Abstract
Background: Multiple myeloma (MM) is a proliferative disease with complex pathogenesis. Most patients will have low body resistance and high inflammatory mediators. Bortezomib is an anti-tumor drug. There are few reports on the clinical efficacy and adverse reactions of bortezomib intervention. This research aimed to explore the effect of bortezomib on inflammation and immune lymphocytes of patients with MM-infected herpes zoster. Objective: The aim of this study is to explore the effect of bortezomib on inflammation and immune lymphocytes, i.e. the expression and correlation of interleukin (IL)-2, IL-10 and tumor necrosis factor-α (TNF-α) in patients with MM-infected herpes zoster (HZ) receiving bortezomib-containing regimen. Methods: From October 2017 to March 2020, 83 MM patients receiving bortezomib-containing regimen were analyzed retrospectively, patients were divided into infection group (28 cases, IG) and non-infection group (55 cases, NG) based on whether or not they are complicated with HZ Pre- and post-treatment. IL-2, IL-10, TNF-α and immune lymphocytes (CD3+, CD4+, CD8+) were tested by AimPlex multifactor flow detection technique, and the Eastern Cooperative Oncology Group (ECOG) performance status scores were compared before therapy. The independent risk factors of patients receiving bortezomib-containing regimen were analyzed via multivariate logistic regression. Results: After therapy, serum IL-2 and TNF-α declined significantly in NG while changed insignificantly in IG. Compared with NG, serum CD3+ and CD4+ in IG increased after treatment, while CD8+ decreased significantly. Before therapy, ECOG score in IG was higher than that in NG. Correlation analysis showed that IL-2 and TNF-α were negatively correlated with CD3+ and CD4+, and positively correlated with CD8+ and ECOG score. IL-10 was the opposite. Multivariate logistic regression analysis identified the independence of declined CD3+, CD4+, CD8+ and IL-10, increased IL-2, TNF-α and ECOG score before treatment as risk factors for HZ. Conclusion: MM patients have a high incidence of HZ. Before treatment, lymphocytopenia, increased IL-2, TNF-α and decreased IL-10 are important risk factors for HZ.
Keywords: Interleukin-2, interleukin-10, tumor necrosis factor-α, bortezomib, multiple myeloma, herpes zoster
Introduction
MM is one of the pervasive malignant tumors in clinical hematological system, and it is also a malignant proliferation tumor of plasma cells, which mostly develops in middle-aged and elderly people [1,2]. The progressive bone destruction is the main manifestation of MM [3]. Some studies have shown that about 80% of MM patients will have obvious bone abnormalities, and bone destruction affects the quality of life of patients [4,5]. At present, chemotherapy is one of the main treatment methods for MM, but chemotherapy cannot completely remove MM cells, so the remaining cells will increase the risk of relapse or drug resistance in patients [6].
Bortezomib is a new anti-tumor drug of proteasome inhibitor [7], which mainly inhibits the activity of nuclear transcription factor (NF-κB) by selectively acting on ubiquitin-protease system, thus inducing the apoptosis of myeloma cells, and promoting bone repair and new bone formation in patients with MM [8]. At present, bortezomib has a substantial curative effect in the clinical treatment of MM, which brings hope for the treatment of MM patients [9]. Studies have revealed that the risk of HZ will increase significantly after bortezomib intervention, which will not only affect the quality of life of patients, but also impact the treatment efficiency for patients with MM [10]. This is because bortezomib acts on CD4+ T lymphocytes, which will cause the impairment of T cell immune function and increase the risk of viral infection [11]. As a viral skin disease, HZ can lead to low body resistance, and invade the nerves of patients, resulting in pain, skin lesions and other related diseases [12]. Studies have unveiled that the action sites of bortezomib are related to NF-κB and T cell immunity, and the abnormal T cell immune function will lead to the infection of HZ virus in patients, so the incidence of HZ in MM patients treated with bortezomib will increase [13]. However, interleukin (IL)-2 and tumor necrosis factor (TNF)-α are important cytokines that reflect the immune function of the body, and participate in the physiological balance of the microenvironment in patients. Once the imbalance occurs, it will induce related immune disorders and abnormalities [14]. For example, studies have shown that the proinflammatory factors IL-6 and TNF-α play an extremely important role in the development and progression of neuropathic pain caused by HZ [15,16]. As an important anti-inflammatory molecule, IL-10 plays a vital role in patients infected with HZ [17]. Both IL-2 and IL-10 play an important role in inducing and maintaining immune tolerance [18]. Studies have also shown that inflammation is essential for the development of HZ, and the level of inflammatory substances is also closely associated with the occurrence of HZ [19]. Therefore, we suspect that serum inflammatory factors can be used as one of the effective analysis indexes in patients with MM-infected HZ after bortezomib intervention.
Therefore, this study was designed to analyze the improvement of bortezomib on inflammation and immune lymphocytes of patients with MM-infected HZ, and to analyze the diagnostic value of IL-2, IL-10 and TNF-α in patients, as well as the risk factors causing HZ.
Materials and methods
Baseline data
From October 2017 to March 2020, 83 patients with MM treated by bortezomib-containing regimen in our hospital were selected. According to whether complicated with HZ, patients were divided into an infection group (28 cases, IG) and a non-infection group (55 cases, NG). This research does not violate ethics, and this plan has been submitted to the hospital ethics committee for review. This research was ratified by the Ethics Committee of our hospital (XT17594RQ1). Both the subjects and their families have been informed and signed the full informed consent.
Inclusion criteria: All of them met the diagnostic criteria of MM [20]; The infected patients developed HZ after 2~3 courses of treatment with bortezomib, and the main manifestations were fever, cough, expectoration, fatigue, anorexia, and burning or tingling sensation in local skin; The moist rales were audible through lung auscultation; General information was complete.
Exclusion criteria were as below: Comorbid with other abnormal blood diseases; complicated with malignant tumour; complicated with infectious diseases; those who received immune pharmaceutical drugs that are not specific to MM diseases; comorbid with mental illness, disturbance of consciousness or mental retardation; incomplete information; those who quitted the experiment halfway and those who were not interviewed.
Sample collection
Before and after treatment, the blood (4 mL) of patients in both groups was drawn and centrifuged at 1500×g and 4°C for 10 min. Supernatant was collected for subsequent experiments.
Factor detection
IL-2, IL-10 and TNF-α (Pinxinnuo Biotechnology Co., Ltd., Tianjin, China, 111155, A111131, A111209) were tested by AimPlex multifactor flow detection technique [21]. Detection methods: The 96-well microporous plate was taken out, and the unused holes were sealed with sealing film. The mixed microspheres (45 μl/well) were added to the experimental wells (the microspheres were whirled forward for 45 s), and the liquid in the wells was removed by suction filter. Sample or standard (45 μL/well) was added: The standard was added directly (45 μL/well). First, 22.5 μL of sample diluent was added to each well, and then 22.5 μL of sample was added. The samples were sealed, shaken (700 r/min) and cultivated at ambient temperature in dark for 60 min. Preparation of 1× secondary antibody mixture: 2× secondary antibody mixture and 2× second diluent were mixed according to 1:1, and the total quantity was prepared according to the number of test wells (25 μL/well). A suction filter was used to remove the fluid from the hole. The 1× washing solution was added (100 μL/well) and washed 3 times, and then the following liquid was absorbed with absorbent paper. The 1× secondary antibody mixture was added (25 μL/well), sealed, shaken (700 r/min) and cultivated at ambient temperature in dark for 30 min. A suction filter was used to remove the fluid from the hole. The 1× washing solution was added (100 μL/well) and washed 3 times, and then the following liquid was absorbed with absorbent paper. Then, SA-PE was added (25 μL/well), sealed, shaken (700 r/min) and cultivated at ambient temperature in dark for 20 min. A suction filter was used to remove the fluid from the hole. The 1× washing solution was added (100 μL/well) and washed 2 times, and then the following liquid was absorbed with absorbent paper. The 1× reading solution was added (100-200 μl/well) (determined according to the sample volume of flow cytometer), and tested on the computer.
Detection of immune lymphocyte
In both groups, T-lymphocyte subsets in peripheral blood of patients were tested by FACSCalibur flow cytometry (Aigesi Biotechnology Co., Ltd., Beijing, China, BD FACSCalibur). The anticoagulated whole blood (100 μL) was put into TruCOUNT test tube, and each 20 μL of CD3+-FITC, CD4+-PE and CD8+-PE (Kemin Biotechnology Co., Ltd., Shanghai, China, DXT-130-098-162, DXT-130-107-623, DXT-130-098-059) antibodies were added respectively, and then mixed evenly and placed at room temperature for 15 minutes. The 370 μl of hemolysin (Qiming Biotechnology Co., Ltd., Shanghai, China, QM4537R/FITC) was added, mixed evenly, and placed at room temperature for 15 min. The samples were detected by flow cytometry, and the values of CD3+, CD4+ and CD8+ in peripheral blood were read. The experimental steps were strictly in accordance with the product specifications.
ECOG scoring index [22]
The patients were scored by 5-point method, which was divided into 5 grades from 0 to 4 to evaluate the physical strength and health status of the patients. A score of 0 means that the activity ability is completely normal, and there is no difference with that before illness; 1 point means that the patient can walk, but he/she can’t do physical work; 2 points means that the patient can’t do any work, and he/she can’t stay in bed for more than 50% of the time when he/she is awake; 3 points means that the patient has limited self-care ability, and he/she must stay in bed for more than 50% of the time when he/she wake up; 4 points means that the patient is completely incapacitated.
Statistical analysis
SPSS25.0 (Beijing Baiao Yijie Technology Co., Ltd., China) was applied for statistical analysis and pictures were drawn by GraphPad Prism 7. The enumeration data were expressed by the number of cases/percentage (n/%). The comparison of enumeration data between the two groups was conducted by Chi-square test. The measuring materials were expressed by (mean ± SD). The comparison of measuring materials between the two groups was conducted by independent sample T-test. Pearson coefficient was applied to analyse the correlation between IL-2, IL-10, TNF-α and CD3+, CD4+, CD8+ and ECOG score. Logistics multivariate regression was adopted to analyse risk factors affecting MM-infected HZ. The difference was statistically significant with P<0.05.
Results
Baseline data
There was no striking difference in gender, average age, body mass index, nationality, clinical stage, tumour type, diet, exercise history, smoking history and drinking history between IG and NG (P>0.05) (Table 1).
Table 1.
Baseline data of patients between the two groups [n (%)]/(mean ± SD)
| Classification | IG (n=28) | NG (n=55) | t/χ2 value | P value |
|---|---|---|---|---|
| Gender | 0.479 | 0.489 | ||
| Male | 17 (60.71) | 29 (52.73) | ||
| Female | 11 (39.29) | 26 (47.27) | ||
| Average age (years old) | 48.23±4.54 | 47.89±4.51 | ||
| Body mass index | 22.45±2.35 | 22.63±2.48 | ||
| Place of residence | 0.053 | 0.819 | ||
| City | 13 (46.43) | 27 (49.09) | ||
| Rural | 15 (53.57) | 28 (50.91) | ||
| Nation | 0.144 | 0.704 | ||
| Han | 17 (60.71) | 31 (56.36) | ||
| Minority nationality | 11 (39.29) | 24 (43.64) | ||
| Clinical stage | 1.014 | 0.314 | ||
| Stage IIIa | 16 (57.11) | 25 (45.45) | ||
| Stage IIIb | 12 (42.86) | 30 (54.55) | ||
| Tumour type | 0.166 | 0.921 | ||
| IgG type | 8 (28.57) | 18 (32.73) | ||
| IgA type | 9 (32.14) | 16 (29.09) | ||
| Light chain type | 11 (39.29) | 21 (38.18) | ||
| Diet | 0.082 | 0.774 | ||
| Light | 9 (32.14) | 16 (29.09) | ||
| Spicy | 19 (67.86) | 39 (70.91) | ||
| Exercise history | 0.144 | 0.705 | ||
| Yes | 18 (64.29) | 33 (60.00) | ||
| No | 10 (35.71) | 22 (40.00) | ||
| Smoking history | 1.023 | 0.312 | ||
| Yes | 19 (67.86) | 31 (56.36) | ||
| No | 9 (32.14) | 24 (43.64) | ||
| Drinking history | 1.006 | 0.316 | ||
| Yes | 17 (60.17) | 27 (49.09) | ||
| No | 11 (39.29) | 28 (50.91) |
Comparison of IL-2, IL-10 and TNF-α between the two groups before and after treatment
Before therapy, IL-2 and TNF-α were higher in IG than in NG (P<0.01), while IL-10 was lower (P<0.01). After therapy, the expression of serum IL-2 and TNF-α in NG declined significantly compared with that before therapy, while IL-10 enhanced significantly. There was no difference in IL-2, IL-10 and TNF-α in IG before and after treatment (P>0.01) (Figure 1).
Figure 1.
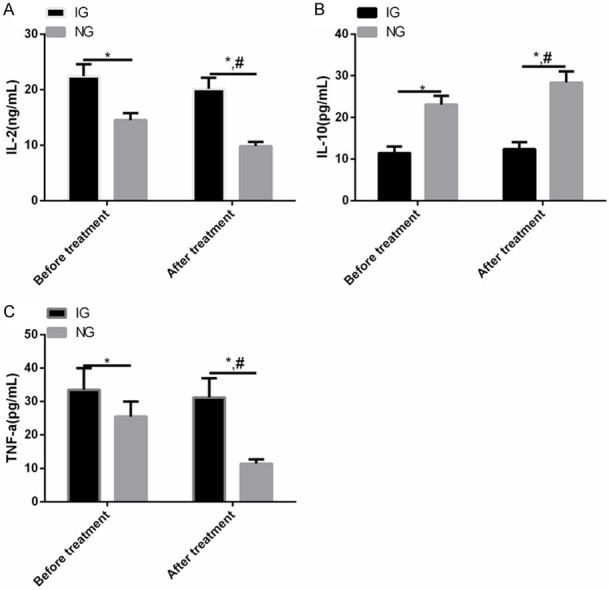
Comparison of IL-2, IL-10 and TNF-α between the two groups before and after treatment. A: IL-2 in IG was higher than that in NG before and after treatment. B: IL-10 in IG was lower than that in NG before and after treatment. C: TNF-α in IG was higher than that in NG before and after treatment. Note: Compared with the two groups, *P<0.01; Compared with IG after treatment, #<0.01.
Comparison of immune lymphocytes between the two groups before and after treatment
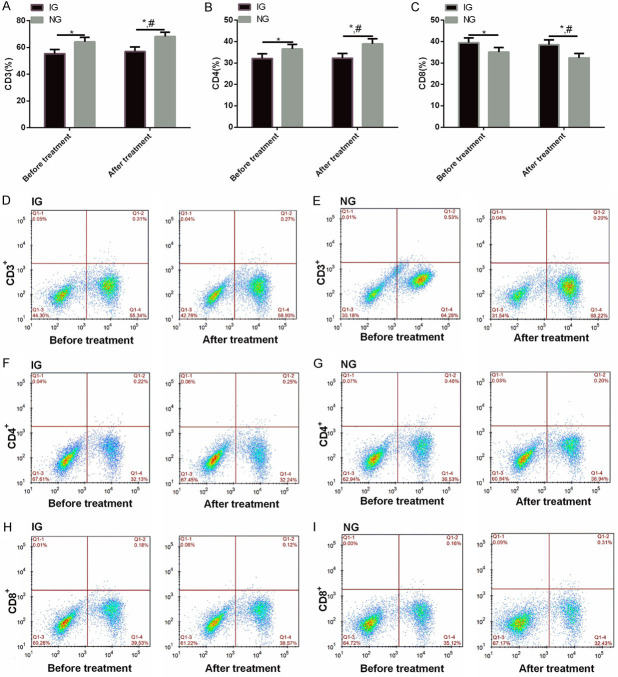
Before therapy, CD3+ and CD4+ in IG were lower than those in NG (P<0.01), while CD8+ was higher than that in NG (P<0.01). After therapy, the expression of serum CD3+ and CD4+ in NG enhanced significantly compared with that before therapy, while CD8+ declined significantly. There was no difference in CD3+, CD4+ and CD8+ in IG before and after treatment (P>0.01) (Figure 2).
Figure 2.
Comparison of immune lymphocytes between the two groups before and after treatment. A: CD3+ in IG was lower than that in NG before and after treatment. B: CD4+ in IG was lower than that in NG before and after treatment. C: CD8+ in IG was higher than that in NG before and after treatment. D, E: Flow cytometry of CD3+ in IG and NG before and after treatment. F, G: Flow cytometry of CD4+ in IG and NG before and after treatment. H, I: Flow cytometry of CD8+ in IG and NG before and after treatment. Note: Compared with the two groups, *P<0.01; Compared with IG after treatment, #<0.01.
Comparison of ECOG scores between the two groups before treatment
Before therapy, the ECOG scores in IG were significantly higher than those in NG (P<0.01) (Table 2).
Table 2.
Comparison of ECOG scores between the two groups before treatment (mean ± SD)
| Group | n | ECOG score |
|---|---|---|
| IG | 28 | 3.06±0.32 |
| NG | 55 | 1.49±0.11 |
| t | - | 32.920 |
| P | - | <0.001 |
Correlation analysis between IL-2, IL-10, TNF-α and CD3+, CD4+, CD8+
In order to explore the correlation between serum IL-2, IL-10, TNF-α and CD3+, CD4+, CD8+, we conducted correlation analysis. The analysis results revealed that serum IL-2 was negatively correlated with CD3+ and CD4+ (r=-0.697, -0.684, P<0.001), and positively correlated with CD8+ (r=0.606, P<0.001). Serum IL-10 was positively correlated with CD3+ and CD4+ (r=0.630, 0.646, P<0.001), and negatively correlated with CD8+ (r=-0.672, P<0.001). Serum TNF-α was negatively correlated with CD3+ and CD4+ (r=-0.680, -0.684, P<0.001), and positively correlated with CD8+ (r=0.680, P<0.001) (Figure 3).
Figure 3.
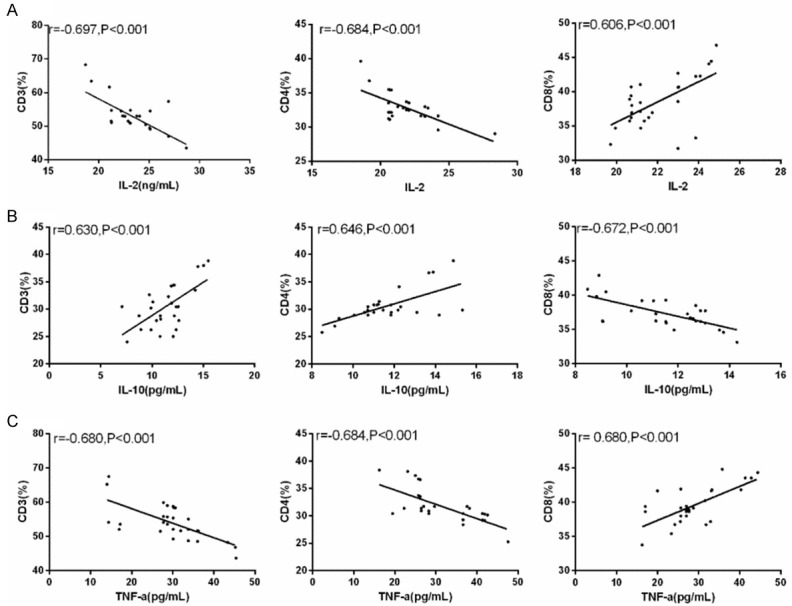
Correlation between IL-2, IL-10, TNF-α and CD3+, CD4+ and CD8+. A: Serum IL-2 was negatively correlated with CD3+ and CD4+ (r=-0.697, -0.684, P<0.001), and positively correlated with CD8+ (r=0.606, P<0.001). B: Serum IL-10 was positively correlated with CD3+ and CD4+ (r=0.630, 0.646, P<0.001), and negatively correlated with CD8+ (r=-0.672, P<0.001). C: Serum TNF-α was negatively correlated with CD3+ and CD4+ (r=-0.680, -0.684, P<0.001), and positively correlated with CD8+ (r=0.680, P<0.001).
Correlation analysis of IL-2, IL-10, TNF-α and ECOG score
In order to explore the correlation between serum IL-2, IL-10, TNF-α and ECOG score, we conducted correlation analysis. The analysis results revealed that IL-2 and TNF-α were positively correlated with ECOG score (r=0.674, 0.611, P<0.001). There was a negative correlation between IL-10 and ECOG score (r=-0.687, P<0.001) (Figure 4).
Figure 4.
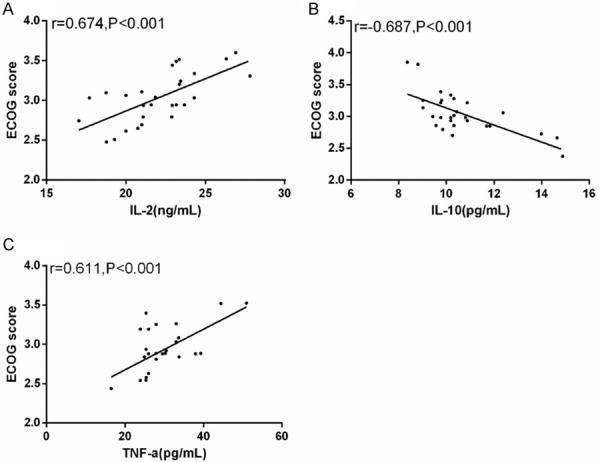
Correlation between IL-2, IL-10, TNF-α and ECOG score. A: Serum IL-2 was positively correlated with ECOG score (r=0.674, P<0.001). B: Serum IL-10 was negatively correlated with ECOG score (r=-0.687, P<0.001). C: Serum TNF-α was positively correlated with ECOG score (r=0.611, P<0.001).
Logistic regression analysis of multiple factors influencing in MM patients infected with HZ after treatment
The factors with differences, such as IL-2, IL-10, TNF-α, CD3+, CD4+, CD8+ and ECOG score, were analysed by multivariate Logistic regression. The findings revealed that IL-2 (P=0.001), IL-10 (P=0.001), TNF-α (P=0.001), CD3+ (P=0.012), CD4+ (P=0.009), CD8+ (P=0.015) and ECOG score (P=0.006) were independent risk factors affecting MM patients infected with HZ after therapy (Tables 3, 4).
Table 3.
Logistic multivariate regression analysis assignment
| Factor | Variable | Assignment |
|---|---|---|
| IL-2 | X1 | Continuous variable |
| IL-10 | X2 | Continuous variable |
| TNF-α | X3 | Continuous variable |
| CD3+ | X4 | Continuous variable |
| CD4+ | X5 | Continuous variable |
| CD8+ | X6 | Continuous variable |
| ECOG score | X7 | Continuous variable |
Table 4.
Logistic regression analysis of multiple factors influencing in MM patients infected with herpes zoster after treatment
| Factor | β | S.E | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| IL-2 | 0.324 | 0.432 | 8.231 | 0.001 | 1.432 | 1.371-2.328 |
| IL-10 | 0.653 | 0.321 | 8.162 | 0.001 | 1.542 | 1.432-3.831 |
| TNF-α | 0.583 | 0.204 | 8.203 | 0.001 | 2.438 | 1.023-4.372 |
| CD3+ | 0.356 | 0.364 | 6.326 | 0.012 | 3.434 | 0.843-3.453 |
| CD4+ | 0.476 | 0.219 | 4.213 | 0.009 | 1.428 | 1.026-2.389 |
| CD8+ | 0.584 | 0.238 | 8.241 | 0.015 | 1.643 | 1.342-2.784 |
| ECOG score | 0.593 | 0.213 | 7.704 | 0.006 | 1.812 | 1.193-5.821 |
Discussion
MM is a disease caused by malignant changes in bone marrow plasma cells, which will affect multiple organs of the patient [23]. In recent years, the incidence of MM is increasing year by year, and the age of onset tends to be younger [24]. Chemotherapy is still the main treatment for MM at present, but the recurrence rate is high [25].
In this research, bortezomib was used to intervene patients with MM. Most patients with MM developed HZ infection after receiving bortezomib intervention [26]. In our study, we have shown that the inflammatory mechanism is involved in the pathogenesis of HZ. Before treatment, IL-2 and TNF-α of patients infected with HZ virus in IG were higher than those in NG, while IL-10 was lower than that in NG. After therapy, the expression of serum IL-2 and TNF-α in NG declined significantly compared with that before therapy, while IL-10 enhanced significantly. However, there was no obvious change in IG. The results showed that the pro-inflammatory factors (IL-2 and TNF-α) were enhanced in patients with MM-infected HZ, while the expression of anti-inflammatory factor IL-10 declined, which indicated that bortezomib intervention could lead to HZ infection in MM patients. Cellular immune function is known to play a vital role in the development and progression of MM [27]. Studies have revealed that there is a severe imbalance of cellular immune function in patients with MM. The main clinical manifestations are characterized by CD4+ decrease and CD8+ increase [28]. However, low immune function can lead to HZ infection in patients with MM [29]. We also showed that before therapy, CD3+ and CD4+ in IG were lower than those in NG, while CD8+ was higher than that in NG. After therapy, the expression of serum CD3+ and CD4+ in NG enhanced significantly compared with that before therapy, while CD8+ declined significantly. However, there was no obvious change in IG. The findings revealed that bortezomib will damage the immune function of patients with MM, and these patients will be infected with HZ. Therefore, the intervention of bortezomib will not enhance the immune function of MM patients who have been infected with HZ. It has been shown that MM patients infected with HZ have low immune function, and also reduce the life ability of patients [30], which is similar to the results of this study. In our study, the ECOG score in IG before treatment was significantly higher than that in NG. This indicated that the activity of patients with MM-infected HZ is reduced due to long-term bed rest, and the chances of infection may be increased due to long-term hospitalization, so the ECOG score is significantly increased.
According to Pearson analysis, IL-2 and TNF-α were negatively correlated with CD3+ and CD4+, and positively correlated with CD8+ and ECOG score. IL-10 was positively correlated with CD3+ and CD4+, and negatively correlated with CD8+ and ECOG score. Studies by Li et al. [31] have shown that MM patients treated with bortezomib have a high incidence of HZ; Before therapy, lymphocytopenia, increased ECOG score, use of cyclophosphamide, and lack of prophylactic antiviral therapy were important risk factors for HZ. Finally, multivariate logistic regression analysis showed that the increase of IL-2, TNF-α, CD8+ and ECOG and the decrease of IL-10, CD3+ and CD4+ were important risk factors for the development of HZ. This suggests that if MM patients have increased IL-2, TNF-α, CD8+, ECOG and decreased IL-10, CD3+, CD4+, bortezomib intervention treatment should be stopped to avoid the infection of HZ. This also validates the results of Li’s study.
Although this study has revealed that serum IL-2, TNF-α and IL-10 have high diagnostic value for patients with MM-infected HZ, there is still some room for improvement. For example, we can supplement the relevant basic research on IL-2, TNF-α and IL-10 to explore the relationship between them and the pathological parameters of patients with MM-infected HZ.
To sum up, patients with MM have a high incidence of HZ. Before treatment, lymphocytopenia, increased IL-2, TNF-α and decreased IL-10 are important risk factors for the occurrence of HZ, which need to be prevented in time.
Disclosure of conflict of interest
None.
References
- 1.Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int. 2016;113:470–476. doi: 10.3238/arztebl.2016.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kritikos N, Priftakis D, Stavrinides S, Kleanthous S, Sarafianou E. Nuclear medicine techniques in merkel cell carcinoma: a case report and review of the literature. Oncol Lett. 2015;10:1610–1616. doi: 10.3892/ol.2015.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bataille R. The multiple myeloma bone eco-system and its relation to oncogenesis. Morphologie. 2015;99:31–37. doi: 10.1016/j.morpho.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Ormond Filho AG, Carneiro BC, Pastore D, Silva IP, Yamashita SR, Consolo FD, Hungria VTM, Sandes AF, Rizzatti EG, Nico MAC. Whole-body imaging of multiple myeloma: diagnostic criteria. Radiographics. 2019;39:1077–1097. doi: 10.1148/rg.2019180096. [DOI] [PubMed] [Google Scholar]
- 5.Ramsenthaler C, Osborne TR, Gao W, Siegert RJ, Edmonds PM, Schey SA, Higginson IJ. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: a multi-centre study. BMC Cancer. 2016;16:427. doi: 10.1186/s12885-016-2410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgren O, Lu SX, Hultcrantz M. MRD testing in multiple myeloma: the main future driver for modern tailored treatment. Semin Hematol. 2018;55:44–50. doi: 10.1053/j.seminhematol.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Vora PA, Patel R, Dharamsi A. Bortezomib-first therapeutic proteasome inhibitor for cancer therapy: a review of patent literature. Recent Pat Anticancer Drug Discov. 2020;15:113–131. doi: 10.2174/1574892815666200401113805. [DOI] [PubMed] [Google Scholar]
- 8.Robak P, Robak T. Bortezomib for the treatment of hematologic malignancies: 15 years later. Drugs R D. 2019;19:73–92. doi: 10.1007/s40268-019-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie H, Gu Y, Wang W, Wang X, Ye X, Xin C, Lu M, Reddy BA, Shu P. Silencing of SENP2 in multiple myeloma induces bortezomib resistance by activating NF-κB through the modulation of IκBα sumoylation. Sci Rep. 2020;10:766. doi: 10.1038/s41598-020-57698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandit A, Leblebjian H, Hammond SP, Laubach JP, Richardson PG, Baden LR, Marty FM, Issa NC. Safety of live-attenuated measles-mumps-rubella and herpes zoster vaccination in multiple myeloma patients on maintenance lenalidomide or bortezomib after autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53:942–945. doi: 10.1038/s41409-018-0112-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Ren HY, Dong YJ, Wang LH, Yin Y, Li Y, Qiu ZX, Cen XN, Shi YJ. Bortezomib regulates the chemotactic characteristics of T cells through downregulation of CXCR3/CXCL9 expression and induction of apoptosis. Int J Hematol. 2012;96:764–772. doi: 10.1007/s12185-012-1195-6. [DOI] [PubMed] [Google Scholar]
- 12.Levin MJ, Weinberg A, Schmid DS. Herpes simplex virus and varicella-zoster virus. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.DMIH2-0017-2015. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Chen J, Han X, Zhang E, Huang X, Guo X, Chen Q, Wu W, Zheng G, He D, Zhao Y, Yang Y, He J, Cai Z. Pirh2 mediates the sensitivity of myeloma cells to bortezomib via canonical NF-kappaB signaling pathway. Protein Cell. 2018;9:770–784. doi: 10.1007/s13238-017-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hozain S, Cottrell J. CDllb+ targeted depletion of macrophages negatively affects bone fracture healing. Bone. 2020;138:115479. doi: 10.1016/j.bone.2020.115479. [DOI] [PubMed] [Google Scholar]
- 15.Peng L, Du B, Sun L, Zhao Y, Zhang X. Short-term efficacy and safety of prednisone in herpes zoster and the effects on IL-6 and IL-10. Exp Ther Med. 2019;18:2893–2900. doi: 10.3892/etm.2019.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim BS, Maverakis E, Alexanian C, Wang JZ, Raychaudhuri SP. Incidence, clinical features, management, and prevention of herpes zoster in patients receiving antitumor necrosis factor therapy: a clinical review. J Cutan Med Surg. 2020;24:278–284. doi: 10.1177/1203475420914622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershon AA, Brooks D, Stevenson DD, Chin WK, Oldstone MBA, Gershon MD. High constitutive interleukin 10 level interferes with the immune response to varicella-zoster virus in elderly recipients of live attenuated zoster vaccine. J Infect Dis. 2019;219:1338–1346. doi: 10.1093/infdis/jiy660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo WJ, Qi H, Deng CY, Zhou HX, Deng SP, Li FR. The roles of IL-2 and IL-10 enhance anti-CD4+5RBmAb immune inhibition in allograft skin. Immunol Res. 2015;61:250–259. doi: 10.1007/s12026-014-8618-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Park GH, Kim MJ, Sim HB, Lee WJ, Lee SJ, Kim SW, Jeon YH, Jang YH, Kim DW. Usefulness of inflammatory markers for the prediction of postherpetic neuralgia in patients with acute herpes zoster. Ann Dermatol. 2018;30:158–163. doi: 10.5021/ad.2018.30.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajkumar SV. Updated diagnostic criteria and staging system for multiple myeloma. Am Soc Clin Oncol Educ Book. 2016;35:e418–423. doi: 10.1200/EDBK_159009. [DOI] [PubMed] [Google Scholar]
- 21.Zhu SR, Chen HY, Wang MJ, Xu YG, Quan RC, Ding XQ, Zhao P, Wang HZ, Guo XQ, Hu XM. Level of regulatory B cells in patients with immune thrombocytopenia and its clinical significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27:175–179. doi: 10.7534/j.issn.1009-2137.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Demirelli B, Babacan NA, Ercelep O, Ozturk MA, Kaya S, Tanrikulu E, Khalil S, Hasanov R, Alan O, Telli TA, Koca S, Aribal ME, Kuzan B, Dane F, Yumuk PF. Modified Glasgow prognostic score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer. 2021;73:230–238. doi: 10.1080/01635581.2020.1749290. [DOI] [PubMed] [Google Scholar]
- 23.Das R, Strowig T, Verma R, Koduru S, Hafemann A, Hopf S, Kocoglu MH, Borsotti C, Zhang L, Branagan A, Eynon E, Manz MG, Flavell RA, Dhodapkar MV. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat Med. 2016;22:1351–1357. doi: 10.1038/nm.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazyka D, Gudzenko N, Dyagil I, Trotsiuk N, Gorokh E, Fedorenko Z, Chumak V, Bakhanova E, Ilienko I, Romanenko A. Incidence of multiple myeloma among cleanup workers of the Chornobyl accident and their survival. Exp Oncol. 2016;38:267–271. [PubMed] [Google Scholar]
- 25.Kosugi H. High-dose chemotherapy with autologous hematopoietic stem cell transplantation for multiple myeloma. Rinsho Ketsueki. 2015;56:279–288. doi: 10.11406/rinketsu.56.279. [DOI] [PubMed] [Google Scholar]
- 26.Kim JW, Min CK, Mun YC, Park Y, Kim BS, Nam SH, Koh Y, Kwon JH, Choe PG, Park WB, Kim I. Varicella-zoster virus-specific cell-mediated immunity and herpes zoster development in multiple myeloma patients receiving bortezomib- or thalidomide-based chemotherapy. J Clin Virol. 2015;73:64–69. doi: 10.1016/j.jcv.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 27.van de Donk N, Usmani SZ. CD3+8 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front Immunol. 2018;9:2134. doi: 10.3389/fimmu.2018.02134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K, van de Donk NW, Weiss BM, Ahmadi T, Lokhorst HM, Mutis T, Sasser AK. Daratumumab depletes CD3+8+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128:384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalafallah AA, Woodgate M, Koshy K, Patrick A. Ophthalmic manifestations of herpes zoster virus in patients with multiple myeloma following bone marrow transplantation. BMJ Case Rep. 2013;2013:bcr2012007625. doi: 10.1136/bcr-2012-007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kibata K, Ito T, Inaba M, Tanaka A, Iwata R, Inagaki-Katashiba N, Phan V, Satake A, Nomura S. The immunomodulatory-drug, lenalidomide, sustains and enhances interferon-alpha production by human plasmacytoid dendritic cells. J Blood Med. 2019;10:217–226. doi: 10.2147/JBM.S206459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G, Zhang QK, Wei XF, Feng YF, Yang WH, Sun YQ. Analysis of risk factors of herpes zoster in patients with multiple myeloma treated with bortezomib. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:1972–1976. doi: 10.19746/j.cnki.issn.1009-2137.2020.06.029. [DOI] [PubMed] [Google Scholar]