Abstract
Objective: To evaluate the diagnostic value of serum human epididymal protein 4 (HE4), carbohydrate antigen 125 (CA125), and risk of ovarian malignancy algorithm (ROMA) in early identification in ovarian cancer. Method: A total of 50 patients with ovarian cancer and 50 patients with benign ovarian tumors admitted to our hospital from January 2019 to January 2020 were included in Group A and Group B, respectively, and 50 healthy adult females during the same period were assigned to the blank group. The serum levels of HE4 and CA125 in each group were determined, and the ROMA of them was calculated according to postmenopausal status. The sensitivity, specificity, and positive diagnosis rate of HE4, CA125, and ROMA were calculated, and ROC curves were drawn to compare the diagnostic value of the three. Results: Group A showed significantly higher serum levels of HE4 and CA125 and a significantly higher ROMA than Group B and the blank group (both P<0.05). No significant difference was found in the serum level of HE4 between Group B and the blank group (P>0.05). The serum level of CA125 and ROMA were significantly higher in Group B when compared with those of the blank group (both P<0.05). The diagnostic sensitivity and positive diagnosis rate of the three indexes, from high to low, were HE4+CA125+ROMA>ROMA>HE4>CA125 (all P<0.05). The diagnostic specificity and the area under the curve (AUC) of the three indexes, from high to low, were HE4+CA125+ROMA>HE4>ROMA>CA125 (all P<0.05). Histologic grading and lymph node metastasis were factors affecting the serum levels of HE4, CA125, and ROMA in patients with ovarian cancer. Conclusion: The combined detection of HE4, CA125, and ROMA is more effective than diagnosis with any single indicator, so the combined diagnosis has a high application value in the early diagnosis of ovarian cancer.
Keywords: HE4, CA125, ROMA, ovarian cancer, application value
Introduction
Ovarian cancer is the most lethal gynecological malignant tumor with a high prevalence in middle and old aged women and a mortality rate higher than that of cervical cancer, posing a great threat to patients’ life and health [1,2]. As the early manifestations of ovarian cancer are rather hidden, the disease may have developed to a middle or advanced stage at the time of diagnosis, resulting in missing of the optimal treatment timing and increased mortality [3-5]. Ultrasound is commonly used in the diagnosis of ovarian tumors. However, it can only determine the presence of a tumor but fails to determine its malignancy. Human epididymal protein 4 (HE4) and carbohydrate antigen 125 (CA125) are also frequently adopted in the diagnosis of ovarian cance [6-8]. CA125, as one of the common clinical tumor markers with a low level in normal conditions, increases to varying degrees in cases of diseases such as ovarian, pancreatic, breast, lung, and stomach cancers, but it has a low specificity, a poor sensitivity, and a high false-positive rate in diagnosing diseases. In addition to its high expression in ovarian cancer, the expression of CA125 also increases in epithelial cancer tissues, benign ovarian tumors, and normal ovarian surface epithelium, which limits its clinical application. HE4 belongs to the whey acidic 4-disulfide central protein family. It was first identified in human epididymal epithelial cells as a small, acidic single-signal peptide encoded by the WFDC2 gene, with high expression in ovarian cancer tissues [9,10], but low or no expression in normal ovarian tissues [11]. The specificity and sensitivity of HE4 for detecting ovarian cancer was 96% and 67%, respectively, and joint determination of HE4 and CA125 has a significantly higher accuracy than mono-determination of CA125 or HE4 levels for the diagnosis of malignant ovarian cancer, with a specificity of 95% and a sensitivity of 76% [12]. The risk of ovarian malignancy algorithm (ROMA) can be used to predict the occurrence of ovarian cancer based on the patient’s menstrual status and a logistic regression model established based on serum CA125 and HE4 levels. A recent study also revealed a low diagnostic sensitivity and specificity of the mono-determination of HE4 level [9]. With an aim to explore a highly sensitive and specific diagnosis method of ovarian cancer, this study analyzed the expression levels of HE4 and CA125 in patients with ovarian cancer, those with benign ovarian tumors, and healthy adult women, and their diagnostic sensitivity and specificity. The novelty of this study lies in the determination of serum CA125 and HE4 levels in patients, which may provide additional diagnostic methods for the diagnosis of ovarian cancer along with the ROMA model.
Materials and methodology
General information
This is a retrospective study. A total of 50 patients with ovarian cancer and 50 patients with benign ovarian tumors admitted to our hospital from January 2019 to January 2020 were included in Group A and Group B, respectively, and 50 healthy adult females during the same period were assigned to the blank group. The ethics committee of our hospital approved the study (2018-11-19). The general data of patients in the two groups were similar, as shown in Table 1 (all P>0.05).
Table 1.
The general data of the three groups
| Group | Age | Duration | Basic Disease | Age | Menopausal Status | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Hypertension | Diabetes | Hyperlipidemia | <50 | ≥50 | Yes | No | |||
| Group A (n=50) | 50.12±6.34 | 6.38±1.27 | 17 | 10 | 7 | 21 | 29 | 32 | 18 |
| Group B (n=50) | 51.09±6.51 | 6.09±1.50 | 15 | 8 | 8 | 24 | 26 | 30 | 20 |
| Blank Group (n=50) | 51.66±6.93 | - | 20 | 39 | 33 | 17 | |||
| F/t/χ2 | 0.696 | 1.043 | 0.762 | 0.078 | 0.298 | 2.265 | 0.402 | ||
| P | 0.5 | 0.299 | 0.683 | 0.962 | 0.862 | 0.322 | 0.818 | ||
Inclusion criteria
(a) Patients with clinical manifestations that were consistent with the ovarian cancer; (b) Patients who were over 18 years old; (c) Patients with normal functions of the heart, lung, and kidney; (d) Patients without a history of drug allergy, drug abuse, or bad habits; (e) Patients and their family members had signed written informed consents. The subjects in the blank group had normal liver and kidney function and normal chest radiographs, without pelvic, breast, and thyroid masses as confirmed by ultrasound examination.
Exclusion criteria
(a) Patients who were unable to cooperate with the investigator due to mental disorder or reluctant for cooperation; (b) Patients with recurrent ovarian tumor; (c) Patients with other tumor diseases. The subjects in the blank group had no underlying diseases such as hypertension and diabetes.
Method
The blood samples of patients in Group A and Group B were collected during routine diagnostic examinations, and those of blank group were collected during early cancer screening. Peripheral blood (5 ml) was collected from each participant after being fasted for eight hours, placed at room temperature for 30 min, and centrifuged at 3000 r/min for 10 min to separate supernatant. The separated supernatant was stored at -80°C. Then the serum level of CA125 was determined using the chemiluminescence method, with 0-35 U/mL as the normal range, and the level of HE4 was determined using enzyme-linked immunosorbent assay, with 140 pmol/L as the normal level. The risk of ovarian cancer in menopausal and premenopausal subjects was calculated using the ROMA Predictive Index (PI), respectively. (1) For premenopausal women: PI=-12.0+2.38×Ln (HE4)+0.0626×Ln (CA125); (2) For menopausal women: PI=-8.09+1.04×Ln (HE4)+0.732×Ln (CA125). The ROMA-based predictive probability of ovarian cancer was expressed as PP (%) (PP (%) = [exp (PI)×100%]/[1+exp (PI)]) [10-13]. PP≥11.4% for premenopausal women and PP≥29.9% for menopausal women indicated a high risk of ovarian cancer.
Outcome measures
The serum levels of HE4 and CA125 were determined, and the ROMA was calculated according to postmenopausal status. Additionally, the sensitivity, specificity, and positive diagnosis rate of HE4, CA125, and ROMA were calculated, and ROC curves were drawn to compare the diagnostic value of HE4, CA125, and ROMA.
Statistics process
SPSS 21.0 was used for data analysis and GraphPad Prism 8.0 was used to visualize the data into graphics. Measurement data were expressed as mean ± SD and analyzed using analysis of variance among three groups. Count data were expressed as [n (%)] and analyzed using the chi-square test. P<0.05 indicates a significant difference.
Results
Age distribution and menopausal status of the three groups
There was no difference in the age and menopausal status among the three groups (both P>0.05), as shown in Table 1.
Serum level of HE4 in the three groups
Group A showed a higher serum level of HE4 than that of Group B and the blank group (326.29±95.48 pmol/L vs. 47.33±12.07 pmol/L vs. 43.61±9.65 pmol/L, P<0.001), and there was no notable difference in serum level of HE4 between Group B and the blank group (P>0.05), as shown in Figure 1.
Figure 1.
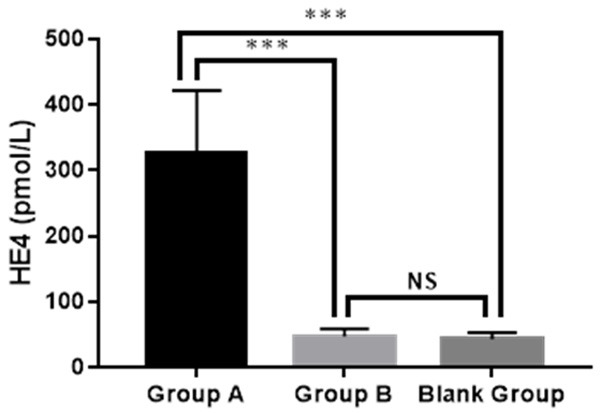
Serum level of HE4 in the three groups. Group A: 326.29±95.48 pmol/L; Group B: 47.33±12.07 pmol/L; Bland group: 43.61±9.65 pmol/L. *** indicated P<0.001, NS indicated no significant difference.
Serum level of CA125 of the three groups
Group A showed a higher serum level of CA125 than Group B and the blank group (426.91±113.03 U/mL vs. 29.35±6.88 U/mL vs. 16.07±5.09 U/mL), and Group B showed a higher serum level of CA125 than the blank group (both P<0.001), as shown in Figure 2.
Figure 2.
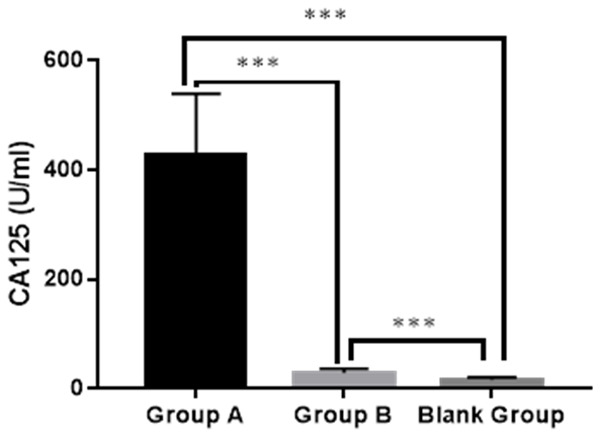
Serum level of CA125 in the three groups. Group A: 426.91±113.03 U/mL; Group B: 29.35±6.88 U/mL; Blank group: 16.07±5.09 U/mL. *** indicated P<0.001.
The ROMA of the three groups
Group A obtained a higher ROMA than Group B and the blank group (65.68±7.43% vs. 9.33±2.11% vs. 6.34±1.47%), and Group B obtained a higher ROMA than the blank group (both P<0.001), as shown in Figure 3.
Figure 3.
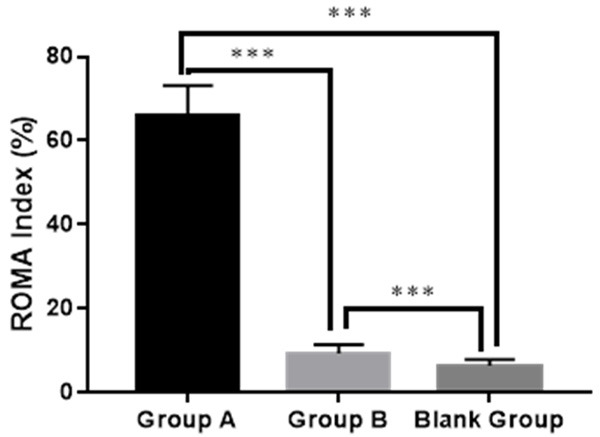
ROMA of the three groups. Group A: 65.68±7.43%; Group B: 9.33±2.11%; Bland group: 6.34±1.47%. *** indicated P<0.001.
Diagnostic value of the four methods
The AUCs of HE4+CA125+ROMA, ROMA, HE4, and CA125 were 0.966, 0.743, 0.862 and 0.606 respectively. From high to low, the sensitivity of the four was HE4+CA125+ROMA>ROMA>HE4>CA125; the specificity of the four was HE4+CA125+ROMA>HE4>ROMA>CA125; the positive rate of the four was HE4+CA125+ROMA>ROMA>HE4>CA125, as shown in Table 2.
Table 2.
The diagnostic value of the four methods (%)
| Sensitivity | Specificity | Positive Diagnosis Rate | |
|---|---|---|---|
| CA125 | 52 | 54 | 54 |
| HE4 | 70a | 86a | 74a |
| ROMA | 84a,b | 72a,b | 84a,b |
| HE4+CA125+ROMA | 94a,b,c | 98a,b,c | 98a,b,c |
| χ2 | 53.12 | 61.65 | 58.9 |
| P | <0.001 | <0.001 | <0.001 |
indicated P<0.05 when compared with CA125;
indicated P<0.05 when compared with HE4;
indicated P<0.05 when compared with ROMA.
AUC of the four methods
The combined diagnosis obtained the highest AUC than the single diagnosis, and the AUC of single diagnosis was HE4>ROMA>CA125, as shown in Figures 4 and 5.
Figure 4.
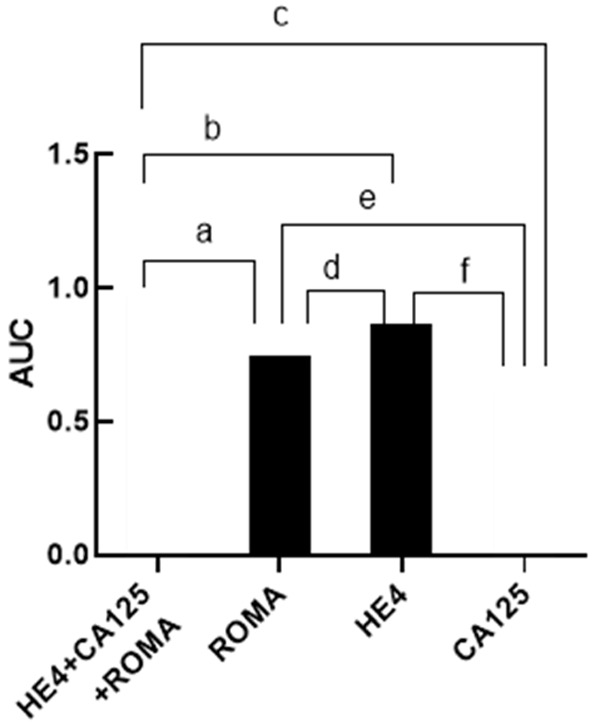
AUC of the four methods. Note: The abscissa from left to right were HE4+CA125+ROMA, ROMA, HE4, and CA125 diagnostic methods. The ordinate represented the AUC of each diagnostic mode. The AUCs of HE4+CA125+ROMA, ROMA, HE4, and CA125 were 0.966, 0.743, 0.862 and 0.606 respectively. a represents the comparison between HE4+CA125+ROMA and ROMA, χ2=20.00, P<0.001; b represents the comparison between HE4+CA125+ROMA and HE4, χ2=6.88, P=0.09; c represents the comparison between HE4+CA125+ROMA and CA125, χ2=38.52, P<0.001; d represents the comparison between ROMA and HE4, χ2=4.47, P=0.04; e represents the comparison between ROMA and CA125, χ2=4.27, P=0.04; f represents the comparison between HE4 and CA125, χ2=16.78, P<0.001.
Figure 5.
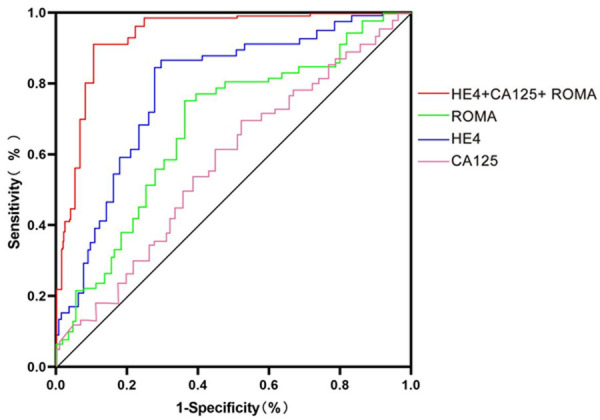
ROC curves of four methods.
Factors affecting serum HE4, CA125, and ROMA levels in patients with ovarian cancer
In the comparison of HE4 and CA125 expression in ovarian cancer patients with different clinicopathological characteristics, serum HE4 and CA125 expression elevated in ovarian cancer patients with clinical stage III+IV, moderate and low histological grade, and lymph node metastasis (P<0.05). No significant difference was found in patients of different ages. The ROMA was higher in patients with ovarian cancer with clinical stage III+IV, moderate or low histological grade, and lymph node metastasis (P<0.05), with no difference among patients of different ages. See Table 3.
Table 3.
Comparison of HE4 and CA125 expression levels and ROMA in ovarian cancer patients with different clinicopathological characteristics
| Clinicopathological characteristics | Case | HE4 (pmol/L) | t | P | CA125 (U/mL) | t | P | ROMA (%) | t | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.574 | 0.325 | 5.464 | 0.366 | 4.568 | 0.377 | ||||
| ≥50 years | 29 | 321.01±90.48 | 424.90±107.36 | 69.23±3.17 | ||||||
| <50 years | 21 | 320.01±94.44 | 425.93±108.03 | 71.15±4.02 | ||||||
| Clinical Stages | 2.544 | 0.001 | 3.157 | 0.001 | 3.479 | 0.001 | ||||
| Stage I-II | 40 | 319.01±89.48 | 421.97±107.36 | 65.23±4.32 | ||||||
| Stage III-IV | 10 | 327.91±95.14 | 430.89±111.03 | 76.52±5.28 | ||||||
| Histological grading | 3.774 | 0..002 | 5.475 | 0.001 | 4.562 | 0..002 | ||||
| High differentiation | 15 | 317.01±88.48 | 423.77±106.44 | 63.21±5.02 | ||||||
| Moderate and low differentiation | 35 | 329.91±96.14 | 431.93±131.03 | 75.04±6.27 | ||||||
| Lymph node metastasis | 5.454 | 0.001 | 6.545 | 0.002 | 7.564 | 0.003 | ||||
| Yes | 40 | 333.91±95.12 | 434.43±122.03 | 75.13±5.83 | ||||||
| no | 10 | 316.35±89.56 | 425.77±111.44 | 66.24±6.12 | ||||||
The factors affecting serum HE4, CA125, and ROMA levels in patients with ovarian cancer were analyzed. Clinical stage, histological grading, and lymph node metastasis that were significant for univariate analysis were used as independent variables, and HE4, CA125, and ROMA of patients with ovarian cancer were used as dependent variables for multifactorial analysis. The results showed that histological grading and lymph node metastasis were factors affecting serum HE4, CA125, and ROMA in patients with ovarian cancer. See Table 4.
Table 4.
Factors affecting serum levels of HE4, CA125 and ROMA in patients with ovarian cancer
| Factors | β | wald | P | OR | 95% CI |
|---|---|---|---|---|---|
| Clinical grading | |||||
| HE4 | 1.116 | 5.544 | 0.452 | 4.777 | 1.634-12.114 |
| CA125 | 1.423 | 6.451 | 0.365 | 4.566 | 1.915-9.878 |
| ROMA | 1.568 | 4.453 | 0.456 | 5.222 | 1.700-12.5.4 |
| Histological grading | |||||
| HE4 | 1.356 | 6.021 | 0.009 | 4.385 | 1.731-12.034 |
| CA125 | 1.603 | 7.415 | 0.005 | 4.502 | 1.912-9.856 |
| ROMA | 1.758 | 10.359 | <0.01 | 5.389 | 1.795-12.132 |
| Lymph node metastasis | |||||
| HE4 | 1.359 | 5.032 | 0.005 | 4.987 | 1.267-10.357 |
| CA125 | 1.412 | 6.051 | 0.017 | 5.013 | 1.345-11.471 |
| ROMA | 1.528 | 7.135 | 0.001 | 5.112 | 1.297-11.364 |
Note: Each assignment factor is a continuous variable.
Discussion
Ovarian cancer, cervical cancer, and breast cancer are common malignant tumors threatening women’s health, of which ovarian cancer has the highest morbidity and mortality [14,15]. HE4 is mainly expressed in some smooth muscle tissues, vascular endothelial cells, or liver and kidney tissues in newborns, with a low positive expression rate in adults. Recent studies have demonstrated the potential role of HE4 in malignant tumorigenesis, as its high expression can promote persistent proliferation and impaired differentiation of endometrial glandular cells and ovarian germinating epithelial cells and thus interfere with the pathological processes of cancer cell adhesion and invasion [16]. CA125 is an important broad-spectrum glycoprotein indicator, and changes in its expression indicate the risk of persistent nuclear spread of cancer cells. The catabolism of the associated glycoproteins on the membrane surface of cancer cells promotes the expression of CA125 along with the abnormal expression of tumor-associated AKT or MAPK signaling pathways in cancer cells of patients [9,10]. ROMA is an effective system for the evaluation of CA125 and HE4, which avoids the disadvantages of a single index in the diagnosis of ovarian malignancies, such as poor sensitivity or specificity. Relevant research has revealed a trend of high expression of CA125 or HE4 in ovarian malignancies, but its relationship with lymph node metastasis or clinical staging of patients is poorly understood. The present study found abnormally high expression of HE4 and CA125 in the serum of patients with ovarian cancer, suggesting their involvement in the progression of ovarian disease. The persistent rise in HE4 and CA125 promotes gene mismatch repair in cancer cells and accelerates the changes in the biological characteristics of cancer cells, thereby exacerbating gynecologic malignancies [11,12]. High expression of HE4 facilitates the formation of the spindle, modification of DNA end structures during the symptomatic process of ovarian epithelial-derived malignancies, which increases the proliferation rate of cancer cells. High expression of CA125 promotes the activation of glycoproteins on the membrane surface of cancer cells, increases the expression of P16 or P56 in cancer cells, and interferes with the interleaved activation of cancer-related signaling pathways. Muinao et al. [13] investigated the alteration of serum tumor-related molecular profiles in ovarian cancer patients with different clinical stages and found that the average increase of HE4 and CA125 could reach more than 25% and 45%, and a more rapid progression of the disease was accompanied with a more advanced clinical stage of the patients and a more significant increase of HE4 and CA125 indexes. Moreover, they found that the expression levels of HE4 and CA125 were higher in patients with lymph node metastasis, advanced clinical stage, and poorly differentiated cancer cells, suggesting that HE4 and CA125 are closely related to the clinicopathological features of ovarian cancer. The reason may be that the high expression of HE4 and CA125 upregulates the expression of adherin 1, an adhesion molecule in the vascular endothelium and lymph node endothelium of cancer cells, which promotes the infiltration of cancer cells into adjacent normal tissues and increases the risk of distant metastasis of cancer cells. In addition, it is also related to the role of HE4 and CA125 in the regulation of differentiation maturation inducers during the early differentiation of tumor cells [17]. ROMA is also closely related to clinicopathological features such as lymph node metastasis, as ROMA is established based on the levels of HE4 and CA125, thus providing a better predictive value. Histologic grading and lymph node metastasis are also strongly associated with CA125 or HE4, and the associated clinicopathologic features are important risk factors affecting the expression of tumor glycoprotein molecules [15]. Therefore, it is clear that the higher levels of HE4, CA125, and ROMA in patients with ovarian cancer are significantly impacted by histological grading and lymph node metastasis [18]. To optimize the early diagnosis of ovarian cancer and further explore CA125 and HE4 in the early diagnosis, CA125, HE4, and ROMA were used for joint diagnosis of ovarian cancer, and the specificity, sensitivity, positive predictive value, and AUC were compared.
In this study, the results showed that Group A showed higher serum levels of HE4 and CA125 than Group B and the blank group, and the serum level of CA125 was higher in Group B when compared to the blank group, but there was no notable difference in HE4 between Group B and the blank group. These results indicated that both CA125 and HE4 increased in patients with ovarian cancer, and CA125 also increased in patients with benign tumors. Moreover, the results demonstrated that the specificity, sensitivity, positive diagnosis rate, and AUC of the combined diagnosis were significantly higher than those of the mono-determination, indicating that the combined diagnosis was superior to the mono-determination. According to the comparison of diagnostic value of the mono-determination of HE4, ROMA, or CA125, the specificity and AUC of the three, from high to low, were HE4>ROMA>CA125, and the sensitivity and positive diagnosis rate of them, from high to low, were ROMA>HE4>CA125, with statistical differences. The ROMA+HE4+CA125 mode was significantly better than the mono-determination of ROMA, HE4, or CA125 in terms of diagnostic effect. This result corroborated the findings of Samborski A [19], who reported that HE4+CA125 was superior to any single tumor marker in the monitoring and diagnosis of female ovarian cancer. The limitations of this study lie in the absence of a prognostic multifactorial analysis and the lack of access to risk factors affecting prognosis, which will be further studied in future research to provide early intervention and improve disease-free progression survival.
The combined diagnosis with HE4+CA125+ROMA is more effective than diagnosis with single markers, which indicates a high application value of the combined diagnosis in the early diagnosis of ovarian cancer.
Disclosure of conflict of interest
None.
References
- 1.Kotsopoulos J, Karlan B, Gronwald J, Hall E, Moller P, Tung N, Zakalik D, Foulkes WD, Rosen B, Neuhausen SL, Sun P, Lubinksi J, Narod SA. Long-term outcomes following a diagnosis of ovarian cancer at the time of preventive oophorectomy among BRCA1 and BRCA2 mutation carriers. Int J Gynecol Cancer. 2020;30:825–830. doi: 10.1136/ijgc-2019-001141. [DOI] [PubMed] [Google Scholar]
- 2.Reade CJ, McVey RM, Tone AA, Finlayson SJ, McAlpine JN, Fung-Kee-Fung M, Ferguson SE. The fallopian tube as the origin of high grade serous ovarian cancer: review of a paradigm shift. J Obstet Gynaecol Can. 2014;36:133–140. doi: 10.1016/S1701-2163(15)30659-9. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez-Castañeda LD, Tovar-Parra D, Quintero G, Amezquita L, Guerrero C, Sanabria D. Isolation and phenotypic characterization of tumor cells of patients with a diagnosis of ovarian cancer. J Cell Physiol. 2020;235:3320–3328. doi: 10.1002/jcp.29220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang SP, Chuang YJ, Lee WB, Tsai YC, Lin CN, Hsu KF, Lee GB. An integrated microfluidic system for rapid, automatic and high-throughput staining of clinical tissue samples for diagnosis of ovarian cancer. Lab Chip. 2020;20:1103–1109. doi: 10.1039/c9lc00979e. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Ni S, Du Z, Li X. A six-CpG-based methylation markers for the diagnosis of ovarian cancer in blood. J Cell Biochem. 2020;121:1409–1419. doi: 10.1002/jcb.29376. [DOI] [PubMed] [Google Scholar]
- 6.Sha R, Badhulika S. Recent advancements in fabrication of nanomaterial based biosensors for diagnosis of ovarian cancer: a comprehensive review. Mikrochim Acta. 2020;187:181. doi: 10.1007/s00604-020-4152-8. [DOI] [PubMed] [Google Scholar]
- 7.Hassanpour S, Baradaran B, de la Guardia M, Baghbanzadeh A, Mosafer J, Hejazi M, Mokhtarzadeh A, Hasanzadeh M. Diagnosis of hepatitis via nanomaterial-based electrochemical, optical or piezoelectrical biosensors: a review on recent advancements. Mikrochim Acta. 2018;185:568. doi: 10.1007/s00604-018-3088-8. [DOI] [PubMed] [Google Scholar]
- 8.Cui R, Wang Y, Li Y, Li Y. Clinical value of ROMA index in diagnosis of ovarian cancer: meta-analysis. Cancer Manag Res. 2019;11:2545–2551. doi: 10.2147/CMAR.S199400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li K, Pei Y, Wu Y, Guo Y, Cui W. Performance of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) in diagnosis of ovarian cancer: a systematic review and meta-analysis. J Ovarian Res. 2020;13:6. doi: 10.1186/s13048-019-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiss A, Timms JF, Smith C, Devetyarov D, Gentry-Maharaj A, Camuzeaux S, Burford B, Nouretdinov I, Ford J, Luo Z, Jacobs I, Menon U, Gammerman A, Cramer R. Highly accurate detection of ovarian cancer using CA125 but limited improvement with serum matrix-assisted laser desorption/ionization time-of-flight mass spectrometry profiling. Int J Gynecol Cancer. 2010;20:1518–1524. [PubMed] [Google Scholar]
- 11.Yu C, Dou T, Liu Y, Liu R. Clinical value of TV-CDS combined with serum tumor markers in diagnosis of ovarian cancer. Oncol Lett. 2020;20:2028–2034. doi: 10.3892/ol.2020.11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brieger KK, Peterson S, Lee AW, Mukherjee B, Bakulski KM, Alimujiang A, Anton-Culver H, Anglesio MS, Bandera EV, Berchuck A, Bowtell DDL, Chenevix-Trench G, Cho KR, Cramer DW, DeFazio A, Doherty JA, Fortner RT, Garsed DW, Gayther SA, Gentry-Maharaj A, Goode EL, Goodman MT, Harris HR, Høgdall E, Huntsman DG, Shen H, Jensen A, Johnatty SE, Jordan SJ, Kjaer SK, Kupryjanczyk J, Lambrechts D, McLean K, Menon U, Modugno F, Moysich K, Ness R, Ramus SJ, Richardson J, Risch H, Rossing MA, Trabert B, Wentzensen N, Ziogas A, Terry KL, Wu AH, Hanley GE, Pharoah P, Webb PM, Pike MC, Pearce CL Ovarian Cancer Association Consortium. Menopausal hormone therapy prior to the diagnosis of ovarian cancer is associated with improved survival. Gynecol Oncol. 2020;158:702–709. doi: 10.1016/j.ygyno.2020.06.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muinao T, Deka Boruah HP, Pal M. Multi-biomarker panel signature as the key to diagnosis of ovarian cancer. Heliyon. 2019;5:e02826. doi: 10.1016/j.heliyon.2019.e02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shipeng G, Yongning C, Yadi Z, Chanyuan LI, Qifan J. Comparison of serum cancer antigen 125, human epididymis protein 4, ROMA, and CPH-I for diagnosis of ovarian cancer in Chinese patients with ovarian mass. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:1393–1401. doi: 10.12122/j.issn.1673-4254.2019.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mapelli P, Incerti E, Fallanca F, Gianolli L, Picchio M. Imaging biomarkers in ovarian cancer: the role of 18F-FDG PET/CT. Q J Nucl Med Mol Imaging. 2016;60:93–102. [PubMed] [Google Scholar]
- 16.Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12:28. doi: 10.1186/s13048-019-0503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Shang W, Zhao H, Rong G, Zhang Y, Xu T, Zhang J, Huang P, Wang F. In silico screening of circulating microRNAs as potential biomarkers for the diagnosis of ovarian cancer. Dis Markers. 2019;2019:7541857. doi: 10.1155/2019/7541857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Liu Y, Liu Q, Yan L, Xue M, Yuan W, Shi M, Feng W, Xu C, Li F. Point-of-care ratiometric fluorescence imaging of tissue for the diagnosis of ovarian cancer. Theranostics. 2019;9:4597–4607. doi: 10.7150/thno.35322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yurkovetsky ZR, Linkov FY, E Malehorn D, Lokshin AE. Multiple biomarker panels for early detection of ovarian cancer. Future Oncol. 2006;2:733–41. doi: 10.2217/14796694.2.6.733. [DOI] [PubMed] [Google Scholar]


