Abstract
Gemcitabine (GEM) is commonly chosen for treating pancreatic cancer. However, its use is limited by toxicity. Earlier in vitro studies with GEM in combination with Bromelain (Brom) and Acetylcysteine (Ac) indicated a substantial reduction in IC50. In this study, immunocytochemistry and Western blot were used to explore the mechanistic effects of Brom and Ac (BromAc®) in vitro. Then, we explored the efficacy and safety of BromAc® only and with GEM in a pancreatic cancer model in vivo. Immunocytochemistry results revealed a reduction in both MUC1 and MUC4 post-treatment. There was a decrease in VEGF, MMP-9, NF-κβ and cleavage of PARP. There was also a decrease in the cell cycle regulators Cyclin B and D as well as TGF-β and the anti-apoptotic Bcl-2. In vivo, the low and high doses of BromAc® alone and with chemotherapy agents were safe. A very significant reduction in pancreatic tumour volume, weight, and ki67 were seen with BromAc® therapy and was equal to treatment with GEM alone and better than treatment with 5-FU. In addition, tumour density was significantly reduced by BromAc®. In conclusion, the anticancer effect of BromAc® is probably related to its mucin depletion activity as well as its effect on proteins involved in cell cycle arrest, apoptosis and modulation of the tumour microenvironment. The in vivo results are encouraging and are considered the first evidence of the efficacy of BromAc® in pancreatic cancer. These results also provide some mechanistic leads of BromAc®.
Keywords: Pancreatic cancer, bromelain, acetylcysteine, BromAc® , gemcitabine, in vitro, in vivo
Introduction
Pancreatic cancer is a lethal malignancy with a very poor prognosis and it is the seventh leading cause of mortality worldwide [1], with prediction to be the second by the year 2030 [2]. In 2018, approximately half a million cases were estimated to be diagnosed with the majority (93%) being fatal [3]. This dismal outcome has been attributed to late diagnosis owing to non-specific symptoms [4]. Pancreatic ductal adenocarcinoma (PDAC) is the usual neoplasm in 80-90% of the patients with a median diagnostic age of approximately 70 years [5].
Current treatment methods involve radiotherapy, thermo-ablation, surgery, chemotherapy and in some cases only palliation [6]. Potential for tumor resectability is determined by the absence or presence of distant metastases and locoregional progression-and 90% of patients are not resectable at diagnosis due to their tumor stage [7]. Over the years a number of chemotherapeutic agents have been used for the treatment of pancreatic cancer such as Gemcitabine (GEM), paclitaxel, 5-FU, Cisplatin, and their combinations have been used with varying success [8,9] although more recently FOLFIRINOX (a combination of 5-FU, leucovorin, irinotecan and oxaliplatin) has been advantageous, owing to noticeable survival increase compared to GEM therapy [10].
Chemo-resistance accounts for the majority of treatment failures and this has been attributed to the heterogeneity of tumor cells in pancreatic cancer and the tumor extracellular matrix [11]. Additionally, many molecular alterations have provided chemotherapy resistance to pancreatic cancer [12]. Resistance Mucins have been identified in several cancers including pancreatic cancers [13]. In PDAC, the expression of several transmembrane mucins and secretory mucins are highly expressed compared to healthy pancreas [14]. Mucins provide the tumor cells with a barrier defense against drug penetration as well as accelerating survival pathways, chemoresistance, metastasis, and accelerated replication [15]. Hence, if mucinous barriers can be degraded then there will be an increase in drug penetration resulting in a higher exposure to chemo-agents with better tumor ablation [16,17] in addition to abrogating other mucin-enhanced tumor survival pathways. This may lead to a better survival. Pancreatic tumors have very dense tumor matrix that are due to presence of collagen and other proteins including hyaluronic acid leading to vascular damage, decreased tumor perfusion and high Intra Tumoral Fluid Pressure (ITFP) which impairs drug delivery [18,19]. The collagen present in the intercellular matrix of tumors has both glycosidic and disulfide linkages that are susceptible to the action of certain agents such as Brom and Ac [20]. Hence, if these barriers to free drug flow can be removed, a better penetration of chemotherapeutic agents into the tumor might be accomplished resulting in a more positive treatment outcome.
Bromelain (Brom), an extract from pineapple (Ananas Comosus) fruit or stem, contains a number of enzymes such as proteases, carbohydrases, hydroxylases, phosphatases, etc. [21] and they have the propensity to hydrolyze the -O- and -N- glycosidic linkages in glycoproteins that are abundant in mucin [22]. Mucins are polymers of glycoproteins with interlinking disulfide linkages [23]. In addition, Brom has also shown anti-cancer properties in several studies and currently it is undergoing clinical evaluation for the treatment of mucinous tumors in a rare cancer known as pseudomyxoma peritonei [24]. Brom is a successful mucolytic in combination with Acetylcysteine (Ac), an antioxidant that can reduce the disulfide bonds found within a mucinous mass [25]. Ac also has anti-cancer properties in several cancers [26]. Further, Brom with its proteolytic activities has the ability to disintegrate dense tumor matrix that are made up of collagen, whilst at the same time, it can also disintegrate hyaluronic acid and hence allow better passage of drugs into the tumors [27].
Remarkably, our previous in vitro studies have demonstrated that a suitable combination of Brom with Ac (BromAc®) has chemotherapeutic efficacy equivalent to GEM in pancreatic and hepatic carcinoma cells. Noticeably, their synergistic combination with GEM enabled a dramatic reduction of the required dosage of GEM [28]. BromAc® addition to GEM was able to potentiate its efficacy in reduction of colon cancer in vivo [29]. If the effective dosage of chemotherapeutic agents can be reduced, then, chemotherapy may be given at shorter intervals without increased toxicity. Seven days rest enable resistant or residual tumor cells to replicate and regain potency in the current treatment regime [30]. Hence, from the encouraging results of our earlier studies [28], we investigated the mechanism of BromAc® actions in vitro, and we proceeded to carry out an in vivo evaluation of these agents with safety and efficacy studies in a nude mouse model of pancreatic cancer.
Materials and method
Cell lines
Human PC cell lines, AsPC-1 and CFPAC-1 (ATCC, Manassas, VA, USA) were maintained as a monolayer in RPMI 1640 medium (Sigma, MO, USA) supplemented with 10% foetal bovine serum (FBS; Wisent, Canada) and 1% antibiotics (100 U/mL penicillin, 100 mg/mL streptomycin; Gibco). Cell lines were incubated in T-75 flasks with 5% CO2 at 37.0°C. Cell lines were routinely passaged at 70% confluence by washing with phosphate buffered saline (PBS), trypsinized for 5.0 min then centrifuged for 5.0 min at 22°C and 1400 rpm after harvesting. Cell count and viability were determined by adding 0.06% trypan blue with an automated cell counter (Thermo Fisher Scientific, California, USA).
Drug preparation
For in vitro studies, bromelain and acetylcysteine were obtained from Sigma-Aldrich (St. Louis, MO, USA). 10 mg/mL solution of bromelain was prepared in TRIS buffer of pH 7.0, adjusted through adding 0.1 M sodium hydroxide and 0.1 M hydrochloric acid. Stock solutions were then filtered through a sterile cap before use. 100 mM acetylcysteine was prepared in TRIS buffer at pH 7.0, adjusted through adding 0.1 M sodium hydroxide and 0.1 M hydrochloric acid. Both were stored at -4.0°C for future use. Bromelain and acetylcysteine were dissolved in RPMI 1640 medium supplemented with 10% foetal bovine serum (FBS) to concentrations of 10,000 µg/mL and 100 mM respectively. Necessary concentrations of bromelain and acetylcysteine were created by diluting with RPMI 1640 media supplemented with 10% FBS.
For in vivo studies, bromelain API was manufactured by Mucpharm Pty Ltd (Australia) as a sterile powder. Bromelain was irradiated to ensure sterility. Acetylcysteine was purchased from Link Pharma, Australia (# AUST-R 170803). Gemcitabine hydrochloride was purchased from Sapphire, Australia (Cat # 000-14954, Vend Cat # 1759-25). 5-FU was purchased from Sigma-Aldrich (Cat # F-6627). For treatment, the stock solutions were freshly made at pH 7.0, adjusted through adding 0.1 M sodium hydroxide and 0.1 M hydrochloric acid. Stock solutions were diluted with 0.9% NaCl according to the final concentrations required.
Immunocytochemistry
Cells were seeded onto sterile glass coverslips and maintained at 37.0°C in an incubator for 24.0 hrs. The cells were then treated with Brom, AC, and a combination for 48.0 hrs. Then the cells were fixed in 4% paraformaldehyde then kept in 1% bovine serum albumin for 1.0 hr. AsPC-1 and CFPAC were incubated at 4.0°C for 12.0 hrs with mouse anti-MUC1 and anti-MUC4 antibodies respectively (Abcam, Cambridge, MA, USA). After washing with PBS, the cells were incubated with the goat anti-mouse IgG secondary antibody (Abcam, Cambridge, MA, USA) for 1.0 hr under dark conditions. After the completion of this step the cells were then counter stained with propidium iodide and visualised with the Olympus IX71 laser scanning confocal microscope (Olympus, Centre Valley, PA, USA) and ×40 oil immersion lens. The Zen program (Carl Zeiss, Cambridge, UK) was used to overlay images.
Western blotting
The effect of Brom and Ac on protein expression was determined through Western blot analysis after 48.0 hrs of treatment. The homogenized cells were lysed with RIPA buffer containing phosphatase and protease inhibitor. Lysates were cleared by centrifuging for 10 min at 4.0°C. Protein concentrations were quantified with the BioRad protein assay (Bio-Rad, Hercules, CA, USA) and resolved through sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to a polyvinyl fluoride membrane (Millipore, Billerica, MA, USA). Subsequently, the membranes were incubated overnight with primary antibodies (Cell Signaling, QLD, Australia) at 4.0°C and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signalling Technology, Danvers, MA, USA) at room temperature for 1.0 hr. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control using the mouse monoclonal anti-GAPDH antibody (Sigma-Aldrich, MO, USA). The membrane was then visualised with ImageQuant LAS4000 Biomolecular imager and ImageQuant software (GE Healthcare, Little Chalfont, Buckinhamshire, UK). Data presented is the density of the protein bands normalised to GADPH. Densitometric measurements were calculated with Image J software.
Safety and efficacy study of BromAc® in combination with cytotoxics in a nude mice model of pancreatic cancer
The animal study was approved by UNSW Animal Care and Ethics Committee (ACEC), Sydney, Australia (approval number: 19/49B) (Supplementary File). Seventy-two 8-week old female Balb/C nude mice (Animal Resources Center, WA, Australia) were used to examine the efficacy of the combination therapy. After 7 days of acclimatization, 2×106 log-phase growing AsPC-1 cells in Matrigel (Cat # E1270, Sigma-Aldrich, Australia) were injected subcutaneously (Day-10). Intraperitoneal treatment was commenced ten days post inoculation to allow the establishment of the disease. Animals were regularly monitored through the study using a standardized method based on the following parameters: body weight, parameters of general wellbeing and indicators of pain and distress classified into four categories (general appearance, natural behavior, provoked behavior and body condition), and tumor volume. Upon completion of the treatment, animals were euthanized, gross appearance of the tumor was examined, and tumors were excised and weighed. The study has been divided into two separate stages: stage 1 (where low doses of GEM and 5-FU have been tested with BromAc®) and stage 2 (where high dose of GEM have been tested with BromAc®) as follows.
Safety and efficacy study of BromAc® in combination with either gemcitabine or 5-FU in a nude mice model of pancreatic cancer-first stage (low doses)
Thirsty-six mice (n=6/group) were used in stage 1. Treatments were administered via intraperitoneal injections for 24 days (D1 to D24): BromAc® 3 times per week, GEM (2 mg/Kg) and 5-FU (15 mg/Kg) once a week (Table 1). Animals were euthanized on Day 24.
Table 1.
Efficacy and safety studies of low and high dose treatment regimens
| Stage | Group | Therapy Type | Treatment |
|---|---|---|---|
| Stage 1 (Low doses) | T1 | Sham | 0.9% sterile saline solution |
| T2 | Combination therapy | Brom (3 mg/kg) + Ac (300 mg/kg) | |
| T3 | Single-agent therapy | GEM (2 mg/kg) | |
| T4 | Combination therapy | GEM (2 mg/kg) | |
| Brom (3 mg/kg) + Ac (300 mg/kg) | |||
| T5 | Single-agent therapy | 5-FU (15 mg/kg) | |
| T6 | Combination therapy | 5-FU (15 mg/kg) | |
| Brom (3 mg/kg) + Ac (300 mg/kg) | |||
| Stage 2 (High doses) | T1 | Sham | 0.9% sterile saline solution |
| T2 | Single-agent therapy | GEM (5 mg/kg) | |
| T3 | Combination therapy | GEM (5 mg/kg) | |
| Brom (3 mg/kg) + Ac (300 mg/kg) | |||
| T4 | Combination therapy | Brom (6 mg/kg) + Ac (500 mg/kg) | |
| T5 | Combination therapy | GEM (2 mg/kg) | |
| Brom (6 mg/kg) + Ac (500 mg/kg) | |||
| T6 | Combination therapy | GEM (5 mg/kg) | |
| Brom (6 mg/kg) + Ac (500 mg/kg) |
The table shows the type of adjuvant and cytotoxic therapies (GEM or 5-FU) in each treatment group. Treatment was delivered by intraperitoneal route.
Safety and efficacy study of BromAc® in combination with gemcitabine in a nude mice model of pancreatic cancer-second stage (high doses)
Thirty-six mice (n=6/group) were used in stage 2. On day 1, treatment regimen was commenced and continued for another 12 days (Table 1). BromAc® was administered every other day (3 times per week; total of 5 doses). GEM (2 or 5 mg/Kg) was administered once/week (total of 2 doses). Animals were euthanized on Day 12.
Histology and immunohistochemistry
Formalin-fixed, paraffin-embedded sections of tumor as well as various organs were stained using H&E standard techniques. For immunohistochemistry, BOND-III Automated IHC Stainer, Leica was used. Sections were blocked for non-specific binding, followed by incubation with anti-human Ki67 (Cell Marque; Rabbit Monoclonal Anti-Human; Clone SP6; Cat # 275R-16; Dilution 1/200), incubated with biotinylated anti-rabbit immunoglobulins, treated with streptavidin peroxidase and counter-stained with hematoxylin. The images were captured using a binocular light microscope with a digital camera.
Statistical analysis
Data were analyzed using GraphPad Prism version 9.0 (GraphPad Software, Inc.). All data were reported as the mean ± SD. Qualitative variables were compared using Student’s t-test. Differences were considered statistically significant when P<0.05.
Results
BromAC® reduced expression of MUC1 and MUC4
Following immunocytochemical analysis, there was strong expression of MUC1 in untreated control AsPC-1 cells with cytoplasmic localisation of the protein (Figure 1). However, the expression MUC1 decreased with treatment of Brom and a combination of both Brom and AC. Untreated CFPAC cells had a high expression of MUC4 but was reduced with both bromelain and AC treatment (Figure 2). For both mucins, the combined use of Brom and Ac resulted in the most marked reduction in the expression of MUC1 and MUC4.
Figure 1.
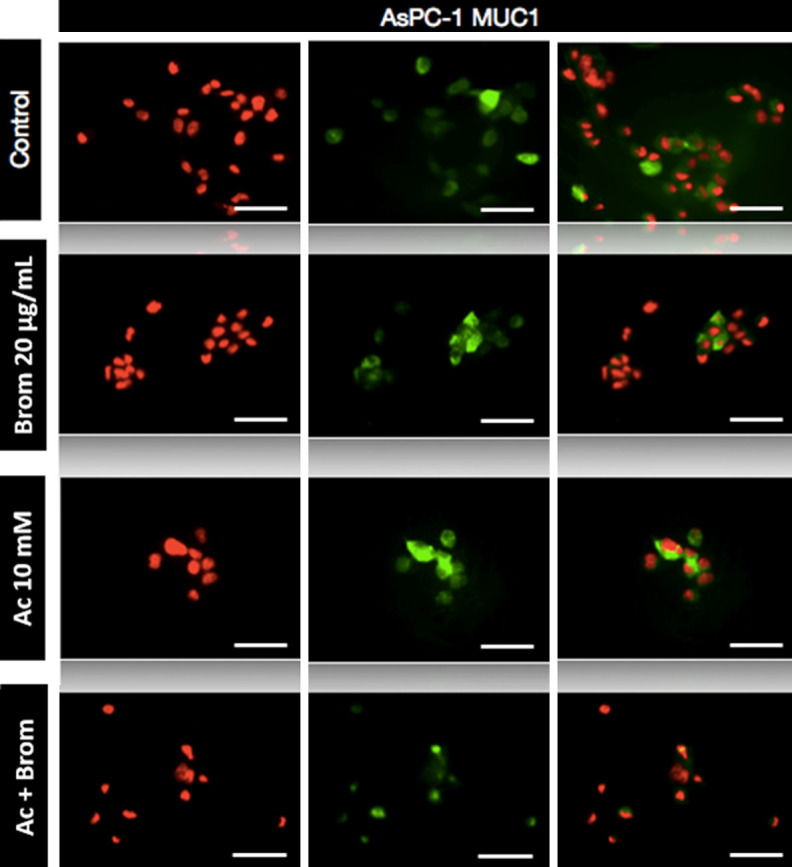
Immunofluorescence staining of AsPC-1 cells. Cells were stained for MUC1 expression after treatment with bromelain and acetylcysteine alone or combination. Immunocytochemistry revealed a reduction of MUC1 post-BromAC® treatment. Fluorescence was viewed using a laser scanning confocal microscope with red corresponding to the nucleus and green to MUC1. The last column shows two-colour merged images. Scale bar: 50 μm. Final magnification, ×600.
Figure 2.
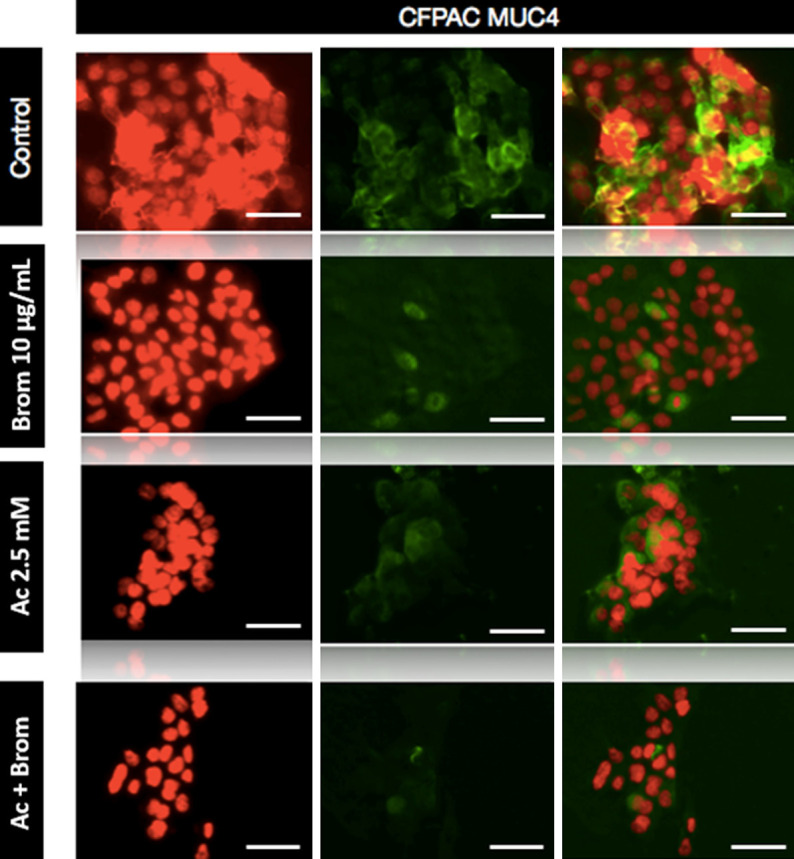
Immunofluorescence staining of CFPAC cells. Cells were stained for MUC4 expression after treatment with bromelain and acetylcysteine alone or combination. Immunocytochemistry revealed a reduction of MUC4 post-BromAC® treatment. Fluorescence was viewed using a laser scanning confocal microscope with red corresponding to the nucleus and green to MUC4. The last column shows two-colour merged images. Scale bar: 50 μm. Final magnification, ×600.
The effect of BromAc® on expression of proteins involved in apoptosis, cell growth and modulation of the tumour microenvironment
Western blot analysis was performed to determine the mechanism of the growth inhibitory effects of BromAc®. AsPC-1 cells were treated with Brom 20 µg/mL and AC 10 mM for 48.0 hrs, where the untreated cells were used as the negative control. As seen in Figure 3, the combination treatment with Brom and AC reduced the expression of vascular endothelial growth factor (VEGF), metalloproteinase-9 (MMP-9), nuclear factor κβ (NF-κβ) and activation of the precursor poly ADP-ribose polymerase (PARP) system. There was also decrease in the cell cycle regulators cyclin B and D, TGFβ and Bcl-2 particularly marked in combination treatment.
Figure 3.
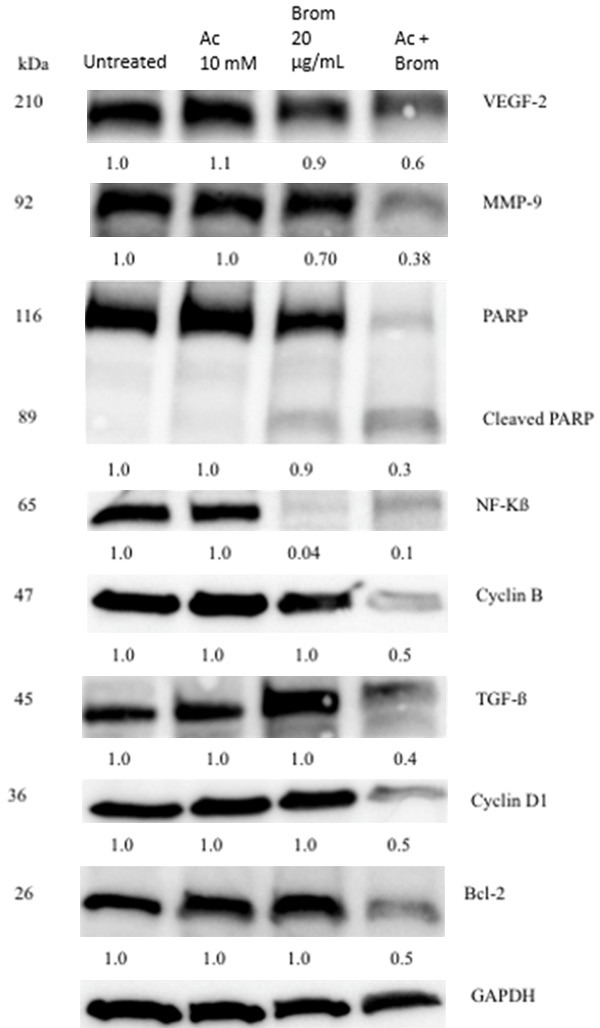
Western blot analysis of proteins involved in oncogenesis in AsPC-1 cells. There was a decrease in VEGF, MMP-9, NF-κβ and cleavage of PARP. There was also a decrease in the cell cycle regulators cyclin B and D as well as TGF-β and the anti-apoptotic Bcl-2. Data shown represents the relative density of protein bands normalised to GAPDH. One-way ANOVA was used to compare the protein expression between the different treatment groups.
In vivo safety and efficacy study-first stage (low doses)
All animals in the treatment groups survived until 24 days (euthanasia). Their weights increased gradually showing steady growth. No treatment-related toxicities were noted (Figure 4A).
Figure 4.
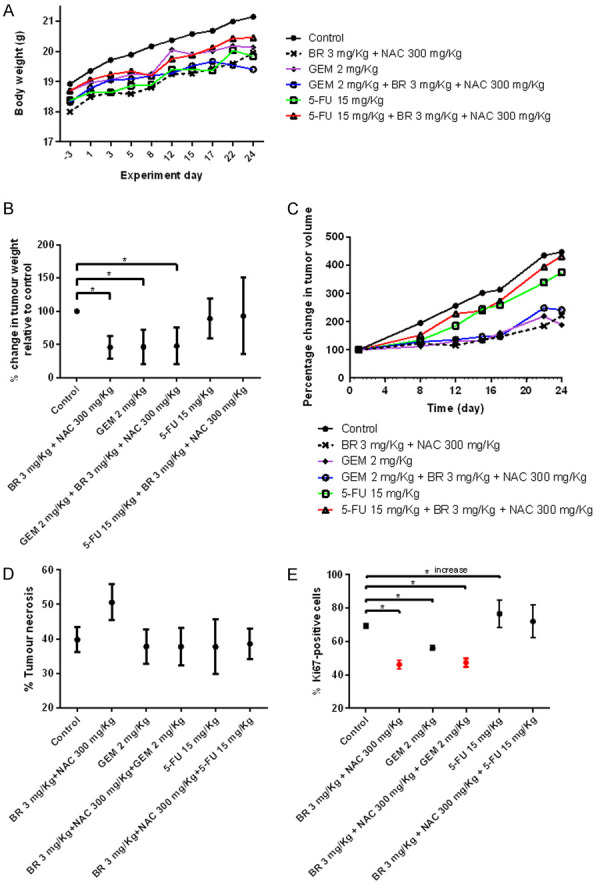
Results of the safety and efficacy in vivo study of BromAc® in combination with cytotoxic therapies in AsPC-1 model of pancreatic cancer-First stage (low doses). A. Graph shows mean body weight fluctuations in subcutaneous AsPC1-tumour bearing nude mice treated with combination therapies. B. It shows percentage change in tumor weight in the treated groups compared to control. C. It shows percentage change in tumor volume. D. Graph showing percentage of tumor necrosis. Necrosis is highest in Brom 3 mg/kg + Ac 300 mg/kg group. E. Graph showing percentage of Ki-67 positive cells. Analysis of immuno-histological images of tumors samples stained using anti-Ki67 antibody. The lowest expression of Ki67 is observed in groups treated with Brom 3 mg/kg + 300 mg/kg alone or with GEM 2 mg/kg that is indicative of reduced cellular replication. Data presented as mean ± SD. Significance level: P<0.05.
5-FU alone or with BromAc® didn’t inhibit tumor growth based on tumor weight at necropsy (Figure 4B; Table 2) whereas BromAc®, gemcitabine, or the combination produced a slightly greater than 50% inhibition, indicating a substantial and similar effect on tumor growth.
Table 2.
Percentage reduction of tumor weight at autopsy in the low dose treatment groups
| Treatment group | Percentage reduction of tumor weight | P-value |
|---|---|---|
| Control | ||
| Brom (3.0 mg/kg) + Ac (300 mg/kg) | 54.10±16.72 | 0.0005 |
| GEM (2.0 mg/kg) | 53.55±25.35 | 0.0035 |
| GEM (2.0 mg/kg) + Brom (3.0 mg/kg) + Ac (300 mg/kg) | 51.91±27.33 | 0.0056 |
| 5-FU (15 mg/kg) | 10.93±30.15 | 0.4153 |
| 5-FU (15 mg/kg) + Brom (3.0 mg/kg) + Ac (300 mg/kg) | 7.10±57.53 | 0.7745 |
Percentage reduction of tumor weight = [tumor weight (control) - tumor weight (treatment)/tumor weight (control)] ×100. Data are presented as mean ± SD; P-values were obtained through t-test; significance level: P<0.05.
Tumor volume measurements over 24 days indicated that three treatment groups, Brom (3.0 mg/kg) + Ac (300 mg/kg), GEM (2.0 mg/kg) and GEM (2 mg/kg) + Brom (3.0 mg/kg) + Ac (300 mg/kg) showed hardly any difference from the base line (day 1) to day 17, after which the volume increased slightly for the three groups followed by a drop at euthanasia for GEM and BromAc® + GEM. Further, the GEM (2 mg/kg) showed a slightly lower tumor volume (Figure 4C).
When the percentage of tumor necrosis was assessed, it indicated that all the treatment groups except that with Brom 3.0 mg/kg + Ac 300 mg/kg had almost equivalent necrosis (<40%) whilst 50% necrosis was observed in this exceptional group indicating that the combination of Brom and Ac in a weight ratio of 0.01: 1.0 accelerated necrosis (Figure 4D). The mean necrosis was numerically higher in the low BromAc® group although results did not reach statistical significance (P=0.18).
The Ki67 expression in the treatment groups indicated that although three different groups such as Brom 3.0 mg/kg + Ac 300 mg/kg, GEM 2.0 mg/kg and Brom 3.0 mg/kg + Ac 300 mg/kg + GEM 2.0 mg/kg had substantial drop compared to controls, much lower values were observed in the first and the last groups, indicating that the presence of Brom and Ac are crucial for controlling the Ki67 (46%, 56%, and 47% respectively compared to control of 69%; P<0.0001) (Figure 4E). However, there was an increase in Ki67 expression in groups treated with either 5-Fu 15.0 mg/kg alone (76%; P=0.0015) or Brom 3.0 mg/kg + Ac 300 mg/kg + 5-FU 15 mg/kg (72%; P=0.2579).
The initial low dose treatment indicated that Brom 3.0 mg/kg + Ac 300 mg/kg, GEM 2.0 mg/kg and Brom 3.0 mg/kg + Ac 300 mg/kg + GEM 2.0 mg/kg had almost equivalent efficacy indicating that any of the dosage forms may be used to derive the present efficacy. This further indicates that at a combination ratio of Brom 3.0 mg/kg + Ac 300 mg/kg, (1:100), no GEM is required for treatment but if GEM is to be added, the dosage can be reduced to an absolute minimum to derive similar maximum therapeutic effect. Hence, this low dose GEM in the presence of Brom and Ac may serve as an effective treatment for pancreatic cancer since more frequent treatment may be instituted (shorter rest interval).
In vivo safety and efficacy study-second stage (high doses)
Body weight measurement indicated again that all the groups exhibited no negative effect on growth and wellbeing (Figure 5A).
Figure 5.
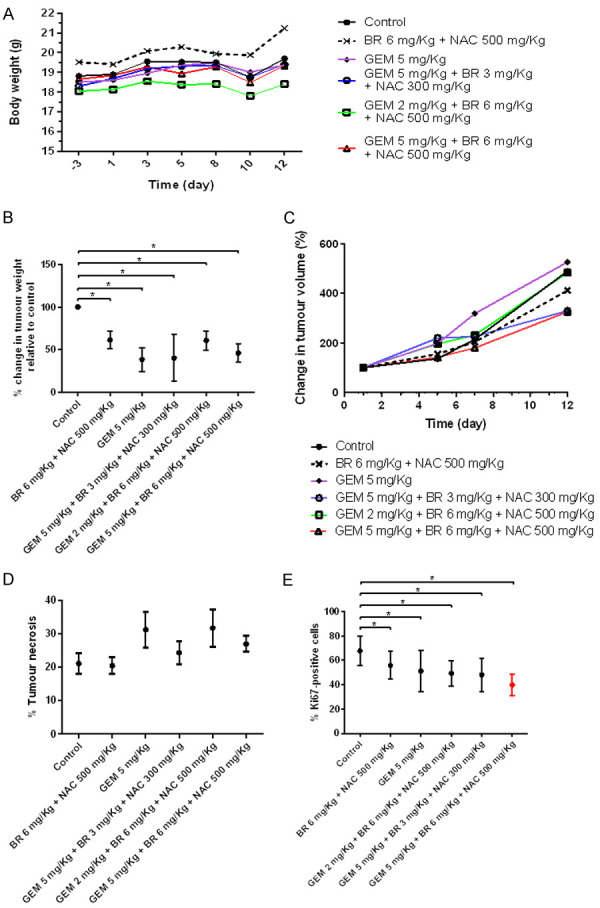
Results of the safety and efficacy in vivo study of BromAc® in combination with GEM in AsPC-1 model of pancreatic cancer-Second stage (high doses). A. Graph shows mean body weight fluctuations in subcutaneous AsPC-1 -tumour bearing nude mice treated with combination therapies. B. It shows percentage change in tumor weight in the treated groups compared to control. C. It shows percentage change in tumor volume. D. Graph showing percentage of tumor necrosis. Necrosis is highest in two groups: GEM 5 mg/kg group and Brom 6 mg/kg + Ac 500 mg/kg + GEM 2 mg/kg group. E. Graph showing percentage of Ki-67 positive cells. Analysis of immuno-histological images of tumors samples stained using anti-Ki67 antibody. The lowest expression of Ki67 is observed in groups treated with Brom 6 mg/kg + 500 mg/kg + GEM 5 mg/kg that is indicative of reduced cellular replication. Data presented as mean ± SD. Significance level: P<0.05.
When percentage change in tumor weight was assessed at euthanasia, GEM 5.0 mg/kg and GEM 5.0 mg/kg + Brom 3.0 mg/kg + Ac 300 mg/kg showed equivalent and the lowest weight (Figure 5B). This was followed by GEM 5.0 mg/kg + Brom 6.0 mg/kg + Ac 500 mg/kg. Table 3 shows that GEM at 5.0 mg/kg, and GEM 5 mg/kg + Brom 3.0 mg/kg + Ac 300 mg/kg have an efficacy of 59.57-61.39% in tumor reduction. A difference of 8-10% is observed between the low and the high dose of GEM. The high GEM dose is 2.5 times greater than the low dose.
Table 3.
Percentage reduction of tumor weight at autopsy in the high dose treatment groups
| Treatment group | Percentage reduction of tumor weight | P-value |
|---|---|---|
| Control | ||
| Brom (6.0 mg/kg) + Ac (500 mg/kg) | 38.53±10.21 | 0.0048 |
| GEM (5.0 mg/kg) | 61.39±13.69 | 0.0006 |
| GEM (5.0 mg/kg) + Brom (3.0 mg/kg) + Ac (300 mg/kg) | 59.57±27.43 | 0.0225 |
| GEM (2.0 mg/kg) + Brom (6.0 mg/kg) + Ac (500 mg/kg) | 38.94±11.11 | 0.0060 |
| GEM (5.0 mg/kg) + Brom (6.0 mg/kg) + Ac (500 mg/kg) | 53.80±10.52 | 0.0020 |
Percentage reduction of tumor weight = [tumor weight (control) - tumor weight (treatment)/tumor weight (control)] ×100. Data are presented as mean ± SD; P-values were obtained through t-test; significance level: P<0.05.
Tumor volume measurement showed that at day 7, GEM 5.0 mg/kg + Brom 6.0 mg/kg + Ac 500 mg/kg and Brom 6.0 mg/kg + Ac 500 mg/kg groups showed a lesser tumor volume compared to the rest. However, at day 12, one of the previously mentioned groups (GEM 5.0 mg/kg + Brom 6.0 mg/kg + Ac 500 mg/kg) in addition to another group (GEM 5.0 mg/kg + Brom 3.0 mg/kg + Ac 300 mg/kg) showed almost equivalent and the lowest tumor volume of the treatments examined (Figure 5C).
Comparison of tumor necrosis to control indicated that Brom 5 mg/kg + Ac 500 mg/kg shared similar outcome, however the other treatment groups showed a higher level of necrosis with groups such as GEM 5.0 mg/kg and GEM 2.0 mg/kg + Brom 6.0 mg/kg + Ac 500 mg/kg showing the highest level of necrosis (P=0.1209) (Figure 5D).
Comparing the Ki67 expression to control indicated that all treatment groups had a significantly lower level of expression (All P<0.0001 except with Brom 6 mg/Kg + Ac 500 mg/Kg, P=0.0014), with GEM 5.0 mg/kg + Brom 6.0 mg/kg + Ac 500 mg/kg expressing the lowest value (Figure 5E).
When the percentage reductions in tumor weights of both the low dose and high dose treatment groups were compared, only two groups (GEM 5.0 mg/kg + Bromelain 3.0 mg/kg + Ac 300 mg/kg & GEM 5.0 mg/kg) showed the highest percentage reduction in tumor weight indicating maximum control over tumor growth.
Tumor density
Tumor density data are shown in Tables 4, 5. It can be seen that the low dose BromAc® group (Brom 3 mg/kg, Ac 300 mg/kg) produced a 10% reduction in density, 5-FU produced an 8% increase, and in the high dose experiment BromAc® produced a 32% decrease in density compared with 34% for GEM alone.
Table 4.
Density of the tumors from the low dose treatment groups determined at autopsy
| Treatment group | Density (g/cm3) | % Change compared to control |
|---|---|---|
| Control | 0.60 | |
| Brom (3 mg/kg) + Ac (300 mg/kg) | 0.54 | 10% decrease |
| GEM (2 mg/kg) | 0.59 | 2.0% decrease |
| GEM (2 mg/kg) + Brom (3 mg/kg) + Ac (300 mg/kg) | 0.63 | 5.0% increase |
| 5-FU 15 mg/kg | 0.65 | 8.0% increase |
| 5-FU (15 mg/kg) + Brom (3 mg/kg) + Ac (300 mg/kg) | 0.60 | No increase |
Density = tumor mass/tumor volume (g/cm3). Percentage change of tumor density = [tumor density (control) - tumor density (treatment)/tumor density (control)] ×100.
Table 5.
Density of the tumors from the high dose treatment groups determined at autopsy
| Treatment group | Density (g/cm3) | % Increase or decrease compared to control |
|---|---|---|
| Control | 0.79 | |
| Brom (6 mg/kg) + Ac (500 mg/kg) | 0.54 | 32% decrease |
| GEM (5 mg/kg) | 0.52 | 34% decrease |
| GEM (5 mg/kg) + Brom (3 mg/kg) + Ac (300 mg/kg) | 0.58 | 27% decrease |
| GEM (2 mg/kg) + Brom (6 mg/kg) + Ac (500 mg/kg) | 0.60 | 24% decrease |
| GEM (5 mg/kg) + Brom (6 mg/kg) + Ac (500 mg/kg) | 0.60 | 24% decrease |
Density = tumor mass/tumor volume (g/cm3). Percentage change of tumor density = [tumor density (control) - tumor density (treatment)/tumor density (control)] ×100.
Organ pathology
No histological evidence of abnormality was seen in liver, spleen, kidney, pancreas, and intestine when control and treated groups were compared (Figure 6), indicating complete safety of the different treatment regimens used over the study period.
Figure 6.
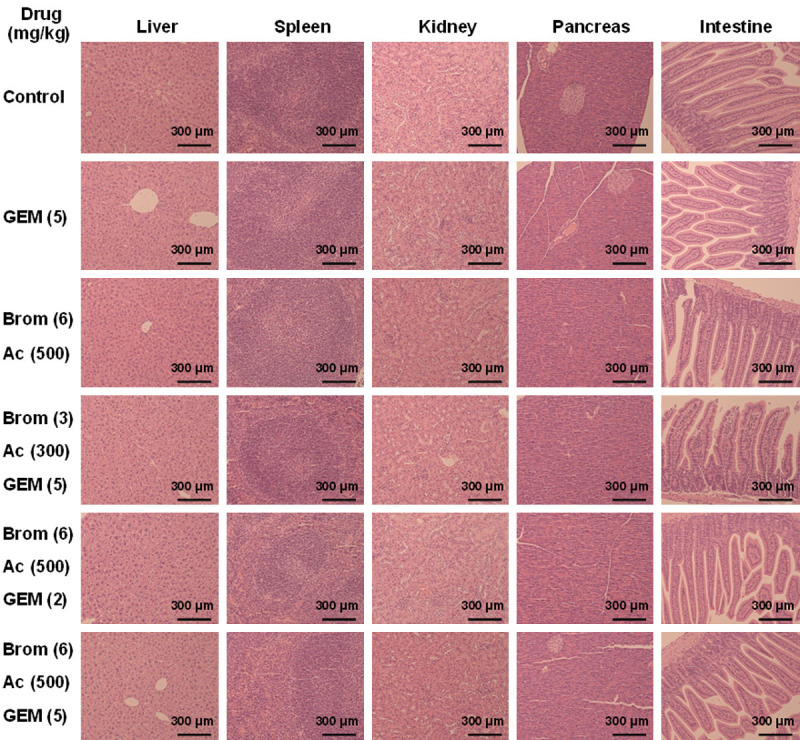
Histology of the various organs that may be affected by treatment with GEM, Bromelain and Acetylcysteine and their combinations at various concentrations. Tissues were hematoxylin and eosin stained. Scale bar: 300 μm. Final magnification, ×100.
Discussion
Immunocytochemistry was performed to determine whether the growth inhibitory effects of BromAC could be attributed to its mucin depleting effects. Mucins are high molecular weight proteins expressed by the respiratory, reproductive, and gastrointestinal epithelium. However, they are aberrantly expressed in pancreatic adenocarcinoma and are implicated in mediating initiation, development, invasion, and resistance of tumour cells [13,31]. We demonstrated the mucin depleting effects of Brom and Ac combination, BromAc®, in gastric cancer and malignant peritoneal mesothelioma [32,33], attributed to the effect of BromAc® on disulphide and glycosidic bonds in mucins [34]. BromAc® has been used recently for treatment of the mucinous pseudomyxoma peritonei (PMP) cancer in clinical trial [24]. Here, our results showed that the effect of Brom in attenuating the expression of MUC1 in AsPC-1 cells, however was the most potent in combination with AC. MUC1 is a membrane bound mucin that promotes the loss of epithelial cell polarity, increases cell proliferation, and upregulates anti-apoptotic pathways [35,36]. Both Brom and AC alone were able to decrease expression of MUC4 in CFPAC cells and were most marked in combination treatment. MUC4 is similarly a transmembrane mucin that is associated with cell proliferation, metastasis, and angiogenesis [37-39].
Through Western blotting, we found that Brom and Ac, particularly in combination, could modulate the tumour microenvironment and induce apoptotic cell death. Treatment resulted in a decreased expression of VEGF, a key mediator of angiogenesis and MMP-9, a matrix metalloproteinase important in metastasis [21]. There was a significant decrease in PARP expression after BromAc® treatment. PARP stimulates cancer progression by boosting cell proliferation and is involved in DNA repair and maintenance of telomerase [40]. Hence, downregulation of PARP expression is an indication of the cancer inhibitory effects of BromAc®. There was also upregulation of cleaved PARP, a marker of apoptosis, which is consistent with results of a previous study [33]. There was also an attenuated expression of NF-κβ with treatment, which is a transcription factor that can be associated with invasion, angiogenesis and chemoresistance in PC [41]. This is consistent with a previous study which observed that BromAc® inactivated NF-κβ in mouse skin papilloma and PC cells, respectively [42]. Combination treatment also reduced the expression of TGF-β and Bcl-2. In addition, Bcl-2 inhibits mitochondrial cytochrome c release and therefore promotes survival [23] whilst aberrant expression of TGF-β is thought to contribute to modulation of the tumour microenvironment to promote metastasis [24]. We also found that BromAc® inhibited cell cycle progression. The cell cycle is controlled by proteins called cyclins, which bind with cyclin dependent kinases (CDKs). Cyclins are important proteins that promote cell cycle progression through activating CDKs. Cyclin B1 binds to CDK1 and forms the mitosis promoting factor (MPF), to stimulates G2/M progression [43]. Similarly, cyclin D1 also simulates cell cycle progression, through phosphorylation of retinoblastoma protein, which releases E2F transcription factors to promote G1/S progression [44]. Reduction of cyclins B and D suggests that BromAc® inhibited cell cycle progression in G1. Finally, inhibition of pro-survival pathways is thought to contribute to cell death, as seen through inhibition of the anti-apoptotic protein Bcl-2.
Perhaps the most important finding is that intraperitoneal administration of BromAc® can profoundly reduce growth of a xenograft of mucinous pancreatic cancer at a distant subcutaneous site and this is a completely novel observation. Whilst we have previously described in vivo inhibitory effect of BromAc® on peritoneal cancers after IP injection [29], this paper indicates that a systemic effect of BromAc® is possible. The second observation is that BromAc® was as effective as GEM in inhibiting tumor growth, and this is the first time that in vivo inhibition of pancreatic cancer growth by BromAc® has been reported. We were disappointed that we did not see synergy between BromAc® + GEM or 5-FU as seen in our in vitro experiments [28]. The reason for this disparity deserves further study. The finding that the tumor growth was significantly and substantially reduced, and that percentage necrosis was high in the BromAc® group supported a strong direct anti-tumor effect. The effect of BromAc® on tumor density is also very interesting and may allow better drug penetration in pancreatic cancer.
In the present study the animals tolerated both the low and high dose treatment without any toxicity as revealed by their body weight gain and other parameters of wellbeing, including immuno-histological evaluations of vital organs at termination of the studies. This is a good indication that both low and high doses as stipulated in the treatment regime can be used for clinical application.
Evaluating efficacy through tumor volume and tumor weight regression indicated that in the low dose groups Brom 3.0 mg/kg + Ac 300 mg/kg, GEM 2.0 mg/kg and GEM 2 mg/kg + Brom 3.0 mg/kg + Ac 300 mg/kg showed similar efficacy. Further, when the percentage change in tumor volume was assessed over the time, the above three groups had similar values, indicating that any of the three treatment regimens could be used with equivalent efficacy. The recommended clinical dosage of GEM for pancreatic cancer is 1000 mg/m2 iv [45], which is equivalent to 27 mg/Kg [46]. The low dose of GEM 2 mg/kg (equivalent to a clinical dose of 0.16 mg/kg [46]) with the addition of BromAc® when compared to 27 mg/Kg clinical dose is equivalent to a reduction of more than 99% GEM and it may enable more frequent treatment with foreseeable better tumor ablation and treatment outcome. This minimal GEM dosage regime in the presence of Ac (antioxidant) may reduce the side effects such as neurotoxicity, cardiotoxicity nephrotoxicity etc. [47,48]. Currently, low dose chemotherapy with gemcitabine is practiced only as palliation [49]. On the other hand, low dose Bromelain and Ac would allow continuous treatment until tumor is completely ablated. This is a major advantage in using BromAc® for treating pancreatic cancer.
Of prime importance is the observation that over treatment period to 17 days, there was almost no tumor growth in the above three treatment groups, indicating the potency of the therapeutic dosage used in controlling the pancreatic cancer growth. Seventeen days translates to almost 2 human years of tumor growth control [50] with this treatment strategy. The tumor necrosis was about 50% in the Brom + Ac group (an increase of 10% compared to controls) indicating that these agents besides modulating other oncoproteins may also modulate the vascular epidermal growth factors [51] that are responsible for angiogenesis and hence the high level of necrosis that contributes to tumor shrinkage [52]. Although necrosis is a common feature in most fast-growing tumors, the level in the other groups of treatment was about 40% and similar to controls.
Treatment with 5-FU gave poor outcome as single agent or in combination. Tumor regression was considerably poor and hence further evaluation at high dose was not carried out. The poor outcome with 5-FU may be due to several reasons. As a prodrug, it has to be phosphorylated into mono-, di- and tri-phosphorylated fluorouracil compounds [53] to be active as a nucleoside. Phosphorylation has been shown to be inhibited by antioxidants such as Ac [54].
It is known that tumor matrix are dense owing to several factors such as their composition that are primarily made up of collagen, fibrin fibers hyaluronic acid etc. and with the accumulation of fluid due to leaky blood vessels and poor lymphatic out flow create a very dense environment that restricts the free entry of chemotherapeutic drugs [55]. In the present treatment the density of the tumor has been evaluated and it was found that treatment groups using Brom 3 mg/kg + Ac 300 mg/kg the density of the tumor fell by 10%; however increasing the dosage to Brom 6 mg/kg + 500 mg/kg Ac, the density was reduced by 32% (a difference of 22%) indicating that Brom and Ac have substantial effect on the tumor matrix and hence the reduction in density. Molecular mechanism that may be at play includes proteolytic action of Brom on collagen, fibrin and other degradable components along with antioxidant action of Ac that has scissoring action on the disulfide bonds linking fibers, proteins etc. [56] or the inhibitory effect of Brom on CD44 [57] which may alter hyaluronic acid turnover in the tumor stroma. To conclude, the BromAc® as anti-cancer agent can be attributed to its mucin-depleting effects as well as its effects on the cellular pathways involved in carcinogenesis. In addition, the present study indicates the safety and efficacy of the treatment regime of BromAc® alone or as an adjuvant with GEM as an effective form of treatment for pancreatic cancer.
Acknowledgements
We would like to thank Mr. John Paul Levi and members of the Pathology Department, St. George Hospital, Kogarah, NSW 2217, Australia for their excellent help in tissue staining. This research is partly funded by Mucpharm Pty Ltd, Australia. Grant number: not applicable.
Disclosure of conflict of interest
DLM is the co-inventor and assignee of the licence for this study and director of the spin-off sponsor company, Mucpharm Pty Ltd. AHM, JA, KP and KK are employees of Mucpharm Pty Ltd. SJV is partly employed by Mucpharm for its cancer development and is supported by an Australian Government Research Training Program Scholarship. VK thanks the Foundation Nuovo Soldati for its fellowship and was partly sponsored for stipend by Mucpharm Pty Ltd.
Supplementary File
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 5.Maire F, Cibot JO, Compagne C, Hentic O, Hammel P, Muller N, Ponsot P, Levy P, Ruszniewski P. Epidemiology of pancreatic cancer in France: descriptive study from the French national hospital database. Eur J Gastroenterol Hepatol. 2017;29:904–908. doi: 10.1097/MEG.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 6.Matsubayashi H, Kiyozumi Y, Ishiwatari H, Uesaka K, Kikuyama M, Ono H. Surveillance of individuals with a family history of pancreatic cancer and inherited cancer syndromes: a strategy for detecting early pancreatic cancers. Diagnostics (Basel) 2019;9:169. doi: 10.3390/diagnostics9040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner M, Wu Z, Krautz C, Pilarsky C, Grutzmann R, Weber GF. Current clinical strategies of pancreatic cancer treatment and open molecular questions. Int J Mol Sci. 2019;20:4543. doi: 10.3390/ijms20184543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zulke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer The CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Olah A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Buchler MW European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 10.Lakatos G, Petranyi A, Szucs A, Nehez L, Harsanyi L, Hegyi P, Bodoky G. Efficacy and safety of FOLFIRINOX in locally advanced pancreatic cancer. A single center experience. Pathol Oncol Res. 2017;23:753–759. doi: 10.1007/s12253-016-0176-0. [DOI] [PubMed] [Google Scholar]
- 11.Delitto D, Black BS, Sorenson HL, Knowlton AE, Thomas RM, Sarosi GA, Moldawer LL, Behrns KE, Liu C, George TJ, Trevino JG, Wallet SM, Hughes SJ. The inflammatory milieu within the pancreatic cancer microenvironment correlates with clinicopathologic parameters, chemoresistance and survival. BMC Cancer. 2015;15:783. doi: 10.1186/s12885-015-1820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajabpour A, Rajaei F, Teimoori-Toolabi L. Molecular alterations contributing to pancreatic cancer chemoresistance. Pancreatology. 2017;17:310–320. doi: 10.1016/j.pan.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, You L, Dai M, Zhao Y. Mucins in pancreatic cancer: a well-established but promising family for diagnosis, prognosis and therapy. J Cell Mol Med. 2020;24:10279–10289. doi: 10.1111/jcmm.15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishn SR, Ganguly K, Kaur S, Batra SK. Ramifications of secreted mucin MUC5AC in malignant journey: a holistic view. Carcinogenesis. 2018;39:633–651. doi: 10.1093/carcin/bgy019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weniger M, Honselmann KC, Liss AS. The extracellular matrix and pancreatic cancer: a complex relationship. Cancers (Basel) 2018;10:316. doi: 10.3390/cancers10090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi IK, Strauss R, Richter M, Yun CO, Lieber A. Strategies to increase drug penetration in solid tumors. Front Oncol. 2013;3:193. doi: 10.3389/fonc.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dauer P, Nomura A, Saluja A, Banerjee S. Microenvironment in determining chemo-resistance in pancreatic cancer: neighborhood matters. Pancreatology. 2017;17:7–12. doi: 10.1016/j.pan.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68:159–171. doi: 10.1136/gutjnl-2018-316451. [DOI] [PubMed] [Google Scholar]
- 19.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrach T, Eckert K, Schulze-Forster K, Nuck R, Grunow D, Maurer HR. Isolation and partial characterization of basic proteinases from stem bromelain. J Protein Chem. 1995;14:41–52. doi: 10.1007/BF01902843. [DOI] [PubMed] [Google Scholar]
- 22.Bansil R, Turner BS. Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid Interface Sci. 2006;11:164–170. [Google Scholar]
- 23.Meldrum OW, Yakubov GE, Bonilla MR, Deshmukh O, McGuckin MA, Gidley MJ. Mucin gel assembly is controlled by a collective action of non-mucin proteins, disulfide bridges, Ca(2+)-mediated links, and hydrogen bonding. Sci Rep. 2018;8:5802. doi: 10.1038/s41598-018-24223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valle SJ, Akhter J, Mekkawy AH, Lodh S, Pillai K, Badar S, Glenn D, Power M, Liauw W, Morris DL. A novel treatment of bromelain and acetylcysteine (BromAc®) in patients with peritoneal mucinous tumours: a phase I first in man study. Eur J Surg Oncol. 2021;47:115–122. doi: 10.1016/j.ejso.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Pillai K, Akhter J, Chua TC, Morris DL. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. Int J Cancer. 2014;134:478–486. doi: 10.1002/ijc.28380. [DOI] [PubMed] [Google Scholar]
- 26.Aluigi MG, De Flora S, D’Agostini F, Albini A, Fassina G. Antiapoptotic and antigenotoxic effects of N-acetylcysteine in human cells of endothelial origin. Anticancer Res. 2000;20:3183–3187. [PubMed] [Google Scholar]
- 27.Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, Yazdi IK, Corbo C, Palomba R, Khaled SZ, Martinez JO, Brown BS, Isenhart L, Tasciotti E. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8:9874–9883. doi: 10.1021/nn502807n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillai K, Mekkawy AH, Akhter J, Badar S, Dong L, Liu AI, Morris DL. Enhancing the potency of chemotherapeutic agents by combination with bromelain and N-acetylcysteine-an in vitro study with pancreatic and hepatic cancer cells. Am J Transl Res. 2020;12:7404–7419. [PMC free article] [PubMed] [Google Scholar]
- 29.Mekkawy AH, Pillai K, Badar S, Akhter J, Ke K, Valle SJ, Morris DL. Addition of bromelain and acetylcysteine to gemcitabine potentiates tumor inhibition in vivo in human colon cancer cell line LS174T. Am J Cancer Res. 2021;11:2252–2263. [PMC free article] [PubMed] [Google Scholar]
- 30.Hegde GV, de la Cruz C, Eastham-Anderson J, Zheng Y, Sweet-Cordero EA, Jackson EL. Residual tumor cells that drive disease relapse after chemotherapy do not have enhanced tumor initiating capacity. PLoS One. 2012;7:e45647. doi: 10.1371/journal.pone.0045647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonckheere N, Skrypek N, Van Seuningen I. Mucins and pancreatic cancer. Cancers (Basel) 2010;2:1794–1812. doi: 10.3390/cancers2041794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Depletion of mucin in mucin-producing human gastrointestinal carcinoma: results from in vitro and in vivo studies with bromelain and N-acetylcysteine. Oncotarget. 2015;6:33329–33344. doi: 10.18632/oncotarget.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs. 2014;25:150–160. doi: 10.1097/CAD.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 34.Pillai K, Akhter J, Chua TC, Morris DL. Anticancer property of bromelain with therapeutic potential in malignant peritoneal mesothelioma. Cancer Invest. 2013;31:241–250. doi: 10.3109/07357907.2013.784777. [DOI] [PubMed] [Google Scholar]
- 35.Tréhoux S, Duchêne B, Jonckheere N, Van Seuningen I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42-44 MAPK, Akt, Bcl-2 and MMP13 pathways. Biochem Biophys Res Commun. 2015;456:757–762. doi: 10.1016/j.bbrc.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama S, Higashi M, Kitamoto S, Oeldorf M, Knippschild U, Kornmann M, Maemura K, Kurahara H, Wiest E, Hamada T, Kitazono I, Goto Y, Tasaki T, Hiraki T, Hatanaka K, Mataki Y, Taguchi H, Hashimoto S, Batra SK, Tanimoto A, Yonezawa S, Hollingsworth MA. Aberrant methylation of MUC1 and MUC4 promoters are potential prognostic biomarkers for pancreatic ductal adenocarcinomas. Oncotarget. 2016;7:42553. doi: 10.18632/oncotarget.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasseur R, Skrypek N, Duchene B, Renaud F, Martinez-Maqueda D, Vincent A, Porchet N, Van Seuningen I, Jonckheere N. The mucin MUC4 is a transcriptional and post-transcriptional target of K-ras oncogene in pancreatic cancer. Implication of MAPK/AP-1, NF-κB and RalB signaling pathways. Biochim Biophys Acta. 2015;1849:1375–1384. doi: 10.1016/j.bbagrm.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Zhi X, Tao J, Xie K, Zhu Y, Li Z, Tang J, Wang W, Xu H, Zhang J, Xu Z. MUC4-induced nuclear translocation of β-catenin: a novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett. 2014;346:104–113. doi: 10.1016/j.canlet.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:108–124. doi: 10.1007/s00534-009-0174-7. [DOI] [PubMed] [Google Scholar]
- 40.Alhusaini A, Cannon A, Maher SG, Reynolds JV, Lynam-Lennon N. Therapeutic potential of PARP inhibitors in the treatment of gastrointestinal cancers. Biomedicines. 2021;9:1024. doi: 10.3390/biomedicines9081024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qanungo S, Uys JD, Manevich Y, Distler AM, Shaner B, Hill EG, Mieyal JJ, Lemasters JJ, Townsend DM, Nieminen AL. N-acetyl-l-cysteine sensitizes pancreatic cancers to gemcitabine by targeting the NFκB pathway. Biomed Pharmacother. 2014;68:855–864. doi: 10.1016/j.biopha.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalra N, Bhui K, Roy P, Srivastava S, George J, Prasad S, Shukla Y. Regulation of p53, nuclear factor kappaB and cyclooxygenase-2 expression by bromelain through targeting mitogen-activated protein kinase pathway in mouse skin. Toxicol Appl Pharmacol. 2008;226:30–37. doi: 10.1016/j.taap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Porter LA, Donoghue DJ. Cyclin B1 and CDK1: nuclear localization and upstream regulators. Prog Cell Cycle Res. 2003;5:335–47. [PubMed] [Google Scholar]
- 44.Han EH, Ng SC, Arber N, Begemann M, Weinstein I. Roles of cyclin D1 and related genes in growth inhibition, senescence and apoptosis. Apoptosis. 1999;4:213–219. doi: 10.1023/a:1009618824145. [DOI] [PubMed] [Google Scholar]
- 45.Gemzar, highlights of prescribing information, The Food and Drug Administration (FDA) Revised: 05/2019. [Google Scholar]
- 46.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilmi M, Ederhy S, Waintraub X, Funck-Brentano C, Cohen A, Vozy A, Lebrun-Vignes B, Moslehi J, Nguyen LS, Salem JE. Cardiotoxicity associated with gemcitabine: literature review and a pharmacovigilance study. Pharmaceuticals (Basel) 2020;13:325. doi: 10.3390/ph13100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hryciuk B, Szymanowski B, Romanowska A, Salt E, Wasag B, Grala B, Jassem J, Duchnowska R. Severe acute toxicity following gemcitabine administration: a report of four cases with cytidine deaminase polymorphisms evaluation. Oncol Lett. 2018;15:1912–1916. doi: 10.3892/ol.2017.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zwitter M, Kovac V, Smrdel U, Vrankar M, Zadnik V. Gemcitabine in brief versus prolonged low-dose infusion, both combined with cisplatin, for advanced non-small cell lung cancer: a randomized phase II clinical trial. J Thorac Oncol. 2009;4:1148–1155. doi: 10.1097/JTO.0b013e3181ae280f. [DOI] [PubMed] [Google Scholar]
- 50.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Redondo P, Bandres E, Solano T, Okroujnov I, Garcia-Foncillas J. Vascular endothelial growth factor (VEGF) and melanoma. N-acetylcysteine downregulates VEGF production in vitro. Cytokine. 2000;12:374–378. doi: 10.1006/cyto.1999.0566. [DOI] [PubMed] [Google Scholar]
- 52.Vayrynen SA, Vayrynen JP, Klintrup K, Makela J, Karttunen TJ, Tuomisto A, Makinen MJ. Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br J Cancer. 2016;114:1334–1342. doi: 10.1038/bjc.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miura K, Kinouchi M, Ishida K, Fujibuchi W, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C, Sasaki I. 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers (Basel) 2010;2:1717–1730. doi: 10.3390/cancers2031717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu Y, Yang G, Zhu F, Peng C, Li W, Li H, Kim HG, Bode AM, Dong Z, Dong Z. Antioxidants decrease the apoptotic effect of 5-Fu in colon cancer by regulating Src-dependent caspase-7 phosphorylation. Cell Death Dis. 2014;5:e983. doi: 10.1038/cddis.2013.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Senthebane DA, Jonker T, Rowe A, Thomford NE, Munro D, Dandara C, Wonkam A, Govender D, Calder B, Soares NC, Blackburn JM, Parker MI, Dzobo K. The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int J Mol Sci. 2018;19:2861. doi: 10.3390/ijms19102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F. N-acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52:751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 57.Harrach T, Gebauer F, Eckert K, Kunze R, Maurer H. Bromelain proteinases modulate the cd44 expression on human molt-4/8 leukemia and sk-mel-28 melanoma-cells in-vitro. Int J Oncol. 1994;5:485–488. doi: 10.3892/ijo.5.3.485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


