Abstract
Parkinson’s disease (PD) is a degenerative disease of the central nervous system (CNS) and is common among the middle-aged and elderly populations. Increasing evidence shows that the gut microbiota may trigger PD through the “gut-microbiota-brain” axis. A previous study revealed that constipation, one of the non-motor symptoms of PD, affects gut microbiota and the progression of PD. However, whether constipation is involved in gut microbiota-associated PD is largely unknown. Therefore, we investigated the relationship between gut microbiota, PD, and constipation in this study. We carried out 16S rRNA sequencing in 15 constipated PD patients (C-PD), 14 non-constipated PD (NC-PD) patients, and 15 healthy controls to evaluate the microbial population. Furthermore, co-occurrence networks were used to assess the gut ecology of the three groups. Spearman analyses were used to analyze the correlation between the differential microbiota and the clinical features. The results showed that there were differences in the composition of the gut microbiota among the C-PD group, the NC-PD group, and the healthy controls. No significant differences were observed in the alpha diversity among the three groups, but the beta diversity differed significantly among the groups. Compared with the healthy controls, the abundance of Hungatella and Collinsella was increased and the abundance of Lachnospira and Fusicatenibacter was reduced in the PD patients’ feces. Compared with the NC-PD group, the relative abundance of Megamonas and Holdemanella were lower, while Hungatella, Streptococcus and Anaerotruncus were enriched in the C-PD group. The co-occurrence network analysis showed that the C-PD group presented a different microbial community relationship compared with the NC-PD group and the healthy controls. Our study provides strong evidence that the gut microbiota may be related to constipation in PD. In addition, our data suggest an association between the differential microbiota genera and the clinical features of PD. Therefore, modulating gut microbiota may be another way to monitor and optimize PD treatment.
Keywords: Parkinson’s disease (PD), 16S rRNA sequencing, gut microbiota, constipation
Introduction
PD is a degenerative disease of the central nervous system (CNS) characterized by motor and non-motor symptoms, which is common in middle-aged and elderly patients. According to published data, the incidence of PD in Chinese people over 65 years old is approximately 1.7% [1]. Previous studies have shown that the core characteristics of PD pathology are the misfolding, abnormal aggregation, and intercellular transmission of α-synuclein (α-Syn) [2,3]. The abnormal aggregation of α-Syn occurs in both the brain and gut. Moreover, increasing evidence shows that the abnormal aggregation of α-Syn in the enteric nerve occurs earlier than it does in the central nervous system [4,5].
As one of the important environmental factors that affects human health, the gut microbiota is considered to be the main cause of α-Syn formation [6,7]. The number of gut microbiota in the gut tract is up to 100 trillion, which is far more than it is in other parts of the human body and about 10 times of the total number of human cells and germ cells [8]. At present, extensive evidence has indicated that the gut microbiota plays a significant role in the pathogenesis of PD [9], most probably through the “gut-microbiota-brain” axis, a two-way connection between the gut microbiota and CNS. Recently, Sampson et al. [10] showed that mice transplanted with gut microbiota from PD patients developed symptoms of dyskinesia, α-Syn aggregation, and inflammation; however, the control mice transplanted with gut microbiota from healthy humans did not display these symptoms. Hence, the gut microbiota may be involved in the pathogenesis of PD, but there are insufficient studies to confirm the possible mechanism.
In fact, in addition to motor symptoms, PD also shows many non-motor symptoms, such as gastrointestinal dysfunction, sleep disorders, and mental illness. Among them, constipation, severely affects patient’s quality of life. Increasing evidence has confirmed that constipation or slow bowel movements may result in dysfunction of the gut microbiota [11].
However, whether constipation is involved in gut microbiota-related PD is largely unknown. As a key factor causing constipation, the stability of the gut microbiota has an important significance and influence on the normal digestion of the human body. A further understanding of the changes in the gut microbiota of patients with PD complicated with constipation is also a key to ensuring the normal life of patients. In this study, we intended to investigate the diversity of the gut microbiota composition in patients with constipated PD (C-PD), non-constipated PD (NC-PD), and the healthy controls, explore the relationship among various microbiota genera by co-occurrence networks, and to determine the significant genera associated with constipation, and analyze the correlation between the differential genera and the clinical features of PD. This associated analysis of constipation and gut microbiota can provide interesting and novel clues to assist in understanding the pathogenesis of PD.
Methods
Study subjects
A total of 15 PD patients with constipation, 14 PD patients without constipation, and 15 healthy controls were recruited from the Affiliated BenQ Hospital of Nanjing Medical University during the period August 2017 to March 2018. The PD patients were all screened from the outpatients and inpatients in the Department of Neurology, and the healthy controls were from the Health Check-up Center. All the participants provided a written informed consent upon enrolment. This study was approved by the Ethics Committee of The Affiliated BenQ Hospital of Nanjing Medical University.
Inclusion criteria: patients diagnosed with idiopathic PD by a neurologist according to the revised PD diagnostic criteria published by the International Movement Disorders Association in 2015.
The exclusion criteria were: (1) patients diagnosed with neurological diseases other than PD; (2) patients with severe comorbidities; (3) patients with a history of gastric/bowel surgery; (4) patients who had used antibiotics or probiotics within the previous 3 months [12]. At the same time, the healthy controls who met any of the exclusion criteria were also not included.
The patients’ basic data, their H-Y (Hoehn-Yahr) grades, their UPDRSIII (Unified Parkinson’s disease rating scale III, unified PD score) analysis scores, their NMSQ (Non-motor Symptoms Questionnaire) scores, their MMSE (Mini-mental State Examination) scores, their PDQ39 (39 PD quality of life questionnaire) scores, and their Wexner scores (evaluation of constipation severity) were collected.
Fecal sample collection and DNA extraction
All the patients or their families were instructed to properly use a special fecal collection device (patented by Shanghai Micro-based Biotechnology Co., Ltd.; the samples can be stored at room temperature for 1 week) to collect approximately 2 g of feces, which were stored in a freezer at -80°C immediately after their collection. The microbiota DNA in the samples was extracted using a QIAamp DNA Stool Mini Kit (QIAGEN, Hilden, Germany), and the integrity of the extracted genomic DNA was measured using 1.2% agarose gel electrophoresis.
16sRNA sequencing and 16SrRNA data analysis
The V4 and V5 regions of 16S the rDNA sequencing were identified using a high-throughput sequencing analysis. The PCR product was recovered using 2% agarose gel, purified with AxyPrep DNA Gel Recovery Kit (AXYGEN), eluted with Tris-HCI, and measured using 2% agarose electrophoresis. According to the standard operating procedures of the Illumina MiSeq platform (Illumin, San Diego, USA), the purified amplified fragments were used to construct a PE 2×300 database. Construction steps: 1. Link the “Y”-shaped connector. 2. Magnetic bead screening to remove the self-linked fragments of the adapter. 3. The PCR amplification reaction was used to enrich the database template. 4. The PCR product was recovered using magnetic beads to obtain the final result. The sequencing was performed on the Illumina MiSeq platform, and a comparative analysis was performed on the NCBI database [13]. After completing a quality control of the sequencing results, an OTU (operational taxonomic unit) cluster analysis, an alpha and beta diversity analysis, a heatmap, and a Wayne diagram were further performed using Qiime 1.9 (REF).
Genera interaction in the ecological networks of the microbial community analysis
To clarify the genera interactions, we constructed co-occurrence networks in the three groups of patients. The WGCNA package of R (Version 3.4.4) was used for a cluster analysis of the genera with high topological overlap into modules. The network was shown using Cytoscape 3.5.1. The threshold was set by Pearson r>0, P<0.1, and the topology overlap was >0.01 [14]. A network analysis was performed on the top 35 genera with significant differences.
Statistical analysis
The data was analyzed using SPSS and R language software. For the data conforming to normal distribution, a group t-test was used for the comparison between two groups. The Wilcox test was used to compare the data that did not follow a normal distribution. For the count data or the composition ratio, chi-squared (x2) tests were used for the comparisons among the groups. One-way analyses of variance followed by LSD post-hoc tests were used to compare multiple groups. The above relevant statistical analysis was carried out using IBM SPSS Statistics 20.0 software.
Results
Cohorts of patients
The statistical analysis showed that there were no significant differences in terms of age, gender, anti-platelet drugs, statins, anti-hypertensive drugs, or smoking rates between the PD group and the healthy controls (P>0.05). In terms of disease severity, the UPDRSIII, NMSQ, PDQ39, and end of dose deterioration scores were significantly higher in the C-PD group compared to the NC-PD group (P=0.031, P=0.001, P=0.005, P=0.008), and no significant differences were observed in the H&Y, MMSE, or dyskinesia scores (P>0.05). In terms of drug use, there was significantly higher dosages of COMT inhibitors (P=0.008) and MAOB inhibitors (P=0.009) in the C-PD group than in the NC-PD group, and the usage of the levodopa and dopamine agonists were similar in the two groups (P>0.05). Compared with the NC-PD group, the C-PD group had a statistically significant difference in age (P=0.044) and a lower proportion of females; there were no significant differences in the combined diseases, anti-platelet drugs, statins, anti-hypertensive drugs, or smoking rates (P>0.05), as shown in Table 1.
Table 1.
Comparison of the general clinical data
| PD patients | Healthy controls | P1 value | Constipation patients | Non-constipation patients | P2 value | |
|---|---|---|---|---|---|---|
| Age (years) | 64.17±8.78 | 60.80±7.33 | 0.210 | 65.14±9.11 | 61.97±7.98 | 0.226 |
| Gender (female) | 9 (31.03%) | 8 (53.33%) | 0.198 | 2 (14.29%) | 15 (50.00%) | 0.044 |
| PD duration (month) | 79.29±46.20 | - | - | 79.29±46.20 | 67.73±42.08 | 0.487 |
| H-Y | 2.32±0.70 | - | - | 2.32±0.70 | 1.90±0.97 | 0.192 |
| UPDRSIII | 22.93±11.69 | - | - | 22.93±11.69 | 14.20±8.91 | 0.031 |
| NMSQ | 14.57±4.20 | - | - | 14.57±4.20 | 8.13±5.15 | 0.001 |
| Wexner | 12.86±4.50 | - | - | 12.86±4.50 | 0.27±0.59 | <0.01 |
| MMSE | 26.79±1.97 | - | - | 26.79±1.97 | 25.80±3.47 | 0.360 |
| PDQ39 score | 51.64±25.02 | - | - | 51.64±25.02 | 26.13±20.06 | 0.005 |
| End of dose deterioration | 9 (64.29%) | - | - | 9 (64.29%) | 2 (13.33%) | 0.008 |
| Dyskinesia | 5 (35.71%) | - | - | 5 (35.71%) | 1 (6.67%) | 0.080 |
| Combined disease | ||||||
| Cerebrovascular disease | 11 (37.93%) | 4 (26.67%) | 0.520 | 5 (35.71%) | 10 (33.33%) | 1.000 |
| Hypertension | 4 (13.79%) | 1 (6.67%) | 0.647 | 2 (14.29%) | 3 (10.00%) | 0.647 |
| Drugs | ||||||
| Levodopa | 14 (100%) | - | - | 14 (100%) | 15 (100%) | 1 |
| Dopamine agonists | 11 (78.57%) | - | - | 11 (78.57%) | 12 (80.00%) | 1 |
| COMT inhibitor | 9 (64.29%) | - | - | 9 (64.29%) | 2 (13.33%) | 0.008 |
| MAOB inhibitor | 10 (71.43%) | - | - | 10 (71.43%) | 3 (20.00%) | 0.009 |
| Antihypertensive drugs | 4 (13.49%) | 1 (6.67%) | 0.647 | 2 (14.29%) | 3 (10.00%) | 0.647 |
| Antiplatelet agent | 11 (37.93%) | 4 (26.67%) | 0.520 | 5 (35.71%) | 10 (33.33%) | 1.000 |
| Statins | 9 (31.03%) | 4 (26.67%) | 1.000 | 5 (35.71%) | 7 (23.33%) | 0.475 |
| Smoking | 9 (31.03%) | 4 (26.67%) | 1.000 | 6 (42.86%) | 7 (23.33%) | 0.288 |
Alterations of gut microbiota composition in the PD patients and the healthy controls
To investigate the gut microbiota composition of the PD patients, we first compared the diversity and composition among the healthy controls, the NC-PD group, and the C-PD group.
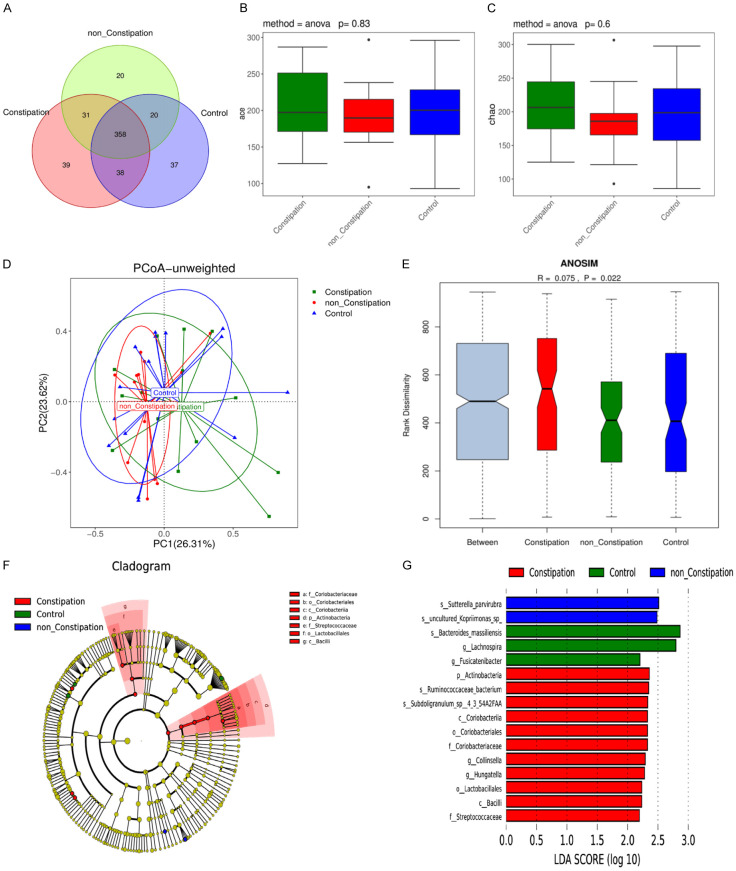
The overlapping OTU of three groups were revealed in a Venn diagram (Figure 1A). The data showed 453, 506 and 466 OTUs detected in the healthy controls, the NC-PD group, and the C-PD group, respectively. The ace index and the chao index of alpha diversity in the three groups were not significantly different (all P>0.05) (Figure 1B, 1C). The PCoA (primary coordinate analysis) of beta diversity calculated based on the unweighted UniFrac distances matrix was used for a cluster analysis of the three distinct groups (Figure 1D), and a significant difference was determined (ANOSIM (analysis of similarities) R=0.075, P=0.022) (Figure 1E). Next, the LEfSe (linear discriminant analysis effect size) analysis revealed that 16 taxa (3 in healthy controls, 2 in the NC-PD group and 11 in the C-PD group) were differentially abundant in the three groups (Figure 1F). Moreover, at the genus level, the abundance of Lachnospira and Fusicatenibacter were lower in the PD patients, and Collinsella and Hungatella were enriched in the PD group compared with the healthy controls (Figure 1G).
Figure 1.
Gut microbiota composition in PD patients and the healthy controls. A. Venn diagram of the OTUs in the three groups. B, C. Alpha diversity based on OUT. D. Primary coordinate analysis based on unweighted UniFrac (PCoA). E. ANOSIM analysis of the Unweighted UniFrac distances among the three groups. F. Lefse tree diagram. G. Difference contribution analysis chart.
The C-PD group showed an alternation of the gut microbiota relative to the NC-PD group
To further check whether constipation was involved in the pathogenesis of PD in the Chinese population, we compared the differences in diversity and composition between the NC-PD group and the C-PD group.
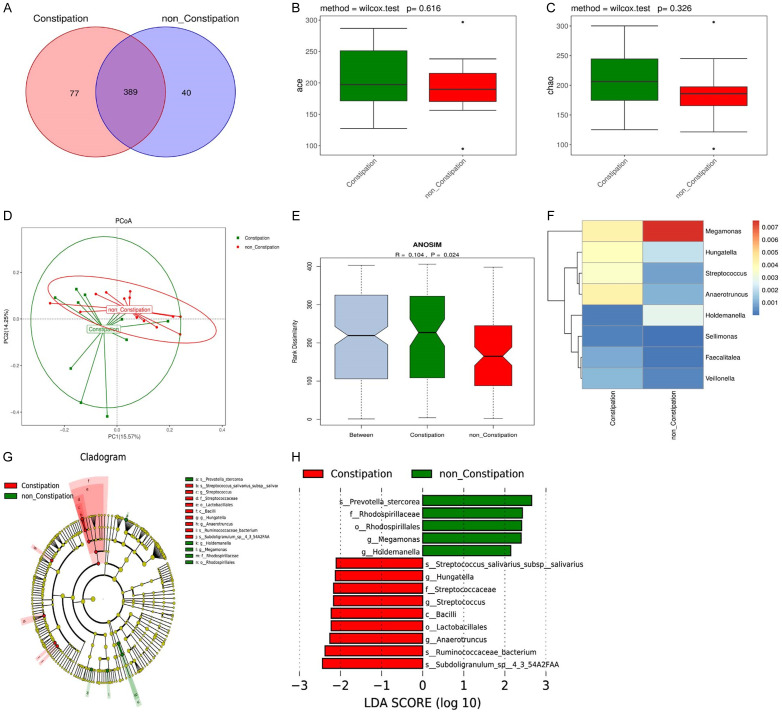
The data demonstrated that 429 and 466 OTUs were detected in the NC-PD group and the C-PD group, respectively (Figure 2A). The ace index and the chao index of alpha diversity between them were not significantly different (all P>0.05) (Figure 2B, 2C). PCoA data calculated on the unweighted UniFrac distances was clustered into two distinct groups (ANOSIM R=0.104, P=0.024) (Figure 2D, 2E). The genera heatmap selected from the differential genera showed that compared with the NC-PD group, the relative abundance of Hungatella, Streptococcus, and Anaerotruncus was significantly higher, but the relative abundance of Megamonas and Holdemanella was lower in the C-PD group (Figure 2F). Next, to confirm the specific taxa related to constipation and specific taxa biomarkers for the constipation diagnosis, an evolutionary branch map was plotted to show the predominant genera and the structure of the gut microbiota in PD patients with or without constipation (Figure 2G). The LEfSe analysis demonstrated that 14 taxa were prominently diverse between the two groups. In these taxa, 10 in red and 4 in green were identified as enriched within the PD patients complicated with and without constipation, respectively. Moreover, at the genus level, the abundance of Megamonas and Holdemanella was lower, but the abundance of Hungatella, Streptococcus and Anaerotruncus was higher in the C-PD group compared with the NC-PD group (Figure 2H).
Figure 2.
Gut microbiota composition in the NC-PD and C-PD groups. A. Venn diagram of the OTUs in two groups. B, C. Alpha diversity based on OUT. D. Primary coordinate analysis based on the unweighted UniFrac (PCoA). E. ANOSIM analysis of the Unweighted UniFrac distances between the two groups. F. Genera heatmap. G. Lefse tree diagram. H. Difference contribution analysis chart.
Aberrant ecological networks of microbial communities occurred differently in the constipation and non-constipation PD patients
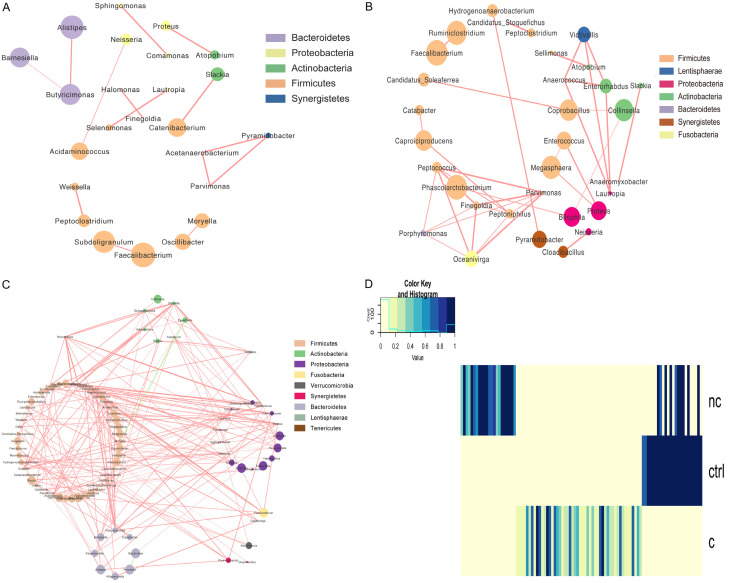
To investigate the relevance among the various genera, the ecological networks of the three groups were visualized. Compared to the NC-PD and C-PD groups, the healthy controls showed a simpler concurrent network with less integrated symbiosis (Figure 3A). Probably due to gastrointestinal dysfunction, the NC-PD group showed a more complex concurrent network than the healthy controls (Figure 3B), and all the genera were positively correlated. The C-PD group showed a multifaceted network in which several genera were clustered and totally grouped into a solo module associated with many other modules (Figure 3C). In this small symbiotic network, only a small number of interactions were negative while most presented a stronger positive relationship, which indicated complex relationships among the different genera (Figure 3D). These networks suggest that the gut microbiota plays a positive role in the maintenance and progress of the related taxa in PD patients.
Figure 3.
Networks to visualize the interactions among the different genera in the three groups. A. Healthy controls. B. Non-constipated patients. C. Constipated patients. The density of the dashed line demonstrates the Pearson coefficient. The size of the node demonstrates the relative abundance. Red links represent positive interactions between nodes, green links represent negative interactions. D. An integration diagram of the three samples.
Associated analysis for differential genera and clinical features
To investigate whether the differential genera of constipation were related to clinical features of PD, we further analyzed the association between microbiota and the clinical features of the PD patients.
As shown in Figure 4, no genera were found to be related to UPDRSIII, H&Y, or MMSE. Wexner scores, which reflect the severity of constipation, displayed a positive correlation with the abundance of Streptococcus, Sellimonas, Veillonella, Anaerrotruncus, Hungatella and Faecalitalea, and a negative correlation with the abundance of Holdemanella and Megamonas. The abundance of Veillonella was positively associated with PDQ39. The abundance of Streptococcus and Veillonella was correlated to the NMSQ scores. Streptococcus, Sellimonas and Veillonella were age-related.
Figure 4.
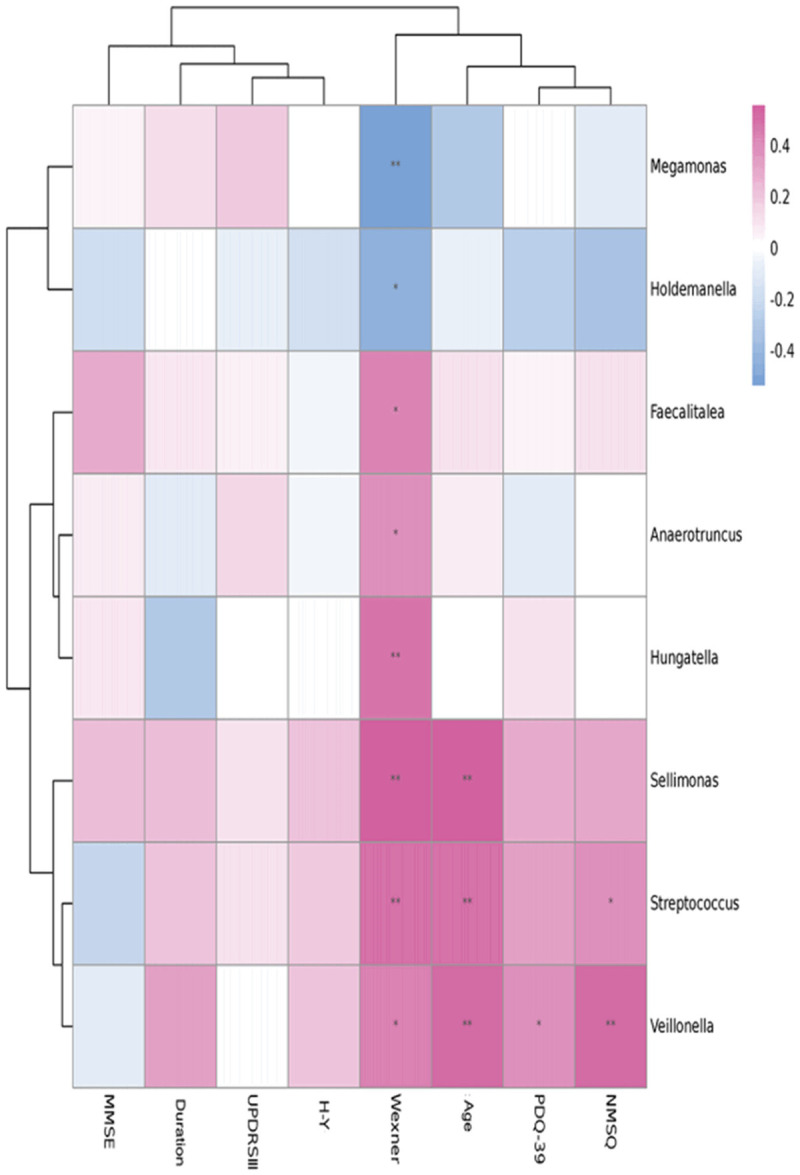
Correlation analysis between the specific gut microbiota and the clinical characteristics of the PD patients. Note: *indicates that the genera abundance is correlated with the clinical characteristics (P<0.05).
Discussion
By comparing the gut microbiota among the healthy controls, the NC-PD group, and the C-PD group, we found that the bacterial diversity level and the composition varied among the three groups. As one of the most common non-motor symptoms, constipation may play a crucial role in the change of gut microbiota in PD patients. The C-PD group was found to harbor higher taxa abundance than the healthy controls and the NC-PD group. Furthermore, the differential genera were associated with the clinical features of PD, indicating that the differential genera might play an important role in the pathogenesis of PD.
The bacterial diversity and composition varied among the three groups. We found that PD patients had more pro-inflammatory genera and fewer potential anti-inflammatory genera. Corrêaoliveira et al. reported that Lachnospira produced SCFAs, which exert anti-inflammatory effects on the immune system through dietary fibers [15], regulate the function of microglia, and repair glial cell damage [12]. A decreased abundance of Lachnospira results in a reduction of the SCFA concentration, leading to an aggravated inflammatory response in the body as well as the impaired function of the microglia, increasing the risk of PD [16]. In this study, we found that the abundance of the anti-inflammatory genus Lachnospira was lower in PD patients compared with the healthy controls, which is consistent with the earlier research. In addition, Stiemsma [17] found that Lachnospira can promote colonic regulatory T cell aggregation and reduce the level of IgE (immunoglobulin E). Since regulatory T cells are shown to be able to slow PD dopaminergic neuronal damage [18], we hypothesized that Lachnospira may also lessen PD dopamine neuronal damage. It has been reported that patients with Crohn’s disease have significantly elevated levels of Actinomyces, and the genus is associated with gastrointestinal function [19]. Therefore, gastrointestinal function may be closely related to the pathogenic process of PD. In addition, PD has been reported to be induced by a latent infection of Actinomycete spores [20]. Hence, the level of Actionomyces may result in a positive change in the pathogenesis of PD. In this research, the relative abundance of Actinomyces was increased in PD patients, which is in agreement with these studies.
In the current study, we found that the C-PD group had fewer anti-inflammatory genera and more pro-inflammatory genera than the NC-PD group. Some studies have found that the genera produce SCFAs to help relieve constipation symptoms [21,22]. Moreover, Shi et al. found that in patients with mixed refractory constipation, the SCFA levels, including acetate, propionate, and butyrate, were negatively correlated with the Wexner score [22], a scale that can reflect the severity of constipation. For example, Megamonas was suggested to produce butyrate and propionate in the human gastrointestinal tract [23], and the abundance of Megamonas was lower in PD patients with constipation compared with the PD patients without constipation, which is consistent with the results in our study. In addition, Shimizu et al. found that Megamonas is negatively associated with the severity of constipation in PD patients complicated with constipation, suggesting that Megamonas may exert anti-inflammatory effects and relieve constipation by producing SCFAs. The abundance of Hungatella was found to be positively related to the severity of constipation in PD patients complicated with constipation in our study. Blachier et al. also found that constipation is related to Hungatella when they treated patients with chronic refractory constipation using fecal microbiota transplantation (FMT) [24]. A significant abundance of Hungatella was found in patients at 1 month after FMT. However, the study did not further investigate the relationship between the change of Hungatella and constipation in patients after FMT, nor did it describe the effect of Hungatella after FMT.
Chronic constipation includes constipation-type irritable bowel syndrome and functional constipation. Currently, there is no consensus that the gut microbiota is involved in chronic constipation, but several studies have found that Bifidobacterium, Clostridium, and Bacteroides are common differential genera in chronic constipation [25,26]. However, no significant difference was observed in Bifidobacterium and Bacteroides between the NC-PD group and the C-PD group in our study. We hypothesized that there were two reasons: First, chronic constipation is mostly a functional change. However, the fecal samples we selected were from the C-PD group, which is different from other studies, and the occurrence and development of the disease may affect the composition of the gut microbiota. Second, the gut microbiota was changed by age [27]. Most previous studies on chronic constipation focused on children and middle-aged patients. The patients in our study were the elderly, so age might also contribute to the inconsistency.
Through a correlation analysis, we found that the abundance of Streptococcus was positively correlated with the Wexner scores, NMSQ scores, and age, but not correlated with UPDRSIII, PDQ39 score, or MMSE. Li et al. found that Streptococcus is increased significantly in PD patients compared to healthy controls, and the abnormal increase could produce neurotoxins and elevate inflammation, resulting in PD [28]. In addition, the increased level of Hungatella in PD was positively correlated with Wexner scores, consistent with the results of Francois Blachier et al. [24].
There are still some limitations in the study. The sample size was small due to the limited sample collection time. The dietary habits of the PD patients were also not analyzed. Another limitation of the current study was that we excluded normal individuals with constipation, an important early warning factor for PD. In the follow-up study, we will expand the sample size to further confirm the significance of the changes in microbiota composition of PD patients, for a better understanding of the pathogenesis of PD.
Our study provides strong evidence that the bacterial diversity level, composition, and the relative abundance varied significantly among the healthy controls, the NC-PD group, and the C-PD group, and the aberrant ecological networks of the microbial communities was designed for the bacterial diversity level and composition of PD patients. In addition, our data suggest an association between differential genera and the clinical features of PD.
Acknowledgements
We greatly thank the volunteers who provided the informed consents and participated in sample collections.
Disclosure of conflict of interest
None.
References
- 1.Zhang ZX, Roman GC, Hong Z, Wu CB, Qu QM, Huang JB, Zhou B, Geng ZP, Wu JX, Wen HB, Zhao H, Zahner GE. Parkinson’s disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365:595–597. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 2.Hansen C, Li JY. Beyond alpha-synuclein transfer: pathology propagation in Parkinson’s disease. Trends Mol Med. 2012;18:248–255. doi: 10.1016/j.molmed.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Hay JC. Alpha-synuclein toxicity in the early secretory pathway: how it drives neurodegeneration in Parkinsons disease. Front Neurosci. 2015;9:433. doi: 10.3389/fnins.2015.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27:709–715. doi: 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 5.Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28:31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]
- 6.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J, Fassbender K, Schwiertz A, Schafer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 8.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 9.Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM, Jagadapillai R, Liu R, Choe K, Shivakumar B, Son F, Jin S, Kerber R, Adame A, Masliah E, Friedland RP. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged fischer 344 rats and caenorhabditis elegans. Sci Rep. 2016;6:34477. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 11.Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sorensen HT. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 12.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiemsma LT, Arrieta MC, Dimitriu PA, Cheng J, Thorson L, Lefebvre DL, Azad MB, Subbarao P, Mandhane P, Becker A, Sears MR, Kollmann TR Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators. Mohn WW, Finlay BB, Turvey SE. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci (Lond) 2016;130:2199–2207. doi: 10.1042/CS20160349. [DOI] [PubMed] [Google Scholar]
- 18.Christiansen JR, Olesen MN, Otzen DE, Romero-Ramos M, Sanchez-Guajardo V. alpha-synuclein vaccination modulates regulatory T cell activation and microglia in the absence of brain pathology. J Neuroinflammation. 2016;13:74. doi: 10.1186/s12974-016-0532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, Inatomi O, Bamba S, Sugimoto M, Andoh A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion. 2016;93:59–65. doi: 10.1159/000441768. [DOI] [PubMed] [Google Scholar]
- 20.Berstad K, Berstad JER. Parkinson’s disease; the hibernating spore hypothesis. Med Hypotheses. 2017;104:48–53. doi: 10.1016/j.mehy.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Chu JR, Kang SY, Kim SE, Lee SJ, Lee YC, Sung MK. Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: a randomized placebo-controlled intervention study. World J Gastroenterol. 2019;25:6129–6144. doi: 10.3748/wjg.v25.i40.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Chen Q, Huang Y, Ni L, Liu J, Jiang J, Li N. Function and clinical implications of short-chain fatty acids in patients with mixed refractory constipation. Colorectal Dis. 2016;18:803–810. doi: 10.1111/codi.13314. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu J, Kubota T, Takada E, Takai K, Fujiwara N, Arimitsu N, Ueda Y, Wakisaka S, Suzuki T, Suzuki N. Relative abundance of megamonas hypermegale and Butyrivibrio species decreased in the intestine and its possible association with the T cell aberration by metabolite alteration in patients with Behcet’s disease (210 characters) Clin Rheumatol. 2019;38:1437–1445. doi: 10.1007/s10067-018-04419-8. [DOI] [PubMed] [Google Scholar]
- 24.Ohara T. Identification of the microbial diversity after fecal microbiota transplantation therapy for chronic intractable constipation using 16s rRNA amplicon sequencing. PLoS One. 2019;14:e0214085. doi: 10.1371/journal.pone.0214085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajilic-Stojanovic M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Chassard C, Dapoigny M, Scott KP, Crouzet L, Del’homme C, Marquet P, Martin JC, Pickering G, Ardid D, Eschalier A, Dubray C, Flint HJ, Bernalier-Donadille A. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 27.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Wu X, Hu X, Wang T, Liang S, Duan Y, Jin F, Qin B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci. 2017;60:1223–1233. doi: 10.1007/s11427-016-9001-4. [DOI] [PubMed] [Google Scholar]