Abstract
Objective: To investigate the clinical efficacy of coenzyme Q10 (CoQ10) plus trimetazidine (TMZ) in treating acute viral myocarditis (AVMC) and the combination’s influence on the oxidative stress markers and the patients’ quality of life (QoL). Methods: This retrospective analysis enrolled 156 patients with AVMC admitted to the Department of Cardiology of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine between February 2018 and February 2019. Based on the treatment method each patient was administered, the patients were classified into a control group (n=72, CoQ10 therapy) and a combination group (n=84, CoQ10+TMZ therapy). The clinical effectiveness was observed in the two groups two weeks after the treatment, and the changes in the patients’ serum inflammatory factor levels, oxidative stress indexes, myocardial enzyme levels, and cardiac function were compared. Results: The combination group had a far superior total effective rate than the control group (90.5% vs. 77.8%, P<0.05). After the treatment, the serum inflammatory factor levels, including tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), and C-reactive protein (CRP), decreased in both groups, and the index levels in the combination group were significantly better than they were in the control group (P<0.05). The oxidative stress indicators, such as superoxide dismutase (SOD), malondialdehyde (MDA) and nitric oxide (NO), improved more significantly in the combination group compared to the control group (P<0.05). The myocardial zymogram creatine kinase (CK), cardiac troponin (cTnI), creatine kinase isoenzyme MB (CK-MB), and lactate dehydrogenase (LDH) levels were reduced in the two groups, with lower levels in the combination group. The left ventricular systolic function and the patients’ QoL were better in the combination group compared with the control group (P<0.05). Conclusions: CoQ10 plus TMZ yields a favorable clinical effectiveness in the treatment of AVMC, and it can effectively promote cardiac function recovery, alleviate oxidative stress and inflammatory reactions, and bolster patients’ QoL.
Keywords: Coenzyme Q10, trimetazidine, viral myocarditis, oxidative stress, inflammatory reaction
Introduction
Acute viral myocarditis (AVMC), a type of myocarditis mainly characterized by non-specific interstitial inflammation of the myocardium and induced by a myocardial virus infection of the heart, can directly damage cardiomyocytes [1,2]. The common viruses are the Coxsackie virus, adenovirus, and the influenza virus [3,4]. Clinically, AVMC is usually manifested as palpitations, chest tightness, shortness of breath, fever, and body aches. However, the disease typically has diverse clinical presentations with varying degrees of severity. Patients with mild disease may present no obvious symptoms, but those with severe disease may suffer from severe arrhythmia, heart failure, cardiogenic shock and sudden death [5,6]. At present, the treatment of AVMC mainly focuses on antiviral medicines and nourishing the myocardium, which help to stabilize the condition. Unlike acute myocarditis, from which approximately half of patients can recover spontaneously with supportive therapy alone, AVMC is a complex process of virus-body interaction, including viral infections, cellular immunity, free radical damage to the myocardium, as well as cytokine-mediated myocardial damage and microvascular injury [7].
Today, immunomodulatory therapy has been used to treat acute myocarditis, including immunoglobulin, immunoadsorption plasma separation and immunosuppression [8]. It is reported that oxidative stress modulation can be a therapeutic target [9]. Oxidative stress injuries play a vital part in some pathological conditions, as an elevated reactive oxygen species (ROS) concentration can directly lead to lipid peroxidation, DNA damage, and increased mitochondrial membrane permeability, as well as exacerbated inflammatory cell infiltration, leading to cell apoptosis [10,11]. Coenzyme Q10 (CoQ10) is an indispensable elements in human life and a strong antioxidant. It is well documented that CoQ10 is critical in mitochondrial respiratory chain electron transfer and adenosine tri-phosphate (ATP) production [12,13]. More recently, data show that CoQ10 plays a beneficial role in cancer, diabetes, heart failure, and a wide spectrum of inflammation-based diseases [14,15]. Trimetazidine (TMZ) has also been extensively used in the treatment of AVMC in recent years, and it helps to promote the energy metabolism of cardiomyocytes and stabilize the condition. Meanwhile, it can reduce the metabolite damage produced by fatty acid β oxidation to the cardiomyocytes, and it can maintain cells’ stable electrical activity states, so it is conducive to correcting intracellular acidosis with a superior therapeutic effect [16]. Accordingly, this study mainly investigated the efficacy of CoQ10 plus TMZ in the treatment of AVMC and the combination’s impacts on oxidative stress and the inflammatory response as well as the patients’ quality of life (QoL).
Methods
Baseline patient data
The clinical data of 156 patients with AVMC treated in the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine from February 2018 to February 2019 were analyzed retrospectively. Based on the different treatment methods, they were classified into a control group (n=72) for CoQ10 therapy and a combination group (n=84) for CoQ10+TMZ therapy. All the enrolled patients showed abnormal ST segments using electrocardiograms (ECG), decreased systolic function using ultrasound, and a positive virus etiology. The exclusion criteria were acute decompensated heart failure, severe arrhythmia, shock, advanced liver disease, or autoimmune diseases. This study was approved by the hospital ethics committee (20181124). Written informed consent was obtained before each patient’s enrolment in the study.
Treatment methods
All the patients were diagnosed promptly after their admission and were administered symptomatic routine treatment (bed rest, low-flow oxygen inhalation, multifunctional ECG monitoring, high-dose vitamin C, energy supplementation, etc.). In addition to this treatment, the patients in the control group were administered 10 mg CoQ10 (Shanghai Meiyou Pharmaceutical Co., Ltd., China, H19999182) 3 times a day for 2 weeks. Meanwhile, the patients in the combination group were administered 20 mg TMZ (Shanxi Challenge & Young Pharmaceutical Group Co., Ltd., China, H20123233) 3 times a day for 2 weeks in addition to the treatment administered to the control group.
Endpoints
(1) Efficacy evaluation: “Markedly effective” indicated the disappearance of the clinical symptoms, with the ECG, heart examination, and laboratory examination results falling within the standard reference range. “Effective” indicated the remission of the clinical symptoms, with the ECG and heart examination indicating a significant improvement, and most of the laboratory examination indexes fell within the standard reference range. “Ineffective” indicated no improvement. Total effective rate = (markedly effective + effective) cases/total cases × 100%. (2) Fasting blood samples were collected from the patients at admission and at two weeks after the treatment. After centrifugation at 3000 × g for 20 min, serum was collected for the determination of superoxide dismutase (SOD; Cat. No. A001-1-2) and malondialdehyde (MDA; Cat. No. A003-1-2) using xanthine oxidase method and nitric oxide (NO; Cat. No. A012-1-2) using the nitrate reductase method, with the kits all purchased from Nanjing Jiancheng Bioengineering Institute, China. (3) The serum tumor necrosis factor-α (TNF-α; Cat. No. FEK1121) and interleukin-8 (IL-8; Cat. No. EK0413) levels were measured using ELISA, and the C-reactive protein (CRP; Cat. No. EK1316) was determined using immunoturbidimetry, with the kits all provided by Wuhan Doctor Biotech Co., Ltd., China. (4) The creatine kinase (CK; Cat. No. E-EL-H1433c), creatine kinase isoenzyme MB (CK-MB; Cat. No. E-EL-H1434c), cardiac troponin (cTnI; Cat. No. E-EL-H0646c) and lactate dehydrogenase (LDH; Cat. No. E-EL-H0556c) levels were determined using ELISA, with the kits all provided by Wuhan Elabscience Co., Ltd., China. (5) Cardiac function: The left ventricular end-diastolic diameters (LVDd), the left ventricular ejection fractions (LVEF), the left ventricular short axis shortening rates (LVFS), and the early and late ventricular filling velocity ratios (the E/A ratios) were measured using a Philips IE33 Color Doppler Ultrasound Scanner (Shanghai Huanxi Medical Equipment Co., Ltd., China, HC00605679) before and after the treatment. (6) Quality of life (QoL): The Short-Form 36 Item Health Survey (SF-36) [17] was used to score the QoL of patients from the eight dimensions of physical functioning, including role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health, with a total of 36 items and a total possible score of 100 points for each dimension. A higher score was associated with less damage and better QoL.
Statistical processing
SPSS 22.0 statistical software and GraphPad Prism 8.0 were utilized for the statistical analysis and the data visualization, respectively. Measurement and counting data were expressed as the means ± standard deviation and percentages (%) and compared using independent samples T tests and Chi-square tests respectively, with P<0.05 as the significance level and 95% as the confidence interval (CI).
Results
Baseline patient data in the two groups
The clinical data of the two groups of patients were collected. The male to female ratio in the control group was 42:30, and the average age was (47.66±4.51) years old. The combination group consisted of 45 males and 39 females with an average age of (48.01±4.84) years old. There were no statistically significant differences in terms of gender, age, body mass index (BMI), course of disease, New York Heart Association (NYHA) classification, disease grading, total cholesterol (TC), triglyceride (TG), blood urea nitrogen (BUN), or serum creatinine (SCr) between the two groups (P>0.05) (Table 1).
Table 1.
General information
| Control group (n=72) | Combination group (n=84) | χ2/t | P | |
|---|---|---|---|---|
| Gender [n (%)] | 0.3564 | 0.5505 | ||
| Male | 42 (58.3) | 45 (53.6) | ||
| Female | 30 (41.7) | 39 (46.4) | ||
| Age (years) | 47.66±4.51 | 48.01±4.84 | 0.4646 | 0.6429 |
| BMI (kg/m2) | 21.64±2.11 | 21.47±2.19 | 0.4915 | 0.6237 |
| Course of disease (d) | 30.8±1.7 | 31.2±1.9 | 1.3756 | 0.1709 |
| NYHA classification | 0.1885 | 0.9101 | ||
| I | 16 (22.2) | 21 (25.0) | ||
| II | 31 (43.1) | 34 (40.5) | ||
| III | 25 (34.7) | 29 (34.5) | ||
| Disease grading | 0.2966 | 0.8622 | ||
| Mild | 22 (30.6) | 29 (34.5) | ||
| Moderate | 33 (45.8) | 37 (44.1) | ||
| Severe | 17 (23.6) | 18 (21.4) | ||
| TC (mmol/L) | 3.84±1.03 | 3.97±1.21 | 0.7159 | 0.4751 |
| TG (mmol/L) | 1.79±0.38 | 1.84±0.47 | 0.7226 | 0.4710 |
| BUN (mmol/L) | 5.11±0.78 | 5.14±0.81 | 0.2346 | 0.8148 |
| SCr (μ(r (/L) | 81.57±8.74 | 82.76±9.14 | 0.8272 | 0.4094 |
Comparison of the clinical efficacy between the two groups
The number of markedly effective, effective and ineffective cases were 29, 27, and 16 respectively in the control group, and they were 50, 26, and 8 in the combination group. The total effective rate in combination group was significantly higher than it was in the control group after the treatment (90.5% vs. 77.8% P<0.05) (Table 2).
Table 2.
Clinical efficacy of the two groups of patients
| Markedly effective | Effective | Ineffective | Total effective rate | |
|---|---|---|---|---|
| Control group (n=72) | 29 (40.3) | 27 (37.5) | 16 (22.2) | 56 (77.8) |
| Combination group (n=84) | 50 (59.5) | 26 (31.0) | 8 (9.5) | 76 (90.5) |
| χ2/t | 4.8021 | |||
| P | 0.0284 |
The oxidative stress indicator levels in the two groups
The serum oxidative stress indexes differed insignificantly between the two groups before the treatment (P>0.05). After the treatment, the SOD increased but the MDA and NO decreased, with more evidently improved parameters in the combination group compared with the control group (P<0.05) (Figure 1).
Figure 1.
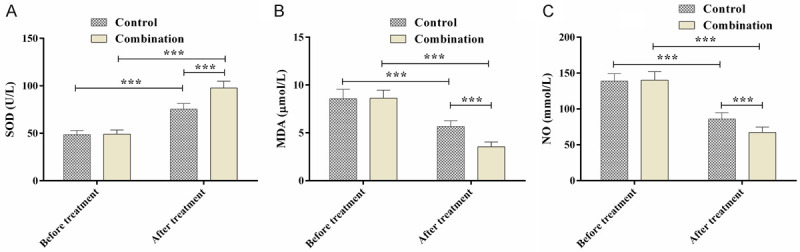
Changes in the oxidative stress indicators. A. SOD activity; B. MDA levels; C. NO levels; ***P<0.001.
Comparison of the inflammatory factors between the two groups
The inflammatory cytokines CRP, TNF-α, and IL-8 showed no significant differences between the two groups before the treatment (P>0.05), but the inflammatory cytokine levels were reduced significantly after the treatment, especially in the combination group (P<0.05) (Figure 2).
Figure 2.
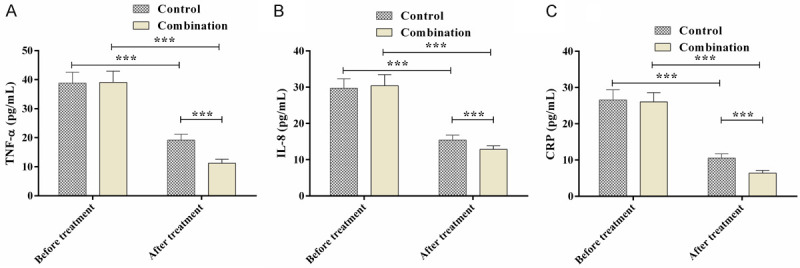
Comparison of the inflammatory factors. A. TNF-α levels; B. IL-8 levels; C. CRP levels; ***P<0.001.
Cardiac function changes in the two groups
No significant differences were observed in the LVDd, LVFS, and E/A ratios between the two groups before and after the treatment. On the other hand, the LVEF, an evaluation of the systolic function, was improved in both groups after the treatment, and the LVEF level in the combination group was significantly higher than it was in the control group (P<0.05) (Table 3).
Table 3.
Changes in the cardiac function
| LVDd (mm) | E/A ratio | |||
|
|
|
|||
| Before treatment | After treatment | Before treatment | After treatment | |
|
| ||||
| Control group (n=72) | 48.26±4.37 | 50.59±4.02 | 1.58±0.19 | 1.59±0.14 |
| Combination group (n=84) | 49.03±4.68 | 51.15±3.87 | 1.61±0.22 | 1.57±0.17 |
| χ2/t | 1.0561 | 0.8850 | 0.9036 | 0.7938 |
| P | 0.2926 | 0.3775 | 0.3676 | 0.4285 |
|
| ||||
| LVEF (%) | LVFS (%) | |||
|
|
|
|||
| Before treatment | After treatment | Before treatment | After treatment | |
|
| ||||
| Control group (n=72) | 46.57±4.81 | 60.87±5.05 | 25.64±1.51 | 26.87±1.69 |
| Combination group (n=84) | 45.87±5.12 | 66.84±5.37 | 26.07±1.74 | 27.11±2.01 |
| χ2/t | 0.8753 | 7.1144 | 1.6346 | 0.7994 |
| P | 0.3828 | <0.0001 | 0.1042 | 0.4253 |
Comparison of the myocardial zymograms between the two groups
Before the treatment, the myocardial zymogram (CK, CK-MB, cTnI and LDH) showed no significant difference between the two groups (P>0.05). After the treatment, the CK, CK-MB, cTnI, and LDH levels were reduced in both groups, especially in the combination group (P<0.05) (Figure 3).
Figure 3.
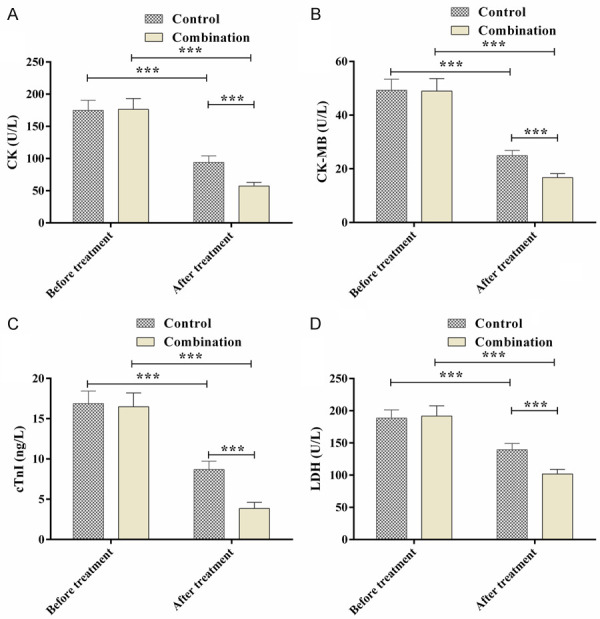
Comparison of the myocardial zymograms. A. CK levels; B. CK-MB levels; C. cTnI levels; D. LDH levels; ***P<0.001.
QoL of the patients in the two groups
The combination group scored higher in role-emotional, somatic functioning, role-physical, social functioning, vitality, bodily pain, mental health and general health than the control group after the treatment (P<0.05) (Table 4).
Table 4.
Quality of life scores
| Role-emotional | Somatic functioning | Role-physical | Social functioning | Vitality | Bodily pain | Mental health | General health | |
|---|---|---|---|---|---|---|---|---|
| Control group (n=72) | 85.15±7.16 | 82.48±6.47 | 83.74±7.02 | 80.97±4.61 | 84.14±5.97 | 79.15±3.84 | 84.26±4.15 | 82.48±4.26 |
| Combination group (n=84) | 92.26±6.57 | 90.15±5.71 | 91.54±6.15 | 90.87±5.12 | 92.46±6.07 | 89.71±4.26 | 93.87±5.71 | 91.84±3.39 |
| χ2/t | 6.4644 | 7.8015 | 7.3973 | 12.6020 | 8.5995 | 16.1483 | 11.8465 | 15.2732 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Discussion
AVMC, a serious cardiovascular disease, mainly refers to localized or diffuse acute inflammatory lesions of the myocardium caused by a viral infection. Patients with AVMC often also suffer from different types of ECG abnormalities, including ST-T changes, sinus tachycardia, ventricular premature beat rate, and other characteristic differences [18]. Affected by the virus, they suffer from cardiomyocyte damage, which further invades the surrounding small blood vessels and produces a large number of oxygen free radicals, resulting in an imbalance of the myocardial immune function and the inflammatory cell infiltration. As such, the cardiomyocytes become degenerated, necrotic, and fibrous, eventually causing myocardial injury [19,20]. For patients with viral myocarditis, elevated myocardial energy consumption directly leads to energy depletion, and at the same time, cardiac hypertrophy can occur, which aggravates the myocardial energy consumption [21]. If not treated in time, the disease will continue to progress, seriously threatening the patients’ lives. Clinically, however, there is currently no specific medicine for the treatment of this disease. Instead, the patients are instructed to rest and undergo treatment to improve their cardiomyocyte metabolism and to treat their complications. Most of the treatment options for viral myocarditis, in addition to standard supportive care, focus on immunosuppression, anti-inflammatories, and the anti-oxidative stress responses [22,23]. CoQ10 and TMZ have established a good foundation for the treatment of chronic heart failure, stable coronary artery disease, and peripheral artery disease [24,25].
This study mainly observed the efficacy of CoQ10 plus TMZ in the treatment of AVMC. The results indicated that the total effective rate of the combination group was significantly higher than the total effective rate in the control group. Moreover, the serum inflammatory factor levels decreased significantly in both groups, and the indexes in the combination group were better than the indexes in the control group. After the episode, AVMC patients will experience changes in their cellular immune T lymphocyte subsets, which promote the synthesis and secretion of inflammatory cytokines in large quantities, resulting in sustained myocardial injury, an important mechanism for the transformation into cardiomyopathy [26]. Evidence has shown that CoQ10 can stabilize the mitochondrial calcium-dependent ion channels, reduce the cell energy consumption, and inhibit myocardial apoptosis [27]. As to TMZ, it is a kind of cell protection drug that not only suppress the β-oxidation of free fatty acids, it also inhibits the formation of ROS and hydrogen peroxide anions and reduces the inflammatory infiltration [28]. Therefore, the combination of the two can better reduce the patients’ inflammatory cytokine levels.
When AVMC occurs, the activity of the antifree radical enzymes in the myocardial tissue decreases, which in turn leads to an incomplete scavenging of the excess free radicals, resulting in a massive accumulation of free radicals and enhanced lipid peroxidation, which damages the cardiomyocytes [29]. MDA is an intermediate product of lipid peroxidation, and its increase indicates enhanced lipid peroxidation [30]. Under pathological conditions, inflammatory cell infiltration and myocardial ischemia produce a flood of oxygen free radicals, and SOD is consumed in large quantities. As a result, the mass of oxygen free radicals accumulated in the body cannot be eliminated in time, which induces a series of lipid peroxidation reactions and leads to an increase in MDA. And enhanced lipid peroxidation and excessive NO can reduce the SOD scavenging activity [31]. In this study, the oxidative stress index levels in the combination group were significantly improved after the treatment. Moreover, the post-treatment myocardial function and left ventricular systolic function in the combination group were better than they were in the control group. CK and CK-MB are important indicators of myocardial injury, and they can reflect the degree of myocardial cell injury to a certain extent [32]. CTnI is unique to the myocardium and can detect minor injuries. When the myocardium is damaged, its level can rise rapidly, so it is closely related to patient prognosis [33]. The study by Huynh et al. showed that CoQ10 can alleviate oxidative stress and left ventricular diastolic dysfunction and promotes the remodeling of the diabetic heart [34], which agrees with our research. And similarly, in the study of Shao et al., CoQ10 combined with TMZ ameliorated the biochemical markers of myocardial injury in patients with AVMC [35]. The reason behind it, we speculate, may be that CoQ10 has a highly effective antioxidant effect, so it can mitigate the oxidative stress injuries caused by myocarditis and prevent the formation of lipid peroxidation free radicals and reduce lipid peroxidation damage by affecting the initiation process of lipid peroxidation. At the same time, TMZ can reduce free radical damage and myocardial oxygen consumption, alleviate ischemia and hypoxic response, and improve left ventricular systolic function. Therefore, the combination of the two may exert a synergistic effect to reduce oxidative stress injuries, thereby effectively improving patients’ cardiac function and accelerating patients’ recoveries. The difference with the preceding research is that we enrolled 156 patients into a control group who received CoQ10 only and a combination group who were treated with CoQ10 combined with TMZ. Moreover, the QoL of patients after the treatment was investigated. The data show that the QoL of the patients in the combination group was also better than the QoL in the control group, suggesting that the combined use of the two drugs can improve patients’ quality of life. Nevertheless, there are still some shortcomings to this study. First, we did not observe the cardiac function changes in the patients at different time periods. Second, basic experiments are needed to explore the specific mechanism of CoQ10 and TMZ in AVMC. These are the key future research directions.
To sum up, CoQ10 plus TMZ is more effective at treating AVMC, and the combination can effectively play an anti-inflammatory role, reducing myocardial injuries and improving patients’ QoL.
Disclosure of conflict of interest
None.
References
- 1.Olejniczak M, Schwartz M, Webber E, Shaffer A, Perry TE. Viral myocarditis-incidence, diagnosis and management. J Cardiothorac Vasc Anesth. 2020;34:1591–1601. doi: 10.1053/j.jvca.2019.12.052. [DOI] [PubMed] [Google Scholar]
- 2.Matshela MR. The role of echocardiography in acute viral myocarditis. Cardiovasc J Afr. 2019;30:239–244. doi: 10.5830/CVJA-2018-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose NR. Viral myocarditis. Curr Opin Rheumatol. 2016;28:383–389. doi: 10.1097/BOR.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis--diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 5.Shauer A, Gotsman I, Keren A, Zwas DR, Hellman Y, Durst R, Admon D. Acute viral myocarditis: current concepts in diagnosis and treatment. Isr Med Assoc J. 2013;15:180–185. [PubMed] [Google Scholar]
- 6.Cooper LT Jr. Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendren NS, Drazner MH, Bozkurt B, Cooper LT Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Sun Y, Su G, Li Y, Shuai X. Intravenous immunoglobulin therapy for acute myocarditis in children and adults. Int Heart J. 2019;60:359–365. doi: 10.1536/ihj.18-299. [DOI] [PubMed] [Google Scholar]
- 9.Anzini M, Merlo M, Sabbadini G, Barbati G, Finocchiaro G, Pinamonti B, Salvi A, Perkan A, Di Lenarda A, Bussani R, Bartunek J, Sinagra G. Long-term evolution and prognostic stratification of biopsy-proven active myocarditis. Circulation. 2013;128:2384–2394. doi: 10.1161/CIRCULATIONAHA.113.003092. [DOI] [PubMed] [Google Scholar]
- 10.Zeng J, Zhu L, Liu J, Zhu T, Xie Z, Sun X, Zhang H. Metformin protects against oxidative stress injury induced by ischemia/reperfusion via regulation of the lncRNA-H19/miR-148a-3p/Rock2 axis. Oxid Med Cell Longev. 2019;2019:8768327. doi: 10.1155/2019/8768327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Wang H, Fang S, Xu C. Roles of endoplasmic reticulum stress and autophagy on H2O2-induced oxidative stress injury in HepG2 cells. Mol Med Rep. 2018;18:4163–4174. doi: 10.3892/mmr.2018.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta MJ, Vazquez Fonseca L, Desbats MA, Cerqua C, Zordan R, Trevisson E, Salviati L. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta. 2016;1857:1079–1085. doi: 10.1016/j.bbabio.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y, Alexis J, Meriano J, Sung HK, Gasser DL, Moley KH, Hekimi S, Casper RF, Jurisicova A. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–895. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SY, Yang KL, Zeng LT, Wu XH, Huang HY. Effectiveness of coenzyme Q10 supplementation for type 2 diabetes mellitus: a systematic review and meta-analysis. Int J Endocrinol. 2018;2018:6484839. doi: 10.1155/2018/6484839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzilli M, Vinereanu D, Lopaschuk G, Chen Y, Dalal JJ, Danchin N, Etriby E, Ferrari R, Gowdak LH, Lopatin Y, Milicic D, Parkhomenko A, Pinto F, Ponikowski P, Seferovic P, Rosano GMC. Trimetazidine in cardiovascular medicine. Int J Cardiol. 2019;293:39–44. doi: 10.1016/j.ijcard.2019.05.063. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhao R, Gu C, Gu Z, Li L, Li Z, Dong C, Zhu J, Fu T, Gao J. The impact of systemic lupus erythematosus on health-related quality of life assessed using the SF-36: a systematic review and meta-analysis. Psychol Health Med. 2019;24:978–991. doi: 10.1080/13548506.2019.1587479. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Miao W, Wang S, Wei M, Su G, Li Z. Acute myocarditis mimicking ST-elevation myocardial infarction: a case report and review of the literature. Exp Ther Med. 2015;10:459–464. doi: 10.3892/etm.2015.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenke A, Holzhauser L, Lobel M, Savvatis K, Wilk S, Weithauser A, Pinkert S, Tschope C, Klingel K, Poller W, Scheibenbogen C, Schultheiss HP, Skurk C. Adiponectin promotes coxsackievirus B3 myocarditis by suppression of acute anti-viral immune responses. Basic Res Cardiol. 2014;109:408. doi: 10.1007/s00395-014-0408-y. [DOI] [PubMed] [Google Scholar]
- 20.De-Pu Z, Li-Sha G, Guang-Yi C, Xiaohong G, Chao X, Cheng Z, Wen-Wu Z, Jia L, Jia-Feng L, Maoping C, Yue-Chun L. The cholinergic anti-inflammatory pathway ameliorates acute viral myocarditis in mice by regulating CD4+ T cell differentiation. Virulence. 2018;9:1364–1376. doi: 10.1080/21505594.2018.1482179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remels AHV, Derks WJA, Cillero-Pastor B, Verhees KJP, Kelders MC, Heggermont W, Carai P, Summer G, Ellis SR, de Theije CC, Heeren RMA, Heymans S, Papageorgiou AP, van Bilsen M. NF-kappaB-mediated metabolic remodelling in the inflamed heart in acute viral myocarditis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2579–2589. doi: 10.1016/j.bbadis.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Van Linthout S, Tschope C. Viral myocarditis: a prime example for endomyocardial biopsy-guided diagnosis and therapy. Curr Opin Cardiol. 2018;33:325–333. doi: 10.1097/HCO.0000000000000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tschope C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hubner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zozina VI, Covantev S, Goroshko OA, Krasnykh LM, Kukes VG. Coenzyme Q10 in cardiovascular and metabolic diseases: current state of the problem. Curr Cardiol Rev. 2018;14:164–174. doi: 10.2174/1573403X14666180416115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy CP, Mullins KV, Kerins DM. The role of trimetazidine in cardiovascular disease: beyond an anti-anginal agent. Eur Heart J Cardiovasc Pharmacother. 2016;2:266–272. doi: 10.1093/ehjcvp/pvv051. [DOI] [PubMed] [Google Scholar]
- 26.Sun C, Zhang X, Yu Y, Li Z, Xie Y. CARD9 mediates T cell inflammatory response in Coxsackievirus B3-induced acute myocarditis. Cardiovasc Pathol. 2020;49:107261. doi: 10.1016/j.carpath.2020.107261. [DOI] [PubMed] [Google Scholar]
- 27.Yang YK, Wang LP, Chen L, Yao XP, Yang KQ, Gao LG, Zhou XL. Coenzyme Q10 treatment of cardiovascular disorders of ageing including heart failure, hypertension and endothelial dysfunction. Clin Chim Acta. 2015;450:83–89. doi: 10.1016/j.cca.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Di Napoli P, Taccardi AA, Barsotti A. Long term cardioprotective action of trimetazidine and potential effect on the inflammatory process in patients with ischaemic dilated cardiomyopathy. Heart. 2005;91:161–165. doi: 10.1136/hrt.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YC, Ge LS, Yang PL, Tang JF, Lin JF, Chen P, Guan XQ. Carvedilol treatment ameliorates acute coxsackievirus B3-induced myocarditis associated with oxidative stress reduction. Eur J Pharmacol. 2010;640:112–116. doi: 10.1016/j.ejphar.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 30.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int J Mol Sci. 2015;16:4600–4614. doi: 10.3390/ijms16034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu C, Zhang T, Zhu B, Cao Z. Diagnostic role of postmortem CK-MB in cardiac death: a systematic review and meta-analysis. Forensic Sci Med Pathol. 2020;16:287–294. doi: 10.1007/s12024-020-00232-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhen J, Li L, Yan J. Advances in biomarkers of myocardial injury in sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:699–702. doi: 10.3760/cma.j.issn.2095-4352.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Huynh K, Kiriazis H, Du XJ, Love JE, Jandeleit-Dahm KA, Forbes JM, McMullen JR, Ritchie RH. Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia. 2012;55:1544–1553. doi: 10.1007/s00125-012-2495-3. [DOI] [PubMed] [Google Scholar]
- 35.Shao L, Ma A, Figtree G, Zhang P. Combination therapy with coenzyme Q10 and trimetazidine in patients with acute viral myocarditis. J Cardiovasc Pharmacol. 2016;68:150–154. doi: 10.1097/FJC.0000000000000396. [DOI] [PubMed] [Google Scholar]


