Abstract
Inflammatory bowel diseases are chronic illnesses that involve intestinal inflammation and are usually diagnosed as Crohn’s disease or ulcerative colitis. As these diseases do not have a cure, the goal of treatment is to induce and maintain remission. Monoclonal antibodies have been recognized as the most advanced therapy to avoid complications and reduce the need for surgical approaches. However, although their effectiveness has been proven by several studies, they can trigger the immune system, induce the occurrence of immunogenicity, which may lead to the loss of response and treatment failure. The purpose of this review is to determine what are the main mechanisms involved in IBD; to assess the recommended treatments; to explore the mechanisms of immunogenicity. We also try to explain the detection and describe the existing advances that make possible the clinical application of these approaches.
Keywords: Inflammatory bowel disease, Crohn’s disease, ulcerative colitis, immunogenicity, monoclonal antibodies
Introduction
Inflammatory bowel disease (IBD) is characterized by a chronic, progressive and recurrent immune-mediated inflammation, which includes the main subtypes of ulcerative colitis (UC) and Crohn’s disease (CD). The etiopathogenesis of IBD is not fully understood, but the known factors include genetics and other internal and external factors, such as environment and lifestyle, and the intestinal microbiota and unregulated immune response [1,2].
Crohn’s disease can affect the entire gastrointestinal tract, which may present itself in the ileum and cecum (40% of presentations), be restricted to the ileum (30%), or restricted to the colon (35%) [3]. The pattern of a segmented lesion with injured areas interspersed with healthy ones is typical, in addition to transmural inflammation. CD can also be distinguished from UC by its behavior (inflammatory, stenosing, and/or fistulising disease), and by non-caseating granulomas [2,4].
Unlike CD, UC involves only the intestinal mucous layer and produces a continuous inflammatory pattern, which is limited to the intestinal colon and rectum. UC injuries caused by inflammation usually start in the rectum and advance proximally throughout the colon [5]. The extent to which these lesions reach determines whether the UC is classified as proctitis, left colitis, or pancolitis and it also indicates severity and possible complications [6,7].
IBD manifestations can be quite wide depending on the severity of the disease and its location. However, the most common clinical presentations include abdominal pain, chronic watery (occasionally bloody) diarrhea, weight loss, fatigue, and fever. Particularly in CD, extraintestinal manifestations, whether on the skin, eyes, or joints, can affect more than 50% of patients [2,7].
The incidence and prevalence of IBD are traditionally higher in urban areas and western countries, such as those in Western Europe and North America [8]. Therefore, an increase in IBD cases tends to correlate with industrial and socio-economic development in countries in Latin America, Asia, Eastern Europe, and Africa. This phenomenon highlights the relevance of lifestyle and the environment as risk factors for the disease [1,2,8].
In general, the development of IBD is based on three pillars that add up and interact with each other: genetics, environmental or external factors, and internal factors, the latter indicates the patients’ microbiota [1].
Immunopathology of IBD
The immune system has been implicated roles in the development of IBD [9], and some studies have demonstrated the effect of the innate and adaptive immune system on intestinal inflammation.
The innate immune system acts as the first line of defense against possible pathogens and is responsible for preventing, controlling, and/or eliminating infections [10]. It is mediated by several cell types, such as monocytes, macrophages, dendritic cells, neutrophils, and natural killer cells [11]. The adaptive immune system is characterized by its specific sensitivity to different molecules, recognizing and reacting to different microbial and non-microbial stimuli. The main cell types associated with this kind of immune response are lymphocytes [10].
All these immune cells secrete cytokines, which are proteins that affect the behavior of other cells [12]. Among the produced cytokines, some have been identified as important in the development or act as maintainers of the inflammatory environment described in IBD.
The tumor necrosis factor α (TNF-α), a mediator of the acute inflammatory response, is produced mainly by macrophages, lymphocytes, dendritic cells, and endothelial cells [10]. The biological activity of TNF-α begins when the cytokine is linked to its receptors TNFR-1 and TNFR-2. This further activates the NF-κB signaling pathway, thereby stimulating the production of other pro-inflammatory cytokines, such as Th1 type cytokines [13,14].
In studies conducted by Friedrich et al. and Geremia et al., a greater amount of TNF-α was found both in the lamina propria and in the intestinal mucosa of patients with IBD [16,17]. The presence of this cytokine induced a pro-inflammatory response, which damaged the gut tissue. In addition, it influenced other cells that present in the mucosa, which in turn leads to its dysregulation.
Similarly, Neurath et al. demonstrated that mice with TNBS-induced colitis produced higher levels of TNF-α. When the gene responsible for this cytokine was inhibited, colitis could not develop in these animals [15]. Furthermore, studies conducted on human patients demonstrated a significant presence of TNF-α producing cells in the gut environment. When antagonists of this cytokine were used, both induction and endoscopic/histological remission status were maintained in these patients [18,19].
Several other studies corroborate Neurath’s findings by showing the chronic elevation of TNF-α in patients with IBD. Braegger et al. quantified the presence of this cytokine in fecal samples from pediatric patients with both active disease and during remission. Their data demonstrated that patients with active CD and UC have higher levels of TNF-α, respectively, and that patients in remission had similar level of TNF-α to those of the controls [20]. Reimund et al. also investigated the production of TNF-α on biopsies of the affected mucosa, but in an adult cohort with CD. Their results demonstrated a higher production of TNF-α in these patients, even in normal mucous membranes that did not show apparent signs of inflammation [21].
Other cytokines have also been shown to act in maintaining the inflammatory state seen in IBD. Among them, IL-12 stimulates IFN-γ production [22] and induces intestinal Th1 immune response [23,24]. It also promotes the development of innate lymphoid cells (ILCs), expands IFN-γ and IL-17A, and produces Th17 cytokines as IL-17 and IL-22 [25-28].
IL-23 has also been described as a participant in IBD inflammation. Disruption of IL-23 leads to resistance to immune-mediated diseases, such as autoimmune encephalomyelitis, and T-cell-induced colitis [29,30]. In an experimental model, IL-23 has been described as essential for the manifestation of chronic intestinal inflammation. It has been demonstrated that IL-23 affects Th17 cells, the main producers of IL-17 and TNF-α [31]. In general, cytokines that play an important role in inducing the inflammation seen in IBD, and is correlated with TNF-α, have the potential to be therapeutic targets.
Anti-TNF-αmonoclonal antibody therapy for IBD
There is currently no specific cure for IBD, so the goal of treatment is to induce and maintain the state of remission in patients, thus controlling the exacerbation of the disease [32,33]. Monoclonal antibodies (MAbs) are antigen-recognizing glycoproteins generated from identical immune cells, which make them identical clones [34]. Thanks to Köhler and Milstein, the Nobel winners in 1975, who described the technique of hybridoma creation, which makes it possible to obtain large amounts of MAbs, that can be utilized clinically [35].
Advances in genetic engineering made it possible to create new MAbs, which are a second class composed of chimeric molecules, and which include a hybridoma formed by human and mouse Ig regions [36].
Among the chimeric MAbs, there is Infliximab (IFX/Remicade®), a TNF-α blocker, which is effective against several pathologies mediated by TNF-α, including CD and UC. In the USA, IFX was approved for use in 1998, which is a chimeric antibody, containing 75% human and 25% murine IgG1. When it binds to soluble TNF-α and to the precursor attached to the membrane, it prevents the activity of this cytokine, reducing the inflammatory response [37,38].
For IBD, IFX, which is administered intravenously, has been utilized to treat moderate to severe cases of both DC and UC that are refractory or intolerant to conventional medications. IFX’s management two phases’ administration: induction and maintenance. Induction doses of 5 mg/kg of body weight are given at week 0, week 2, and week 6. Thereafter, the maintenance phase is initiated and infusions are performed every 8 weeks, with the same dose as for induction [5,39].
The effectiveness of IFX has already been described by several studies, including one performed by Rutgeerts et al., which analyzed the efficacy of repeated infusions, and whether this administration would maintain the remission in patients with active CD. IFX has shown tolerance and efficiency in managing the clinical symptoms of patients that didn’t respond to conventional therapy [40]. Similarly, Sandborn also studied the role of IFX, by analyzing patients who had active, moderate, or severe UC, and who received IFX treatment. Their results demonstrated that patients using MAb required less colectomies when compared to patients who received the placebo [41].
In an attempt to minimize the incidence of anti-drug antibodies (ADAs), humanized MAbs were developed by using the hybridoma technique. However, a 100% human equivalent began to be used in the antibody constant region that was previously murine [42]. In general, humanized MAbs tend to produce fewer ADAs than the chimeric ones, but the incidence of ADAs is not wholly eliminated [34,43].
Adalimumab (Humira®), approved by the FDA in 2002, was initially used to treat rheumatological disorders [5]. The medication is an IGg1 that has high affinity and specificity to soluble TNF-α [44], which prevents the action of this cytokine. Adalimumab is commonly used as an option for IBD patients who had previously used IFX, and has been shown to be well tolerated along with a clinical benefit [45]. Adalimumab administration, similar to IFX, is divided into induction and maintenance phases. However, it is a medication for subcutaneous application, distinct from IFX, which is intravenous. In the induction phase, the patient receives a dose of 160 mg on week 0, 80 mg on week 2, and then 40 mg every 2 weeks. The dose during the maintenance phase is 40 mg every 2 weeks [44].
Several studies have proven the capacity of Adalimumab to induce and maintain remission in both CD and UC. Hanauer et al. included in their study patients with moderate to severe CD who had never received previously anti-TNF-α. The medication regimen was administrated according to the drug description leaflet. The results showed that remission rates were achieved in 4 weeks, being higher than placebo [46]. The role of Adalimumab was also studied in UC, as was the study by Colombel et al., in which researchers followed patients with moderate to severe forms for 4 years. The results indicated that the medication led to remission, and to mucosa healing, which increased the quality of life of these patients [47].
Another MAbs that also work by blocking the action of TNF-α is Certolizumab pegol (Cimzia®). Besides having the potential to be used in the treatment of IBD, this medication is currently used for treating CD. This MAb, also humanized, is a pegylated Fab fragment, thus differing structurally from both IFX and Adalimumab, and it acts by inhibiting the activation of the TNF-α membrane receptor by neutralizing both the soluble form of the cytokine and its transmembrane form [48,49]. Certolizumab pegol is administered subcutaneously and also requires phases of induction and maintenance. In the induction phase, 400 mg are administered on week 0, week 2, and week 4. Afterward, the patient receives 400mg every 4 weeks during maintenance phase [48].
Several researchers have analyzed the role of Certolizumab pegol to evaluate its effectiveness. Sandborn et al. conducted a randomized, placebo-controlled, and double-blind study to evaluate the efficacy of this medication. Patients with moderate to severe CD, who received Certolizumab pegol according to the drug description leaflet, were included. The results showed that the medication was associated with an improved response, but there was no increase in the remission rates [50]. Stein et al. analyzed the use of Certolizumab pegol as a re-induction therapy and demonstrated that most patients who received re-induction doses failed to achieve a sustained clinical response. In fact, previous treatment with another anti-TNF-α showed a reduced response, suggesting that the use of Certolizumab pegol is effective as initial therapy [51].
Despite the effectiveness, some patients show a loss of response to anti-TNF-α over time [52]. Studies indicate that the loss of response to IFX in patients with CD is 13%, and 25% to Adalimumab [53,54].
One way to continue the use of anti-TNF-α and to decrease the formation of ADAs is to associate the biological medication with immunosuppressants, such as azathioprine. In the SONIC study, patients received IFX, azathioprine, or a combination of both. The results showed that patients who received both medications demonstrated a superior remission without the need for corticosteroids when compared to those who used IFX as monotherapy. Also, serum levels of IFX were higher in patients receiving combination therapy, demonstrating the benefit of concomitant use of the two medications [18].
Similarly, the DIAMOND study analyzed the same context, but with Adalimumab. Again, patients using Adalimumab, azathioprine, and both concomitant medications were included. Although the study showed no differences regarding clinical remission, it did confirm endoscopic improvement in those patients who used the two combined medications, when compared to the monotherapy of Adalimumab [55].
Immunogenicity
As mentioned above, the use of MAbs in the treatment of IBD has brought several advances and has significantly improved the quality of patients’ life. Despite advances in genetic engineering and the improved quality of MAbs, it has shown an increase in the number of adverse effects/reactions to these medications related to loss of response [56].
The process by which a medication can lead the immune system to respond against it, thereby compromising its effectiveness or generating toxic compounds, is called immunogenicity [57]. Its occurrence is closely associated to the disappearance of the medication in the bloodstream and, consequently, results in therapeutic failure [58-60].
Besides the immune response that is triggered by the medications, the humoral immune response is also activated. Active T helper lymphocytes cause stimulation, proliferation, and further differentiation of B cells that are linked to antigens, in this case, MAbs. Plasma cells are generated from that differentiation, which is responsible for the production of specific antibodies against MAbs [10].
Several factors can lead to the formation of ADAs, such as the frequency of medication administration, the route that the drug uses to be metabolized, particularities of the medication biology itself, and factors intrinsic to the patient (e.g.: genetic factors, the use of other medications) [61,62]. In general, the production of ADAs directly interferes with the effect of the drug or produces changes that neutralize its effectiveness, and may even generate adverse reactions [63,64].
Neutralizing ADAs (ntADAs) act in a way that directly interferes with the biological activity of the medication. ADAs associated with TNF-α blockers render the medication ineffective. These ADAs against idiotypes can prevent the binding of the medication with its complement in the structure of TNF-α, which is normally associated with the Fab portion of the antibody. Furthermore, another way in which ADAs function is neutralized occurs when ADAs can bind to different parts of the MAb, modifying their morphological structure, thus preventing their binding to TNF-α [65]. On the other hand, non-neutralizing ADAs (bADAs), cause the removal of the medication by inducing its filtration by the spleen, in addition to facilitating the binding of the ADA to the phagocytic cells that remove them. Both ntADAs and bADAs, when linked to Mab, may form a complex composed of drug-ADA that is called immune-complex [65].
Immuno-complexes (ICs) can activate dendritic cell maturation. In addition, they can influence the promotion of cytotoxic T lymphocytes and make them behave like opsonins, thus activating the complementary system C1q pathway [10]. The formation of ICs happens continuously whenever the ADA finds the MAb in the bloodstream [66].
In addition to the roles mentioned above, some IC molecules can generate type III hypersensitivity reactions that are observable when these complexes are deposited in diffuse capillaries. This and by the ability of ICs to cross-link with complement receptors and Fcγ receptors can cause vascular thrombosis and increase inflammation in that region [67].
The formation of antibodies has come under increasing scrutiny as one of the mechanisms that could explain the reduced efficacy or even the total therapeutic failure of some drugs. Among the main acute clinical adverse effects associated with MAbs are flushing, shortness of breath, hives, and chest tightness. All can occur with the presence of ADA. These symptoms are seen mainly in patients who produce anti-IFX antibodies [68]. Some types of serious reactions can happen, such as delayed-type hypersensitivity after the infusion, characterized by fever, rash, itching, myalgia, headaches [69].
Baert et al. analyzed the formation of ADA in patients with CD receiving IFX infusions. After the first infusion, about 40% of them developed anti-IFX antibodies, and after the fifth infusion, this number increased to 61%. He also noted that the presence of these antibodies was associated with a higher incidence of some type of reaction during the infusion, and also with a less effective clinical response [68]. Similarly, Cheifetz also analyzed the incidence of reactions to IFX infusion in CD patients. About 5% of them experienced reactions during the infusions, and less than 1% experienced a delayed reaction [31].
A way to decrease the chances of MAbs to trigger the immune system is to concomitantly add immunomodulatory medications, such as azathioprine, 6-mercaptopurine, or methotrexate, thus avoiding loss of response. Several studies analyzed the impact of adding these medications, and whether in their presence the amount of anti-drug antibodies decreased [18,71-73]. Although the mechanism that allows the effects of immunosuppressants on immunogenicity to happen is not yet known, Krieckaert et al. hypothesize that it is possibly due to the suppression of the immune response. This may block the expansion of immunoreactive cells, which leads to reduced formation of antibodies against the drugs [74].
Vermeire et al. sought to analyze the impact of azathioprine and methotrexate, administered concomitantly with infliximab, in patients with CD. In addition to the lower incidence of antibodies against infliximab in those patients who used immunomodulators, patients who did not receive the concomitant medication had very low serum MAb levels [75]. Correspondingly, a similar pattern was observed with Adalimumab. When an immunosuppressive agent such as azathioprine or methotrexate was used, less ADA was produced and, consequently, a lower incidence of adverse effects occurred [74]. The same was not observed when corticosteroids or aminosalicylates were used [56].
Bodini also analyzed the formation of ADAs in patients using Adalimumab. CD patients who had not previously received IFX, who were in remission, and who were in the medication maintenance phase were included. The results showed that the development and presence of ADAs influenced the serum level of Adalimumab, and that this led to the promotion of clinical relapse [76]. Similarly, Yarur et al. investigated the association between levels of ADAs with levels of Adalimumab and CD endoscopic activity. They found that patients with active disease showed high serum levels of anti-TNF-α due to the inability of the medication to neutralize the production of TNF-α in the tissue [77].
In addition to the formation of ADAs as one of the main causes of MAbs therapeutic failure, researchers also sought to analyze the formation of ICs as one of the contributing factors to this deficient response. Few studies have analyzed the role of ICs specifically in IBD, and further studies are needed to contribute to a better understanding. Most of what is known came from studies on rheumatological diseases. A significant number of patients with rheumatoid arthritis develop ADA after receiving both IFX and Adalimumab in the first 6 months of therapy [78-80]. Many of the adverse effects are believed to be related to the formation of ICs induced by the use of medication [62]. In an experimental study performed by Arnoult et al., mice received doses of IFX or Adalimumab intending to analyze the IC formation induced by these medications. Their results demonstrated that both knockout and wild-type animals formed an immune response against MAbs, which suggests that ICs play a determining role in the therapeutic response [81].
Measurement of immunogenicity compounds
As immunogenicity is correlated with changes in drug efficacy, as well as patient safety, the quantification of drug levels along with the measurement of ADAs makes it possible to identify if the patient is responding adequately to the therapy. Depending on the amounts of drug and ADAs levels, they can help to identify a possible pathway that is leading to this clinical non-response [82].
With the inclusion of anti-TNF-α as a therapy for IBD, these biological compounds may act as a trigger for the immune system, so immunogenicity may occur. Studies conducted by Vermeire et al. and Strand et al. analyzed the relationship among the MAbs used for both CD and UC treatment and their capability to induce immunogenicity. Their data have demonstrated that the immunogenicity rate varies from 0.0% to 65.3% for IFX and 0.3% to 38.0% for adalimumab [75,83].
Strategies to determine the response to the medication can be made empirically, by observing the patient’s reaction, or by evaluating serum drug levels and their correlation to the measurement of ADAs [84]. This latter strategy is called Therapeutic Drug monitoring (TDM) and the literature has shown satisfactory results concerning the cost-benefit ratio of its application [85,86].
With TDM, not only did patients experience lower hospitalization rates (22% versus 35% in the group without monitoring), but there were also 24% savings from using optimized treatment [84]. TDM can be performed reactively, when the patient already shows the first signs of loss of response. This demonstrates the need to investigate possible immunogenicity, and then to choose a more appropriate medication. TDM can also be mannered proactively, in which the patient still responds positively to the treatment. However, in order to maintain its effectiveness, an analysis must be made to avoid subtherapeutic or supratherapeutic drug levels, preventing relapses [85].
Several studies seek to understand the best way to perform TDM, proactively or reactively. Initially, the guidelines supported the reactive practice, with the intention to optimize doses when there was loss of response. However, because this strategy does not maximize its best use, proactive TDM has been given greater consideration [86]. The TAXIT (Trough Concentration Adapted Infliximab Treatment) trial demonstrated that when TDM was performed proactively, it was associated with lower frequencies of undetectable IFX levels, and consequently a lower chance of relapse [87]. Similarly, in a study by Amiot et al., the authors demonstrated that in cases of remission, IFX de-escalation has better results when using TDM instead of symptoms and tests such as C-Reactive Protein (CRP) [88]. Concerning more severe cases and those that present higher drug clearance, proactive TDM also proved to be indicated more often [89].
One of the most used techniques for these purposes is the enzyme-linked immunosorbent assay (ELISA). This methodology is a technique that makes possible the detection and quantification of soluble substances, including antibodies. Its main advantage is its high sensitivity and specificity. Despite being an excellent method, among its disadvantages are the need for specialized technicians to perform it and the fact that its reagents need to be handled with care since their degradation occurs mainly when exposed to sources of heat or light [90].
Several companies already produce commercial ELISA kits to detect serum levels of MAbs and ADAs, such as Lisa-Tracker® (Theradiag, Marne-la-Vallée, France) and Promonitor® (Proteomika S.L., subsidiary of Progenika Biopharma S.A., Spain). The tests differ from each other in terms of their kit methodology, such as the Lisa-Tracker®, which is an ELISA that uses its wells pre-coated with TNF-α, while Promonitor®, as a capture ELISA, has its wells covered with anti-TNF monoclonal antibody bound to recombinant TNF-α [91,92], but both are effective and reliable.
New technologies have emerged to improve ELISA tests, making them more sensitive and faster to perform. One of these improvements is the Multiplex, a system that is similar to the ELISA, and derived from it. However, it uses magnetic beads that allow for multiple analyses in a single experiment. Despite being a more refined and improved technique, its use is more common for research than in the clinic itself [93].
There are also commercial Multiplex kits that allow the quantification of serum levels of different MAbs in the same experiment, such as SIL-Infliximab and SIL-Adalimumab (MilliporeSigma®, a Merck® brand). This technique allowed for improvements in both reproducibility and accuracy in the quantification of MAbs of IFX and Adalimumab in routines [94].
Likewise, it is also possible to use the ELISA technique to measure circulating ICs in both serum and blood plasma. Since the formation of ICs is associated with several pathologies such as autoimmune, rheumatological diseases, bacterial and viral infections, in addition to allergies. The measurement of these complexes is extremely important [95-100]. As previously stated, despite their effectiveness, they are often performed only in the laboratory environment. The need for specific equipment and reagents makes them unviable in the clinic.
With this purpose in mind, researchers have been working towards simplifying these tests, so that they can be made outside laboratories, with fewer reagents, while maintaining their quality and sensitivity. In the context of IBD, these advances have produced rapid-result tests. Through the lateral flow test (LFT) technique, it is possible to detect the presence of the desired substance without the need for the use of specialized equipment. This technique, commonly used in everyday life as in pregnancy tests, where the beta HCG is measured in a strip, is a simple, economical method that gives its result in a short time [101].
One of the tests already in use is the measurement of calprotectin, a protein derived from neutrophils. It is not metabolized by the intestine, remains intact in the stool, and is considered a marker of gastrointestinal inflammation [102]. Its presence in the feces can indicate disease activity, relapse, or healing of the mucosa, and can also aid in identifying the best appropriate management for that patient [103,104]. Through LFT, is possible to quantify the presence of calprotectin in the patient sample. If the amount of this protein is low, it indicates that the patient does not have active inflammatory disease, so more invasive tests such as colonoscopies can be avoided. If the situation is the opposite, and the protein is presented in high quantities, imaging and/or endoscopic exams must be performed, mainly to identify the site of inflammation.
LFT tests are now commercially available to measure the serum level of some MAbs such as IFX and Adalimumab, like those from Bülhmann® (Schönenbuch, Switzerland) [105]. These tests do not require complex apparatus, and all the necessary reagents come with the kit, which is composed of the diluent and a cassette in which the diluted sample is added. In 15 minutes, the amount of medication that the patient has in the peripheral circulation is available.
Several studies have shown that although these tests are rapid, their efficiency and applicability are similar to the results obtained by ELISA measurements, such as those performed by Laserna-Mendieta et al. and Gomes et al. [88,106]. Likewise, this company has also recently developed kits for measuring the ADAs generated by these MAbs. The technique works in the same way as the LFT serum dosage tests, with the same methodology and execution.
Anti-TNF-α through levels monitoring in clinical practice
Monitoring drug levels and the presence of ADAs is possible and has been widely used in clinical practice. However, it is important to know the ideal moment to perform its evaluation. Vermeire et al. claim that the moment of measurement can influence the detection rate of both drug and ADAs levels. They observed that some assays are not able to detect the presence of anti-drug antibody because the concentration of the drug at the time of collection (before the administration of the next dose) is lower [75].
Lower levels of MAbs, as well as the presence of ADAs, have been identified as the most important factors in the secondary loss of response during treatment of patients with anti-TNF-α therapy [68,107].
Clinically, the intensification of drug dosage regimen is frequently performed empirically, based on the patients’ symptoms. The continuation of the treatment already established is maintained until there is no response and the patient needs to be submitted to another class of medication to regain the ideal response. Therefore, the use of TDM for serum levels and the detection of ADAs may require a personalized approach to identify an early failure of the anti-TNF-α agent [108]. With this information, the best medical conduct can be determined, benefiting the patient with a specific therapeutic adjustment of the drug (Figure 1).
Figure 1.
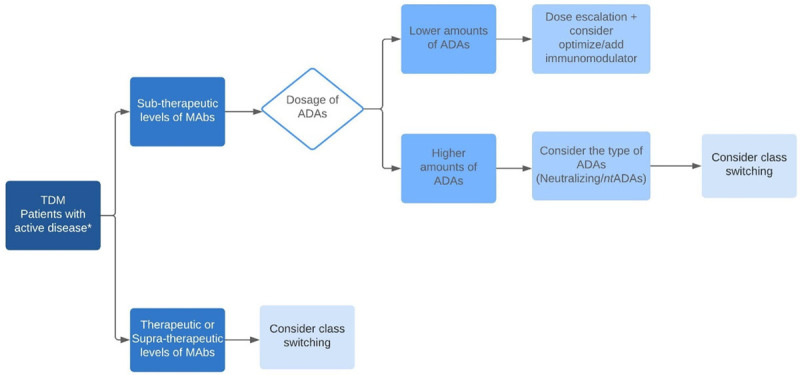
Monitoring Drug Levels and ADAs formation. Clinical management, when accompanied by TDM, contributes to conducting the patients’ treatment, attending to their clinical needs more appropriately, and improving their quality of life. * Non-responders, determined by endoscopic and radiological examination.
The presence of antibodies is not always related to the low levels of the drug and a worse outcome. This finding can be justified by the existence of two types of antibodies, the transient antibody and the persistent antibody. The persistent antibody does not become extinct over time. However, the transient antibody is inconsistent and certainly does not affect drug levels [109].
Bodini et al. performed a study that analyzed the usefulness of assessing minimum drug levels early. They observed that the presence of persistent antibodies, when compared to temporary antibodies, affects serum concentrations of MAbs, and that the patients who developed persistent antibodies more frequently showed lower response during treatment [110]. Patients with persistent antibodies may develop low drug levels, and this will affect the patient’s clinical outcome. However, temporary antibodies appear to be biologically inactive [111].
Mitrev et al. demonstrated that undetectable levels of anti-TNF-α facilitated sustained remission after a period of drug pause, associated with endoscopic remission, and normal levels of inflammatory biomarkers [107]. Although drug monitoring is used to improve anti-TNF-α treatment, it has not been used to identify adverse reactions to the anti-TNF-α agent.
Concentrations of drug levels can be classified as therapeutic or subtherapeutic, and antibody levels as detectable or undetectable. These classifications are used to assess whether there was a loss of response due to immunogenicity or as a result of non-immune mediated pharmacokinetics or pharmacodynamics [112].
Although there are several commercial tests, some of them present benefits, such as rapid quantification and the ease of handling equipment and materials. Other types of tests may demand specialized technicians and require a longer time to be performed. Some of the disadvantages presented by rapid tests have been described in a cohort of patients under the use of IFX and have demonstrated especially their inability to accurately detect samples with positive antibodies [113]. Another issue concerning rapid tests is that this method might be used to quantify levels of antibodies in serum samples with undetectable levels of IFX. However, it wasn’t effective in quantifying samples with IFX concentrations ≥0.4 µg/ml [114].
In a comparison between the established ELISA assay and rapid tests used to measure IFX levels, it was observed that these tests cannot be interchanged to measure drug levels in IBD patients [113].
Although ELISA assays and rapid tests are not interchangeable, the mathematical correlation of the minimum levels of IFX measured in the tests does hold well. However, the comparison between assays should be performed with caution, because just a good mathematical correlation is not sufficient by itself [115].
From bench to bedside: from labs to clinics
The importance of basic research for obtaining knowledge, understanding processes, in addition to developing and proving hypotheses has already been described. However, there is a big barrier between having the knowledge and actually applying it, especially in the clinic. Hence, translational research, in which the knowledge obtained from basic research, mainly concerning molecular approaches, is one of the most pursued nowadays and can be effectively used in clinical tests.
Translational research makes it possible to develop new drugs, medications, and the identification of biomarkers that can directly improve the patient’s life. Both basic and applied research complement each other. Basic research provides the knowledge to be analyzed translationally. On the other hand, clinical experience can help to identify molecules and targets more quickly.
The creation of tests for monitoring diseases is a product of translational research (Figure 2). For these tests to be developed, many previous experiments, which identified the molecules, their pathways, and their mode of action, needed to be analyzed in isolation, so that, when brought together, they may achieve the desired result.
Figure 2.
From Bench to Bedside. New translational technologies make it possible for the knowledge obtained by basic research on immunogenicity to have a direct positive impact on the treatment of IBD patients who are undergoing biological therapy.
To be able to provide a fast test result, technology is already being used in the clinic. The speed and the ease of performing the test outside of a laboratory or at the bedside bring several benefits to the patients, who can have their treatment performed in a personalized and faster way, using more specific medications, or even analyzing the need for a complementary invasive investigation. The development of rapid tests, especially the TDM type that does not require a laboratory bench, brings numerous advantages both in terms of clinical and financial benefits. Measuring the individual need of the patient and adjusting the doses to those that are necessary, the treatment can be more accurate and effective, and its cost can decrease because unnecessary doses will be avoided.
Despite the various advances that have been already achieved, there is still a need for new translational studies to be performed. They must guarantee the effectiveness of these tests so that the result obtained is reliable because the patients’ kind of treatment will be directly affected by it. Since IBD are still incurable diseases, translational technologies become excellent contributors to provide a better quality of life for these patients.
Conclusion
In conclusion, the present review correlated the occurrence of clinical therapeutic failure in patients with IBD that use monoclonal antibodies as treatment. By characterizing immunogenicity, we explained the main factors that lead to loss of response, such as the formation of anti-drug antibodies and immune complexes. Our review also demonstrated the importance of monitoring the drug therapy, diagnosing early, or even preventing the occurrence of immunogenicity in these patients.
Acknowledgements
We thank Prof. Tristan Torriani for revising the English version of our manuscript. This work was supported by the National Council for Scientific and Technological Development (CNPq) [Grant number #301388/2018-0 for R.F.L.]. L.M.G. (author) was supported by São Paulo Research Foundation (FAPESP) [Grant number #2020/01924-5].
Disclosure of conflict of interest
None.
Abbreviations
- ADAs
Anti-Drug Antibodies
- bADAs
Non-Neutralizing ADAs
- CRP
C-Reactive Protein
- FDA
Food and Drug Administration
- HCG
Human Chorionic Gonadotropin
- IBD
Inflammatory Bowel Disease
- ICs
Immuno-complexes
- IFN-γ
Interferon-gamma
- IFX
Infliximab
- Ig
Immunoglobulin
- IgG1
Immunoglobulin G1
- IL-12
Interleukin-12
- IL-17
Interleukin-17
- IL-17A
Interleukin-17A
- IL-22
Interleukin-22
- IL-23
Interleukin-23
- ILCs
Innate Lymphoid Cells
- LFT
Lateral Flow Test
- ntADAs
Neutralizing ADAs
- TDM
Therapeutic Drug Monitoring
- Th1
T helper 1
- Th17
T helper 17
- TNBS
Trinitrobenzenesulfonic Acid
- TNFR-1
TNF receptor-1
- TNFR-2
TNF receptor-2
- TNF-α
Tumor Necrosis Factor-Alpha
- UC
Ulcerative Colitis
References
- 1.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 2.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389:1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 3.Goldman L, Aussielo D. C ecil: tratado de Medicina Interna. Elsevier. 2005 [Google Scholar]
- 4.Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92:1088–1103. doi: 10.1016/j.mayocp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Cardozo WS, Sobrado CW. Doença inflamatória intestinal. Editora Manole. 2015 [Google Scholar]
- 6.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 7.Flynn S, Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am. 2019;99:1051–1062. doi: 10.1016/j.suc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 8.GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807–4812. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas AK, Lichtmann AH, Pillai S. Philadelphia: Elsevier Saunders; 2018. Cellular and molecular immunology. [Google Scholar]
- 11.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 12.Murphy K, Travers P, Walport M, Janeway C. New York: Garland Science; 2012. Janeway’s immunobiology. [Google Scholar]
- 13.MacEwan DJ. TNF ligands and receptors--a matter of life and death. Br J Pharmacol. 2002;135:855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leppkes M, Roulis M, Neurath MF, Kollias G, Becker C. Pleiotropic functions of TNF-α in the regulation of the intestinal epithelial response to inflammation. Int Immunol. 2014;26:509–515. doi: 10.1093/intimm/dxu051. [DOI] [PubMed] [Google Scholar]
- 15.Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Büschenfelde KH, Strober W, Kollias G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol. 1997;27:1743–1750. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;16:992–1006. doi: 10.1016/j.immuni.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 19.Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S, Rutgeerts P. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400. e393. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 20.Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 21.Reimund JM, Wittersheim C, Dumont S, Muller CD, Kenney JS, Baumann R, Poindron P, Duclos B. Increased production of tumour necrosis factor-alpha interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn’s disease. Gut. 1996;39:684–689. doi: 10.1136/gut.39.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monteleone G, Parrello T, Luzza F, Pallone F. Response of human intestinal lamina propria T lymphocytes to interleukin 12: additive effects of interleukin 15 and 7. Gut. 1998;43:620–628. doi: 10.1136/gut.43.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn’s disease. Am J Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]
- 25.Sarra M, Monteleone I, Stolfi C, Fantini MC, Sileri P, Sica G, Tersigni R, Macdonald TT, Pallone F, Monteleone G. Interferon-gamma-expressing cells are a major source of interleukin-21 in inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:1332–1339. doi: 10.1002/ibd.21238. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs DF, Wannacher L. Rio de Janeiro: Guanabara Koogan; 2010. Farmacologia clínica: fundamentos da terapêutica racional. [Google Scholar]
- 27.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjösberg JM, Spits H. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 28.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 30.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheifetz AS. Management of active Crohn disease. JAMA. 2013;309:2150–2158. doi: 10.1001/jama.2013.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 33.Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD) Pharmacol Rep. 2011;63:629–642. doi: 10.1016/s1734-1140(11)70575-8. [DOI] [PubMed] [Google Scholar]
- 34.Scientific Writing Team of Nuventra Pharma Sciences. Monoclonal antibodies: past, present and future. 2018 [Google Scholar]
- 35.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 36.Kurosawa K, Lin W, Ohta K. Chimeric antibodies. Methods Mol Biol. 2014;1060:139–148. doi: 10.1007/978-1-62703-586-6_8. [DOI] [PubMed] [Google Scholar]
- 37.Grupo de estudos da doença inflamatória intestinal do Brasil. Consenso sobre tratamento da doença inflamatória intestinal. Arquivos de Gastroenterologia. 2010 [Google Scholar]
- 38.Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brasil Gdeddiid. Consenso sobre tratamento da doença inflamatória intestinal. Arquivos de Gastroenterologia. 2010;47:313–325. [Google Scholar]
- 40.Rutgeerts P, D’Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, Mayer L, Van Hogezand RA, Braakman T, DeWoody KL, Schaible TF, Van Deventer SJ. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–769. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 41.Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A, Johanns J, Lu J, Horgan K, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250–1260. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 42.Safdari Y, Farajnia S, Asgharzadeh M, Khalili M. Antibody humanization methods - a review and update. Biotechnol Genet Eng Rev. 2013;29:175–186. doi: 10.1080/02648725.2013.801235. [DOI] [PubMed] [Google Scholar]
- 43.Waldmann H. Human monoclonal antibodies: the benefits of humanization. Methods Mol Biol. 2019;1904:1–10. doi: 10.1007/978-1-4939-8958-4_1. [DOI] [PubMed] [Google Scholar]
- 44.Abbvie Farmacêutica. Humira (Adalimumabe) [bula do medicamento] [Google Scholar]
- 45.Sandborn WJ, Hanauer S, Loftus EV Jr, Tremaine WJ, Kane S, Cohen R, Hanson K, Johnson T, Schmitt D, Jeche R. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn’s disease. Am J Gastroenterol. 2004;99:1984–1989. doi: 10.1111/j.1572-0241.2004.40462.x. [DOI] [PubMed] [Google Scholar]
- 46.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 47.Colombel JF, Sandborn WJ, Ghosh S, Wolf DC, Panaccione R, Feagan B, Reinisch W, Robinson AM, Lazar A, Kron M, Huang B, Skup M, Thakkar RB. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: data from ULTRA 1, 2, and 3. Am J Gastroenterol. 2014;109:1771–1780. doi: 10.1038/ajg.2014.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ministério da Saúde. Cimzia® Certolizumab Pegol [Google Scholar]
- 49.Yamazaki H, So R, Matsuoka K, Kobayashi T, Shinzaki S, Matsuura M, Okabayashi S, Kataoka Y, Tsujimoto Y, Furukawa TA, Watanabe N. Certolizumab pegol for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2019;8:Cd012893. doi: 10.1002/14651858.CD012893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 51.Stein AC, Rubin DT, Hanauer SB, Cohen RD. Incidence and predictors of clinical response, re-induction dose, and maintenance dose escalation with certolizumab pegol in Crohn’s disease. Inflamm Bowel Dis. 2014;20:1722–1728. doi: 10.1097/MIB.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 52.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987–995. doi: 10.1111/j.1365-2036.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- 53.Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 54.Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106:674–684. doi: 10.1038/ajg.2011.60. [DOI] [PubMed] [Google Scholar]
- 55.Nakase H, Motoya S, Matsumoto T, Watanabe K, Hisamatsu T, Yoshimura N, Ishida T, Kato S, Nakagawa T, Esaki M, Nagahori M, Matsui T, Naito Y, Kanai T, Suzuki Y, Nojima M, Watanabe M, Hibi T. Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalised pharmacokinetics in patients with Crohn’s disease: a subanalysis of the DIAMOND trial. Aliment Pharmacol Ther. 2017;46:873–882. doi: 10.1111/apt.14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber RW. Adverse reactions to biological modifiers. Curr Opin Allergy Clin Immunol. 2004;4:277–283. doi: 10.1097/01.all.0000136757.58818.10. [DOI] [PubMed] [Google Scholar]
- 57.Purcell RT, Lockey RF. Immunologic responses to therapeutic biologic agents. J Investig Allergol Clin Immunol. 2008;18:335–342. [PubMed] [Google Scholar]
- 58.Willrich MA, Murray DL, Snyder MR. Tumor necrosis factor inhibitors: clinical utility in autoimmune diseases. Transl Res. 2015;165:270–282. doi: 10.1016/j.trsl.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Aarden L, Ruuls SR, Wolbink G. Immunogenicity of anti-tumor necrosis factor antibodies-toward improved methods of anti-antibody measurement. Curr Opin Immunol. 2008;20:431–435. doi: 10.1016/j.coi.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Bendtzen K. Personalized medicine: theranostics (therapeutics diagnostics) essential for rational use of tumor necrosis factor-alpha antagonists. Discov Med. 2013;15:201–211. [PubMed] [Google Scholar]
- 61.Bloem K, Hernández-Breijo B, Martínez-Feito A, Rispens T. Immunogenicity of therapeutic antibodies: monitoring antidrug antibodies in a clinical context. Ther Drug Monit. 2017;39:327–332. doi: 10.1097/FTD.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 62.Krishna M, Nadler SG. Immunogenicity to biotherapeutics - the role of anti-drug immune complexes. Front Immunol. 2016;7:21. doi: 10.3389/fimmu.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 64.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 65.Bendtzen K. Immunogenicity of anti-TNF-α biotherapies: II. clinical relevance of methods used for anti-drug antibody detection. Front Immunol. 2015;6:109. doi: 10.3389/fimmu.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voice JK, Lachmann PJ. Neutrophil Fc gamma and complement receptors involved in binding soluble IgG immune complexes and in specific granule release induced by soluble IgG immune complexes. Eur J Immunol. 1997;27:2514–2523. doi: 10.1002/eji.1830271008. [DOI] [PubMed] [Google Scholar]
- 67.Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009;120:2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baert F, Noman M, Vermeire S, Van Assche G, D’ Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 69.Han PD, Cohen RD. Managing immunogenic responses to infliximab: treatment implications for patients with Crohn’s disease. Drugs. 2004;64:1767–1777. doi: 10.2165/00003495-200464160-00004. [DOI] [PubMed] [Google Scholar]
- 70.Danese S, Fiorino G, Peyrin-Biroulet L. Positioning therapies in ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:1280–1290. doi: 10.1016/j.cgh.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, Dijkmans BA, Tak PP, Wolbink GJ. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66:921–6. doi: 10.1136/ard.2006.065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Colombel JF, Panaccione R, D’Haens G, Li J, Rosenfeld MR, Kent JD, Pollack PF. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–38. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 73.West RL, Zelinkova Z, Wolbink GJ, Kuipers EJ, Stokkers PC, van der Woude CJ. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2008;28:1122–6. doi: 10.1111/j.1365-2036.2008.03828.x. [DOI] [PubMed] [Google Scholar]
- 74.Krieckaert CL, Bartelds GM, Lems WF, Wolbink GJ. The effect of immunomodulators on the immunogenicity of TNF-blocking therapeutic monoclonal antibodies: a review. Arthritis Res Ther. 2010;12:217. doi: 10.1186/ar3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol. 2018;11:1756283x17750355. doi: 10.1177/1756283X17750355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bodini G, Savarino V, Dulbecco P, Baldissarro I, Savarino E. The influence of anti-adalimumab antibodies on adalimumab trough levels, TNF-α levels and clinical outcome. Journal of Crohn’s and Colitis. 2014;8:S42. [Google Scholar]
- 77.Yarur AJ, Jain A, Sussman DA, Barkin JS, Quintero MA, Princen F, Kirkland R, Deshpande AR, Singh S, Abreu MT. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut. 2016;65:249–255. doi: 10.1136/gutjnl-2014-308099. [DOI] [PubMed] [Google Scholar]
- 78.Anderson PJ. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum. 2005;34:19–22. doi: 10.1016/j.semarthrit.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, Dijkmans BA, Aarden L, Wolbink GJ. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 80.Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, van Riel PL, Bendtzen K. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1739–1745. doi: 10.1136/ard.2008.092833. [DOI] [PubMed] [Google Scholar]
- 81.Arnoult C, Brachet G, Cadena Castaneda D, Azzopardi N, Passot C, Desvignes C, Paintaud G, Heuzé-Vourc’h N, Watier H, Gouilleux-Gruart V. Crucial role for immune complexes but Not FcRn in Immunization against anti-TNF-α antibodies after a single injection in mice. J Immunol. 2017;199:418–424. doi: 10.4049/jimmunol.1601246. [DOI] [PubMed] [Google Scholar]
- 82.Mojtahed Poor S, Ulshöfer T, Gabriel LA, Henke M, Köhm M, Behrens F, Geisslinger G, Parnham MJ, Burkhardt H, Schiffmann S. Immunogenicity assay development and validation for biological therapy as exemplified by ustekinumab. Clin Exp Immunol. 2019;196:259–275. doi: 10.1111/cei.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strand V, Goncalves J, Isaacs JD. Immunogenicity of biologic agents in rheumatology. Nat Rev Rheumatol. 2021;17:81–97. doi: 10.1038/s41584-020-00540-8. [DOI] [PubMed] [Google Scholar]
- 84.Guidi L, Pugliese D, Panici Tonucci T, Berrino A, Tolusso B, Basile M, Cantoro L, Balestrieri P, Civitelli F, Bertani L, Marzo M, Felice C, Gremese E, Costa F, Viola F, Cicala M, Kohn A, Gasbarrini A, Rapaccini GL, Ruggeri M, Armuzzi A. Therapeutic drug monitoring is more cost-effective than a clinically based approach in the management of loss of response to infliximab in inflammatory bowel disease: an observational multicentre study. J Crohns Colitis. 2018;12:1079–1088. doi: 10.1093/ecco-jcc/jjy076. [DOI] [PubMed] [Google Scholar]
- 85.Mitrev N, Vande Casteele N, Seow CH, Andrews JM, Connor SJ, Moore GT, Barclay M, Begun J, Bryant R, Chan W, Corte C, Ghaly S, Lemberg DA, Kariyawasam V, Lewindon P, Martin J, Mountifield R, Radford-Smith G, Slobodian P, Sparrow M, Toong C, van Langenberg D, Ward MG, Leong RW. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46:1037–1053. doi: 10.1111/apt.14368. [DOI] [PubMed] [Google Scholar]
- 86.Vermeire S, Dreesen E, Papamichael K, Dubinsky MC. How, when, and for whom should we perform therapeutic drug monitoring? Clin Gastroenterol Hepatol. 2020;18:1291–1299. doi: 10.1016/j.cgh.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 87.Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, Simoens S, Rutgeerts P, Gils A, Vermeire S. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–9. doi: 10.1053/j.gastro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 88.Amiot A, Hulin A, Belhassan M, Andre C, Gagniere C, Le Baleur Y, Farcet JP, Delchier JC, Hüe S. Therapeutic drug monitoring is predictive of loss of response after de-escalation of infliximab therapy in patients with inflammatory bowel disease in clinical remission. Clin Res Hepatol Gastroenterol. 2016;40:90–8. doi: 10.1016/j.clinre.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 89.Cheifetz AS, Abreu MT, Afif W, Cross RK, Dubinsky MC, Loftus EV Jr, Osterman MT, Saroufim A, Siegel CA, Yarur AJ, Melmed GY, Papamichael K. A comprehensive literature review and expert consensus statement on therapeutic drug monitoring of biologics in inflammatory bowel disease. Am J Gastroenterol. 2021;116:2014–2025. doi: 10.14309/ajg.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crowther JR. Springer; 2009. The ELISA guidebook. [DOI] [PubMed] [Google Scholar]
- 91.Theradiag. https://www.clsdiagnostics.com/files/lisa-trackerv06-2017-uk-6pg.pdf. Lisa-Tracker Datasheet.
- 92.Proteomika Biopharma. Progenika. http://promonitor.progenika.com/images/stories/PromonitorBrochure_EN.pdf. Promonitor Datasheet.
- 93.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jalili PR, Ray K, Sigma M. Multiplex Quantification of Infliximab and Adalimumab in Human Serum by LC -MS/MS Using Full-Length Stable Isotope Labeled Internal Standards. [Internet] https://www.sigmaaldrich.com/BR/pt/technical-documents/technical-article/protein-biology/protein-mass-spectrometry/multiplex-quantification-of-infliximab-and-adalimumab. Accessed in January 12th, 2021.
- 95.Nydegger UE, Lambert PH, Gerber H, Miescher PA. Circulating immune complexes in the serum in systemic lupus erythematosus and in carriers of hepatitis B antigen. Quantitation by binding to radiolabeled C1q. J Clin Invest. 1974;54:297–309. doi: 10.1172/JCI107765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Genovese MC, Davis JS 4th. Soluble immune complexes in human disease. CRC Crit Rev Clin Lab Sci. 1980;12:123–170. doi: 10.3109/10408368009108728. [DOI] [PubMed] [Google Scholar]
- 97.Use and abuse of laboratory tests in clinical immunology: critical considerations of eight widely used diagnostic procedures. Report of a joint IUIS/WHO meeting on assessment of tests used in clinical immunology. Clin Immunol Immunopathol. 1982;24:122–138. doi: 10.1016/0090-1229(82)90095-2. [DOI] [PubMed] [Google Scholar]
- 98.Bernstein KA, Kahl LE, Balow JE, Lefkowith JB. Serologic markers of lupus nephritis in patients: use of a tissue-based ELISA and evidence for immunopathogenic heterogeneity. Clin Exp Immunol. 1994;98:60–65. doi: 10.1111/j.1365-2249.1994.tb06607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ritzmann SE, Daniels JC. Immune complexes: characteristics, clinical correlations, and interpretive approaches in the clinical laboratory. Clin Chem. 1982;28:1259–1271. [PubMed] [Google Scholar]
- 100.Sagnelli E, Felaco FM, Triolo G, Vernace SJ, Filippini P, Piccinino F, Behrens U, Paronetto F. Circulating complement fixing immune complexes in chronic hepatitis. Use of anti-C3 enzyme immunoassay to define antibody class and nature of antigen. J Clin Lab Immunol. 1983;12:11–15. [PubMed] [Google Scholar]
- 101.Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60:111–120. doi: 10.1042/EBC20150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793–798. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 103.Lin JF, Chen JM, Zuo JH, Yu A, Xiao ZJ, Deng FH, Nie B, Jiang B. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407–1415. doi: 10.1097/MIB.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 104.Takashima S, Kato J, Hiraoka S, Nakarai A, Takei D, Inokuchi T, Sugihara Y, Takahara M, Harada K, Okada H, Tanaka T, Yamamoto K. Evaluation of Mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am J Gastroenterol. 2015;110:873–880. doi: 10.1038/ajg.2015.66. [DOI] [PubMed] [Google Scholar]
- 105.Bühlmann. Quantum Blue TDM. https://www.buhlmannlabs.ch/products-solutions/gastroenterology/quantum-blue/quantum-blue-tdm/
- 106.Laserna-Mendieta EJ, Salvador-Martín S, Arias-González L, Ruiz-Ponce M, Menchén LA, Sánchez C, López-Fernández LA, Lucendo AJ. Comparison of a new rapid method for the determination of adalimumab serum levels with two established ELISA kits. Clin Chem Lab Med. 2019;57:1906–1914. doi: 10.1515/cclm-2019-0202. [DOI] [PubMed] [Google Scholar]
- 107.Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf. 2017;16:303–317. doi: 10.1080/14740338.2017.1269169. [DOI] [PubMed] [Google Scholar]
- 108.Yanai H, Lichtenstein L, Assa A, Mazor Y, Weiss B, Levine A, Ron Y, Kopylov U, Bujanover Y, Rosenbach Y, Ungar B, Eliakim R, Chowers Y, Shamir R, Fraser G, Dotan I, Ben-Horin S. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015;13:522–530. e522. doi: 10.1016/j.cgh.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 109.Vande Casteele N, Gils A, Singh S, Ohrmund L, Hauenstein S, Rutgeerts P, Vermeire S. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962–971. doi: 10.1038/ajg.2013.12. [DOI] [PubMed] [Google Scholar]
- 110.Bodini G, Giannini EG, Savarino V, Del Nero L, Lo Pumo S, Brunacci M, De Bortoli N, Jain A, Tolone S, Savarino E. Infliximab trough levels and persistent vs transient antibodies measured early after induction predict long-term clinical remission in patients with inflammatory bowel disease. Dig Liver Dis. 2018;50:452–456. doi: 10.1016/j.dld.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 111.Steenholdt C, Al-khalaf M, Brynskov J, Bendtzen K, Thomsen O, Ainsworth MA. Clinical implications of variations in anti-infliximab antibody levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2209–2217. doi: 10.1002/ibd.22910. [DOI] [PubMed] [Google Scholar]
- 112.Steenholdt C, Brynskov J, Thomsen O, Munck LK, Fallingborg J, Christensen LA, Pedersen G, Kjeldsen J, Jacobsen BA, Oxholm AS, Kjellberg J, Bendtzen K, Ainsworth MA. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–927. doi: 10.1136/gutjnl-2013-305279. [DOI] [PubMed] [Google Scholar]
- 113.Lim MH, Aluzaite K, Schultz M, Casey P. Infliximab trough levels: a comparison between the quantum blue infliximab assay and the established ELISA. J Gastroenterol Hepatol. 2020;35:1302–1306. doi: 10.1111/jgh.14964. [DOI] [PubMed] [Google Scholar]
- 114.Rocha C, Lago P, Fernandes S, Correia L, Portela F, Vieira AI, Patita M, Arroja B, Ministro P, Alves C, Dias CC, Magro F. Rapid test detection of anti-infliximab antibodies: performance comparison with three different immunoassays. Therap Adv Gastroenterol. 2020;13:1756284820965790. doi: 10.1177/1756284820965790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bertin D, Serrero M, Grimaud JC, Desjeux A, Desplat-Jégo S. Monitoring of infliximab trough levels and anti-infliximab antibodies in inflammatory bowel diseases: a comparison of three commercially available ELISA kits. Cytokine. 2020;126:154859. doi: 10.1016/j.cyto.2019.154859. [DOI] [PubMed] [Google Scholar]


