Abstract
Purpose: This study investigated liver enzymes, bile acid metabolism, and liver fibrosis in nonalcoholic fatty liver disease (NAFLD) to evaluate the therapeutic effects of microecological preparations on fatty liver. Methods: Liver enzymes, liver fibrosis, and bile acids were assessed in 40 healthy volunteers and 124 NAFLD patients. All patients were retested for liver enzymes, bile acids, and liver fibrosis after two months of bifid triple viable capsule therapy. Results: (1) Prior to treatment, alanine aminotransferase, aspartate aminotransferase, glutamyl transpeptidase, FibroScan liver stiffness, total bile acid, chenodeoxycholic acid, deoxycholic acid, glycocholic acid, glycochenodeoxycholic acid, glycodeoxycholic acid, taurocholic acid, taurochenodeoxycholic acid, taurodeoxycholic acid, and taurolithocholic acid increased with the severity of NAFLD (P<0.05). Primary/secondary bile acids increased in patients compared to healthy controls; free/conjugated bile acids decreased (P<0.05). (2) We detected a positive correlation between total bile acid, cholic acid, chenodeoxycholic acid, deoxycholic acid, ursodeoxycholic acid, glycocholic acid, glycochenodeoxycholic acid, glycodeoxycholic acid, taurocholic acid, taurochenodeoxycholic acid, taurodeoxycholic acid, taurolithocholic acid, tauroursodeoxycholic acid, and FibroScan liver stiffness. (3) Following treatment, liver enzymes decreased. Bile acids were impacted by decreasing primary/secondary bile acids and increasing free/conjugated bile acids. Improvements were observed in the fibrosis of mild fatty liver. No effects were observed for moderate and severe fatty liver. Conclusions: Liver enzymes, bile acids, and liver fibrosis were correlated with the severity of NAFLD. There were positive correlations between bile acids and liver fibrosis. Bifid triple viable capsules could decrease liver enzymes and impact bile acid metabolism but failed to effectively improve liver fibrosis.
Keywords: Nonalcoholic fatty liver disease, bile acids, liver fibrosis, bifid triple viable capsules
Introduction
Nonalcoholic fatty liver disease (NAFLD) is involved in the clinicopathological syndromes of hepatocellular macrovesicular steatosis caused by nonalcoholic and other definite liver damage [1]. NAFLD includes nonalcoholic hepatic steatosis, nonalcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma (HCC) [2,3]. NAFLD has become the most widespread chronic hepatic disease and the leading cause of elevated liver enzymes in medical examinations in China [1,4]. The nosogenesis of NAFLD is unclear. The “two hit hypothesis” centered on oxidative stress and lipid peroxidation proposed in 1998 is generally accepted [5]. In recent years, the “multiple parallel hits hypothesis” has been put forward, in which genetic and environmental factors (insulin resistance, adipocytokine imbalance, and intestinal flora disturbance) accelerate the occurrence and advancement of NAFLD [6,7].
Bile acids take a significant part in fat synthesis and metabolism. Bile acid metabolism mainly depends on enterohepatic circulation [8]. Enterohepatic circulation disorders caused by hepatic and gall diseases are the main factors of abnormal blood bile acids, and variations in the serum bile acid spectrum affect the synthesis, secretion, and metabolism of bile acids from liver [9,10]. A series of pathological changes during NAFLD lead to damaged liver cell function, resulting in increased bile acid levels entering the blood through the hepatic sinusoid, coupled to increased enterohepatic circulation [4]. Clinical measurements of bile acids involve serum total bile acid, which has low specificity for the diagnosis of hepatobiliary disease. Ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS) is a rapidly developing quantitative analysis technique. Due to its high selectivity, high sensitivity, high throughput sample processing, and high degree of automation, UPLC-MS/MS has emerged as the preferred method for biological sample detection. It can be used to measure all subtypes of bile acids in the serum simultaneously [11].
To date, clinically available NAFLD drugs are limited. In recent years, studies have highlighted how intestinal microecological imbalances are correlated with the occurrence and evolution of NAFLD, confirming their potential as a therapeutic strategy [12]. Enteric microorganisms and bile acids are interdependent. Microorganisms can alter bile acids composition in the host and regulate their conversion [13,14]. Microecological preparations are viable bacteria made by normal microorganisms or substances promoting the growth of microorganisms that are beneficial to the host. They can maintain microecological balance, prevent and treat diseases, and improve the health of the host. Bifid triple viable capsules are a type of microecological preparation. More than 700 million bifid triple viable capsules have been consumed by over 8 million Chinese patients including functional gastrointestinal disorder, diarrhea, constipation, and inflammatory bowel disease since 1995. Studies regarding the regulation of bile acid metabolism by bifid triple viable capsules in NAFLD are limited. It is uncertain if bifid triple viable capsules can improve hepatic fibrosis in NAFLD patients.
In this study, UPLC-MS/MS was employed to simultaneously detect fifteen subtypes of blood bile acids in healthy and NAFLD patients. We analyzed the correlation of bile acids and liver fibrosis in NAFLD patients. We preliminarily evaluated the therapeutic effects of microecological preparations in NAFLD by observing changes in liver enzymes, bile acids, and hepatic fibrosis after two months of treatment using bifid triple viable capsules.
Materials and methods
Subjects
From August 2015 to April 2017, 124 patients with NAFLD of the Affiliated Suzhou Hospital of Nanjing Medical University were enrolled. Patients were 25 to 65 years old and included 75 males and 49 females. The standard of diagnosis and severity of NAFLD were in accordance with the 2018 Chinese Guidelines of Prevention and Treatment for NAFLD [15]. Liver biopsy is the golden standard for diagnosing NAFLD. It is not universally available because of its invasive attribute. In total, 124 patients were diagnosed with NAFLD according to ultrasound (US) or computed tomography (CT) and divided into 50 mild cases (accounting for 40.32%, from 25 to 65 years old, including 31 males and 19 females), 44 moderate cases (accounting for 35.48%, from 28 to 65 years old, including 26 males and 18 females) and 30 severe cases (accounting for 24.20%, from 25 to 64 years old, including 18 males and 12 females). In some patients, the diagnosis was confirmed by liver biopsy (Figure 1).
Figure 1.

Liver biopsy pathology. A. Represents observation of liver tissue by low-power optical microscopy at 100 times magnification. B. Represents observation of liver tissue by high-power optical microscopy at 200× magnification. Bar = 100 μm.
Forty healthy people were chosen as controls and were matched with NAFLD patients in accordance with age (from 20 to 65 years old) and sex (24 males and 16 females). The healthy controls had no liver disease and exhibited normal liver function and structure. All subjects signed informed consents.
Exclusion criteria: (1) Excessive drinking (ethanol ingestion >30 g per day in males, >20 g per day in females); (2) Other liver diseases including viral hepatitis and drug-induced hepatic diseases; (3) Fatty liver caused by inflammatory bowel disease or Cushing’s syndrome; (4) Pregnant and lactating women; (5) History of acute gastroenteritis and other digestive system diseases in the past 2 months; (6) History of medication that could disturb the observations or absorption of the therapeutic drug (such as glucocorticoids, rifaximin) in the past 2 months; (7) Allergies or intolerant to the study drugs; and (8) Serious psychoneurosis and subjects with poor compliance.
Groups
There were four groups: healthy controls (n = 40, Group 1), mild fatty liver (n = 50, Group 2), moderate fatty liver (n = 44, Group 3), and severe fatty liver (n = 30, Group 4).
Study design
Prior to treatment: (1) The height and weight of all subjects were measured. Body mass index [BMI = weight (in kilograms)/height (in meters)2] was calculated on the day of selection. (2) Blood analysis was performed on all subjects on selection day, including assessments of renal function [creatinine (Cr)], liver function [alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl transpeptidase (GGT), total bilirubin (TB), total bile acid (TBA)], albumin (ALB), lipid metabolism [total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C)], and glucose metabolism [fasting plasma glucose (FBG), fasting serum insulin (FINS), and insulin resistance (HOMA-IR = FPG×FINS/22.5)]. (3) A 5 MHz US diagnostic instrument and MX8000 CT were used for liver US and liver/spleen CT ratios, respectively. (4) The FibroScan values of the liver were determined using a FibroScan 502 US diagnostic instrument on the day of selection. In NAFLD, FibroScan liver stiffness measurement (LSM) ≥15.0 kPa is deemed to liver cirrhosis, 11.0 kPa≤LSM<15.0 kPa is deemed to progressive liver fibrosis, LSM<10.0 kPa can exclude cirrhosis, LSM<8.0 kPa can exclude progressive liver fibrosis, and LSM in 8.0 to 11.0 kPa needs to undergo liver biopsy for the status of liver fibrosis [16]. (5) A total of 15 subtypes of blood bile acids of all subjects were detected through UPLC-MS/MS, including 5 free bile acids [cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA)]. Ten conjugated bile acids formed by the combination of glycine and taurine [glycocholic acid (GCA), glycochenodeoxycholic acid (GCDCA), glycodeoxycholic acid (GDCA), glycolithocholic acid (GLCA), glycoursodeoxycholic acid (GUDCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), taurodeoxycholic acid (TDCA), taurolithocholic acid (TLCA), and tauroursodeoxycholic acid (TUDCA)] on the day of selection.
After treatment, all patients with NAFLD were retested for liver enzymes (ALT, AST, and GGT), serum total bile acid, 15 subtypes of bile acids, and LSM by FibroScan after two months of bifid triple viable capsules [Bifico, 210 mg/one capsule (each capsule contained Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis, and the viable bacteria number of each capsule was no less than 1.0×107 cfu), 3 times a day, 420 mg each time, Shanghai Xinyi Pharmaceutical Company, Shanghai, China]. All patients received no other treatments that could affect bile acid metabolism and liver fibrosis during the treatment period.
Our research was a retrospective study. Ethical clearance by the institutional ethics committee of Suzhou Municipal Hospital was obtained for this study (KL901066) which complied with the Declaration of Helsinki. The study was conducted in human subjects, and the study caused no harm or risk to the subjects. The study protected the rights and privacy of subjects.
Statistical analysis
Data are shown as percentages for categorical data and the mean ± SD for quantitative data. Data were analyzed using SPSS20.0 and Prism 6.0. Measurement data were assessed through the Kolmogorov-Smirnov test of normality and Levene test of homogeneity of variance. P values <0.05 were determined statistically significant.
Results
Comparison of demographics, blood analysis, CT, FibroScan data, and bile acids prior to treatments
Prior to treatment, no obvious differences in age, sex, height, Cr, or ALB (P>0.05) were observed. Weight, BMI, ALT, AST, GGT, TB, TG, TC, HDL, LDL, FBG, FINS, HOMA-IR, liver/spleen CT ratio, and LSM differed among the groups prior to treatment (P<0.001). According to pairwise comparisons among the groups, ALT, AST, GGT, weight, BMI, TG, TC, LDL, FINS, HOMA IR, liver/spleen CT ratio, and LSM increased with the seriousness of NAFLD (P<0.05), HDL decreased (P<0.05). No significant differences were observed in FBG between Group 1 and Group 2 (P>0.05), but differences were observed between other groups through pairwise comparisons (P<0.05). There were no significant differences in TB between Groups 1-3 (P>0.05) but, Group 4 differed from the other groups (P<0.05) (Table 1).
Table 1.
Comparison of demographics, blood analysis, CT, and FibroScan data prior to treatment
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | F/χ2 value | P value |
|---|---|---|---|---|---|---|
| Cases | 40 | 50 | 44 | 30 | ||
| Age (yr) | 43.23±10.32 | 45.78±9.79 | 44.64±10.13 | 44.97±10.03 | 0.487 | 0.692 |
| Gender (M/F) | 24/16 | 31/19 | 26/18 | 18/12 | 0.090 | 0.993 |
| Weight (kg) | 61.21±7.87 | 66.89±7.37 | 77.67±9.00 | 86.65±9.32 | 66.512 | <0.001 |
| Height (m) | 1.71±0.08 | 1.70±0.07 | 1.70±0.07 | 1.69±0.07 | 0.309 | 0.819 |
| BMI (kg/m2) | 20.90±1.43 | 22.99±1.60 | 26.98±2.05 | 30.09±1.70 | 206.955 | <0.001 |
| Cr (µmmol/L) | 62.29±10.15 | 65.24±10.65 | 65.32±9.48 | 64.71±10.02 | 0.822 | 0.483 |
| ALT (U/L) | 23.29±8.90 | 51.95±12.56 | 80.78±13.48 | 107.05±13.57 | 314.269 | <0.001 |
| AST (U/L) | 19.19±8.62 | 37.61±10.17 | 64.95±11.03 | 83.45±11.89 | 273.965 | <0.001 |
| GGT (U/L) | 23.19±9.96 | 44.38±11.39 | 82.26±13.70 | 100.74±14.47 | 300.529 | <0.001 |
| TB (µmol/L) | 10.84±3.77 | 11.69±3.72 | 12.43±3.99 | 15.55±4.53 | 8.936 | <0.001 |
| ALB (g/L) | 47.77±4.00 | 48.08±4.03 | 46.87±3.26 | 47.40±3.42 | 0.894 | 0.445 |
| TG (mmol/L) | 1.03±0.35 | 1.90±0.40 | 2.61±0.50 | 3.48±0.53 | 195.923 | <0.001 |
| TC (mmol/L) | 4.11±0.81 | 5.17±1.06 | 6.29±0.94 | 7.23±1.15 | 67.504 | <0.001 |
| HDL (mmol/L) | 1.35±0.35 | 1.21±0.24 | 1.08±0.25 | 0.92±0.27 | 15.908 | <0.001 |
| LDL (mmol/L) | 1.77±0.59 | 2.56±0.80 | 3.51±0.95 | 4.40±0.90 | 69.123 | <0.001 |
| FBG (mmol/L) | 5.06±0.66 | 5.38±0.78 | 5.71±0.84 | 6.18±0.76 | 13.799 | <0.001 |
| FINS (mU/L) | 7.30±1.81 | 11.45±2.50 | 18.31±2.23 | 22.87±2.44 | 343.172 | <0.001 |
| HOMA-IR | 1.63±0.43 | 2.74±0.71 | 4.62±0.72 | 6.25±0.74 | 342.558 | <0.001 |
| liver/spleen CT ratio | 1.12±0.06 | 0.84±0.08 | 0.60±0.05 | 0.30±0.08 | 932.026 | <0.001 |
| LSM (Kpa) | 5.88±0.79 | 7.13±0.87 | 9.06±1.26 | 13.61±1.49 | 320.957 | <0.001 |
Notes: Group 1 is healthy controls, Group 2 is mild fatty liver, Group 3 is moderate fatty liver, and Group 4 is severe fatty liver. Abbreviations: BMI: body mass index; Cr: creatinine; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: glutamyl transpeptidase; TB: total bilirubin; ALB: albumin; TG: triglyceride; TC: total cholesterol; HDL: high density lipoprotein cholesterol; LDL: low density lipoprotein cholesterol; FBG: fasting plasma glucose; FINS: fasting serum insulin; HOMA-IR: homeostasis model assessment of insulin resistance; LSM: liver stiffness measurement.
Prior to treatment, the comparison of free bile acids among all the groups showed no obvious distinctions in LCA (P = 0.278). Significant differences in CA, CDCA, DCA, and UDCA among the four groups were observed (P<0.001), among which CDCA and DCA gradually increased with NAFLD severity. LCA and UDCA showed no obvious distinctions between Groups 1 and 2 (P>0.05). Pairwise comparisons showed obvious distinctions in contrast to the other groups (P<0.05) (Figure 2; Table 2).
Figure 2.
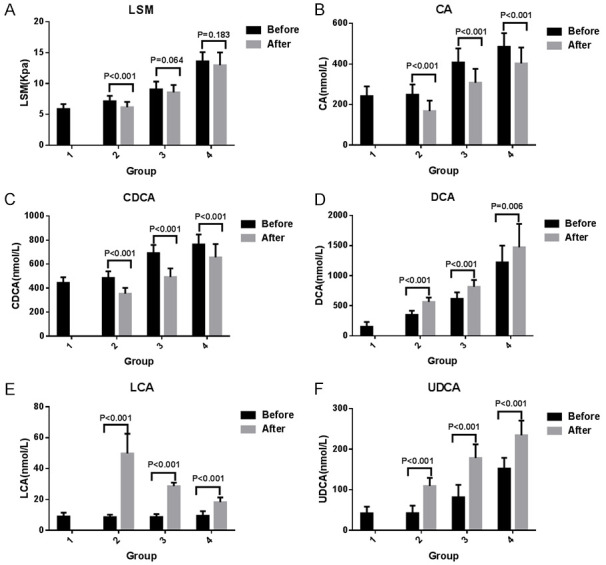
Comparison of LSM and free bile acid pre- and posttreatment. A-F. Represent a comparison of LSM, CA, CDCA, DCA, LCA, and UDCA before and after treatment. Group 1 included healthy controls, Group 2 included mild fatty liver, Group 3 included moderate fatty liver, and Group 4 included severe fatty liver. Abbreviations: LSM: liver stiffness measurement; CA: cholic acid; CDCA: chenodeoxycholic acid; DCA: deoxycholic acid; LCA: lithocholic acid; UDCA: ursodeoxycholic acid.
Table 2.
Pairwise comparison of total bile acid and the 15 subtypes of bile acids prior to treatment
| Variables | P1,2 | P1,3 | P1,4 | P2,3 | P2,4 | P3,4 |
|---|---|---|---|---|---|---|
| TBA (µmmol/L) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CA (nmmol/L) | 0.579 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CDCA (nmmol/L) | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| DCA (nmmol/L) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| LCA (nmmol/L) | 0.397 | 0.574 | 0.301 | 0.783 | 0.064 | 0.116 |
| UDCA (nmmol/L) | 0.937 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| GCA (nmmol/L) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| GCDCA (nmmol/L) | 0.049 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| GDCA (nmmol/L) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| GLCA (nmmol/L) | 0.887 | 0.544 | 0.106 | 0.431 | 0.069 | 0.275 |
| GUDCA (nmmol/L) | 0.617 | 0.866 | 0.826 | 0.490 | 0.819 | 0.704 |
| TCA (nmmol/L) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| TCDCA (nmmol/L) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| TDCA (nmmol/L) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| TLCA (nmmol/L) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| TUDCA (nmmol/L) | 0.246 | 0.204 | 0.001 | 0.878 | 0.011 | 0.019 |
| Free/Conjugated | <0.001 | <0.001 | <0.001 | 0.131 | 0.090 | 0.969 |
| Primary/Secondary | 0.054 | 0.020 | 0.007 | 0.062 | 0.021 | 0.529 |
Notes: Group 1 is healthy controls, Group 2 is mild fatty liver, Group 3 is moderate fatty liver, and Group 4 is severe fatty liver. P1,2 indicates comparison between Groups 1 and 2, P1,3 means comparison between Groups 1 and 3, P1,4 compares Groups 1 and 4, P2,3 refers to a comparison between Groups 2 and 3, P2,4 indicates a comparison between Groups 2 and 4, P3,4 describes the comparison between Groups 3 and 4. Abbreviations: TBA: total bile acid; CA: cholic acid; CDCA: chenodeoxycholic acid; DCA: deoxycholic acid; LCA: lithocholic acid; UDCA: ursodeoxycholic acid; GCA: glycocholic acid; GCDCA: glycochenodeoxycholic acid; GDCA: glycodeoxycholic acid; GLCA: glycolithocholic acid; GUDCA: glycoursodeoxycholic acid; TCA: taurocholic acid; TCDCA: taurochenodeoxycholic acid; TDCA: taurodeoxycholic acid; TLCA: taurolithocholic acid; TUDCA: tauroursodeoxycholic acid.
Prior to treatment, the comparison of glycine-conjugated bile acids among the four groups showed no obvious distinctions in GLCA (P = 0.281) and GUDCA (P = 0.910). Significant differences in GCA, GCDCA, and GDCA among the four groups was observed (P<0.05), which increased with the severity of NAFLD (Figure 3; Table 2).
Figure 3.

Comparison of conjugated bile acid pre- and posttreatment. A-J. Represent a comparison of GCA, GCDCA, GDCA, GLCA, GUDCA, TCA, TCDCA, TDCA, TLCA, and TUDCA before and after treatment. Group 1 included healthy controls, Group 2 included mild fatty liver, Group 3 included moderate fatty liver, and Group 4 included severe fatty liver. Abbreviations: GCA: glycocholic acid; GCDCA: glycochenodeoxycholic acid; GDCA: glycodeoxycholic acid; GLCA: glycolithocholic acid; GUDCA: glycoursodeoxycholic acid; TCA: taurocholic acid; TCDCA: taurochenodeoxycholic acid; TDCA: taurodeoxycholic acid; TLCA: taurolithocholic acid; TUDCA: tauroursodeoxycholic acid.
Prior to treatment, the comparison of all taurine-conjugated bile acids among the four groups revealed distinctions (P<0.05). TCA, TCDCA, TDCA, and TLCA increased with the severity of NAFLD. TUDCA significantly differed from Group 4 and the other three groups (P<0.05). Pairwise comparisons showed no obvious distinctions among the other groups (P>0.05) (Figure 3; Table 2).
Prior to treatment, there were obvious distinctions in TBA, free/conjugated bile acid, and primary/secondary bile acid among the four groups (P<0.05). Among them, TBA increased with the severity of NAFLD. Free/conjugated bile acids showed obvious distinctions (P<0.05) between Groups 1 and 2 and Groups 3 and 4. Pairwise contrast between the other groups showed no obvious distinctions (P>0.05). Primary/secondary bile acids did not significantly differ between Groups 1 and 2, Groups 2 and 3, or Groups 3 and 4 (P>0.05). Significant differences between Groups 1 and 3, Groups 1 and 4, and Groups 2 and 4 were observed (P<0.05) (Figure 4; Table 2).
Figure 4.

Comparison of TBA, free/conjugated bile acid, and primary/secondary bile acid pre- and posttreatment. A-C. Represent a comparison of TBA, free/conjugated bile acid, and primary/secondary bile acid before and after treatment. Group 1 included healthy controls, Group 2 included mild fatty liver, Group 3 included moderate fatty liver, and Group 4 included severe fatty liver. Abbreviations: TBA: total bile acid.
Quantitative data were compared with one-way ANOVA. Categorical data were analyzed by the chi-square test. Pairwise contrast between multiple groups was conducted by SNK-q and LSD-t ANOVA methods.
Correlation analysis between serum bile acids and LSM in patients with NAFLD prior to treatment
In NAFLD patients, a positive pertinence between TBA and LSM was observed (r = 0.937, P<0.001). Positive correlations between CA (r = 0.619, P<0.001), CDCA (r = 0.684, P<0.001), DCA (r = 0.898, P<0.001), UDCA (r = 0.725, P<0.001), GCA (r = 0.893, P<0.001), GCDCA (r = 0.890, P<0.001), GDCA (r = 0.810, P<0.001), TCA (r = 0.936, P<0.001), TCDCA (r = 0.907, P<0.001), TDCA (r = 0.911, P<0.001), TLCA (r = 0.765, P<0.001), TUDCA (r = 0.261, P = 0.003), and LSM were also observed. We observed no correlation between LCA (r = 0.091, P = 0.316), GLCA (r = 0.058, P = 0.524), GUDCA (r = 0.027, P = 0.769), or LSM (Figures 5, 6).
Figure 5.

Correlation between total bile acid, free bile acid and LSM in patients with NAFLD before treatment. A-F. Represent the correlation between total bile acid, CA, CDCA, DCA, LCA, UDCA, and LSM. Abbreviations: LSM: liver stiffness measurement; TBA: total bile acid; CA: cholic acid; CDCA: chenodeoxycholic acid; DCA: deoxycholic acid; LCA: lithocholic acid; UDCA: ursodeoxycholic acid.
Figure 6.

Correlation between conjugated bile acid and LSM in patients with NAFLD before treatment. A-J. Represent the correlation between GCA, GCDCA, GDCA, GLCA, GUDCA, TCA, TCDCA, TDCA, TLCA, TUDCA, and LSM. Abbreviations: LSM: liver stiffness measurement; GCA: glycocholic acid; GCDCA: glycochenodeoxycholic acid; GDCA: glycodeoxycholic acid; GLCA: glycolithocholic acid; GUDCA: glycoursodeoxycholic acid; TCA: taurocholic acid; TCDCA: taurochenodeoxycholic acid; TDCA: taurodeoxycholic acid; TLCA: taurolithocholic acid; TUDCA: tauroursodeoxycholic acid.
Correlations were analyzed through partial correlation analysis. Pearson’s correlation coefficients were used for bivariate normal data distributions.
Comparison of liver enzymes, bile acids, and liver stiffness in patients with NAFLD post treatment
In Group 2, contrast of GLCA, TDCA, TLCA, and TUDCA revealed no obvious distinctions pre and post treatment (P>0.05). A decrease in ALT, AST, GGT, LSM, CA, CDCA, GCA, GCDCA, GDCA, GUDCA, TCA, TCDCA, TBA, and primary/secondary bile acid following treatment was observed (P<0.05). In contrast, DCA, LCA, UDCA, and free/conjugated bile acid increased after treatment (P<0.05) (Figures 2, 3, 4 and 7).
Figure 7.

Comparison of liver enzymatic activity pre- and posttreatment. A-C. Represent a comparison of ALT, AST, and GGT before and after treatment. Group 1 included healthy controls, Group 2 included mild fatty liver, Group 3 included moderate fatty liver, and Group 4 included severe fatty liver. Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: glutamyl transpeptidase.
In Group 3, no significant differences in the comparison of LSM, GLCA, TLCA, and TUDCA before and after treatment were observed (P>0.05). A decrease in ALT, AST, GGT, CA, CDCA, GCA, GCDCA, GDCA, GUDCA, TCA, TCDCA, TDCA, TBA, and primary/secondary bile acid occurred following the treatment (P<0.05). DCA, LCA, UDCA, and free/conjugated bile acid increased posttreatment (P<0.05) (Figures 2, 3, 4 and 7).
In Group 4, the comparison of LSM, GLCA, GUDCA, TLCA, and TUDCA showed no obvious distinctions pre and post treatment (P>0.05). Decreases in ALT, AST, GGT, CA, CDCA, GCA, GCDCA, GDCA, TCA, TCDCA, TDCA, TBA, and primary/secondary bile acid following treatment were observed (P<0.05). DCA, LCA, UDCA, and free/conjugated bile acid also increased after treatment (P<0.05) (Figures 2, 3, 4 and 7).
Data were compared with t-tests.
Discussion
Bile acids participate in the fat metabolism affecting energy metabolism, the inflammatory response and the progress of hepatic diseases as important signal regulators [17]. Combined with farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (GPBAR1), bile acids adjust immune equilibrium and the inflammatory response of the body, and affects hepatocyte steatosis, cell injury, and apoptosis [18,19]. FXR is a bile acid receptor that downregulates the de novo synthesis of triacylglycerides and upregulates fatty acid oxidation, inhibiting the synthesis and transport of very low-density lipoprotein (VLDL), slowing liver steatosis, and promoting the transport of peripheral fats to the liver through the activation of endothelial lipoprotein lipase [20]. FXR activation improves glucose metabolism by inhibiting liver gluconeogenesis and glycogenolysis, increasing insulin sensitivity in both fat and skeletal muscle [21]. FXR reduces the release of proinflammatory cytokines through NF-κB suppression [17]. GPBAR1 activation increases the consumption of excess energy, promoting fat consumption and heat production, improving insulin sensitivity, and inhibiting the release of inflammatory cytokines in hepatic Kupffer cells [22]. Abnormal lipid metabolism, hepatocellular fat accumulation, and liver inflammation caused by bile acid cholestasis are important factors in the pathogenesis of NAFLD [23]. Compared to healthy people, the size of bile acid pool and its component in NAFLD patients significantly change, indicating that abnormal bile acid metabolism could affect the occurrence and progression of NAFLD [24]. We found that serum TBA, free bile acids, including CDCA and DCA, and conjugated bile acids, covering GCA, GCDCA, GDCA, TCA, TCDCA, TDCA, and TLCA, increased with the optimal seriousness of NAFLD (P<0.05). Free/conjugated bile acids decreased, and primary/secondary bile acids increased in NAFLD in contrast to the health. Partial differences in free/conjugated and primary/secondary bile acids were found according to disease grade (P<0.05). Guoxiang Xie [25], Nisreen Nimer [26], and their coworkers found that bile acids levels covering CA, DCA, TCA, TDCA, and TCDCA, increased significantly in NAFLD with respect to normal liver subjects by a metabolomics approach, which was partially consistent with our study. Different bile acids have different hydrophobicity. LCA, DCA, CDCA, and GCDCA have strong hydrophobicity. It is believed that the stronger the hydrophobicity is, the greater the cytotoxicity [27]. Increased concentrations of hydrophobic bile acids can lead to cholestatic liver injury, damage the liver cell membrane, and cause mitochondrial damage in hepatocytes [28]. Conjugated and free bile acids have differential effects on FXR because of their degree of dissociation. Conjugated bile acids strongly inhibit FXR because of their low levels of dissociation. The lower the degree of dissociation, the stronger the inhibitory effects [18]. The activation of GPBAR1 by secondary bile acids was reported to be stronger than that of primary bile acids. When GPBAR1 is activated by secondary bile acids, it can induce phosphorylation of a single amino acid (Ser291) in NLRP3 inflammasomes by activating the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway, playing an anti-inflammatory role [29]. The increase in serum bile acids in NAFLD is mainly in the form of conjugated and primary bile acids, which agrees with the results of our study.
Liver fibrosis is a vital factor in the assessment of NAFLD progress [30]. Fibroscans quantitatively assess steatosis and liver fibrosis stages, representing a noninvasive method of hepatic fat detection recommended by international guidelines [16]. We found that LSM increased with the seriousness of NAFLD. NAFLD patients showed a positive correlation among TBA, CA, CDCA, DCA, UDCA, GCA, GCDCA, GDCA, TCA, TCDCA, TDCA, TLCA, TUDCA, and LSM. As previously mentioned, bile acids are involved in liver inflammation and energy metabolism through a variety of pathways. During the continuous liver injury and repair induced by chronic inflammation, the metabolic network of the liver is altered, affecting numerous endogenous small molecular metabolites and their upstream and downstream biological pathways. Disrupted metabolic pathways, in turn, exacerbate the progression of inflammation-associated liver fibrosis, leading to a vicious cycle [31]. Bile acids were closely connected with the degree of liver fibrosis in NAFLD.
In recent years, developments in microecology have led to the notion of “gut-liver axis”, revealing the correlation between intestinal microecology and NAFLD. As intestinal beneficial bacteria, Lactobacillus and Bifidobacteria occupy absolute dominance in the digestive tract. Numerous studies have shown that alteration of intestinal flora was present in NAFLD, which was manifested by a decrease in beneficial bacteria (Bifidobacteria, Lactobacillus, and Akkermansia muciniphila) and an increase in harmful bacteria [32]. The imbalance of intestinal flora leads to an increase in intestinal mucosal barrier permeability. The intestinal flora and enterogenic endotoxin can directly enter the portal vein, interfere with bile acid metabolism, and stimulate Kupffer cells to secrete a large number of inflammatory substances, not only leading to liver cell injury, apoptosis, and necrosis but also aggravating intestinal mucosal barrier damage. The result is a vicious cycle of NAFLD [33]. The imbalance of intestinal flora leads to an increase in various proinflammatory cytokines, chemokines, and bacterial metabolites (bile acid, short-chain fatty acid, and ethanol). This activates hepatic stellate cells and stimulates platelets to release a large amount of transforming growth factor-β. Activation of hepatic stellate cells and release of transforming growth factor-β can produce extracellular matrix and ultimately result in liver fibrosis [34,35]. This shows that intestinal microbiota normalization may turn into a therapeutic choice for NAFLD. Bifid triple viable capsules are live bacteria prepared by Bifidobacterium, Lactobacillus acidophilus, and Enterococcus faecalis in an appropriate proportion which contain the bacteria that NAFLD patients lack. Some studies in China have found that the number of Bifidobacteria and Lactobacillus increased and the number of harmful bacteria (such as Staphylococcus and Enterobacterium) decreased significantly in NAFLD after taking bifid triple viable capsules orally. Beneficial bacteria, such as Bifidobacteria and Lactobacillus, directly and indirectly restrain the growth of harmful bacteria by consolidating the intestinal mucosal barrier, competing with harmful bacteria for nutrients and producing metabolites, and eventually restoring the proportion of intestinal microbiota [36]. The recovery of intestinal flora can reduce enterogenic endotoxemia, regulate hepatocyte immune stress, and relieve hepatocyte inflammation. This effect can regulate lipid metabolism, reduce hepatic fat accumulation, and alleviate hepatocellular damage [37]. Improvement of hepatocellular damage reduces the release of liver enzymes into blood, reducing liver enzyme levels in NAFLD.
Enteric microorganisms and bile acids are suggested to be interdependent [38]. Bile acids maintain the normal intestinal barrier through the mitosis-activated protein kinase (MAPK) and FXR pathways, inhibiting excessive enteric microorganism growth and participating in the regulation of enteric microorganism composition and lipid and glucose homeostasis in the gut-liver axis [39]. Enteric microorganisms also change the component of bile acids and antagonize FXR in the intestinal tract, leading to metabolic dysfunction, obesity, and insulin resistance [2,40,41]. Most conjugated bile acids are actively reabsorbed by apical sodium-dependent bile acid transporters at the terminal ileum through the portal vein to the liver. Intestinal bacteria can inhibit the release of apical sodium-dependent bile acid transporters by stimulating the GATA4 transcription factor, reducing the reabsorption of conjugated bile acids and reducing their levels in blood [42]. Numerous microbial floras in the intestinal tract, such as Bifidobacterium and Lactobacillus, can produce bile salt hydrolases that can hydrolyze conjugated bile acids to free bile acids [43]. Bifidobacteria and other beneficial intestinal bacteria can also dehydroxylate primary bile acids to secondary bile acids through a variety of enzymatic reactions, such as bacterial hydroxysteroid dehydrogenase, reducing the toxicity of bile acids [44]. We found that bifid triple viable capsules could regulate the bile acid metabolism of NAFLD by reducing total bile acids and primary/secondary bile acids and increasing free/conjugated bile acids, consistent with observations in other studies [45].
There is no recognized effective drug that can be recommended for liver fibrosis. According to the theoretical basis and results of the study mentioned above, we speculated that taking bifid triple viable capsules could improve the degree of liver fibrosis in NAFLD. A randomized trial found that probiotic and prebiotic could alter the intestinal flora but fail to improve liver fat or fibrosis [46]. In our study, oral bifid triple viable capsules could improve the severity of hepatic fibrosis in patients with mild NAFLD, but failed to improve fibrosis in patients with moderate or severe fatty liver. There were three possibilities for these findings. The level of liver fibrosis in mild NAFLD was generally moderate and could be improved after active treatments. The degree of liver fibrosis in some patients with moderate and severe NAFLD might have reached the progressive stage, which was difficult to reverse. Bifid triple viable capsules were merely probiotics that indirectly improved hepatocellular damage by regulating intestinal flora. The therapeutic effect might be poor when taking bifid triple viable capsules alone. The intestinal flora are a large and diverse flora library that can be influenced by many factors. The crosstalk between probiotics and original bacterial inhabitants in the gut was unknow. The research results may have fallen short of expectations.
The sample size was small, and the time of therapy was short. More studies including larger sample sizes, liver biopsies, intestinal flora, and randomized controlled trials are needed to assess the gravity of fatty liver fibrosis and improve the evaluation of therapeutic effects.
Conclusions
This study revealed that liver enzymes, serum bile acids and liver fibrosis were related to the severity of NAFLD. We observed a positive correlation between bile acids and liver fibrosis. Following treatment with bifid triple viable capsules, a decrease in liver enzymes and primary/secondary bile acids and an increase in free/conjugated bile acids were observed, which improved liver fibrosis in mild fatty liver but failed to improve moderate and severe fatty livers.
Acknowledgements
We sincerely thank Dr. Chunli Zhou, Dr. Jiwei Cao, The Affiliated Suzhou Hospital of Nanjing Medical University and all participants and institutions for assistance to this study.
Disclosure of conflict of interest
None.
References
- 1.Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, Fan J, Goh KL, Hamaguchi M, Hashimoto E, Kim SU, Lesmana LA, Lin YC, Liu CJ, Ni YH, Sollano J, Wong SK, Wong GL, Chan HL, Farrell G. Asia-pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 2.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 3.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 4.Molinaro A, Wahlström A, Marschall HU. Role of bile acids in metabolic control. Trends Endocrinol Metab. 2018;29:31–41. doi: 10.1016/j.tem.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 6.Poeta M, Pierri L, Vajro P. Gut-liver axis derangement in non-alcoholic fatty liver disease. Children (Basel) 2017;4:66. doi: 10.3390/children4080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas JT, Francque S, Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol. 2016;78:181–205. doi: 10.1146/annurev-physiol-021115-105331. [DOI] [PubMed] [Google Scholar]
- 8.van Zutphen T, Bertolini A, de Vries HD, Bloks VW, de Boer JF, Jonker JW, Kuipers F. Potential of intestine-selective FXR modulation for treatment of metabolic disease. Handb Exp Pharmacol. 2019;256:207–234. doi: 10.1007/164_2019_233. [DOI] [PubMed] [Google Scholar]
- 9.Vítek L, Haluzík M. The role of bile acids in metabolic regulation. J Endocrinol. 2016;228:R85–96. doi: 10.1530/JOE-15-0469. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Dawson PA. Animal models to study bile acid metabolism. Biochim Biophys Acta Mol Basis Dis. 2019;1865:895–911. doi: 10.1016/j.bbadis.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E, Lu J, Chen D, Zhu D, Wang Y, Zhang Y, Zhou N, Wang J, Li J, Li L. Dynamic changes of plasma metabolites in pigs with GalN-induced acute liver failure using GC-MS and UPLC-MS. Biomed Pharmacother. 2017;93:480–489. doi: 10.1016/j.biopha.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 12.Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci. 2016;17:928. doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Jiang S, Zhao X, Li H, Lin W, Li B, Lu J, Sun Y, Yuan J. Community-metabolome correlations of gut microbiota from child-turcotte-pugh of A and B patients. Front Microbiol. 2016;7:1856. doi: 10.3389/fmicb.2016.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Zhu C, Shao L, Ye J, Shen Y, Ren Y. Role of bile acids in dysbiosis and treatment of nonalcoholic fatty liver disease. Mediators Inflamm. 2019;2019:7659509. doi: 10.1155/2019/7659509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update. Zhonghua Gan Zang Bing Za Zhi. 2018;26:195–203. doi: 10.3760/cma.j.issn.1007-3418.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Consensus on clinical application of transient elastography detecting liver fibrosis: a 2018 update. Zhonghua Gan Zang Bing Za Zhi. 2019;27:182–191. doi: 10.3760/cma.j.issn.1007-3418.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Liu H, Zhang M, Guo GL. Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B. 2016;6:409–412. doi: 10.1016/j.apsb.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan L, Lai R, Ma N, Dong Y, Li Y, Wu Q, Qiao J, Lu H, Gong L, Tao Z, Chen J, Xie Q, Ren J. miR-552-3p modulates transcriptional activities of FXR and LXR to ameliorate hepatic glycolipid metabolism disorder. J Hepatol. 2021;74:8–19. doi: 10.1016/j.jhep.2020.07.048. [DOI] [PubMed] [Google Scholar]
- 19.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, Lu L, Wang D. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694. e1673. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 21.Kim SG, Kim BK, Kim K, Fang S. Bile acid nuclear receptor farnesoid X receptor: therapeutic target for nonalcoholic fatty liver disease. Endocrinol Metab (Seoul) 2016;31:500–504. doi: 10.3803/EnM.2016.31.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carino A, Biagioli M, Marchianò S, Fiorucci C, Zampella A, Monti MC, Scarpelli P, Ricci P, Distrutti E, Fiorucci S. Ursodeoxycholic acid is a GPBAR1 agonist and resets liver/intestinal FXR signaling in a model of diet-induced dysbiosis and NASH. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:1422–1437. doi: 10.1016/j.bbalip.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Malhi H, Camilleri M. Modulating bile acid pathways and TGR5 receptors for treating liver and GI diseases. Curr Opin Pharmacol. 2017;37:80–86. doi: 10.1016/j.coph.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie G, Wang X, Huang F, Zhao A, Chen W, Yan J, Zhang Y, Lei S, Ge K, Zheng X, Liu J, Su M, Liu P, Jia W. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int J Cancer. 2016;139:1764–1775. doi: 10.1002/ijc.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimer N, Choucair I, Wang Z, Nemet I, Li L, Gukasyan J, Weeks TL, Alkhouri N, Zein N, Tang WHW, Fischbach MA, Brown JM, Allayee H, Dasarathy S, Gogonea V, Hazen SL. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism. 2021;116:154457. doi: 10.1016/j.metabol.2020.154457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanyal AJ, Ling L, Beuers U, DePaoli AM, Lieu HD, Harrison SA, Hirschfield GM. Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Rep. 2021;3:100255. doi: 10.1016/j.jhepr.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang JYL. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017;1:3–9. doi: 10.1016/j.livres.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiorucci S, Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med. 2015;21:702–714. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 30.De Chiara F, Thomsen KL, Habtesion A, Jones H, Davies N, Gracia-Sancho J, Manicardi N, Hall A, Andreola F, Paish HL, Reed LH, Watson AA, Leslie J, Oakley F, Rombouts K, Mookerjee RP, Mann J, Jalan R. Ammonia scavenging prevents progression of fibrosis in experimental nonalcoholic fatty liver disease. Hepatology. 2020;71:874–892. doi: 10.1002/hep.30890. [DOI] [PubMed] [Google Scholar]
- 31.McPhail MJW, Shawcross DL, Lewis MR, Coltart I, Want EJ, Antoniades CG, Veselkov K, Triantafyllou E, Patel V, Pop O, Gomez-Romero M, Kyriakides M, Zia R, Abeles RD, Crossey MME, Jassem W, O’Grady J, Heaton N, Auzinger G, Bernal W, Quaglia A, Coen M, Nicholson JK, Wendon JA, Holmes E, Taylor-Robinson SD. Multivariate metabotyping of plasma predicts survival in patients with decompensated cirrhosis. J Hepatol. 2016;64:1058–1067. doi: 10.1016/j.jhep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Huang B, Jiang X, Chen W, Zhang J, Wei Y, Chen Y, Lian M, Bian Z, Miao Q, Peng Y, Fang J, Wang Q, Tang R, Gershwin ME, Ma X. Mucosal-associated invariant T cells improve nonalcoholic fatty liver disease through regulating macrophage polarization. Front Immunol. 2018;9:1994. doi: 10.3389/fimmu.2018.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau LHS, Wong SH. Microbiota, obesity and NAFLD. Adv Exp Med Biol. 2018;1061:111–125. doi: 10.1007/978-981-10-8684-7_9. [DOI] [PubMed] [Google Scholar]
- 34.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Beraza N. Fibrosis and the intestinal microbiome; a focus on chronic liver disease. Curr Opin Pharmacol. 2019;49:76–81. doi: 10.1016/j.coph.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Musoles R, García Tejedor A, Laparra JM. Immunonutritional contribution of gut microbiota to fatty liver disease. Nutr Hosp. 2020;37:193–206. doi: 10.20960/nh.02775. [DOI] [PubMed] [Google Scholar]
- 37.Houghton D, Stewart CJ, Day CP, Trenell M. Gut microbiota and lifestyle interventions in NAFLD. Int J Mol Sci. 2016;17:447. doi: 10.3390/ijms17040447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He X, Ji G, Jia W, Li H. Gut microbiota and nonalcoholic fatty liver disease: insights on mechanism and application of metabolomics. Int J Mol Sci. 2016;17:300. doi: 10.3390/ijms17030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang DY, Zhu L, Liu HN, Tseng YJ, Weng SQ, Liu TT, Dong L, Shen XZ. The protective effect and mechanism of the FXR agonist obeticholic acid via targeting gut microbiota in non-alcoholic fatty liver disease. Drug Des Devel Ther. 2019;13:2249–2270. doi: 10.2147/DDDT.S207277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng X, Li S, Li Y, Gan RY, Li HB. Gut microbiota’s relationship with liver disease and role in hepatoprotection by dietary natural products and probiotics. Nutrient. 2018;10:1457. doi: 10.3390/nu10101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Out C, Patankar JV, Doktorova M, Boesjes M, Bos T, de Boer S, Havinga R, Wolters H, Boverhof R, van Dijk TH, Smoczek A, Bleich A, Sachdev V, Kratky D, Kuipers F, Verkade HJ, Groen AK. Gut microbiota inhibit Asbt-dependent intestinal bile acid reabsorption via Gata4. J Hepatol. 2015;63:697–704. doi: 10.1016/j.jhep.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alemán JO, Bokulich NA, Swann JR, Walker JM, De Rosa JC, Battaglia T, Costabile A, Pechlivanis A, Liang Y, Breslow JL, Blaser MJ, Holt PR. Fecal microbiota and bile acid interactions with systemic and adipose tissue metabolism in diet-induced weight loss of obese postmenopausal women. J Transl Med. 2018;16:244. doi: 10.1186/s12967-018-1619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor SA, Green RM. Bile acids, microbiota, and metabolism. Hepatology. 2018;68:1229–1231. doi: 10.1002/hep.30078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Thomsen M, Vitetta L. Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics. J Cell Biochem. 2019;120:2713–2720. doi: 10.1002/jcb.27635. [DOI] [PubMed] [Google Scholar]
- 46.Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, Childs CE, Del Fabbro S, Bilson J, Moyses HE, Clough GF, Sethi JK, Patel J, Wright M, Breen DJ, Peebles C, Darekar A, Aspinall R, Fowell AJ, Dowman JK, Nobili V, Targher G, Delzenne NM, Bindels LB, Calder PC, Byrne CD. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology. 2020;158:1597–1610. e7. doi: 10.1053/j.gastro.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


