Abstract
Objective: To investigate the role of early enteral nutrition support (ENN) on the improvement of immune function and physical recovery of patients with colon carcinoma. Methods: The patients with colon carcinoma treated in our hospital from November 2018 to November 2019 were obtained and randomly grouped into the control group (CG) and the early enteral nutrition support group (ENN group). The changes of nutritional status and immune function related indexes, the changes of inflammatory reaction indexes, and the physical recovery and complication rate were compared between groups. Results: Before operation, there was no evident difference between the two groups in nutrition index level (serum transferrin, albumin, prealbumin and hemoglobin), immune function index level (IgA, IgG, IgM, CD4+, CD8+, CD4+/CD8+) and inflammatory reaction indices (CRP, PGE, IL-6) (P>0.05). After operation, weight loss, incision cicatrized time, postoperative defecation time, getting up after operation and length of hospital stay of the ENN group were better than those of the CG. The change of nutritional indexes was also evidently better in the ENN group. Immune function was evidently improved compared with the CG. The level of inflammatory reaction factors was also evidently lower in the ENN group, and the incidence of postoperative complications was evidently lower than that of the CG, and the physical recovery was also better than that of the CG (P<0.05). Conclusion: ENN for patients with colon carcinoma can improve their immune function, improve their nutritional level and promote their physical recovery.
Keywords: Colon carcinoma, early enteral nutrition, immune function, nutrition level, recovery
Introduction
Colon carcinoma [1] is a common malignant tumor, and its pathogenesis is not clear, but many research reports suggest that it is related to risk factors such as high pressure in life, lack of exercise, more entertainment, lack of fresh vegetables and fruits, and eating too much fatty food [2]. It is often divided into the categories of lump, infiltration and ulcer in the clinic, and is divided into adenocarcinoma, mucinous carcinoma and undifferentiated carcinoma histologically, among which adenocarcinoma is the most common and undifferentiated carcinoma has the worst prognosis. In the clinic, the symptoms of colon carcinoma are usually distinguished by the left and right colon, and abdominal pain, abdominal mass, intestinal obstruction, and changes in stool habits and stool characteristics are common symptoms [3,4]. The proliferation of malignant tumor cells causes the body’s nutrition to be constantly consumed. In addition, anorexia, pain and other reactions can lead to different degrees of malnutrition and decreased immune function in tumor patients. Postoperative fasting further aggravates the negative nitrogen balance and affects immune function [5]. At present, the main treatment for colon carcinoma is still radical surgery [6]. Intestinal obstruction is not only a symptom of colon carcinoma patients, but is also a complication of colon carcinoma. It usually causes poor stool discharge or inability to defecate, abdominal distension, nausea and vomiting, resulting in poor appetite or inability to eat, which will cause malnutrition and decreased immunity for a long time. In particular, intestinal obstruction caused by colon carcinoma is often complicated with chronic blood loss and anemia, thus aggravating the above symptoms and resulting in slow healing of the postoperative incision, increased incidence of anastomotic leakage and other complications, and it affects various organs of the whole body, increasing surgical risk and postoperative mortality [7,8].
Parenteral nutrition [9] has always been used as the main nutritional method after colon carcinoma surgery. However, according to reports, long-term use of parenteral nutrition, especially during fasting after surgery, will cause intestinal mucosa atrophy and damage the intestinal mucosal barrier. In recent years, it is advocated to use enteral nutrition in the early postoperative period [10]. Early postoperative enteral nutrition for patients with gastrointestinal carcinoma is superior to parenteral nutrition in promoting the recovery of immune suppression caused by surgical stress, especially in cellular immunity [11]. Therefore, the early use of enteral nutrition combined with parenteral nutrition after colon carcinoma surgery is helpful for patients’ recovery [12].
In this study, the above two methods were used after colon carcinoma surgery, and the effects of the two methods on immune function and physical recovery of patients were compared.
Data and methods
Research participants
A total of 97 patients with colon carcinoma from November 2018 to November 2019 were randomly grouped into the control group (CG) and the early enteral nutrition support group (EEN group). There were 40 cases in the CG, including 22 men and 18 women, aged (58.6±5.1) years. There were 57 cases in the ENN group, including 34 men and 23 women, aged (59.1±5.7) years. Inclusion criteria were as follows: patients were diagnosed by colonoscopy before operation and confirmed by pathology after operation; patients did not receive radiotherapy and chemotherapy before operation; no albumin or immunopotentiator was used within 2 weeks before operation; patients or their families signed the informed consent; the study was approved by the Ethics Committee. Exclusion criteria were as follows: patients with severe liver and kidney dysfunction; patients with congenital abnormal amino acid metabolism or those complicated with other metabolic diseases and allergic diseases; patients with diabetes and thyroid dysfunction; patients with pyloric obstruction or digestive tract obstruction. There was no evident difference in the general data between the two groups (P<0.05).
Methods
Patients in both groups were given radical operation for colon carcinoma by doctors in the same operation group. After operation, patients were given nutritional support with equal calories and nitrogen standards. Non-protein calories were 125.5 kJ·kg-1·d-1, and nitrogen was 0.2 g·kg-1·d-1.
In the ENN group, no stomach tube was placed. After 6-24 h after the operation, the patients were given 250 mL of 5% glucose saline orally. If the patients had no adverse digestive tract reactions, they were given enteral nutrition emulsion the next day, and the total amount was controlled at 30 mL·kg-1·d-1. In the initial stage, insufficient liquid was supplemented by intravenous infusion of glucose saline.
The CG received parenteral nutrition support via the central vein, and was given 10% glucose solution (carbohydrate), 11.4% amino acid (nitrogen), 20% fat emulsion, fat-soluble vitamins, water-soluble vitamins, various trace elements, and insulin. The ratio of insulin to sugar was 1:5 to make a 3 L bag of parenteral nutrition solution, 2500 mL/d, and it was slowly infused within 24 h.
Outcome measures
(1) Clinical indicators and gastrointestinal function recovery time: weight loss, incision cicatrized time, recovery time for anal exsufflation, defecation and length of hospital stay were observed.
(2) Cellular immune function of patients: Peripheral blood of patients in each group was collected before operation, 1 day and 7 days after operation. T lymphocyte subsets (CD4+, CD8+, CD4+/CD8+) in the peripheral blood of two groups were measured by flow cytometry, and immunoglobulins (IgA, IgG, IgM) were measured by immunoturbidimetry.
(3) Detection of inflammatory and stress indicators: the levels of C-reactive protein, PGE and IL-6 before and after operation in the two groups were detected.
(4) Nutritional indicators of patients: serum transferrin (TF), serum albumin (ALB), prealbumin (PAB) and hemoglobin (Hb) before and after operation in both groups were observed.
(5) Postoperative complications: The incidence of postoperative complications was observed 7 days after operation.
Statistical analysis
SPSS 21.0 was applied for data processing, and the measurement data were expressed as the mean (average of at least three independent experiments) ± standard deviation. t test was applied for comparison between groups. The counting data were represented as (n, %) % and analyzed by chi-square test. GraphPad Prism 6 was applied for figure illustration. The difference was statistically significant with P<0.05.
Results
General information
There was no significant difference in the patients’ general data such as sex, age, weight, Dukes stage, tumor location, history of alcoholism and smoking between the two groups (P<0.05) (Table 1).
Table 1.
General data
| CG (n=40) | EEN group (n=57) | χ2/t | P | |
|---|---|---|---|---|
| Gender | 0.2082 | 0.6482 | ||
| Male | 22 (55) | 34 (59.6) | ||
| Female | 18 (45) | 23 (40.4) | ||
| Age | 58.6±5.1 | 59.1±5.7 | 0.4438 | 0.6582 |
| Weight (kg) | 53.1±6.2 | 54.3±6.5 | 0.9121 | 0.3640 |
| Dukes staging | 0.9562 | 0.6200 | ||
| A | 1 (2.5) | 2 (3.5) | ||
| B | 33 (82.5) | 50 (87.7) | ||
| C | 6 (15) | 5 (8.8) | ||
| Tumor location | 0.2599 | 0.8781 | ||
| Sigmoid colon carcinoma | 12 (30) | 15 (26.3) | ||
| Left colon carcinoma | 19 (47.5) | 30 (52.6) | ||
| Right colon carcinoma | 9 (22.5) | 12 (21.2) | ||
| History of alcoholism | 0.0177 | 0.8942 | ||
| Yes | 23 (57.5) | 32 (56.1) | ||
| No | 17 (42.5) | 25 (43.9) | ||
| History of smoking | 0.1303 | 0.7182 | ||
| Yes | 26 (65) | 35 (61.4) | ||
| No | 14 (35) | 22 (38.6) |
Clinical indicators of both groups of patients
The weight loss, incision cicatrized time, postoperative defecation time, getting up and length of hospital stay in the ENN group were better than those in the CG (P<0.05) (Table 2).
Table 2.
Comparison of clinical indexes between two groups
| Weight loss (kg) | Incision cicatrized time (d) | Exhaust time after operation (h) | Getting up after operation (h) | Length of hospital stay (d) | |
|---|---|---|---|---|---|
| CG (n=40) | 3.26±0.51 | 9.86±1.02 | 41.83±10.62 | 40.21±11.62 | 11.25±2.03 |
| ENN group (n=57) | 3.51±0.48 | 11.36±1.18 | 54.82±11.03 | 53.64±11.09 | 14.52±3.15 |
| χ2/t | 2.4608 | 6.5100 | 5.7972 | 5.7567 | 5.7733 |
| P | 0.0157 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Nutritional indicators of both groups of patients
Before operation, there was no evident difference in serum transferrin, albumin, prealbumin and hemoglobin levels between the two groups (P<0.05). After operation, the indexes of the EEN group were evidently higher than those of the CG (P<0.05) (Table 3).
Table 3.
Comparison of nutritional indexes between the two groups
| TF (g/L) | ALB (g/L) | PA (mg/L) | Hb (g/L) | ||
|---|---|---|---|---|---|
| Preoperative | CG (n=40) | 1.20±0.33 | 35.12±3.12 | 129.32±9.26 | 105.62±3.03 |
| ENN group (n=57) | 1.21±0.35 | 34.32±3.26 | 128.84±8.95 | 105.23±3.10 | |
| χ2/t | 0.1418 | 1.2108 | 0.2563 | 0.6156 | |
| P | 0.8875 | 0.2290 | 0.7982 | 0.5396 | |
| Postoperative | CG (n=40) | 1.65±0.62 | 33.32±3.03 | 135.63±9.13 | 116.23±2.59 |
| ENN group (n=57) | 1.97±0.58 | 37.42±3.68 | 153.23±10.25 | 125.32±2.36 | |
| χ2/t | 2.5998 | 5.7984 | 8.7019 | 17.9364 | |
| P | 0.0108 | <0.0001 | <0.0001 | <0.0001 | |
Inflammation indicators of both groups of patients
There was no evident difference in CRP, PGE and IL-6 between the two groups (P<0.05). After operation, the indexes of patients in the two groups decreased evidently, and the decrease in the EEN group was more than that in the CG (P<0.05) (Figure 1).
Figure 1.
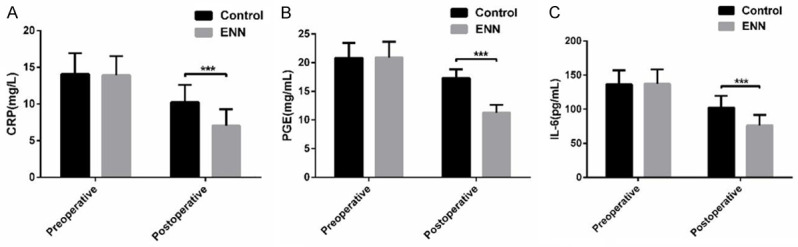
Comparison of inflammation indexes between two groups of patients. A: Comparison of CRP level between two groups; B: Comparison of PGE level between two groups; C: Comparison of IL-6 levels between the two groups. ***indicates compared with the CG, P<0.001.
Immunological indexes of two groups of patients
The immunological indexes of two groups before operation, 1 day after operation and 7 days after operation were compared. The results showed that there was no evident difference between the two groups before operation (P<0.05). One day after operation, the levels of IgA, IgM, IgG, CD4+ and CD4+/CD8+ in the EEN group were evidently better than those in the CG. Seven days after operation, all indexes were evidently better in the ENN group (P<0.05) (Table 4).
Table 4.
Comparison of immunological indexes between two groups of patients
| CG (n=40) | EEN group (n=57) | χ2/t | P | ||
|---|---|---|---|---|---|
| IgA (g/L) | Preoperative | 1.75±0.35 | 1.76±0.31 | 0.1482 | 0.8825 |
| One day after operation | 1.33±0.29 | 1.72±0.30 | 6.3892 | <0.0001 | |
| Seven days after operation | 1.30±0.25 | 1.79±0.33 | 7.9253 | <0.0001 | |
| IgM (g/L) | Preoperative | 1.18±0.26 | 1.17±0.23 | 0.1991 | 0.8421 |
| One day after operation | 1.02±0.16 | 1.13±0.20 | 2.8885 | 0.0047 | |
| Seven days after operation | 1.07±0.19 | 1.19±0.34 | 2.0199 | 0.0462 | |
| IgG (g/L) | Preoperative | 10.43±1.33 | 10.45±1.28 | 0.0745 | 0.9407 |
| One day after operation | 7.80±1.03 | 10.26±1.22 | 10.4088 | <0.0001 | |
| Seven days after operation | 7.93±1.16 | 10.53±1.32 | 24.7633 | <0.0001 | |
| CD4+ (%) | Preoperative | 42.31±6.98 | 42.38±7.06 | 0.0484 | 0.9615 |
| One day after operation | 35.21±6.51 | 40.21±7.02 | 3.5537 | 0.0005 | |
| Seven days after operation | 38.26±6.72 | 42.56±7.13 | 2.9933 | 0.0035 | |
| CD8+ (%) | Preoperative | 22.30±2.35 | 22.25±2.29 | 0.1047 | 0.9168 |
| One day after operation | 23.12±2.68 | 22.42±2.36 | 1.3595 | 0.1772 | |
| Seven days after operation | 20.51±3.03 | 17.69±3.52 | 4.1086 | <0.0001 | |
| CD4+/CD8+ (%) | Preoperative | 1.89±0.18 | 1.90±0.21 | 0.2445 | 0.8073 |
| One day after operation | 1.52±0.23 | 1.79±0.18 | 6.4793 | <0.0001 | |
| Seven days after operation | 1.86±0.31 | 2.40±0.36 | 7.6919 | <0.0001 | |
Incidence of postoperative complications in both groups
Postoperative complications mainly included nausea, vomiting, abdominal pain, abdominal distension, diarrhea and constipation. The total incidence of complications in the EEN group (7%) was lower than that in the CG (22.5%) (P<0.05) (Table 5).
Table 5.
Comparison of postoperative complications between the two groups
| Nausea | Vomiting | Abdominal pain | Abdominal distension | Diarrhea | Constipation | Total incidence rate | |
|---|---|---|---|---|---|---|---|
| CG (n=40) | 2 (5) | 1 (2.5) | 2 (5) | 1 (2.5) | 2 (5) | 1 (2.5) | 9 (22.5) |
| ENN group (n=57) | 0 | 1 (1.75) | 1 (1.75) | 0 | 1 (1.75) | 1 (1.75) | 4 (7.0) |
| χ2/t | 4.8551 | ||||||
| P | 0.0276 |
Discussion
Patients with colon carcinoma often suffer from malnutrition and low immune function due to insufficient nutrition intake before operation and metabolic disorders and energy consumption caused by the tumor itself [13]. After the operation, the metabolic disorder is further aggravated due to operative stress, protein is decomposed and the energy consumption is increased, thus causing the body to be in an immunosuppression state [14]. Perioperative high-quality nursing can reduce trauma stress and improve prognosis. Early enteral nutrition can improve overall nutrition, enhance immunity and promote rehabilitation [15].
By comparing the recovery of colon carcinoma patients with parenteral nutrition support and ENN, the results showed that there was no evident difference in the nutritional index level, immune function index level and inflammatory reaction index between the two groups before operation. After operation, the weight loss, incision cicatrized time, postoperative defecation time, getting up after operation and length of hospital stay of the ENN group were all better than those of the CG. Anal exhaust time and bowel sound recovery time can reflect the intestinal function recovery of patients after colon carcinoma operation. Anal exhaust indicates that the intestinal tract has recovered the functions of peristalsis, secretion and absorption, and the intestinal environment is stable. Enteral nutrition can not only stimulate the intestinal tract to promote peristalsis, but also improve the blood circulation of the intestinal mucosa, eliminate the intestinal mucosa atrophy caused by intestinal obstruction and increase the absorption of nutrients [16,17]. The length of stay indirectly reflects the recovery of patients. The changes of nutritional indexes (serum transferrin, albumin, prealbumin and hemoglobin) were also evidently better in the ENN group (P<0.05). Immune function (IgA, IgG, IgM, CD4+, CD8+, CD4+/CD8+) was evidently improved compared with the CG. Serum Alb and PA are common indicators that directly reflect the nutritional status of the body [18]. These indicators are often decreased in patients with carcinoma after surgery, indicating that patients are in a state of malnutrition. Malnutrition can aggravate immune dysfunction of the body, indicating that the functions of lymphocytes and B cells are inhibited, the functions of B cells are decreased, and the synthesis of immunoglobulin is decreased due to insufficient secretion of autoantibodies, revealing that the content of IgG, IgM and IgA in peripheral blood are decreased [19]. This result is similar to the research result of Xu et al. [20], which also suggests that nutritional support plays an active role in improving immune function and relieving inflammation after operation of colon cancer patients, and accelerates the recovery of gastrointestinal function. T lymphocyte dysfunction is characterized by CD4+, CD4+/CD8+ ratio decreasing and CD8+ increasing [21]. The results showed that CD4+ and CD4+/CD8+ ratio in the EEN group were higher than those in the CG, which indicated that early postoperative enteral nutrition could improve the immune function of colon carcinoma patients, which may be related to enteral nutrition directly providing nutrition for intestinal mucosa. More than half of human lymphoid tissues exist in the gastrointestinal mucosa, and enteral nutrient solution guarantees the energy supply of lymphoid tissues and immune cells, and promotes the recovery of postoperative immune function of patients [22]. The levels of inflammatory reaction factors (CRP, PGE, IL-6) were also evidently lower in the ENN group. C-reactive protein is an index reflecting the degree of inflammatory reaction of the body. Obvious malnutrition can aggravate the inflammation and even cause multiple organ dysfunction, which is the reason for patients undergoing early nutritional intervention after surgery [23,24]. Long-term use of parenteral nutrition can lead to disuse atrophy of gastrointestinal function and abnormal mucosal function in patients, and even enterogenous infection in severe cases, while enteral nutrition can help patients recover gastrointestinal function, enhance the nutritional status of the body and reduce pathogenic bacteria from entering blood through intestinal mucosa [25]. The incidence of postoperative complications in the EEN group was evidently lower than that in the CG, and the physical recovery was also better in the ENN group. The most serious and complicated complication after colon carcinoma operation is anastomotic leakage. Prevention of anastomotic leakage after colon carcinoma operation is the key to treatment success. Anastomotic leakage often occurs in the 3-7 days after operation and has typical symptoms and signs, such as fever, abdominal pain, abdominal distension, abdominal tenderness and rebound pain, and coffee fecal odor drainage from the abdominal drainage tube. Studies have shown that enteral nutrition can reduce metabolic stress and lung infection. Compared with parenteral nutrition, enteral nutrition can not only reduce the mortality of patients, but also reduce the incidence of complications [26]. This study also has some deficiencies. For example, the early enteral nutrition support has been widely used, but the sample size we selected is too small, and the support for the conclusion is not enough. More studies and an increase of sample size will be carried out in subsequent studies.
To sum up, early postoperative enteral nutrition support for patients with colon carcinoma can improve immune function, improve nutrition level, promote the recovery of intestinal function and accelerate the recovery of patients.
Disclosure of conflict of interest
None.
References
- 1.Hatano Y, Fukuda S, Hisamatsu K, Hirata A, Hara A, Tomita H. Multifaceted interpretation of colon cancer stem cells. Int J Mol Sci. 2017;18:1446. doi: 10.3390/ijms18071446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulrich CM, Himbert C, Holowatyj AN, Hursting SD. Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nat Rev Gastroenterol Hepatol. 2018;15:683–698. doi: 10.1038/s41575-018-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16:359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappell MS. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North Am. 2008;37:1–24. v. doi: 10.1016/j.gtc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Bonetto A, Rupert JE, Barreto R, Zimmers TA. The colon-26 carcinoma tumor-bearing mouse as a model for the study of cancer cachexia. J Vis Exp. 2016:54893. doi: 10.3791/54893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim NK, Kim YW, Han YD, Cho MS, Hur H, Min BS, Lee KY. Complete mesocolic excision and central vascular ligation for colon cancer: principle, anatomy, surgical technique, and outcomes. Surg Oncol. 2016;25:252–262. doi: 10.1016/j.suronc.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Chen TM, Huang YT, Wang GC. Outcome of colon cancer initially presenting as colon perforation and obstruction. World J Surg Oncol. 2017;15:164. doi: 10.1186/s12957-017-1228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Deng S, Gu J, Li J, Wu K, Zheng H, Cheng P, Zhang J, Zhao G, Tao K, Wang G, Cai K. Clinical effectiveness of endoscopic stent placement in treatment of acute intestinal obstruction caused by colorectal cancer. Med Sci Monit. 2019;25:5350–5355. doi: 10.12659/MSM.914623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Copeland EM 3rd, Pimiento JM, Dudrick SJ. Total parenteral nutrition and cancer: from the beginning. Surg Clin North Am. 2011;91:727–736. vii. doi: 10.1016/j.suc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Rosania R, Chiapponi C, Malfertheiner P, Venerito M. Nutrition in patients with gastric cancer: an update. Gastrointest Tumors. 2016;2:178–187. doi: 10.1159/000445188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abunnaja S, Cuviello A, Sanchez JA. Enteral and parenteral nutrition in the perioperative period: state of the art. Nutrients. 2013;5:608–623. doi: 10.3390/nu5020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perinel J, Mariette C, Dousset B, Sielezneff I, Gainant A, Mabrut JY, Bin-Dorel S, Bechwaty ME, Delaunay D, Bernard L, Sauvanet A, Pocard M, Buc E, Adham M. Early enteral versus total parenteral nutrition in patients undergoing pancreaticoduodenectomy: a randomized multicenter controlled trial (Nutri-DPC) Ann Surg. 2016;264:731–737. doi: 10.1097/SLA.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 13.Almasaudi AS, McSorley ST, Dolan RD, Edwards CA, McMillan DC. The relation between Malnutrition Universal Screening Tool (MUST), computed tomography-derived body composition, systemic inflammation, and clinical outcomes in patients undergoing surgery for colorectal cancer. Am J Clin Nutr. 2019;110:1327–1334. doi: 10.1093/ajcn/nqz230. [DOI] [PubMed] [Google Scholar]
- 14.Berkovich L, Ghinea R, Greemland I, Majdop S, Shpitz B, Mishaeli M, Avital S. Inhibition of TNFalpha in peritoneal fluids of patients following colorectal resection attenuates the postoperative stress-related increase in colon cancer cell migration: a prospective, in vitro study. Surg Oncol. 2018;27:479–484. doi: 10.1016/j.suronc.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Wei L, Huo X, Ding Y, Zhou X, Liu D. Effects of early postoperative enteral nutrition versus usual care on serum albumin, prealbumin, transferrin, time to first flatus and postoperative hospital stay for patients with colorectal cancer: a systematic review and metaanalysis. Contemp Nurse. 2018;54:561–577. doi: 10.1080/10376178.2018.1513809. [DOI] [PubMed] [Google Scholar]
- 16.Yuan HC, Xiang Q, Zhang N, Qin WJ, Cai W. Acupuncture combined with early enteral nutrition on patients with postoperative laparoscopic common bile duct exploration: a prospective randomized Trial. Chin J Integr Med. 2020;26:769–775. doi: 10.1007/s11655-019-3048-0. [DOI] [PubMed] [Google Scholar]
- 17.Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. Impact of early enteral and parenteral nutrition on prealbumin and high-sensitivity C-reactive protein after gastric surgery. Genet Mol Res. 2015;14:7130–7135. doi: 10.4238/2015.June.29.6. [DOI] [PubMed] [Google Scholar]
- 18.Hong X, Yan J, Xu L, Shen S, Zeng X, Chen L. Relationship between nutritional status and frailty in hospitalized older patients. Clin Interv Aging. 2019;14:105–111. doi: 10.2147/CIA.S189040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Wang C. Effect of omega-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy: a meta-analysis of randomized control trials in China. Medicine (Baltimore) 2018;97:e0472. doi: 10.1097/MD.0000000000010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu R, Ding Z, Zhao P, Tang L, Tang X, Xiao S. The effects of early post-operative soluble dietary fiber enteral nutrition for colon cancer. Nutrients. 2016;8:584. doi: 10.3390/nu8090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y, Yong C, Ren K, Li D, Yuan H. Effects of post-surgical parenteral nutrition on patients with gastric cancer. Cell Physiol Biochem. 2018;49:1320–1328. doi: 10.1159/000493410. [DOI] [PubMed] [Google Scholar]
- 22.Li K, Xu Y, Hu Y, Liu Y, Chen X, Zhou Y. Effect of enteral immunonutrition on immune, inflammatory markers and nutritional status in gastric cancer patients undergoing gastrectomy: a randomized double-blinded controlled trial. J Invest Surg. 2020;33:950–959. doi: 10.1080/08941939.2019.1569736. [DOI] [PubMed] [Google Scholar]
- 23.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow R, Bruera E, Chiu L, Chow S, Chiu N, Lam H, McDonald R, DeAngelis C, Vuong S, Ganesh V, Chow E. Enteral and parenteral nutrition in cancer patients: a systematic review and meta-analysis. Ann Palliat Med. 2016;5:30–41. doi: 10.3978/j.issn.2224-5820.2016.01.01. [DOI] [PubMed] [Google Scholar]
- 26.Peng J, Cai J, Niu ZX, Chen LQ. Early enteral nutrition compared with parenteral nutrition for esophageal cancer patients after esophagectomy: a meta-analysis. Dis Esophagus. 2016;29:333–341. doi: 10.1111/dote.12337. [DOI] [PubMed] [Google Scholar]


