Abstract
Objective: To investigate the therapeutic effect and safety of different doses of apatinib mesylate combined with chemotherapy in the treatment of advanced oral cancer. Methods: Totally 100 patients with advanced oral cancer admitted to our hospital from January 2019 to July 2020 were retrospectively analyzed and divided into a control group (500 mg apatinib mesylate combined with chemotherapy) and an experimental group (250 mg apatinib mesylate combined with chemotherapy). The two groups were compared in terms of the incidence of adverse reactions, treatment effective rate, disease control rate, objective response rate, Karnofsky performance status (KPS) score (quality of life), score of the mental status scale in non-psychiatric settings (MSSNS), survival rates and vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor 2 (VEGFR-2) after treatment. In addition, logistic regression was used to analyze the influencing factors for KPS<85 after oral cancer treatment. Results: The treatment effective rate, disease control rate, objective response rate, KPS score (quality of life), survival rates in the experimental group were all significantly improved compared to those in the control group (all P<0.05), and the incidence of adverse reactions, MSSNS score, and the levels of VEGF and VEGFR-2 after treatment in the experimental group were significantly lower than those in the control group (all P<0.05). Furthermore, a history of smoking, a history of drinking, a tooth brushing index <3, the frequency of teeth cleansing ≤1 time per year, a history of oral diseases >3 times, and poor nutritional status were independent risk factors for KPS<85 after oral cancer treatment. Conclusion: Apatinib mesylate (250 mg) combined with chemotherapy can reach optimal efficacy with highest safety but least adverse effects for patients with advanced oral cancer.
Keywords: Apatinib mesylate, chemotherapy, advanced oral cancer, safety, effectiveness, different doses
Introduction
Oral cancer generally refers to the malignant tumors in the oral cavity, including tongue cancer, gum cancer, etc. Patients with oral cancer suffer long-lasting or repeated oral ulcers and feel pain during eating and speaking, which greatly reduces their quality of life [1-3]. Generally, the preferred treatment for such patients is surgery. However, surgery is more suitable for patients with early tumors, as they are more likely to have complete resection that can effectively inhibit cancer recurrence because of their smaller tumor tissues [4-6]. For patients with advanced oral cancer, chemotherapy is usually recommended to inhibit and inactivate tumor cells, which aims to slow down the progression, inhibit tumor cell growth and prolong the survival of patients [7-9]. Apatinib mesylate is a drug intended for the treatment of advanced cancers. At present, it is commonly used with a high dose for treatment. Despite obvious therapeutic effect, its side effects are relatively extensive, so, many patients fail to adapt to the high-dose treatment. This study mainly evaluated the therapeutic effect and safety of 250 mg and 500 mg apatinib mesylate combined with chemotherapy in the treatment of patients with (advanced) oral cancer.
Materials and methods
Materials
A total of 100 patients with advanced oral cancer admitted to our hospital from January 2019 to July 2020 were enrolled and divided into a control group and an experimental group based on the treatment method, with 50 cases in each group. Patients in the control group were 29-72 years old, and those in the experimental group were 30-71 years old. This study was approved by the hospital ethics committee (2018-12-11).
Inclusion/exclusion criteria
Inclusion criteria
(1) Patients with clinical manifestations of oral cancer in the middle or late stages; (2) Patient at an age of ≥18 years old; (3) Patients with normal liver and kidney functions; (4) Patients without other organic diseases, and history of allergies; (5) Patients voluntarily participated in the study and signed an informed consent form.
Exclusion criteria
(1) Patients with a consciousness disorder or unable to cooperate with the research; (2) Patients with other organic diseases; (3) Patients with early oral cancer.
Methods
Patients in the control group were treated with 500 mg apatinib mesylate (producer: Jiangsu Hengrui Pharmaceutical Co., Ltd.; SFDA approval number: H20140104; specification: 0.25 g) combined with chemotherapy, 2 tablets each time, once a day, until they developed severe intolerance. In contrast, patients in the experimental group received 250 mg apatinib mesylate combined with chemotherapy, 1 tablet each time, once a day, until they developed severe intolerance [10-12].
Both groups of patients received conventional chemotherapy. Specifically, Tigio capsules (producer: Jiangsu Hengrui Pharmaceutical Co., Ltd.; SFDA approval number: H20100135; Specification: 20 mg) were administered according to their body surface area: 40 mg each time and twice a day for those with the body surface less than 1.25 m2; 40 mg in the morning and 60 mg in the evening (twice a day) for those with the body surface area of 1.25-1.50 m2; 60 mg each time and twice a day for those with the body surface area larger than 1.5 m2.
Indicators
The two groups were compared in the following items: the incidence of adverse reactions, treatment effective rate, disease control rate, objective response rate, Karnofsky performance status (KPS) score (quality of life) [11], score of the mental status scale in non-psychiatric settings (MSSNS) [12], survival rates within 1 month, 2 months, and 3 months after treatment and the levels of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor 2 (VEGFR-2) after treatment [13].
Treatment efficacy was classified into markedly effective, effective and ineffective. Markedly effective: complete relief with focus cleared; Effective: partial relief without enlarged target lesion; Ineffective: no relief with increased lesion.
The lower the expression levels of VEGF and VEGFR-2, the greater the probability of survival of the patient.
The response evaluation criteria for solid tumors (RECIST) include: Complete response (CR): disappearance of all target lesions; Partial response (PR): at least a 30% decrease in the sum of the longest diameter of the measured lesions; Progressive disease (PD): a 20% or greater increase in the sum of the longest diameter of measured lesions or more new lesions; Stable disease (SD): insufficient lesion shrinkage to qualify for PR nor sufficient increase to qualify for PD [13-15]. Objective response rate (ORR) = (CR + PR)/total number*100%; disease control rate (DCR) = (CR + PR + SD)/total number*100%.
KPS has a full score of 100 points, and the higher the score, the better the health condition and the higher the tolerance to the side effects of treatment.
The MSSNS score has a dividing line of 60 points with a score below 60 indicating normal mental state, a score of 60 to 70 indicating mildly abnormal mental state, and a score of more than 70 indicating abnormal mental state.
Statistical analysis
The data in this study were all processed by the statistical analysis software SPSS20.0. The measurement data were expressed as “mean ± standard deviation” (x±S), and their comparison among multiple groups was performed by the single-factor analysis of variance or repeated measures analysis of variance, and pairwise comparison by the LSD-t-test. The count data were expressed by percentage (%), and their comparison among multiple groups was analyzed by χ2. In addition, Logistic regression was used to analyze the influencing factors for KPS<85 after oral cancer treatment. P<0.05 means that the difference is statistically significant.
Results
Comparison of general information
There was no statistical significance in general data such as gender, age, and course of disease between the two groups (P>0.05). See Table 1.
Table 1.
Statistical comparison of materials (x̅±s)
| Group | Experimental Group | Control Group | X2/t | P | |
|---|---|---|---|---|---|
| Gender (male/female) | 25/25 | 26/24 | 0.36 | 0.55 | |
| Age (year-old) | 47.62±4.25 | 47.31±4.53 | 0.35 | 0.73 | |
| Height (cm) | 169.82±9.86 | 169.76±9.26 | 0.03 | 0.98 | |
| Weight (kg) | 75.59±6.02 | 75.00±5.96 | 0.49 | 0.62 | |
| Course of Disease (months) | 3.39±0.69 | 3.32±0.73 | 0.49 | 0.62 | |
| Hypertension (number of cases) | 10 | 11 | 0.06 | 0.81 | |
| Diabetes (number of cases) | 10 | 7 | 0.64 | 0.42 | |
| Hyperlipidemia (number of cases) | 4 | 6 | 0.44 | 0.51 | |
| Tumor Types | Tongue Cancer | 22 | 20 | 0.16 | 0.69 |
| Gum Cancer | 25 | 26 | 0.04 | 0.84 | |
| Lip Cancer | 3 | 4 | 0.15 | 0.70 | |
Comparison of treatment efficacy between the two groups
Compared with the control group, the experimental group yielded a more favorable outcome in terms of the effective rate of treatment (P<0.05). See Table 2.
Table 2.
Comparison of effective rate of treatment between the two groups
| Group | Markedly effective | Effective | Ineffective | Effective rate [n (%)] |
|---|---|---|---|---|
| Experimental group | 33 | 12 | 5 | 45 (90) |
| Control group | 16 | 18 | 16 | 34 (68) |
| X2 | 7.29 | |||
| P | 0.007 |
Comparison of the incidence of adverse reactions between the two groups
The experimental group showed a lower incidence rate of adverse reactions than the control group (P<0.05). See Table 3.
Table 3.
Comparison of the incidence rate of adverse reactions between the two groups
| Group | Proteinuria | Leukopenia | Hypertension | Total Incidence Rate (%) |
|---|---|---|---|---|
| Experimental Group | 2 | 0 | 0 | 4% |
| Control Group | 5 | 1 | 3 | 18% |
| X2 | 5.01 | |||
| P | 0.03 |
Comparison of disease control rate and objective response rate between the two groups
The disease control rate and objective response rate of the experimental group were both significantly higher than those of the control group (both P<0.05). See Table 4.
Table 4.
Comparison of disease control rate and objective response rate between the two groups
| Group | CR | PR | SD | PD | Disease Control Rate (%) | Objective Response Rate (%) |
|---|---|---|---|---|---|---|
| Experimental Group | 2 | 38 | 9 | 1 | 98% | 80% |
| Control Group | 0 | 29 | 10 | 11 | 78% | 58% |
| X2 | 9.47 | 5.66 | ||||
| P | 0.002 | 0.017 |
Comparison of KPS scores (quality of life) and MSSNS scores between the two groups
A higher KPS score was obtained in the experimental group than that in the control group, and the experimental group showed a lower MSSNS score, as compared to the control group (P<0.05). See Figure 1.
Figure 1.
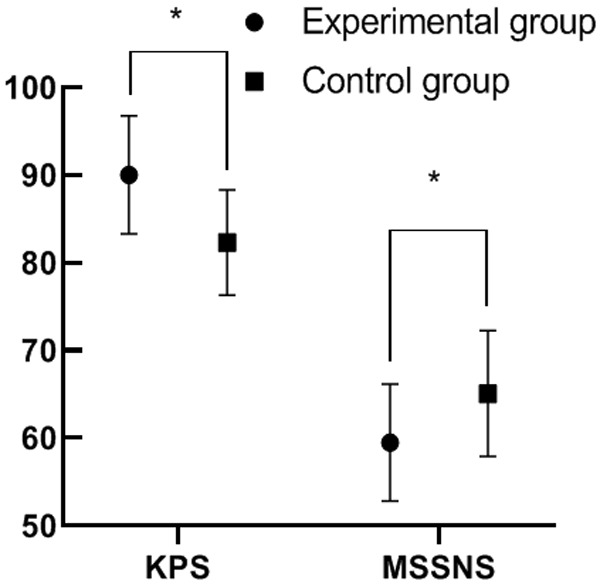
Comparison of KPS scores (quality of life) and MSSNS scores between the two groups. Note: The abscissa indicates the KPS and MSSNS scores from left to right, and the ordinate indicates the points. Comparison of the KPS score of the experimental group (90.03±6.73) with that of the control group (82.30±6.00), t=6.06, *P<0.05, and the comparison result was statistically significant; Comparison of the MSSNS score of the experimental group (59.48±6.66) with that of the control group (65.07±7.19), t=4.03, *P=0.003, and the comparison result was statistically significant.
Comparison of survival rates within 1 month, 2 months, and 3 months after treatment between the two groups
Results in Figure 2 present a superior survival rate in the experimental group than that in the control group 1, 2 and 3 months after treatment (all P<0.05).
Figure 2.
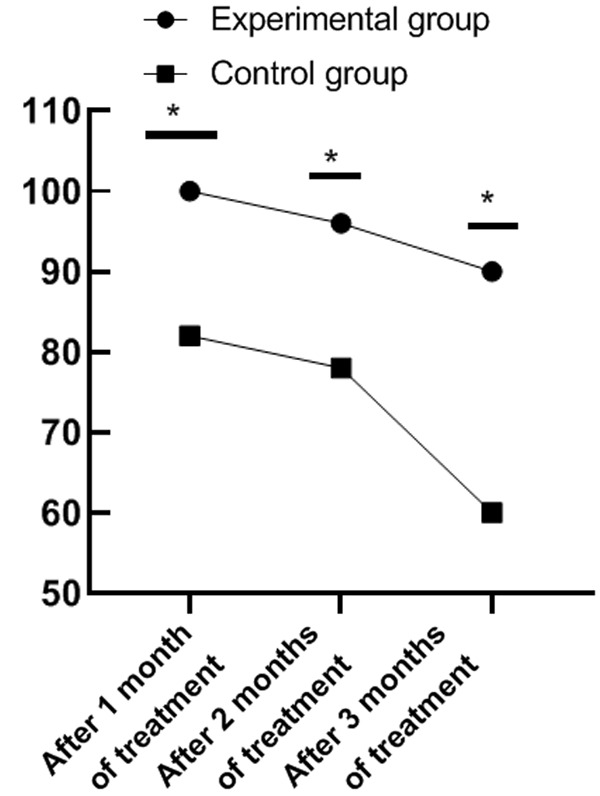
Comparison of survival rates in January, February, and March after treatment between the two groups. Note: The abscissa represents 1 month, 2 months and 3 months after treatment, and the ordinate represents the survival rate of patients. One month after treatment, the survival rate of patients in the experimental group was 100% and that in the control group was 82%, X2=9.89, *P=0.002, so the comparison result was statistically significant; Two months after treatment, the survival rate of patients in the experimental group was 96% and that in the control group was 78%, X2=7.16, *P=0.007, so the comparison result was statistically significant; Three months after treatment, the survival rate of patients in the experimental group was 90% and that in the control group was 60%, X2=12.00, *P=0.001, so the comparison result was statistically significant.
Comparison of VEGF and VEGFR-2 levels after treatment between the two groups
After treatment, the levels of VEGF and VEGFR-2 in the experimental group were lower than those in the control group (both P<0.05). See Figure 3.
Figure 3.
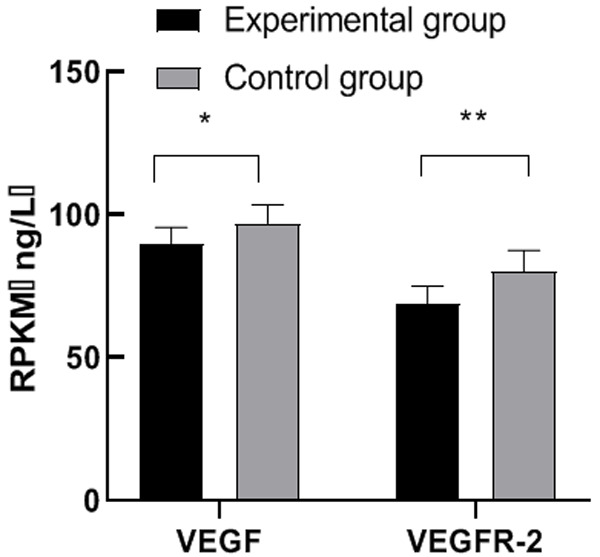
Comparison of VEGF and VEGFR-2 levels after treatment between the two groups. Note: The abscissa represents VEGF and VEGFR-2 from left to right, and the ordinate represents the expression level. Comparison between the VEGF level of the experimental group (89.56±5.88) ng/L and that of the control group (96.88±6.57) ng/L, t=5.87, *P<0.05, and the comparison result was statistically significant; Comparison between the VEGFR-2 level of the experimental group (68.74±6.33) ng/L and that of the control group (80.12±7.42) ng/L, t=8.25, ** P<0.01, and the comparison result was statistically significant.
Univariate analysis of factors for KPS<85 after oral cancer treatment
After oral cancer treatment, the two groups of patients with different KPS scores had a statistically significant difference in smoking history, drinking history, tooth brushing frequency, cleaning treatment, tooth cleaning at the time of treatment, oral disease history, and nutritional status indicators (all P<0.05, Table 5).
Table 5.
Univariate analysis of related influencing factors of KPS<85 score after oral treatment
| Index | KPS≥85 score | KPS<85 score | X2 | P-value |
|---|---|---|---|---|
| Occupation | 1.356 | 0.964 | ||
| Physical labour | 26 | 24 | ||
| Mental labour | 24 | 26 | ||
| Smoking | ||||
| Yes | 12 | 37 | 2.365 | 0.001 |
| No | 38 | 13 | ||
| Drinking | 2.964 | 0.002 | ||
| Yes | 11 | 36 | ||
| No | 39 | 14 | ||
| Teeth brushing index | 5.364 | 0.001 | ||
| <3 | 13 | 40 | ||
| ≥3 | 37 | 10 | ||
| Frequency of teeth cleaning (year) | 9.458 | 0.002 | ||
| <1 | 14 | 42 | ||
| ≥1 | 36 | 8 | ||
| Teeth cleaning | 8.452 | 0.001 | ||
| yes | 47 | 16 | ||
| no | 3 | 34 | ||
| Times of oral disease visits | 6.354 | 0.002 | ||
| <3 | 36 | 11 | ||
| ≥3 | 14 | 39 | ||
| Nutritional status | 4.368 | 0.004 | ||
| Normal | 38 | 16 | ||
| Poor | 12 | 34 |
Multivariate analysis of factors for KPS<85 after oral cancer treatment
A history of smoking, a history of drinking, a tooth brushing index <3, frequency of cleansing treatments ≤1 time per year, a history of oral diseases> 3 times, and poor nutritional status were independent risk factors for KPS<85 after oral cancer treatment (all P<0.05, Table 6).
Table 6.
Multivariate analysis of related influencing factors of KPS<85 score after oral treatment
| Index | β | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Smoking | 1.074 | 0.465 | 5.324 | 0.026 | 2.147 | 0.897-5.364 |
| Drinking | 1.136 | 0.911 | 1.632 | 0.001 | 2.364 | 1.236-10.355 |
| Teeth brushing <3 | 1.425 | 0.934 | 2.364 | 0.007 | 4.156 | 1.638-13.652 |
| Frequency of teeth cleaning ≤1 | 1.365 | 0.998 | 2.345 | 0.002 | 4.637 | 1.758-15.364 |
| Oral disease visits >3 | 0.836 | 0.364 | 1.524 | 0.031 | 2.311 | 0.564-5.116 |
| Poor nutritional status | 1.431 | 1.456 | 0.948 | 0.021 | 4.634 | 1.421-12.365 |
Discussion
Oral cancer has a relatively high incidence rate, and its manifestations are obvious. Its early symptoms include recurrent oral ulcers and difficulty in opening the mouth. Patients with middle or late oral cancer suffer long-lasting oral ulcers, and there are usually color changes and purulent of the ulcer part [16-18]. Patients with early oral cancer can be treated with tumor resection, which can effectively remove the tumor tissues and inhibit the spread of the tumor cells. However, for those with oral cancer in the middle or late stage, it is difficult to remove the extensive tumor tissues by one-time surgery [19-21]. Therefore, those patients usually undergo chemotherapy to inhibit the growth and development of tumor. Chemotherapy can be combined with other drugs to inhibit the growth of tumor cells, such as apatinib mesylate, a drug specifically used for the treatment of advanced cancer. In clinical application, it has been found that apatinib mesylate can effectively improve the therapeutic effect with a sound clinical efficacy. However, under the most commonly used method of administration of apatinib emesylate, many patients have suffered severe intolerance and some serious complications. Under such circumstances, although the overdose of apatinib mesylate brings the disease under control, it will cause other complications [22-24]. This paper mainly investigated the efficacy and safety of different doses of apatinib mesylate combined with chemotherapy in patients with advanced oral cancer. The goal is to find the safest and most effective dosage of apatinib mesylate.
The results showed that the treatment effect, disease control rate, objective response rate, adverse reactions, survival rate of patients, and patient’s quality of life were all significantly improved in the experimental group treated with 250 mg apatinib mesylate combined with chemotherapy compared with the control group treated with 500 mg apatinib mesylate combined with chemotherapy. Apatinib mesylate has the advantages of high precision and high efficiency in the treatment of advanced cancer, but due to its irritation, some patients may have serious intolerance. Besides, in this study, apatinib mesylate was administrated until the patient had a severe intolerance to it, which meant that the patient would be treated with apatinib mesylate for a longer time when he/she experienced a longer period before having a severe intolerance reaction, and the treatment for oral cancer would be more effective correspondingly. Therefore, the use of a larger dose of apatinib mesylate will shorten the treatment time for some poorly tolerated patients. A shorter acting time of apatinib mesylate indicates greater compromises of its clinical efficacy. In this study, the 250 mg dose of apatinib mesylate was compared with the 500 mg dose regime, and the results showed that the former demonstrated better overall treatment effect and higher safety. By analyzing the adverse reactions, it is possible to carry out treatment against possible adverse reactions and to investigate the safety of the treatment. Caiping Nie et al. [25] once proposed that the treatment effect of apatinib mesylate combined with chemotherapy for patients with advanced oral cancer was good, and the optimal therapeutic effect and safety were achieved when the dose of apatinib mesylate was 250 mg. The conclusion is consistent with the results of the present study. Moreover, a history of smoking, a history of drinking, a tooth brushing index <3, the frequency of teeth cleansing ≤1 time per year, a history of oral diseases >3 times, and poor nutritional status were independent risk factors for KPS<85 after oral cancer treatment.
In summary, 250 mg apatinib mesylate combined with chemotherapy can reach optimal efficacy with highest safety and least adverse effects on patients with advanced oral cancer.
Disclosure of conflict of interest
None.
References
- 1.Kawasaki R, Sasaki Y, Nishimura T, Katagiri K, Morita KI, Sekine Y, Sawada SI, Mukai SA, Akiyoshi K. Magnetically navigated protein transduction in vivo using iron oxide-nanogel chaperone hybrid. Adv Healthc Mater. 2021;10:e2001988. doi: 10.1002/adhm.202001988. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja US, Shetty DC, Rathore A, Dhillon M. Occult prostate carcinoma with metastasis to the mandible presenting as numb chin syndrome. J Oral Biol Craniofac Res. 2021;11:393–395. doi: 10.1016/j.jobcr.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu ZY, Qin R, Tian GY, Zhang Z, Chen M, He H, Xi Y, Wang Y. Apatinib combined with S-1 as second-line therapy in advanced gastric cancer. Medicine (Baltimore) 2021;100:e25630. doi: 10.1097/MD.0000000000025630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mota de Oliveira M, Peterle GT, Monteiro da Silva Couto CV, de Lima Maia L, Kühl A, Gasparini Dos Santos J, Moysés RA, Trivilin LO, Borçoi AR, Archanjo AB, Evangelista Monteiro de Assis AL, Nunes FD, Santos MD, Álvares da Silva AM. PAI-1 expression in intratumoral inflammatory infiltrate contributes to lymph node metastasis in oral cancer: a cross-sectional study. Ann Med Surg (Lond) 2021;65:102303. doi: 10.1016/j.amsu.2021.102303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyzas P. Management of the cN0 neck in early oral cancer: time to revise the guidance? Br J Oral Maxillofac Surg. 2021;59:387–388. doi: 10.1016/j.bjoms.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Glombik D, Oxelbark Å, Sundqvist P, Carlsson J, Lambe M, Drevin L, Davidsson S, Kirrander P. Risk of second HPV-associated cancers in men with penile cancer. Acta Oncol. 2021;60:888–890. doi: 10.1080/0284186X.2021.1885056. [DOI] [PubMed] [Google Scholar]
- 7.Zheng W, Zhou Q, Yuan C. Nanoparticles for oral cancer diagnosis and therapy. Bioinorg Chem Appl. 2021;2021:9977131. doi: 10.1155/2021/9977131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Richards CA, Cameron A, Collin J, Hughes CW, Main BG. Slow to heal or slow to diagnose cancer? British Dental J. 2021;230:518–522. doi: 10.1038/s41415-021-2837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao H, Chen XY, Tan XD. Effectiveness and safety of apatinib in the treatment of osteosarcoma: a single-arm meta-analysis among Chinese patients. BMC Cancer. 2021;21:55. doi: 10.1186/s12885-021-08154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Z, Fei W, Sun HR, Huang YK, Gao JP, Huang H. Apatinib combined with PD-L1 blockade synergistically enhances antitumor immune responses and promotes HEV formation in gastric cancer. J Cancer Res Clin Oncol. 2021;147:2209–2222. doi: 10.1007/s00432-021-03633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalid MA, Achakzai IK, Ahmed Khan S, Majid Z, Hanif FM, Iqbal J, Laeeq SM, Luck NH. The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench. 2018;11:301–305. [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Xu L, Wang Y, Gao H. Comprehensive nursing intervention combined with early activityactivity applied in ventilator-associated pneumonia and its influence on blood gas index. Am J Transl Res. 2021;13:5647–5652. [PMC free article] [PubMed] [Google Scholar]
- 13.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang DX, Yang X, Long JY, Lin JZ, Mao JZ, Xie FC, Wang YC, Wang YY, Xun ZY, Bai Y, Yang XB, Guan M, Pan J, Seery S, Sang XT, Zhao HT. The effectiveness and safety of apatinib plus camrelizumab in patients with previously treated advanced biliary tract cancer: a prospective clinical study. Front Oncol. 2021;18:8–10. doi: 10.3389/fonc.2021.646979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saidak Z, Lailler C, Testelin S, Chauffert B, Clatot F, Galmiche A. Contribution of genomics to the surgical management and study of oral cancer. Ann Surg Oncol. 2021 doi: 10.1245/s10434-021-09904-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Wei X, Wang X, Zheng X, Chang B, Shen L, Zhu H, Yang M, Li S, Zheng X. NDUFA4L2 promotes glioblastoma progression, is associated with poor survival, and can be effectively targeted by apatinib. Cell Death Dis. 2021;12:377. doi: 10.1038/s41419-021-03646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Q, Ma Y, Shen F, Wang Q, Song X, Jiang W, Xie S. Case of bullous pemphigoid induced by apatinib mesylate. Br J Clin Pharmacol. 2021;87:2158–2159. doi: 10.1111/bcp.14583. [DOI] [PubMed] [Google Scholar]
- 18.Sun C, Xu YH, Wang X, Guo Y, Qiu S, Shao GG, Yang ZG, Liu YP, Zhang P, Ma KW. Successful treatment of Afatinib plus Apatinib using for a lung adenocarcinoma patient with HER-2 V659D mutation: a rare case report. Anti-cancer drugs. 2021;32:38. doi: 10.1097/CAD.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YC, Deng XZ, Ding Z, Kang J, Wu B, Guo BM, Fan YB. Preoperative neoadjuvant targeted therapy with apatinib for inoperable differentiated thyroid cancer: a case report. Medicine. 2021;100:20. doi: 10.1097/MD.0000000000025191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu ZY, Hu C, Yu JF, Du Y, Hu P, Yu GF, Hu CG, Zhang Y, Mao W, Chen SQ, Cheng XD. Effectiveness of conversion surgery following apatinib plus paclitaxel/S1 for advanced gastric cancer with unresectable factors: a multicenter, single-arm, phase II trial. Front Pharmacol. 2021;100:178–181. doi: 10.3389/fphar.2021.642511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long ZY, Huang MQ, Liu KT, Li MH, Li J, Zhang HM, Wang Z, Lu YJ. Assessment of efficiency and safety of apatinib in advanced bone and soft tissue sarcomas: a systematic review and meta-analysis. Front Oncol. 2021;35:17. doi: 10.3389/fonc.2021.662318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SY, Wu QJ, Liu D, Yu HJ, Zhong YH, Zhou YF. Successful management of adenoid cystic carcinoma of the lacrimal sac with apatinib combined with concurrent chemoradiotherapy: a case report. Ann Palliat Med. 2021;27:1733. doi: 10.21037/apm-20-1934. [DOI] [PubMed] [Google Scholar]
- 23.Chi DM, Chen BQ, Guo SP, Bai KH, Ma HL, Hu YH, Li QQ, Zhu YJ. Oral maintenance therapy using apatinib combined with S-1/capecitabine for esophageal squamous cell carcinoma with residual disease after definitive chemoradiotherapy. Aging. 2021;13:58–61. doi: 10.18632/aging.202652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng YY, Song X, Jia RB. Case report: favorable response to the tyrosine kinase inhibitor apatinib in recurrent merkel cell carcinoma. Front Oncol. 2021;27:18–21. doi: 10.3389/fonc.2021.625360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie CP, Fan QX, Wang F. Clinical effectiveness and safety analysis of apatinib mesylate in the treatment of advanced oral cancer. Cancer Progress. 2018;16:1758–1761. [Google Scholar]


