Abstract
The clinical course of Pneumocystis pneumonia in liver transplant recipients has not been well investigated. Therefore, we collected and analyzed the clinical, epidemiological, and molecular data from patients with Pneumocystis pneumonia as well as paired controls (Chinese Clinical Trial Registry, ChiCTR2100046028; www.chictr.org.cn). There were a total of ten patients diagnosed with Pneumocystis pneumonia containing prospectively included six patients and retrospectively collected four patients, of which seven were transferred to the surgical intensive care unit and four died. The transmission map revealed that inter-patient transmission of Pneumocystis jirovecii was impossible; P. jirovecii detection was negative in all air samples. It was positive only in one sample from the twelve healthcare workers who had close contact with diseased patients. Five out of 79 liver transplant recipients during the outbreak were colonized with Pneumocystis jirovecii compared to 2 out of 94 after the outbreak upon admission (P>0.05). Liver transplant recipients with Pneumocystis pneumonia had totally different genotypes based on multilocus sequence typing. Additionally, we found an unreported mutation in the cytochrome b gene. The absolute CD19+ B-cell counts (odds ratio: 1.028; 95% confidence interval: 1.000-1.057; P=0.049) were defined to be the only significant independent risk factor. At a cut-off value of 117.16/µL, the sensitivity and specificity were 100% and 70%, respectively. Pneumocystis pneumonia is a severe complication following liver transplantation. The outbreak may not be caused by nosocomial transmission. A decrease in absolute CD19+ B-cell counts may be associated with the development of Pneumocystis pneumonia.
Keywords: Pneumocystis pneumonia, liver transplant recipients, multilocus sequence typing, transmission routes, risk factors
Introduction
Pneumocystis jirovecii (P. jirovecii), an opportunistic fungal microorganism, can cause Pneumocystis pneumonia (PCP), resulting in significant mortality [1-5]. PCP used to occur mainly in patients diagnosed with human immunodeficiency virus (HIV) due to lymphocyte deficiency [1,2]. PCP is now a growing concern in non-HIV patients, especially in patients who have undergone solid organ transplantation because post-transplant PCP generally presents as severe pneumonia and may cause nosocomial outbreaks [3,4,6-8]. Risk factors for PCP have been suggested among transplant recipients, mostly in renal transplant patients [9-11]. However, due to its low incidence, only a few studies with respect to PCP in liver transplant recipients have been reported [12-16], and the predictive value of circulating lymphocytes has not been further explored in this cohort (Table 1).
Table 1.
PCP in liver transplant recipients
| Authors | Year | Cases | Male | Age (mean) | Time from LT to PCP diagnosis (mean) | PCP prophylaxis | Death in relation to PCP | Risk factors |
|---|---|---|---|---|---|---|---|---|
| Rostved et al. | 2013 | 14 | 10 | 51 | 27 d | 0 | 1 | CMV viremia |
| Sarwra et al. | 2013 | 7 | 2 | 57 | 7 m | 0 | 5 | - |
| Choi et al. | 2013 | 8 | 4 | 52 | 9.5 m | 8 | 4 | A rejection episode |
| Desoubeaux et al. | 2016 | 4 | 4 | 55 | 113 d | 0 | 2 | - |
| Miguel Montanes et al. | 2018 | 15 | 11 | 49 | 102 d | 0 | 2 | Lymphopenia |
LT, liver transplantation; PCP, Pneumocystis pneumonia; CMV, Cytomegalovirus.
Moreover, as it is not possible to culture P. jirovecii with routine methods, PCP diagnosis depends on molecular detection of P. jirovecii deoxyribonucleic acid (DNA) by quantitative polymerase chain reaction (qPCR) [17,18]. Nevertheless, this approach is not suitable for an epidemiological investigation of a PCP outbreak. Exploring an outbreak transmission route relies on spatiotemporal and strain-typing measures because genotyping based on multilocus sequence typing has been proposed as the standard tool for strain characterization in virtue of excellent discriminatory power and reproducibility [17]. Presently, the exact route of transmission among PCP patients is not clearly understood. In PCP outbreaks, epidemiological research with genotyping has suggested an inter-human airborne transmission route in patients with different primary diseases [7,8,19-21]. However, a systematic study of the transmission route and potential sources has not been reported in liver transplant recipients.
In this study, we retrospectively collected data from a cluster of four patients infected with PCP between July 2015 and July 2016 and performed a prospective study on the new onset of PCP patients from April 2018. The clinical, epidemiological, and molecular characteristics of the PCP patients and controls were systematically analyzed to investigate the clinical course, potential possible transmission routes, and risk factors.
Materials and methods
Study design
Beijing Chaoyang Hospital has a total of 1,900 beds and performs more than 100 liver transplantations per year. No patients with PCP had been observed during the previous ten years. Between July 2015 and July 2016, a cluster of four patients was diagnosed with PCP. We retrospectively collected their clinical data and performed a prospective study on PCP patients from the first onset in April 2018 (patient no. 5) to determine the clinical course, transmission routes, and risk factors. If there had been no new onset of PCP patients for more than six months after the diagnosis of the last PCP patient, we considered it was an end of an outbreak due to a lack of a definition.
PCP patients were defined when the following criteria were met: (i) clinical symptoms including fever, cough, and dyspnea; (ii) radiographic findings of a new alveolar or interstitial infiltrate on a chest computerized tomography; (iii) either sputum or bronchoalveolar lavage fluid (BALF) positive for P. jirovecii [18].
The study was approved by the Institutional Review Board of Beijing Chaoyang Hospital (No. 2016-2-19-38) in accordance with the Helsinki declaration of 1975, as revised in 1983. Written informed consent was obtained from all participants in the prospective study.
Transmission map
We collected and analyzed data concerning PCP patients’ interactions within the hospital from the first admission of the fifth patient until the diagnosis of PCP in the last patient to determine the possible patient-patient transmission. The outpatient clinic, the hepatology ward, and the surgical intensive care unit (SICU) were located at different positions in the hospital. Outpatients had no direct contact with inpatients. The hepatology wards for pre-transplant and post-liver transplant recipients were on the same floor but in different corridors. Patients had to share a ward before liver transplantation and were transferred to a single ward from the SICU following surgery.
Air sampling
Due to a lack of an air sampling device, we used the traditional passive sedimentation method according to the Hospital Sanitary Disinfection Standard (GB15982-2012) to collect the specimen with a medium plate about 9 cm in diameter [22,23]. Only the SICU had laminar airflow with high-efficiency particulate air filtration. The windows and doors of the room were closed and the patients stayed still for at least 10 min before sampling. The medium plates filled with sterile saline were placed at different sites. If the room was ≤30 m2, three sites (inner, middle, and outer) were selected along a diagonal line. Otherwise, five sites were chosen including four corners and the middle point. All sites were one meter away from the floor and the walls. During sampling, the plates were opened for 30 min. After the diagnosis of PCP, air samples were collected in each ward as soon as possible, as well as in a room not used for patient care as a negative control.
Screening for colonization in healthcare workers and patients
To explore the impact of liver transplant recipients with PCP among healthcare workers (HCW) and other patients, we examined and compared their prevalence. Colonization was defined when HCW or patients had P. Jirovecii but did not develop PCP. HCW, who had been working for more than two years at our center, were prospectively sampled for P. Jirovecii detection. HCW were divided into two groups, consisting of those who had close contact with PCP patients and those who had no occupational contact with PCP patients as a negative control group.
Meanwhile, liver transplant recipients upon admission were prospectively sampled to determine the frequency of colonization with P. Jirovecii during the outbreak and six months after the outbreak for another consecutive six months. For HCW and transplant recipients with respiratory symptoms, a BALF sample or a sputum sample was taken. Otherwise, a nasal sample was taken.
DNA extraction and P. jirovecii detection
For each sample, the collection liquid was centrifuged at 10,000 rpm for 3 min, and then the supernatant was carefully removed to leave a 0.5 mL pellet for qPCR processing. DNA was extracted (Smart LabAssist-32) by using the QIAamp DNA Mini Kit (QIAGEN) according to the manufacturer’s recommendations. Four µL of DNA extract, negative control, and positive control were added to 36 µL of reaction separately. Cycling conditions were as follows: 1 cycle of 2 min at 37°C, 2 min at 94°C; 40 cycles of 15 s at 93°C and 60 s at 60°C; single point fluorescence detected at 60°C (ABI7500 PCR System). P. jirovecii detection was devised to target kex1 gene. Positive DNA samples of P. jirovecii were subsequently stored at -20°C for typing.
Pneumocystis genotyping
Positive DNA samples of P. jirovecii from immunocompromised patients with different primary diseases were included as a control group at Beijing Chaoyang Hospital, in addition to samples from PCP cases, HCW, and colonized patients. This control group included patients diagnosed with PCP during the same period as PCP cases. Each case was matched to 2 control patients randomly to compare their genotyping (Table 2). Samples were sequenced blindly by a commercial company. A combination of three loci belonging to the superoxide dismutase (SOD), the mitochondrial large subunit of ribosomal RNA (mtLSU rRNA), and the cytochrome b (CYB) genes were chosen [17,18,24-26]. Nested PCR was used for amplification (ETC811, Eastwin Life Sciences). Each PCR mixture contained 1 ul template, 1 ul (10 pmol/ul) each of forward primer and reverse primer, 15 ul Super Mix and water to a 30 ul final volume. The cycling conditions were 10 cycles of 96°C for 5 min, 96°C for 20 s, 52-62°C for 30 s, 72°C for 1 min followed by 30 cycles of 96°C for 20 s, 52°C for 30 s, and 72°C for 1 min, with a final extension step at 72°C for 5 min. Then, sequencing was performed on an ABI 3730XL DNA sequencer (Applied Biosystems) using a sequencing ready reaction kit (ABI). Each sequencing mixture contained 2 ul Mix (Bigdye3.1, 5X sequencing buffer, H2O), 2 ul purified template, and 1 ul (5 mmol/L) primer. The cycling conditions were 95°C for 15 s, followed by 35 cycles of 95°C for 15 s, 50°C for 5 s, and 60°C for 90 s. Consensus sequences were aligned with reference sequences (GenBank accession numbers AF146753.1 for SOD, M58605.1 for mtLSUrRNA, and AF320344.1 for CYB). P. jirovecii isolates with one or more nucleotide differences were considered to be different strains.
Table 2.
Multilocus sequence typing
| Locus | Primers (specific amplification) | Amplicon size | Polymorphisms |
|---|---|---|---|
| SOD | F1: AATAAGGGTTTAATTAGTCTT | 762 bp | 110, 191, 215 |
| R1: CTTACATTCCATATATTTTCA | 670 bp | ||
| F2: GAACCTTATCTTTCTCAT | |||
| R2: TCATTTTAGTATTTAGTCTC | |||
| mtLSU rRNA | F1: GTGAAAGCCCAGAGTCCC | 1216 bp | 54-57, 80, 85, 248, 288 |
| R1: CCGAGTTCCTTCGCAATA | 738 bp | ||
| F2: CTTTTGCATAATGGGTCAG | |||
| R2: TTTTCGGCGAATAGGATTT | |||
| CYB | F1: TATGGTTCATTATCAGGACTG | 1016 bp | 279, 299, 348, 362, 369, 516, 547, 566, 675, 742, 832-833, 838 |
| R1: ACCAGAGGAATAACAACTAAG | 942 bp | ||
| F2: ACAGATTATTACGGGTGT | |||
| R2: GTAGCATATTGTCCAAGC |
SOD, the superoxide dismutase; mtLSU rRNA, mitochondrial large subunit of ribosomal RNA; CYB, cytochrome b.
Risk factor analysis
For risk factors analysis, each PCP case was matched to 3 controls that had not developed PCP following liver transplantation. They were matched by gender, age (±3 years), primary diseases for transplantation (malignant or benign), and model for end-stage liver disease score (±3, one day before transplantation). Preoperative parameters, operative parameters, and postoperative parameters of these patients were collected and analyzed.
Antibodies and flow cytometric measurement
Peripheral blood (50 μL) from PCP patients within three days after diagnosis and from controls with an equal follow-up time was stained with multitest antibodies Anti-Human CD45-FITC/CD3-PC5/CD4-RD1/CD8-ECD for T-cells, and Anti-Human CD45-PC5 and Anti-Human CD19 PC-7 for B-cells (Beckman Coulter) at room temperature in the dark for 15 min, respectively, followed by lysis with OptiLyse C (Beckman Coulter) at room temperature in the dark for 10 min. After that, 0.2 μL Isoton III Diluent was added to the mixture. Finally, flow-count fluorospheres (50 μL) were added prior to collection on a flow cytometer. A fluorescence activated cell sorter Cytomics™ FC500 Flow Cytometer from Beckman Coulter was used for measurement, and data were analyzed using CXP Analysis (Beckman Coulter).
Statistical analysis
The Fisher’s exact test was used for categorical variables while the independent samples t-test and Mann-Whitney U test were employed for normal quantitative variables and non-normal quantitative variables, respectively. Variables with a P-value <0.1 on univariate analysis were included in the multivariable model. Multivariable conditional logistic regression was performed to determine independent risk factors for PCP. Results of the multivariable conditional logistic regression are presented as odds ratios and 95% confidence intervals. A P-value <0.05 was considered statistically significant. A receiver operating characteristic curve was built to assess the diagnostic performance of the predictor(s) associated with PCP patients. Values were expressed as mean ± standard deviation for quantitative variables. Data analyses were carried out by using SPSS 19.0 computer software (IBM Corp., Armonk, NY, USA).
Results
General characteristics and outcome
A total of ten patients were diagnosed with PCP following liver transplantation between July 2015 and January 2019 according to the criteria described above at Beijing Chaoyang Hospital. Among these patients, four cases were retrospectively collected and six cases prospectively included, which we considered an outbreak (from April 2018 to January 2019). As there had been no new onset of PCP patients for more than six months since February 2019, we defined it as the end of the outbreak.
The characteristics of PCP patients are summarized in Table 3. The overall incidence rate of 10 PCP patients newly diagnosed out of 375 transplant recipients who were followed up over this period was 2.6%. Tacrolimus-based immunosuppressive regimens were applied to nine patients in comparison to cyclosporin A to one patient. None of the patients were subjected to PCP prophylaxis at the time of diagnosis. Nine patients (9/10) with post-transplantation PCP were readmitted to the hospital, of which seven (7/9) were transferred to SICU with 85.7% (6/7) in need of mechanical ventilation, and 42.9% (3/7) in need of extracorporeal membrane oxygenation support. All patients were treated with Trimethoprim/Sulfamethoxazole. Four patients died of respiratory failure. The overall in-hospital mortality was 40% (4/10). The mortality was 57.1% (4/7) in patients requiring ICU treatment.
Table 3.
The characteristics of PCP cases involved in an outbreak
| Patients | Age range | Primary diseases | Date of diagnosis | Time since transplant (w) | Sample type | Outcome |
|---|---|---|---|---|---|---|
| P1 | 66-70 | HCC | 7.19.2015 | 9 | sputum | Dead |
| P2 | 61-65 | HCC | 9.9.2015 | 8 | sputum | Dead |
| P3 | 51-55 | HCC | 3.24.2016 | 21 | sputum | Alive |
| P4 | 61-65 | Cirrhosis | 7.26.2016 | 15 | BALF | Dead |
| P5 | 36-40 | Cirrhosis | 4.1.2018 | 51 | sputum | Alive |
| P6 | 26-30 | Cirrhosis | 6.21.2018 | 21 | BALF | Dead |
| P7 | 51-55 | Cirrhosis | 8.17.2018 | 1 | sputum | Alive |
| P8 | 56-60 | HCC | 10.3.2018 | 19 | sputum | Alive |
| P9 | 61-65 | HCC | 12.15.2018 | 17 | sputum | Alive |
| P10 | 61-65 | HCC | 1.14.2019 | 10 | sputum | Alive |
PCP, Pneumocystis pneumonia; HCC, hepatic cell carcinoma; BALF, bronchoalveolar lavage fluid.
Transmission map
Based on the visual investigation, no previous PCP patients had a chance to contact the following PCP patients before diagnosis during hospitalization. Both patients no. 8 and no. 9, and patients no. 9 and no. 10 might have met at the outpatient clinic, respectively; however, they had an appointment on different dates after we checked their records. Moreover, PCP patients lived in different areas of China and did not know each other before admission. Therefore, the transmission map revealed that inter-patient transmission of P. jirovecii was impossible among these patients (Figure 1).
Figure 1.
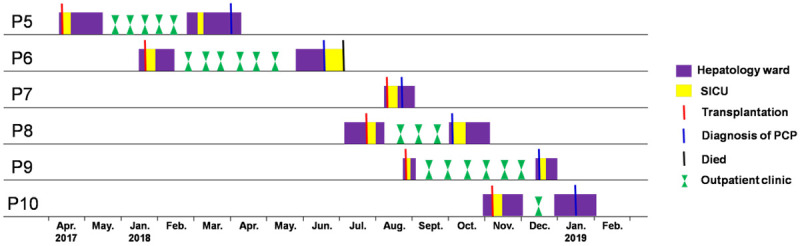
Transmission map of P. jirovecii among liver transplant recipients. The x-axis shows dates, and the y-axis shows patient numbers. No encounters were found among liver transplant recipients for P. jirovecii transmission. SICU, surgical intensive care unit; PCP, Pneumocystis pneumonia.
P. jirovecii detection of air samples, HCW, and colonized patients
First, we wanted to check whether P. jirovecii could be transmitted through the air. After the diagnosis of PCP, air samples were collected in each ward as soon as possible, and in a room not used for patient care as a negative control. A total of 21 air samples from seven rooms (three for each and three wards with laminar airflow) were collected using the traditional passive sedimentation method. We found that P. jirovecii detection was negative in all air samples.
Then, HCW were investigated as a potential source of P. Jirovecii infection. All HCW had an annual check-up and were in good condition. Since none of them had any respiratory symptoms during the period, HCW had to provide a nasal sample with a moist sterile cotton-swab (no BALF samples or sputum samples). P. Jirovecii detection was done in samples from twenty HCW (one for each), of which twelve were from those HCW who had close contact with PCP patients, and eight from the negative control group. P. jirovecii detection was positive only in one HCW encountering PCP patients (1/12).
Finally, we decided to determine the frequency of colonization with P. Jirovecii in liver transplant recipients. Samples from liver transplant recipients (79 vs. 94) upon admission were prospectively collected for six consecutive months since the diagnosis of the No. 6 PCP patient and August 2019 (six months after the diagnosis of the tenth PCP patient), respectively. There was no new onset of PCP patients between August 2019 and April 2020. The characteristics of liver transplant recipients are summarized in Table 4. BALF samples (n=7 and 11), sputum samples (n=24 and 32), and nasal samples (n=48 and 51) were taken from these patients during and after the outbreak, respectively. Five patients sampled during the outbreak were positive for P. jirovecii detection and classified as colonized in comparison to two patients sampled after the outbreak (P>0.05).
Table 4.
Characteristics of sampled liver transplant recipients
| Characteristics | During the outbreak | After the outbreak | P value |
|---|---|---|---|
| Number | 79 | 94 | |
| Sex | 0.260 | ||
| Male | 63 | 81 | |
| Female | 16 | 13 | |
| Age | 53.09±10.24 | 53.33±9.33 | 0.871 |
| Primary diseases | 0.757 | ||
| Benign | 38 | 43 | |
| Malignant | 41 | 51 | |
| Sample type | 0.661 | ||
| BALF samples | 7 | 11 | |
| Sputum samples | 24 | 32 | |
| Nasal samples | 48 | 51 | |
| P. jirovecii detection | 0.313 | ||
| Positive | 5 | 2 | |
| Negative | 74 | 92 |
P. jirovecii typing
Positive P. jirovecii samples from 6 PCP cases (from P5 to P10), 12 unrelated controls (renal transplantation n=2, renal failure n=2, multiple myeloma n =1, severe pneumonia n=2, Behcet’s disease n=1, dermatomyositis n=1, antineutrophil cytoplasmic antibody-associated vasculitis n=1, and chronic obstructive pulmonary disease n=2), 1 colonized HCW and 7 colonized liver transplant recipients were genotyped. Among these samples, genotyping failed for 15 samples because of a weak signal. Results for four PCP cases (P6, P8, P9, and P10) and seven unrelated controls (C4-C6 and C8-C11) are reported in Table 5.
Table 5.
Multilocus sequence typing analysis of samples from liver transplant recipients with PCP and PCP controls
| Polymorphisms | Patient number | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| P6 | P8 | P9 | P10 | C4 | C5 | C6 | C8 | C9 | C10 | C11 | |
| SOD genotype | SOD2 | SOD1 | Mixed (SOD2/SOD1) | SOD2 | |||||||
| 110 | TT | / | CC | / | / | / | / | / | CT | TT | / |
| 191 | TT | / | TT | / | / | / | / | / | TT | TT | / |
| 215 | CC | / | TT | / | / | / | / | / | TC | CC | / |
| mtLSU rRNA genotype | Allele 8 | Allele 8 | Allele 3 | Allele 8 | Allele 8 | Allele 8 | |||||
| 54-57 | 4xA/4xA | / | / | / | / | 4xA/4xA | 4xA/4xA | 4xA/4xA | 4xA/4xA | 4xA/4xA | / |
| 80 | CC | CC | CC | CC | CC | CC | CC | CC | CC | CC | CC |
| 85 | TT | CT | TT | CC | TT | TT | CC | TT | TT | TT | CC |
| 248 | CC | CT | CC | CC | CC | CC | TT | CC | CC | CC | TT |
| 288 | AA | / | / | / | / | AA | AA | AA | AA | AA | / |
| CYB genotype | Mixed (CYB10/CYB11) | CYB2 | Mixed (CYB1/CYB6) | CYB1 | CYB2 | CYB2 | |||||
| 279 | CC | / | / | / | / | CC | CC | CC | CC | CC | / |
| 299 | CC | / | / | / | / | CC | CC | CC | CC | CC | / |
| 348 | AA | / | / | / | / | AA | AA | AA | AA | AA | / |
| 362 | CC | / | / | / | / | CC | CC | CC | CC | CC | / |
| 369 | GG | / | / | / | / | GG | GG | GG | GG | GG | / |
| 516 | CC | / | / | / | / | CC | CT | CC | CC | CC | / |
| 547 | CC | / | / | / | / | CC | CC | CC | CC | CC | / |
| 566 | CT | / | / | / | / | CC | CC | CC | CC | CC | / |
| 675 | AA | / | / | / | / | AA | AA | AA | AA | AA | / |
| 742 | CC | / | / | / | / | CC | CC | CC | CC | CC | / |
| 832-833 | TT/TT | / | / | / | / | TT/TT | TT/TT | TT/TT | TT/TT | TT/TT | / |
| 838 | CT | / | / | / | / | TT | CC | CC | TT | TT | / |
PCP, Pneumocystis pneumonia; SOD, superoxide dismutase; mtLSU rRNA, mitochondrial large subunit of ribosomal RNA; CYB, cytochrome b; P, transplant recipients with PCP; C, immunocompromised patients with PCP as a control group. /, sequence typing failure; _, new genotypes are underlined.
SOD2, Allele 8, and mixed (CYB10/CYB11) were all identified in one transplant recipient and SOD1 in another transplant patient. SOD2 and mixed (SOD2/SOD1) were identified in 1 patient from the control group, Allele 3 and Allele 8 in 1 and 4 patients, mixed (CYB1/CYB6), CYB1 and CYB2 in 1, 1, and 3 patients, separately. Taking into account of mtLSUrRNA and SOD typing, the four liver transplant recipients had totally different genotypes from each other though the nucleotide positions were not completely sequenced at the 2 loci. Moreover, one transplant patient harbored a previously unreported combination of C566T, C832-833TT, C838C/T mutation at the CYB gene (CYB10/CYB11), whereas none of the controls carried the mutation [27,28].
Risk factors for PCP patients
Data from ten PCP patients and 30 transplant recipients without PCP were collected and compared, consisting of preoperative parameters, operative parameters, and postoperative parameters (Table 6). There were no significant differences in preoperative parameters such as age, sex, primary diseases, Child score, model for end-stage liver disease score, body mass index, diabetes, smoking, drinking, heart disease, respiratory disease, albumin, creatinine, bilirubin, INR, lymphocyte count and lymphocyte percentage (P>0.05). Similarly, operative parameters such as operating time, warm ischemia time, cold storage time, bleeding, transfusion, surgical type (piggyback), and anhepatic phase did not show statistically significant difference (P>0.05). Finally, postoperative parameters such as acute rejection, CMV, respiratory infection, bile leak, delayed graft function, bleeding, abdominal infection, immunosuppressants, ICU stay time, CD3+ T-cell percentage, CD4+ T-cell percentage, CD8+ T-cell percentage, and CD19+ B-cell percentage were similar between patients with and without PCP (P>0.05).
Table 6.
Risk factors for the development of PCP in transplant recipients
| Parameters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| With PCP | Without PCP | P | OR | CI | P | |
| Preoperative | ||||||
| Sex (male/female) | 9/1 | 27/3 | 1.000 | |||
| Age | 55.50±13.00 | 55.93±10.57 | 0.916 | |||
| Primary disease (benign) | 3 | 9 | 1.000 | |||
| Child score | 7.90±2.73 | 8.23±2.84 | 0.747 | |||
| MELD score | 14.90±9.77 | 15.50±10.60 | 0.875 | |||
| BMI | 24.51±3.09 | 24.70±3.50 | 0.876 | |||
| Diabetes | 2 | 9 | 0.696 | |||
| Smoking | 3 | 10 | 1.000 | |||
| Drinking | 2 | 10 | 0.693 | |||
| Heart disease | 1 | 2 | 1.000 | |||
| Respiratory disease | 1 | 1 | 0.442 | |||
| Albumin (g/L) | 37.08±6.60 | 35.82±7.03 | 0.620 | |||
| Creatinine (µmol/L) | 72.72±19.82 | 97.61±92.67 | 0.662* | |||
| Bilirubin (µmol/L) | 234.43±348.05 | 148.97±221.42 | 0.888* | |||
| INR | 1.38±0.55 | 1.38±0.56 | 0.978 | |||
| Lymphocyte count (/L) | 1.24±0.68 | 1.11±0.68 | 0.626 | |||
| Lymphocyte percentage (%) | 22.54±10.13 | 21.98±8.80 | 0.868 | |||
| Operative | ||||||
| Operating time (min) | 549.50±219.15 | 500.13±111.00 | 0.354 | |||
| Warm ischemia time (min) | 2.70±1.06 | 2.57±0.86 | 0.802* | |||
| Cold storage time (h) | 7.20±1.03 | 7.43±0.94 | 0.376* | |||
| Bleeding (ml) | 740.00±245.85 | 790.00±326.26 | 0.660 | |||
| Transfusion (ml) | 600.00±432.05 | 693.33±734.82 | 0.707 | |||
| Surgical type (piggyback) | 10 | 29 | 1.000 | |||
| Anhepatic phase (ml) | 58.80±30.33 | 74.00±36.72 | 0.246 | |||
| Postoperative | ||||||
| Acute rejection | 3 | 3 | 0.153 | |||
| CMV | 2 | 4 | 0.629 | |||
| Respiratory infection | 4 | 7 | 0.418 | |||
| Bile leak | 0 | 1 | 1.000 | |||
| Delayed graft function | 1 | 0 | 0.250 | |||
| Bleeding | 0 | 0 | - | |||
| Abdominal infection | 2 | 4 | 0.629 | |||
| Immunosuppressants | 0.906 | |||||
| Tacrolimus | 9 | 28 | ||||
| Sirolimus | 4 | 9 | ||||
| MMF | 4 | 14 | ||||
| Cyclosporin A | 1 | 2 | ||||
| Prednisone | 1 | 1 | ||||
| Tacrolimus concentration (ng/ml) | 7.57±2.62 | 5.52±2.14 | 0.023 | 0.770 | 0.435-1.366 | 0.372 |
| ICU stay time (h) | 91.90±34.92 | 125.23±70.44 | 0.161 | |||
| #CD3+ T-cell counts (/µL) | 306.81±222.40 | 655.00±323.44 | 0.003 | 0.996 | 0.953-1.041 | 0.854 |
| #CD3+ T-cell percentage (%) | 62.53±16.41 | 67.58±13.99 | 0.349 | |||
| #CD4+ T-cell counts (/µL) | 168.15±152.02 | 365.01±220.06 | 0.012 | 1.004 | 0.955-1.056 | 0.872 |
| #CD4+ T-cell percentage (%) | 31.64±11.42 | 37.28±11.60 | 0.189 | |||
| #CD8+ T-cell counts (/µL) | 132.32±85.59 | 261.34±175.18 | 0.033 | 1.009 | 0.963-1.056 | 0.710 |
| #CD8+ T-cell percentage (%) | 29.82±15.61 | 27.66±15.28 | 0.701 | |||
| #CD19+ B-cell counts (/µL) | 48.09±39.11 | 164.68±89.50 | 0.000 | 1.028 | 1.000-1.057 | 0.049 |
| #CD19+ B-cell percentage (%) | 12.34±10.03 | 18.54±10.63 | 0.114 | |||
PCP, Pneumocystis pneumonia; OR, odds ratio; CI, 95% confidence interval; MELD, model for end-stage liver disease; BMI, body mass index; INR, international normalized ratio; CMV, cytomegalovirus; MMF, Mycophenolate mofetil; ICU, intensive care unit.
Mann-Whitney U was used.
PCP cases tested right after diagnosis; controls tested following the same period as PCP patients.
However, Tacrolimus concentration was significantly higher while absolute CD3+ T-cell counts, absolute CD4+ T-cell counts, absolute CD8+ T-cell counts, and absolute CD19+ B-cell counts were significantly lower in patients with PCP than those in controls (P<0.05).
Using multivariable conditional logistic regression, we found that absolute CD19+ B-cell counts (odds ratios: 1.028; 95% confidence intervals: 1.000-1.057; P=0.049) were the only independent risk factor. The area under the receiver operating characteristic curve was 0.910 for the prediction of PCP among patients following transplantation. The cut-off value was 117.16/µL and corresponding sensitivity and specificity were 100% and 70%, respectively.
Discussion
In this study we found that PCP was a severe complication following liver transplantation. The outbreak of PCP in liver transplant recipients at our center was not caused by nosocomial transmission according to the analysis of their clinical, epidemiological, and molecular characteristics though PCP patients, colonized patients, and HCW with close contact with PCP patients were a potential source of P. jirovecii transmission. Moreover, a decrease in absolute CD19+ B-cell counts might be associated with the development of PCP in liver transplant recipients.
Previously, PCP was most frequently associated with HIV patients. In contrast, its incidence has been decreasing recently [1,2]. Growing evidence has shown an increase in PCP incidence and mortality in liver transplant recipients during the last decades, which has contributed to the increasing number of PCP outbreaks [4]. In our study, all liver transplant recipients with PCP required hospital admission, of which 77.8% were transferred to intensive care units and 60% required mechanical ventilation. Our data also showed that PCP is related to considerable mortality; 40% of liver transplant recipients with PCP died despite appropriate care and treatment. Presently, chemoprophylaxis is recommended among transplant recipients to reduce its incidence. It should be noted that we did not apply chemoprophylaxis for liver transplant patients because the incidence of PCP in our institution was 2.6%. Whereas, routine anti-PCP prophylaxis is suggested for centers with an incidence rate of at least 3%-5% or for individuals with recurrent PCP [29]. A systematic review and meta-analysis of randomized controlled trials suggested that prophylaxis for PCP should be considered when the risk for PCP in adults is higher than 3.5% [30]. Moreover, liver transplant recipients may avoid prophylaxis when receiving low levels of immunosuppression [31]. So far, no subsequent PCP case has been detected and the outbreak has been considered a sporadic event. Hence, no changes will be made in our clinical practices.
An outbreak of PCP in immunocompromised patients has been repeatedly reported to be caused by nosocomial transmission via four proposed sources, including (i) directly contacting PCP patients; (ii) contacting the same HCW who had close contact with PCP patients; (iii) contacting the same colonized patients who might or might not have close contact with previous PCP patients; (iv) airborne transmission of environmental aerosols contaminated with P. jirovecii from PCP patients [7,8,19-21]. Therefore, we conducted the systematic research to investigate the potential sources of transmission. First, from the transmission map, there was no spatiotemporal possibility for these PCP patients to meet each other before diagnosis either during hospitalization or at the outpatient clinic, which led to the rejection of this route. Then, air sample detection was all negative. There were two main reasons that accounted for this phenomenon: the traditional passive sedimentation method which was less sensitive compared with an air sampler, and laminar airflow rooms which made it difficult to collect P. jirovecii. Finally, colonized HCW and colonized patients were found to have a higher occurrence of P. jirovecii when compared with controls. Additionally, we had to admit that PCR on swabs was less sensitive than lower respiratory samples, which may have resulted in possible false-negative results. Invasive investigations could improve the active screening of P. jirovecii infected patients in case of high suspicion.
Furthermore, positive P. jirovecii samples were genotyped. Apart from sequence typing failure in some samples or loci, the results were still sufficient to differentiate the P. jirovecii strains. To our surprise, PCP patients showed completely different P. jirovecii strains, which were partially distinct from those detected in unrelated controls from other departments because of incomplete sequencing. This meant PCP patients were infected from different sources. It is likely due to: (1) the PCP patients had been the asymptomatic carriers before admission; (2) the MLST approach was not sensitive to detection of mixed bases [32]. Moreover, we found an unreported mutation at the CYB gene (CYB10/CYB11) only in 1 PCP patient [27,28].
Next, risk factors for the development of PCP were analyzed. In our study, we identified decreased CD19+ B-cell counts to be the only risk factor. Nevertheless, in contrast to published studies that have reported several predictors such as age ≥65, CMV, acute rejection, and decreased CD4+ T-cell counts, no such increased risk of PCP was observed in our study [9-11]. In our analysis, we could not confirm age as a relevant risk factor. The mean age of the cluster was 55 years with two patients ≥65 years. The presence of CMV viremia was associated with PCP. Basically, CMV infection may simply reflect the degree of immunosuppression. Rostved et al. described a high rate of CMV co-infection but could not detect increased rates of CMV prior to diagnosis of PCP [12]. Acute rejection implies the activation of the immune system while PCP occurs in immunocompromised patients. Therefore, acute rejection precedes PCP. It is the anti-rejection treatment that suppresses the immune system leading to lymphocyte deficiency, especially decreased CD4+ T-cell counts [33-35]. In essence, acute rejection is a reflection of decreased CD4+ T-cell counts. Allograft dysfunction could increase the risk of PCP [36]. Decreased CD4+ T-cell counts are a clearly defined risk factor for PCP in HIV patients, while no clear threshold could be defined for CD4+ T-cell counts in liver transplant recipients [37].
A growing number of studies have stressed the key role CD19+ B-cells have played in the immune system. Rong et al. found that Pneumocystis burden in B cell-deficient mice progressively increased. Additionally, clearance of Pneumocystis was delayed in B cell-activating factor receptor-deficient mice, which had few B cells and Pneumocystis-specific IgG and IgM antibodies [38]. Hernandez-Novoa et al. reported that CD40L-KO mice were highly susceptible to developing severe Pneumocystis pneumonia due to a decrease in CD19+ B-cell compared with immunocompetent mice [39], which may involve interaction between B cells and CD4+ T cells via the CD40-CD40L pathway [40]. In the lungs of corticosteroid-treated PCP mice, the repression of gene expression, corresponding to B cell immunity, including B cell signaling, homeostasis, and Ig production, was prominent. When the B cell immunity was suppressed by corticosteroid treatment, it was likely that PCP was stimulated to develop [41]. Hence, the CD19+ B-cells exhibite an antifungal effect against P. jirovecii. In conjunction with these data, our study advocates the assumption that a decreased number of CD19+ B-cell in the blood may be the risk factor for PCP.
The main limitation of this study is that it represents the experience of a single center with a small number of patients. However, it is really difficult to collect more samples due to its lower incidence. Therefore, future studies, preferably multi-center controlled clinical trials, are needed to further validate our initial report.
Acknowledgements
This work was supported by Open Project of Beijing Key Laboratory of Tolerance Induction and Organ Protection in Transplantation (2017YZNS01) and National Natural Science Foundation of China (81601392). Oliver Witzke is supported by an unrestricted grant of the Rudolf-Ackermann-Stiftung (Stiftung für Klinische Infektiologie). Benjamin Wilde is funded by the Dr. Werner Jackstädt-Stiftung.
Disclosure of conflict of interest
O.W. has received research grants for clinical studies, speaker’s fees, honoraria and travel expenses from Amgen, Astellas, Bristol-Myers Squibb, Chiesi, Hexal, Janssen-Cilag, MSD, Novartis, Roche, Pfizer, and Sanofi.
References
- 1.Curtis JR, Yarnold PR, Schwartz DN, Weinstein RA, Bennett CL. Improvements in outcomes of acute respiratory failure for patients with human immunodeficiency virus-related Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 2000;162:393–398. doi: 10.1164/ajrccm.162.2.9909014. [DOI] [PubMed] [Google Scholar]
- 2.Curtis JR, Greenberg DL, Hudson LD, Fisher LD, Krone MR, Collier AC. Changing use of intensive care for HIV-infected patients with Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 1994;150:1305–1310. doi: 10.1164/ajrccm.150.5.7952557. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt JJ, Lueck C, Ziesing S, Stoll M, Haller H, Gottlieb J, Eder M, Welte T, Hoeper MM, Scherag A, David S. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit Care. 2018;22:307. doi: 10.1186/s13054-018-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosseini-Moghaddam SM, Shokoohi M, Singh G, Dufresne SF, Boucher A, Jevnikar A, Prasad GVR, Shoker A, Kabbani D, Hebert MJ, Cardinal H, Houde I, Humar A, Kumar D. A multicenter case-control study of the effect of acute rejection and cytomegalovirus infection on pneumocystis pneumonia in solid organ transplant recipients. Clin Infect Dis. 2019;68:1320–1326. doi: 10.1093/cid/ciy682. [DOI] [PubMed] [Google Scholar]
- 5.Cordonnier C, Alanio A, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Hauser PM, Lagrou K, Melchers WJ, Helweg-Larsen J, Matos O, Bretagne S, Maertens J Fifth European Conference on Infections in Leukemia (ECIL-5; a joint venture of The European Group for Blood and Marrow Transplantation (EBMT), The European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and The European LeukemiaNet (ELN) Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71:2379–2385. [Google Scholar]
- 6.Chapman JR, Marriott DJ, Chen SC, MacDonald PS. Post-transplant Pneumocystis jirovecii pneumonia--a re-emerged public health problem? Kidney Int. 2013;84:240–243. doi: 10.1038/ki.2013.212. [DOI] [PubMed] [Google Scholar]
- 7.Le Gal S, Damiani C, Rouille A, Grall A, Treguer L, Virmaux M, Moalic E, Quinio D, Moal MC, Berthou C, Saliou P, Le Meur Y, Totet A, Nevez G. A cluster of Pneumocystis infections among renal transplant recipients: molecular evidence of colonized patients as potential infectious sources of Pneumocystis jirovecii. Clin Infect Dis. 2012;54:e62–71. doi: 10.1093/cid/cir996. [DOI] [PubMed] [Google Scholar]
- 8.Yiannakis EP, Boswell TC. Systematic review of outbreaks of Pneumocystis jirovecii pneumonia: evidence that P. jirovecii is a transmissible organism and the implications for healthcare infection control. J Hosp Infect. 2016;93:1–8. doi: 10.1016/j.jhin.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Iriart X, Challan Belval T, Fillaux J, Esposito L, Lavergne RA, Cardeau-Desangles I, Roques O, Del Bello A, Cointault O, Lavayssiere L, Chauvin P, Menard S, Magnaval JF, Cassaing S, Rostaing L, Kamar N, Berry A. Risk factors of Pneumocystis pneumonia in solid organ recipients in the era of the common use of posttransplantation prophylaxis. Am J Transplant. 2015;15:190–199. doi: 10.1111/ajt.12947. [DOI] [PubMed] [Google Scholar]
- 10.De Castro N, Xu F, Porcher R, Pavie J, Molina JM, Peraldi MN. Pneumocystis jirovecii pneumonia in renal transplant recipients occurring after discontinuation of prophylaxis: a case-control study. Clin Microbiol Infect. 2010;16:1375–1377. doi: 10.1111/j.1469-0691.2009.03143.x. [DOI] [PubMed] [Google Scholar]
- 11.Arend SM, Westendorp RG, Kroon FP, van’t Wout JW, Vandenbroucke JP, van Es LA, van der Woude FJ. Rejection treatment and cytomegalovirus infection as risk factors for Pneumocystis carinii pneumonia in renal transplant recipients. Clin Infect Dis. 1996;22:920–925. doi: 10.1093/clinids/22.6.920. [DOI] [PubMed] [Google Scholar]
- 12.Rostved AA, Sassi M, Kurtzhals JA, Sorensen SS, Rasmussen A, Ross C, Gogineni E, Huber C, Kutty G, Kovacs JA, Helweg-Larsen J. Outbreak of pneumocystis pneumonia in renal and liver transplant patients caused by genotypically distinct strains of Pneumocystis jirovecii. Transplantation. 2013;96:834–842. doi: 10.1097/TP.0b013e3182a1618c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miguel Montanes R, Elkrief L, Hajage D, Houssel P, Fantin B, Francoz C, Dreyfuss D, Ricard JD, Durand F. An outbreak of Pneumocytis jirovecii pneumonia among liver transplant recipients. Transpl Infect Dis. 2018;20:e12956. doi: 10.1111/tid.12956. [DOI] [PubMed] [Google Scholar]
- 14.Choi YI, Hwang S, Park GC, Namgoong JM, Jung DH, Song GW, Ha TY, Moon DB, Kim KH, Ahn CS, Lee SG. Clinical outcomes of Pneumocystis carinii pneumonia in adult liver transplant recipients. Transplant Proc. 2013;45:3057–3060. doi: 10.1016/j.transproceed.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 15.Desoubeaux G, Dominique M, Morio F, Thepault RA, Franck-Martel C, Tellier AC, Ferrandiere M, Hennequin C, Bernard L, Salame E, Bailly E, Chandenier J. Epidemiological outbreaks of pneumocystis jirovecii pneumonia are not limited to kidney transplant recipients: genotyping confirms common source of transmission in a liver transplantation unit. J Clin Microbiol. 2016;54:1314–1320. doi: 10.1128/JCM.00133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarwar S, Carey B, Hegarty JE, McCormick PA. Low incidence of Pneumocystis jirovecii pneumonia in an unprophylaxed liver transplant cohort. Transpl Infect Dis. 2013;15:510–515. doi: 10.1111/tid.12117. [DOI] [PubMed] [Google Scholar]
- 17.Maschmeyer G, Helweg-Larsen J, Pagano L, Robin C, Cordonnier C, Schellongowski P 6th European Conference on Infections in Leukemia (ECIL-6), a joint venture of The European Group for Blood and Marrow Transplantation (EBMT), The European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and The European LeukemiaNet (ELN) ECIL guidelines for treatment of Pneumocystis jirovecii pneumonia in non-HIV-infected haematology patients. J Antimicrob Chemother. 2016;71:2405–2413. doi: 10.1093/jac/dkw158. [DOI] [PubMed] [Google Scholar]
- 18.Cooley L, Dendle C, Wolf J, Teh BW, Chen SC, Boutlis C, Thursky KA. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014;44:1350–1363. doi: 10.1111/imj.12599. [DOI] [PubMed] [Google Scholar]
- 19.Miller RF, Lindley AR, Copas A, Ambrose HE, Davies RJ, Wakefield AE. Genotypic variation in Pneumocystis jirovecii isolates in Britain. Thorax. 2005;60:679–682. doi: 10.1136/thx.2004.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmoldt S, Schuhegger R, Wendler T, Huber I, Sollner H, Hogardt M, Arbogast H, Heesemann J, Bader L, Sing A. Molecular evidence of nosocomial Pneumocystis jirovecii transmission among 16 patients after kidney transplantation. J Clin Microbiol. 2008;46:966–971. doi: 10.1128/JCM.02016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller RF, Ambrose HE, Wakefield AE. Pneumocystis carinii f. sp. hominis DNA in immunocompetent health care workers in contact with patients with P. carinii pneumonia. J Clin Microbiol. 2001;39:3877–3882. doi: 10.1128/JCM.39.11.3877-3882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling S, Hui L. Evaluation of the complexity of indoor air in hospital wards based on PM2.5, real-time PCR, adenosine triphosphate bioluminescence assay, microbial culture and mass spectrometry. BMC Infect Dis. 2019;19:646. doi: 10.1186/s12879-019-4249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas D, Galler H, Fritz C, Hasler C, Habib J, Reinthaler FF. Comparative study of impaction and sedimentation in an aerosol chamber using defined fungal spore and bacterial concentrations. PLoS One. 2017;12:e0187039. doi: 10.1371/journal.pone.0187039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denis CM, Mazars E, Guyot K, Odberg-Ferragut C, Viscogliosi E, Dei-Cas E, Wakefield AE. Genetic divergence at the SODA locus of six different formae speciales of Pneumocystis carinii. Med Mycol. 2000;38:289–300. doi: 10.1080/mmy.38.4.289.300. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair K, Wakefield AE, Banerji S, Hopkin JM. Pneumocystis carinii organisms derived from rat and human hosts are genetically distinct. Mol Biochem Parasitol. 1991;45:183–184. doi: 10.1016/0166-6851(91)90042-5. [DOI] [PubMed] [Google Scholar]
- 26.Kazanjian P, Armstrong W, Hossler PA, Lee CH, Huang L, Beard CB, Carter J, Crane L, Duchin J, Burman W, Richardson J, Meshnick SR. Pneumocystis carinii cytochrome b mutations are associated with atovaquone exposure in patients with AIDS. J Infect Dis. 2001;183:819–822. doi: 10.1086/318835. [DOI] [PubMed] [Google Scholar]
- 27.Maitte C, Leterrier M, Le Pape P, Miegeville M, Morio F. Multilocus sequence typing of Pneumocystis jirovecii from clinical samples: how many and which loci should be used? J Clin Microbiol. 2013;51:2843–2849. doi: 10.1128/JCM.01073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esteves F, Gaspar J, Tavares A, Moser I, Antunes F, Mansinho K, Matos O. Population structure of Pneumocystis jirovecii isolated from immunodeficiency virus-positive patients. Infect Genet Evol. 2010;10:192–199. doi: 10.1016/j.meegid.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis. 2001;33:1397–1405. doi: 10.1086/323129. [DOI] [PubMed] [Google Scholar]
- 30.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:1052–1059. doi: 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 31.Trotter JF, Levi M, Steinberg T, Lancaster J. Absence of Pneumocystis jiroveci pneumonia in liver transplantation recipients receiving short-term (3-month) prophylaxis. Transpl Infect Dis. 2008;10:369–371. doi: 10.1111/j.1399-3062.2008.00318.x. [DOI] [PubMed] [Google Scholar]
- 32.Delliere S, Gits-Muselli M, Bretagne S, Alanio A. Outbreak-causing fungi: pneumocystis jirovecii. Mycopathologia. 2019;185:783–800. doi: 10.1007/s11046-019-00408-w. [DOI] [PubMed] [Google Scholar]
- 33.Carter JT, Melcher ML, Carlson LL, Roland ME, Stock PG. Thymoglobulin-associated Cd4+ T-cell depletion and infection risk in HIV-infected renal transplant recipients. Am J Transplant. 2006;6:753–760. doi: 10.1111/j.1600-6143.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 34.Woodle ES, Xu D, Zivin RA, Auger J, Charette J, O’Laughlin R, Peace D, Jollife LK, Haverty T, Bluestone JA, Thistlethwaite JR Jr. Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3gamma1(Ala-Ala) in the treatment of acute renal allograft rejection. Transplantation. 1999;68:608–616. doi: 10.1097/00007890-199909150-00003. [DOI] [PubMed] [Google Scholar]
- 35.Hourmant M, Babinet F, Cantarovich D, Latour M, Carcagne J, Vie H, Bonneville M, Moreau JF, Carosella E, Bignon JD, et al. Polyclonal rabbit gamma globulins against a human cytotoxic CD4 T cell clone. II. Use in prevention of rejection in kidney transplantation: a pilot study. Transplantation. 1989;48:260–263. doi: 10.1097/00007890-198908000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Phipps LM, Chen SC, Kable K, Halliday CL, Firacative C, Meyer W, Wong G, Nankivell BJ. Nosocomial Pneumocystis jirovecii pneumonia: lessons from a cluster in kidney transplant recipients. Transplantation. 2011;92:1327–1334. doi: 10.1097/TP.0b013e3182384b57. [DOI] [PubMed] [Google Scholar]
- 37.Messiaen PE, Cuyx S, Dejagere T, van der Hilst JC. The role of CD4 cell count as discriminatory measure to guide chemoprophylaxis against Pneumocystis jirovecii pneumonia in human immunodeficiency virus-negative immunocompromised patients: a systematic review. Transpl Infect Dis. 2017;19 doi: 10.1111/tid.12651. [DOI] [PubMed] [Google Scholar]
- 38.Rong HM, Li T, Zhang C, Wang D, Hu Y, Zhai K, Shi HZ, Tong ZH. IL-10-producing B cells regulate Th1/Th17-cell immune responses in Pneumocystis pneumonia. Am J Physiol Lung Cell Mol Physiol. 2019;316:L291–L301. doi: 10.1152/ajplung.00210.2018. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Novoa B, Bishop L, Logun C, Munson PJ, Elnekave E, Rangel ZG, Barb J, Danner RL, Kovacs JA. Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice. J Leukoc Biol. 2008;84:420–430. doi: 10.1189/jlb.1207816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiley JA, Harmsen AG. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J Immunol. 1995;155:3525–3529. [PubMed] [Google Scholar]
- 41.Hu Y, Wang D, Zhai K, Tong Z. Transcriptomic analysis reveals significant B lymphocyte suppression in corticosteroid-treated hosts with pneumocystis pneumonia. Am J Respir Cell Mol Biol. 2017;56:322–331. doi: 10.1165/rcmb.2015-0356OC. [DOI] [PubMed] [Google Scholar]


