daily living, which agreed with our results. In the subsequent detection of STMs, we observed obviously reduced VEGF, EGF and TGF-β in both arms after treatment, especially in the research group, indicating that Bz+TMZ can more effectively inhibit tumor deterioration and growth and reduce the levels of STMs in patients, which may be related to the mechanism of Bz in inhibiting angiogenesis. Similarly, Kwiatkowski SC et al. [29] revealed that Bz can significantly reduce VEGF and TGF-β in patients with recurrent malignant gliomas, and effectively control tumor growth and recurrence. In terms of prognosis, our research identified statistically higher 1-year and 2-year survival rates as well as MST in the research group, indicating that Bz+TMZ can validly improve the long-term survival rate and extend patient survival, which may be related to the efficacy of the combination therapy in improving patients’ QoL and delaying tumor progression. Finally, we compared the treatment satisfaction, and found that the satisfaction was significantly higher in the research group, indicating that the patients were more willing to accept the Bz+TMZ regimen. Nagane M et al. [30] reported that Bz can significantly improve the 1-year survival rate, MST and DCR of patients with recurrent malignant gliomas, which was in accordance with our research results.
To sum up, Bz+TMZ for patients with recurrent malignant gliomas can significantly improve the RR, DCR, long-term survival rate and treatment satisfaction of patients, bolster their QoL and prognosis, and reduce the levels of STMs without increasing the incidence of ARs. Although this study has achieved encouraging results, it still has some shortcomings. For example, the risk factors can be analyzed to monitor patients’ recovery after treatment, and the sample size should be expanded to increase the credibility of the study. In the future, we will carry out research from the above angles.
Disclosure of conflict of interest
None.
References
- 1.Stepp H, Stummer W. 5-ALA in the management of malignant glioma. Lasers Surg Med. 2018;50:399–419. doi: 10.1002/lsm.22933. [DOI] [PubMed] [Google Scholar]
- 2.Yan C, Wang J, Yang Y, Ma W, Chen X. Molecular biomarker-guided anti-angiogenic targeted therapy for malignant glioma. J Cell Mol Med. 2019;23:4876–4882. doi: 10.1111/jcmm.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan YL, Wang X, Xing JS, Lou JC, Ma XC, Zhang B. The potential roles of dopamine in malignant glioma. Acta Neurol Belg. 2017;117:613–621. doi: 10.1007/s13760-016-0730-2. [DOI] [PubMed] [Google Scholar]
- 4.Siegel C, Armstrong TS. Nursing guide to management of major symptoms in patients with malignant glioma. Semin Oncol Nurs. 2018;34:513–527. doi: 10.1016/j.soncn.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Laub CK, Stefanik J, Doherty L. Approved treatments for patients with recurrent high-grade gliomas. Semin Oncol Nurs. 2018;34:486–493. doi: 10.1016/j.soncn.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Balvers RK, Dirven CM, Leenstra S, Lamfers ML. Malignant glioma in vitro models: on the utilization of stem-like cells. Curr Cancer Drug Targets. 2017;17:255–266. doi: 10.2174/1568009616666160813191809. [DOI] [PubMed] [Google Scholar]
- 7.Hua L, Wakimoto H. Oncolytic herpes simplex virus therapy for malignant glioma: current approaches to successful clinical application. Expert Opin Biol Ther. 2019;19:845–854. doi: 10.1080/14712598.2019.1614557. [DOI] [PubMed] [Google Scholar]
- 8.Winter SF, Loebel F, Dietrich J. Role of ketogenic metabolic therapy in malignant glioma: a systematic review. Crit Rev Oncol Hematol. 2017;112:41–58. doi: 10.1016/j.critrevonc.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, Wei Z, Wang L, Kong L, Sun H, Ping Q, Mo R, Zhang C. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 10.Chelliah SS, Paul EAL, Kamarudin MNA, Parhar I. Challenges and perspectives of standard therapy and drug development in high-grade gliomas. Molecules. 2021;26:1169. doi: 10.3390/molecules26041169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omar AI, Mason WP. Temozolomide: the evidence for its therapeutic efficacy in malignant astrocytomas. Core Evid. 2010;4:93–111. doi: 10.2147/ce.s6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bahr O, Phuphanich S, Sepulveda JM, De Souza P, Sahebjam S, Carleton M, Tatsuoka K, Taitt C, Zwirtes R, Sampson J, Weller M. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkmate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seystahl K, Hentschel B, Loew S, Gramatzki D, Felsberg J, Herrlinger U, Westphal M, Schackert G, Thon N, Tatagiba M, Pietsch T, Reifenberger G, Loffler M, Wick W, Weller M, German Glioma N. Bevacizumab versus alkylating chemotherapy in recurrent glioblastoma. J Cancer Res Clin Oncol. 2020;146:659–670. doi: 10.1007/s00432-019-03086-9. [DOI] [PubMed] [Google Scholar]
- 14.Kim MM, Umemura Y, Leung D. Bevacizumab and glioblastoma: past, present, and future directions. Cancer J. 2018;24:180–186. doi: 10.1097/PPO.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong Z, Yan C, Zhu R, Wang J, Wang Y, Wang Y, Wang R, Feng F, Ma W. Imaging biomarkers guided anti-angiogenic therapy for malignant gliomas. Neuroimage Clin. 2018;20:51–60. doi: 10.1016/j.nicl.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol. 2013;34:944–50. S1–11. doi: 10.3174/ajnr.A3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, Schlegel J, Meyer B, Combs SE. Modification and optimization of an established prognostic score after re-irradiation of recurrent glioma. PLoS One. 2017;12:e0180457. doi: 10.1371/journal.pone.0180457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters KB, West MJ, Hornsby WE, Waner E, Coan AD, McSherry F, Herndon JE 2nd, Friedman HS, Desjardins A, Jones LW. Impact of health-related quality of life and fatigue on survival of recurrent high-grade glioma patients. J Neurooncol. 2014;120:499–506. doi: 10.1007/s11060-014-1574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SW, Cho KR, Choi JW, Kong DS, Seol HJ, Nam DH, Lee JI. Pattern of disease progression following stereotactic radiosurgery in malignant glioma patients. J Clin Neurosci. 2020;76:61–66. doi: 10.1016/j.jocn.2020.04.047. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Inbar O, Lee CC, Mousavi SH, Kano H, Mathieu D, Meola A, Nakaji P, Honea N, Johnson M, Abbassy M, Mohammadi AM, Silva D, Yang HC, Grills I, Kondziolka D, Barnett GH, Lunsford LD, Sheehan J. Stereotactic radiosurgery for intracranial hemangiopericytomas: a multicenter study. J Neurosurg. 2017;126:744–754. doi: 10.3171/2016.1.JNS152860. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez A, Tatter SB. Laser ablation of recurrent malignant gliomas: current status and future perspective. Neurosurgery. 2016;79(Suppl 1):S35–S39. doi: 10.1227/NEU.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 23.Shah AH, Burks JD, Buttrick SS, Debs L, Ivan ME, Komotar RJ. Laser interstitial thermal therapy as a primary treatment for deep inaccessible gliomas. Neurosurgery. 2019;84:768–777. doi: 10.1093/neuros/nyy238. [DOI] [PubMed] [Google Scholar]
- 24.Curry RC, Dahiya S, Alva Venur V, Raizer JJ, Ahluwalia MS. Bevacizumab in high-grade gliomas: past, present, and future. Expert Rev Anticancer Ther. 2015;15:387–397. doi: 10.1586/14737140.2015.1028376. [DOI] [PubMed] [Google Scholar]
- 25.Xu T, Chen J, Lu Y, Wolff JE. Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis. BMC Cancer. 2010;10:252. doi: 10.1186/1471-2407-10-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschi E, Lamberti G, Visani M, Paccapelo A, Mura A, Tallini G, Pession A, De Biase D, Minichillo S, Tosoni A, Di Battista M, Cubeddu A, Bartolini S, Brandes AA. Temozolomide rechallenge in recurrent glioblastoma: when is it useful? Future Oncol. 2018;14:1063–1069. doi: 10.2217/fon-2017-0681. [DOI] [PubMed] [Google Scholar]
- 27.Rades D, Witteler J, Leppert J, Schild SE. Re-Irradiation for recurrent glioblastoma multiforme. Anticancer Res. 2020;40:7077–7081. doi: 10.21873/anticanres.14735. [DOI] [PubMed] [Google Scholar]
- 28.Maitre P, Gupta T, Maitre M, Goda J, Krishnatry R, Chatterjee A, Sridhar E, Sahay A, Mokal S, Moiyadi A, Shetty P, Patil V, Jalali R. Prospective longitudinal assessment of quality of life and activities of daily living as patient-reported outcome measures in recurrent/progressive glioma treated with high-dose salvage re-irradiation. Clin Oncol (R Coll Radiol) 2021;33:e155–e165. doi: 10.1016/j.clon.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Kwiatkowski SC, Guerrero PA, Hirota S, Chen Z, Morales JE, Aghi M, McCarty JH. Neuropilin-1 modulates TGFbeta signaling to drive glioblastoma growth and recurrence after anti-angiogenic therapy. PLoS One. 2017;12:e0185065. doi: 10.1371/journal.pone.0185065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagane M, Nishikawa R, Narita Y, Kobayashi H, Takano S, Shinoura N, Aoki T, Sugiyama K, Kuratsu J, Muragaki Y, Sawamura Y, Matsutani M. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012;42:887–895. doi: 10.1093/jjco/hys121. [DOI] [PMC free article] [PubMed] [Google Scholar]


