Abstract
As a type of nanosized membranous vesicles secreted by living cells, extracellular vesicles (EVs) mediate intercellular communications with excellent physicochemical stability and biocompatibility. By delivering biologically active molecules including proteins, nucleic acids and lipids, EVs participate in many physiological and pathological processes. Increasing studies have suggested that EVs may be biomarkers for liquid biopsy of retinal diseases due to the ability to transfer through the blood-retinal barrier. EVs also represent a novel cell-free strategy to repair tissue damage in regenerative medicine. Evidence has indicated that EVs can be engineered and modified to enhance their efficacy. In this review, an overview of the characteristics, isolation, and identification of EVs is provided. Moreover, recent advances with EVs in the diagnosis and treatment of retinal diseases and the engineering approaches to elevate their effects are introduced, and opportunities and challenges for clinical application are discussed.
Keywords: Extracellular vesicles, retinal diseases, diagnosis, therapy, engineered extracellular vesicles
Introduction
Retinal degeneration is the main cause of vision decline and blindness, seriously affecting the life quality of patients [1,2]. Formed by a complex cell network, the retina is responsible for the photoelectric signal conversion in the eye [3]. Currently, fundus imaging and optical coherence tomography imaging provide opportunities to detect a significant retinal lesion [4]. However, the discovery of early noninvasive biomarkers to monitor retinal diseases progress still needs further investigation. Moreover, although the retinal therapy strategies including anti-angiogenic drugs and laser surgery have achieved encouraging progress, their therapeutic outcomes remain unsatisfactory, often with complications and side effects [5]. Therefore, developing novel and effective diagnosis and treatment approaches of retinal diseases is necessary.
During EV formation, various bioactive molecules including proteins, nucleic acids and lipids are loaded into the EV. As natural delivery vehicles with little toxicity and immunogenicity, EVs transmit cargoes to mediate cell-to-cell communication, thereby contributing to the pathophysiologic regulation of many diseases [6]. EVs are important indicators of liquid biopsy and exist in biologic fluids such as blood, urine, semen and saliva [7]. Moreover, produced by a paracrine mechanism, EVs mediate the reparative effects of cell transplantation [8]. Studies indicate that EVs are a promising candidate for the cell-free therapy of refractory diseases [9-11]. The unique feature of EVs to protect cargoes from degradation makes them a novel nanomaterial, that can be engineered to further enhance their targeting and functions [12]. Collectively, these biologic effects endow EVs great promise for diagnosis and treatment approaches in nanomedicine.
In this review, we elaborate on recent research on EVs in retinal diseases and summarize their roles as novel biomarkers and therapeutic options. The engineering strategies of EVs and their challenges of clinical use are also discussed, to show future research directions.
Overview of EVs
Classification, biogenesis, and uptake of EVs
Previous studies suggested that EVs were delivery vehicles carrying metabolic wastes produced by cells. Since an important role of EVs to regulate immunologic function was revealed, researchers began to focus on their biological effects [13]. There are mainly three subsets of EVs recognized, namely exosomes, microvesicles (MVs), and apoptotic bodies. Generated by the fusion of multivesicular bodies (MVBs) and plasma membranes, exosomes are a relatively smaller component of EVs, with the diameter of 30-150 nm and a density of 1.13-1.19 g/mL [14]. MVs are slightly larger EVs produced when the plasma membranes of cells bud outward, with the diameter up to 1000 nm [15]. The diameter of an apoptotic body is the largest among all types of EVs, usually above 1000 nm [16]. Many attempts have been made to investigate the roles of exosomes and MVs in intercellular communication, but with few studies on the value of apoptotic bodies in nanomedicine.
The biogenesis of exosomes has several stages including endocytosis, MVB formation, and exosome release. Formed by the endocytosis of plasma membranes, multiple intraluminal vesicles (ILVs) fuse to generate endosomes. The loading of bioactive molecules such as microRNA (miRNA), lipids, and proteins promotes the production of MVBs [17]. After the fusion of MVBs and plasma membranes, exosomes are released outside. The biologic effects of exosomes are closely associated with the cargo sorting, which is usually regulated by the endosomal sorting complexes required for the transport (ESCRT)-dependent mechanism. Specifically, ESCRT-0-induces the retention of the ubiquitinated proteins to initiate this progress. Then ESCRT-l/ll triggers the restricted membrane to access the cavity, followed by the ESCRT-lll-mediated spiral structure formation and the neck bud shrinkage. Finally, ATPase vacuolar protein sorting-4 drives membrane rupture [18]. Alternatively, the MVBs formation can also be independent of ESCRT mechanism. Recent evidence reveals that syntenin-mediated ILVs germination is regulated by the small GTPase ADP ribosylation factor 6 and its effector protein phospholipase D2 [19].
The formation of MVs is usually caused by the increased expression of intracellular calcium. Due to the asymmetric membrane lipids of resting cells, phosphatidylcholine and sphingomyelin are located externally, while amine-containing lipids such as phosphatidylserine and phosphatidylethanolamine are in the inner leaflet [20]. Calcium ions mediate the movement of lipids to promote membrane budding [17]. In addition, MVs are modified by reorganizing the cytoskeleton during the release process. EVs transport their cargoes to recipient cells mainly through endocytosis, direct fusion with the plasma membrane, and receptor-ligand interaction [21] (Figure 1).
Figure 1.
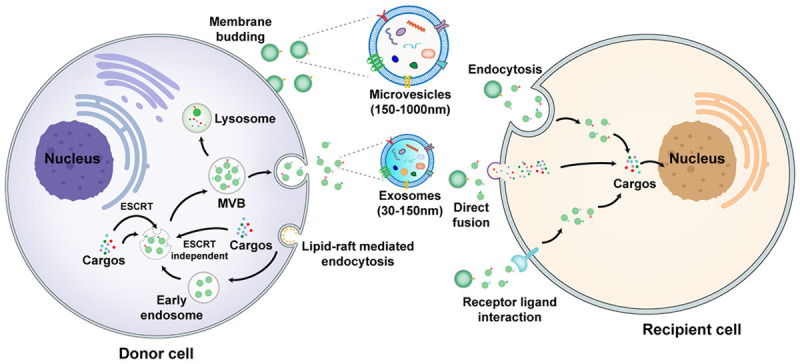
The biogenesis, release, and uptake of exosomes and microvesicles. Lipid-raft-mediated endocytosis of the plasma membrane results in formation of early endosomes, followed by production of MVBs through the ESCRT-dependent or ESCRT-independent pathways. MVBs fuse with the plasma membrane to release exosomes. Microvesicles are derived from outward budding of the plasma membrane. EVs transfer cargoes to recipient cells through endocytosis, direct fusion, and receptor ligand interaction. MVBs: multivesicular bodies, ESCRT: endosomal sorting complexes required for transport.
The isolation of EVs
According to the unique properties of EVs, several separation techniques have been established, including differential centrifugation, density gradient centrifugation, size exclusion chromatography, ultrafiltration, immune-affinity capture, and polymer precipitation (Figure 2). Among them, some methods can distinguish subtypes of EVs to extract exosomes. For differential centrifugation, numerous impurities such as intact cells, dead cells, and cell debris existing in the sample are initially removed by a series of low-speed centrifugations (300-10,000 × g), followed by ultracentrifugation (100,000 × g) to enrich smaller EVs including exosomes. However, due to the fact that the size of exosomes is heterogeneous and may overlap with MVs, the purity of exosomes isolated by this method is low. According to the density of exosomes, density gradient centrifugation adopts proper media to further isolate exosomes on the basis of differential centrifugation [22]. Although exosomes are purified, this method remains cumbersome and requires special equipment. Comparatively, size exclusion chromatography involves the usage of the sepharose-packed columns that adsorb small vesicles temporarily, while large particles are eluted because they cannot enter the pore, thus achieving the separation of exosomes [23]. Ultrafiltration depends on the size of the nanopore membrane to extract exosomes from the biofluids [24]. These two methods are simple and convenient, whereas the obtained exosomes still lack specificity. Based on the protein markers of different EVs subtypes, immune-affinity capture isolates exosomes by binding the corresponding antibodies to magnetic beads, exhibiting the advantage of specific extraction with high purity [25]. However, owing to the disadvantages including high cost and low yield, this method is not suitable for clinical application. Recently, many commercial kits have become available to isolate exosomes conveniently. After removing impurities by low-speed centrifugations, polyethylene glycol (PEG) is used to separate insoluble components including exosomes from the sample [22]. Nevertheless, the presence of proteins and other EVs subtypes in the precipitates reduces the purity of exosomes. Therefore, it is difficult to meet completely the requirements of high purity, convenience, good integrity and high yield at present. With the development of nanotechnology, multiple innovative methods for efficient and specific isolation of exosomes from complex body fluids have emerged. Studies suggest that a microfluidic filtering method represents one promising strategy for exosomes separation with the advantage of efficiency and automation. For instance, Lee et al. combine acoustic and microfluidic technologies to isolate exosomes from red blood cells with high purity [26]. Based on microfluidic technology, Liu et al. adopt biocompatible polymers to control viscous elasticity and achieve continuous and label-free separation of exosomes [27]. Moreover, combination of the immune-affinity capture method and microfluidic technology displays the ability to isolate EVs efficiently and specifically [28]. The application of several nanomaterials provides another useful method for EV extraction. For example, nanowires decorated with the antibodies of exosomal markers can be employed to capture exosomes in the sample, with high specificity and sensitivity [29]. Taken together, the appropriate isolation method selected is related to the concentration requirements of EVs [30].
Figure 2.
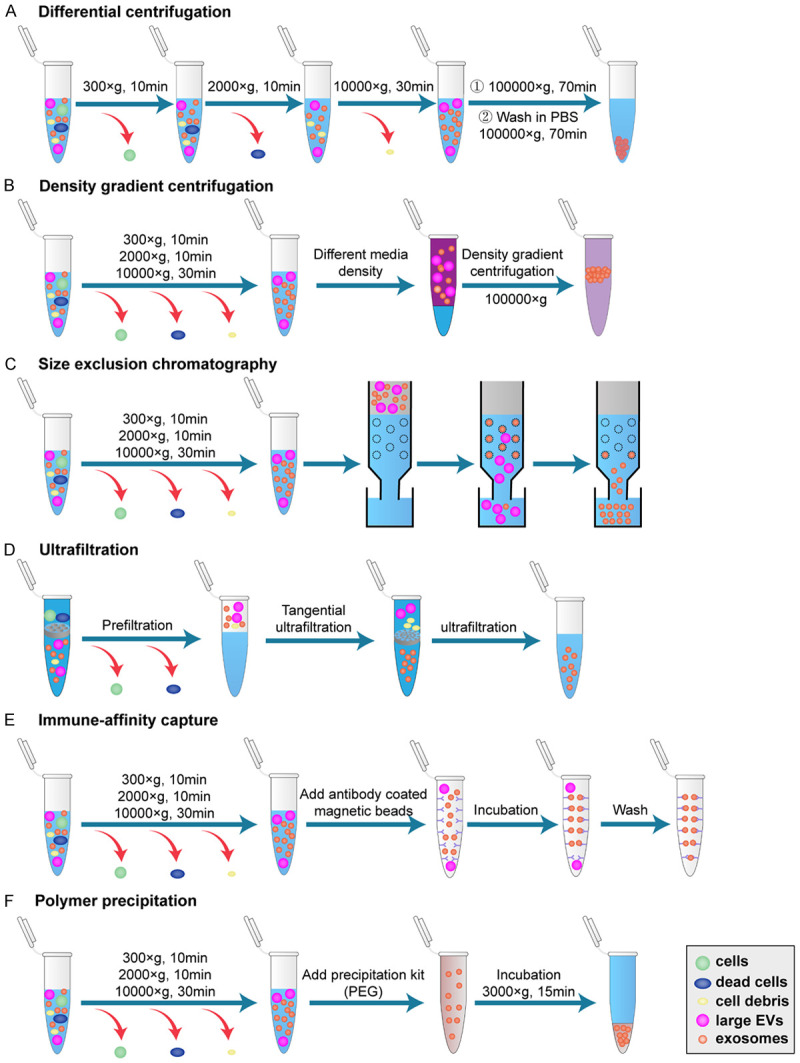
Traditional isolation methods of exosomes. According to the differing properties of exosomes, several extraction methods have been established, including differential centrifugation (A), density gradient centrifugation (B), size exclusion chromatography (C), ultrafiltration (D), immune-affinity capture (E), and polymer precipitation (F). The main processes of each method are shown. PBS: phosphate buffer saline, PEG: polyethylene glycol, EVs: extracellular vesicles.
Components and identification of EVs
The ingredients of EVs reflect the state of donor cells (Figure 3). As the major component of cargoes, proteins in EVs can be divided into non-specific proteins and specific proteins. Non-specific proteins provide important targets for EV identification, mainly including cytoskeletal proteins (tubulin, actin, and filament binding proteins), membrane fusion proteins (Annexin, Alix, and tumor susceptibility gene 101), signal transduction proteins (protein kinase, G protein), tetraspanins (CD9, CD63 and CD81), chaperone proteins (heat shock protein 70 and heat shock protein 90), phospholipase and lipid-related proteins [31]. On the other hand, different donor cell-derived EVs contain various specific proteins, which can serve as biomarkers of several diseases. Moreover, EVs transport multiple nucleic acids, such as mRNA, miRNA, long noncoding RNA (lncRNA), circular RNA (circRNA), genomic DNA, and mitochondrial DNA, to regulate gene expression of recipient cells [6]. The lipids in EVs are involved in the regulation of endocytosis, metabolic balance, inflammation, and tumor microenvironment [32].
Figure 3.
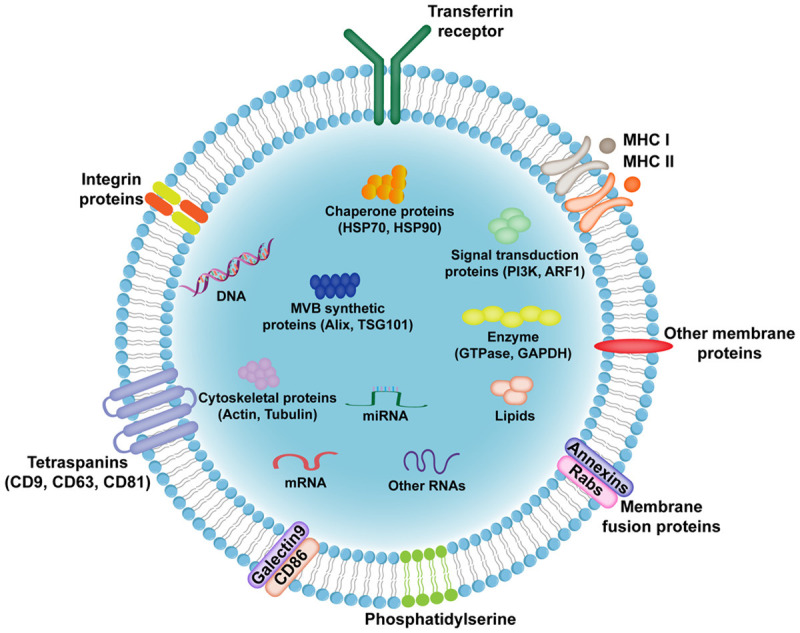
Main components of EVs. The phospholipid bilayer of EVs protects cargoes from degradation. Multiple membrane proteins exert different functions such as signal transduction, immune response, and metabolic regulation. EVs contain various bioactive molecules including proteins, nucleic acids and lipids. MHC: major histocompatibility complex, HSP: heat shock protein, MVB: multivesicular body, TSG101: tumor susceptibility gene 101, PI3K: phosphatidylinositol 3-kinase, ARF1: ADP ribosylation factor 1, GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
The identification of EVs is a necessary process after extraction due to the significant heterogeneity among EVs. Many detection technologies have been established based on the morphology, particle size, particle number, and molecular composition of EVs. After fixation and dehydration, EVs may shrink and present as cup-like vesicles under transmission electron microscopy or scanning electron microscopy [33]. Spherical EVs with phospholipid bilayers can be observed by cryo-electron microscopy [34]. Nanoparticle tracking analysis (NTA) is widely used for the particle size and concentration detection of EVs according to the Brownian motion of vesicles [35]. In addition, tunable resistive pulse sensing detection technology recognizes the instantaneous change of the ion current generated by EVs, thus analyzing their concentration and size distribution [36]. Compared with NTA, this method is usually applied to detect relatively larger vesicles with diameter greater than 150 nm. Traditional flow cytometry does not work well for accurate analysis for the particle size of smaller EVs due to the limited detection range [37]. Stoner et al. have developed a novel strategy to measure the size of individual exosomes by using fluorescent dye di-8-ANEPPS [38]. For protein detection, mass spectrometry analysis, enzyme-linked immunosorbent assay, and western blot are commonly adopted. Recently, researchers have reported novel protein analysis methods including microfluidic micronuclear magnetic resonance [39] and protein sensor technology platform [40]. Moreover, polymerase chain reaction (PCR) amplification and its derivatization methods are usually used to detect the expression of nucleic acids in EVs [41]. Mass spectrometry is a major approach for lipidomic analysis [42].
EVs in retinal disease diagnosis
The inherent potential of EVs to transport bioactive molecules between cells has resulted in the extensive exploration of their biologic effects in pathologic and physiologic processes. In 1992, Caldwell et al. demonstrated that abnormal vesicles combined with extracellular collagen fibers, thus affecting the communication between peripheral cells and endothelial cells in the diabetic retina [43]. Since then, studies have suggested that EVs derived from injured cells can promote retinal disease progression. Importantly, the protection of cargoes caused by the plasma membrane and the ability to transport through the blood-retinal barrier make EVs promising biomarkers of retinal diseases (Table 1).
Table 1.
Diagnostic use of EVs in retinal disease
| Cargo | Retinal disease | Source | Functions | Ref. |
|---|---|---|---|---|
| cPWWP2A | DR | Pericytes | Improve pericyte-endothelial cell crosstalk and alleviate vascular dysfunction by binding with miR-579 | [49] |
| miR-15a | DR | Pancreatic β cells | Aggravate oxidative stress and apoptosis to accelerate DR progression | [50] |
| CCR5 | DR | Plasma | Distinguish the severity of DR | [53] |
| IgG | DR | Plasma | Activate the classical complement pathway to promote retinal vascular damage | [54] |
| TNFAIP8 | DR | Plasma | Promote retinal endothelial cells proliferation, migration and angiogenesis | [55] |
| miR-21-3p, miR-30b-5p, miR-150-5p | DR | Serum | Induce retinal damage by promoting abnormal angiogenesis | [57] |
| MiR-26b-5p | DR | Serum | Promote endothelial dysfunction | [58] |
| MiR-302a, miR-122 | AMD | RPE cells | Ameliorate retinal angiogenesis | [67] |
| HDAC6 | AMD | RPE cells | Impair retinal barrier function | [68] |
| VEGFR2 | AMD | RPE cells | Accelerate retinal neovascularization | [69] |
| Cathepsin D, Cytokeratin | AMD | Aqueous humor | Enhance autophagy level and promote epithelial- mesenchymal transition to aggravate AMD | [70] |
| MiR-301-3p, miR-361-5p, miR-424-5p | AMD | Serum | Inhibit tube formation | [71] |
| miR-486-5p, miR-626, miR-885-5p | AMD | Serum | Regulate the apoptosis and neovascularization pathways to participate in AMD progress | [72] |
EVs: extracellular vesicles; Ref.: references; DR: diabetic retinopathy; CCR5: C-C chemokine receptor type 5; TNFAIP8: tumor necrosis factor-α-induced protein 8; AMD: age-related macular degeneration; RPE: retinal pigment epithelium; HDAC6: histone deacetylase 6; VEGFR2: vascular endothelial growth factor receptor 2.
Diabetic retinopathy (DR)
As a severe microvascular complication of diabetes, DR has been the main cause of vision decline and blindness in adults [44]. Hyperglycemia induces an increased retinal oxidative stress and inflammatory infiltrate resulting in the apoptosis of retinal cells [45]. Distinguished by the formation of new blood vessels, DR is divided into non-proliferative DR and proliferative DR [46]. The duration and the severity of diabetes are the major risk factors of DR, so strict control of the blood glucose level can apply an effective strategy for retinal protection [47]. However, the progression of DR can also be independent of the glucose metabolism disorder, implying that the application of diabetes-relevant biomarkers is not exhaustive to predict DR [48]. Due to the irreversibility and rapid development of DR, there is an urgent need to find novel diagnosis and treatment approaches.
Under hyperglycemic conditions, EVs-mediated communications between retinal cells contribute to DR progression. The investigation of specific molecules in EVs to reflect DR development has advanced much. For instance, Liu et al. demonstrate that high-glucose stimulation promotes the secretion of exosomes carrying cPWWP2A, which participates in the regulation of retinal vessels by acting as a sponge of miR-579 [49]. Moreover, exosomes derived from pancreatic β cells transfer miR-15a to induce elevated retinal oxidative stress and apoptosis by targeting AKT3 [50]. However, the isolation of specific donor cell-released EVs in vivo is still complicated. Recent evidence shows that plasma-derived EVs of DR patients can cause peripheral cell detachment, endothelial cell migration, retinal leakage, and neovascularization [51]. Circulating EV analysis reveals that the levels of cytokines and angiogenic factors are significantly upregulated compared to healthy subjects [52]. C-C chemokine receptor 5 (CCR5) is enriched in EVs and accelerates retinal injury in DR [53]. Increased plasma exosomes containing IgG mediate the activation of the classical complement pathway, leading to the deterioration of retinal vessels [54]. In addition, the expression of tumor necrosis factor-α-induced protein 8 (TNFAIP8), which promotes retinal vascular endothelial cell proliferation, migration, and tube formation, is elevated in plasma EVs of DR patients [55]. Noncoding RNAs, especially miRNAs, have emerged as important regulators of ocular neovascularization [56]. The diagnostic potential of miR-21-3p, miR-30b-5p, miR-150-5p, and miR-26b-5p was revealed by microarray analysis [57,58]. These findings indicate that EVs regulate DR progression by delivering multiple cargoes, representing a novel diagnostic strategy for the disease.
Age-related macular degeneration (AMD)
AMD is a leading cause of severe visual impairment and blindness among people aged over 60 [59]. The accumulation of extracellular deposition, known as drusen, results in the gradual death of retinal pigment epithelium (RPE) cells and photoreceptor cells [60]. The feature of the late stage is geographic atrophy and neovascularization [61]. Although the genetic risk model score displays accuracy in the identification of high-risk groups, a large proportion of AMD patients are still ignored, implying that genetics is not the only factor to determine the progression of AMD [62]. Studies have shown that age, environment, and behavioral factors are also involved in the development of the disease [63]. Establishing effective diagnostic strategies provides promise for early therapy of AMD.
Several studies have revealed that EVs play important roles in the formation of drusen. For example, exosomes-mediated intercellular communications in oxidative stress microenvironment downregulate CD46 and CD59 expressions to induce complement dysfunction, resulting in the aggravated damage of RPE cells [64]. Microparticles (MPs) from injured RPE cells promote the degeneration of healthy RPE cells [65]. Importantly, aged RPE cells increase the autophagy level to cause the production of drusen by exosome-transferred intracellular proteins [66]. The ability of EVs to promote AMD progression leads to the extensive exploration of their cargoes. Reportedly, bioactive molecules such as proteins and nucleic acids in EVs can be novel biomarkers of AMD. Retinal oxidative stress induces the downregulation of miR-302a and miR-122 in EVs secreted by RPE cells [67]. Injured RPE cells transmit EVs containing histone deacetylase 6 (HDAC6) to impair retinal barrier function [68]. Enhanced expression of vascular endothelial growth factor receptor 2 (VEGFR2) in RPE cells-derived exosomes is responsible for the retinal neovascularization [69]. In addition, the ability of cathepsin D and cytokeratin in exosomes from the aqueous humor of AMD patients to reflect therapeutic effects after treatment makes them promising candidates for AMD diagnosis and prognosis [70]. Furthermore, researchers have evaluated the possibility of molecules in circulating EVs to serve as ideal biomarkers of AMD. Grassmann et al. demonstrate that miR-301-3p, miR-361-5p, and miR-424-5p carried by circulating exosomes are highly associated with neovascular AMD [71]. Another study of Elbay et al. suggests that the changed expressions of miR-486-5p, miR-626, and miR-885-5p in serum-derived exosomes of AMD patients yield a potential strategy to monitor disease progression [72]. In addition to miRNAs, there are few studies on other types of biomarkers in circulating EVs. The use of EVs for early diagnosis of AMD still needs further investigation.
Retinitis pigmentosa (RP)
RP is a common dystrophic retinopathy characterized by photoreceptor cell degeneration-inducing decreased night vision and visual field [73]. RP is recognized as an inherited disease, whereas the clinical symptoms, onset time, and progression rate are heterogeneous [74]. RP involves various inheritance patterns including X-linked RP and autosomal dominant or recessive RP [75]. Although research on the pathogenesis of RP is constantly advancing, there are still few specific biomarkers and efficient treatments to predict and repair this retinal injury.
Multiple vesicles formed by membrane eversion and extrusion assemble outside the injured photoreceptor cells [76]. Further studies have shown that accumulated EVs mediate the transport of rhodopsin which regulates the function of photoreceptor cells [77,78]. Moreover, the internalization of degenerated rod photoreceptor-derived MVs by RPE cells leads to the abnormal distribution of rhodopsin [79]. Ingredient analysis reveals that EVs from denatured photoreceptor cells contain more miRNAs and angiogenic substances [80]. The inhibition of EVs effectively protects photoreceptor cell survival [81]. The intercellular communication mediated by EVs promotes RP progression, whereas specific molecules in EVs to monitor this disease have not yet been reported.
Retinopathy of prematurity (ROP)
ROP is the main cause of blindness in children due to incomplete development of vision-related tissues and organs [82]. Gestational age and birth weight exhibit a significant correlation with the severity of ROP [83]. Characterized by functional and structural disorders of retinal vessels, ROP has stages of hyperoxia-induced vascular growth stagnation and subsequent neovascularization [84]. Current treatment strategies including laser surgery and intravitreal injection of antiangiogenic drugs may result in serious infections and side effects [85]. The discovery of novel approaches for monitoring and improving this retinal injury should ensure lifelong visual quality for premature infants. As a novel cell-free therapy for ROP, the protective value of EVs will be discussed in the next section, but more studies are needed to support the diagnostic use of EVs in ROP.
Glaucoma
Characterized by retinal ganglion cells (RGC) loss, optic nerve degeneration, and visual field defects, glaucoma is an important cause of irreversible blindness [86]. Open-angle glaucoma and closed-angle glaucoma are two common subtypes of glaucoma [87]. Due to the increased production and decreased outflow of aqueous humor, elevated intraocular pressure (IOP) causes gradual damage to RGCs, leading to interrupted transmission of retinal input [88]. Homeostatic IOP contributes to maintain the structure and function of the eye. Currently, reducing IOP and protecting RGC survival are the main strategies for the treatment of glaucoma. However, effective control of this risk factor cannot always alleviate disease development. Many patients with primary glaucoma may have no changes in IOP, indicating that several other factors are also involved in RGC loss [89]. It is necessary to develop novel approaches to predict and prevent glaucoma progression.
Owing to its value in draining aqueous humor, the trabecular meshwork (TM) is considered an important target to reduce IOP. Studies indicate that in a pathologic environment, TM-derived EVs contain a series of bioactive molecules that can act as early diagnostic biomarkers of glaucoma. For instance, miRNA microarray analysis reveals that 23 miRNAs are upregulated and 3 miRNAs are downregulated in EVs secreted by human TM cells after TGF-β stimulation [90]. TM-derived exosomes show enrichment in myocilin, resulting in the occurrence of glaucoma [91]. Moreover, EV-mediated intercellular communication promotes pathologic extracellular matrix accumulation in the TM [92]. Lerner et al. found that miR-29b in exosomes derived from non-pigmented ciliary epithelial cells participates in extracellular matrix remodeling by inhibiting Wnt signaling and reducing COL3A1 expression in TM cells [93]. In addition, Aires et al. suggest that exosomes from retinal microglial cells increase the release of pro-inflammatory cytokines, promote retinal cell apoptosis, and mediate RGC loss in glaucoma [94]. Interestingly, recent evidence shows that as the main component of aqueous humor, exosomes play important roles in controlling the dynamic balance of aqueous humor [95]. The aggravation of glaucoma may be closely associated with changed cargoes in aqueous humor-derived exosomes, which can be further analyzed to provide a new strategy for early diagnosis.
EVs in retinal disease therapy
Therapy of retinal diseases mainly relies on surgical treatment and drug intervention. Due to the risk of serious sideeffects, the exploration of a novel therapeutic approach has emerged as a research priority. Studies have indicated that stem cell-based transplantation exerts reparative functions in several retinal diseases [96,97]. Although the use of stem cells obtained from the patient can avoid transplant rejection, possible tumorigenicity and safety issues still limit the clinical use of this method. As a paracrine mechanism of cell therapy, EVs are promising candidates for the treatment of retinal diseases [87]. Efforts have been made to analyze the therapeutic efficacy of EVs derived from multiple cells (Figure 4).
Figure 4.
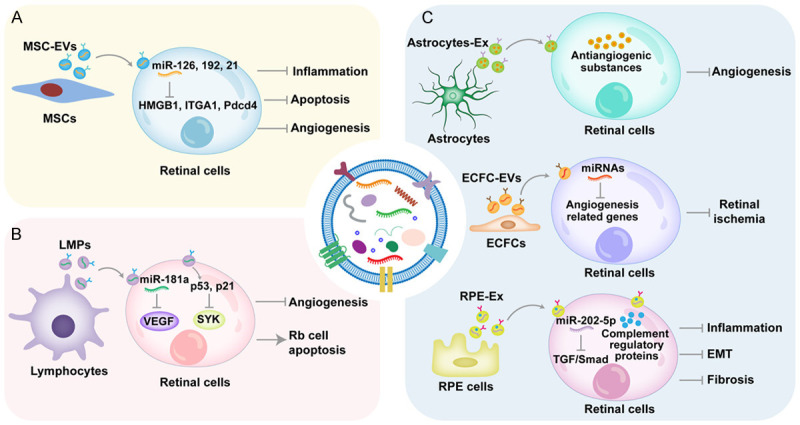
Therapeutic value of EVs in retinal diseases. A. MSC-EVs alleviate retinal inflammation, apoptosis, and angiogenesis mainly through the delivery of miRNAs. B. LMPs can ameliorate retinal neovascularization and induce Rb cell apoptosis. C. EVs derived from retinal cells, including astrocytes, endothelial colony-forming cells, and RPE cells, maintain retinal function to relieve retinal disease progression. MSCs: mesenchymal stem cells, MSC-EVs: extracellular vesicles derived from mesenchymal stem cells, HMGB1: high mobility group box 1, ITGA1: integrin subunit α1, Pdcd4: programmed cell death 4, LMPs: lymphocyte-derived microparticles, VEGF: vascular endothelial growth factor, SYK: spleen tyrosine kinase, Rb: retinoblastoma, Astrocytes-Ex: exosomes derived from astrocytes, ECFCs: endothelial colony-forming cells, ECFC-EVs: extracellular vesicles derived from endothelial colony-forming cells, RPE: retinal pigment epithelium, RPE-Ex: exosomes derived from retinal pigment epithelium cells, TGF: transforming growth factor, EMT: endothelial-to-mesenchymal transition.
Mesenchymal stem cell (MSC)-derived EVs
MSCs, multipotent adult stem cells with the abilities of self-renewal and multidirectional differentiation, function in tissue regeneration Their advantages including low immunogenicity, convenient extraction, and great differentiation potential have given MSCs widespread attention [11]. Emerging evidence shows that EVs derived from MSCs (MSC-EVs) mediate the therapeutic effects of MSCs and avoid the cell therapy-induced tumorigenicity [98,99]. For example, MSC-EVs ameliorate retinal inflammation by miR-126-induced inhibition of high mobility group box 1 (HMGB1) signaling pathway [100]. In addition, miR-192 in MSC-EVs shows the capacity to alleviate DR progression by targeting integrin subunit α1 (ITGA1) [101]. In clinical trials of macular hole patients, the intravitreal injection of exosomes derived from MSCs (MSC-Ex) recovers retinal structure and function effectively and safely [102]. As an important projection neuron, degenerated RGCs promote the progression of several retinal diseases. Recent studies indicate that MSC-Ex can protect RGC survival, maintain their function, and stimulate axon regeneration [103,104]. Moreover, MSC-EVs alleviate retinal ischemia by protecting retinal function and ameliorating inflammation and apoptosis [105]. However, MSC-EVs have also been described to promote retinal injury in DR. MSCs cultured in high-glucose conditions release exosomes to accelerate retinal vascular disorders, indicating that the biologic function of EVs is determined by the state of MSCs [106]. Therefore, the application of MSC-EVs in the therapy of retinal diseases requires further study.
Lymphocyte-derived MPs
Previous studies have indicated that lymphocyte-derived MPs (LMPs) display significant alleviation of hyperproliferative cells. The report that LMPs alleviate Müller cell proliferation and tube formation provides evidence that LMPs are effective agents of retinal diseases [107]. Moreover, miR-181a, selectively enriched in LMPs, blocks VEGF signaling pathway in human retinal endothelial cells, thus relieving retinal neovascularization [108]. Retinoblastoma (Rb) is a malignant tumor derived from photoreceptor precursor cells and usually occurs in children under 3 years. Qiu et al. demonstrate that LMPs inhibit spleen tyrosine kinase expression and promote Rb cell apoptosis by activating p53 and p21 genes [109]. These findings indicate that LMPs exert therapeutic effects on retinal diseases mainly characterized by angiogenesis.
Retinal cell-derived EVs
Under a pathologic microenvironment, EVs derived from retinal cells have been considered a double-edged sword. The promotional role of EVs in retinal diseases has been mentioned, whereas many retinal cells-derived EVs also show the ability to maintain retinal homeostasis. In the laser-induced choroidal neovascularization model, retinal astrocyte-released exosomes transport antiangiogenic cargoes to alleviate neovascularization [110]. Moreover, EVs from endothelial colony-forming cells contain diverse miRNAs to target angiogenesis-related genes, leading to a decrease in ischemic area and amelioration of retinal vessels in an oxygen-induced retinopathy (OIR) model [111]. Complement regulatory proteins in exosomes derived from RPE cells mediate the protection of adjacent cells and the inhibition of retinal inflammation [112]. In addition, miR-202-5p in exosomes from RPE cells suppresses retinal fibrosis and endothelial-to-mesenchymal transition by blocking the transforming growth factor/Smad signaling pathway in DR [113]. These results indicate that retinal cell-derived EVs may be novel therapy for retinal diseases.
Engineering strategies of EVs
Appropriate modification of EVs is a promising approach to increase their diagnostic potential and therapeutic efficiency [12]. Strategies of EV engineering mainly include cargo loading and membrane modification (Figure 5). According to previous studies, detailed methods of these strategies are summarized (Table 2).
Figure 5.
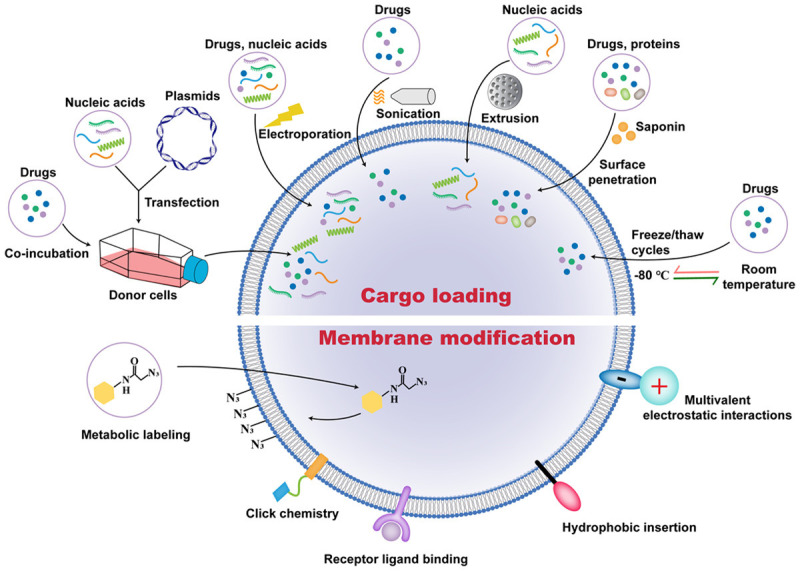
Approaches to EVs engineering. Co-incubation and transfection are applied to load cargoes into donor cells, followed by the secretion of EVs containing the corresponding substances. Electroporation, sonication, extrusion, saponin-mediated surface penetration, and freeze/thaw cycles methods are used to load cargoes into EVs. Moreover, membrane modification strategies including metabolic labeling, click chemistry, receptor ligand binding, hydrophobic insertion, and multivalent electrostatic interactions are used to increase the targeting of EVs.
Table 2.
Evaluation of strategies for EV engineering
| Strategy | Method | Principle | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Active loading | Transfection | Liposomes-mediated delivery | Simple, maintain the integrity of EVs | Low specificity and efficacy, toxicity | [116] |
| Co-incubation | The interaction between cargos and the lipid bilayer | Simple, maintain the integrity of EVs | Inefficient loading, low specificity | [117] | |
| Passive loading | Electroporation | Permeation pores in the membrane created by transient voltage | Rapid, high efficiency | EVs aggregation, impair EVs integrity | [120,121] |
| Sonication | Membrane deformation | High efficiency | EVs aggregation, impair EVs integrity | [122] | |
| Mechanical extrusion | Mechanical force | Efficient loading | Change the membrane structure | [123] | |
| Saponin-mediated surface penetration | Remove cholesterol to form pores in the membrane | Efficient loading | Toxicity | [124] | |
| Freeze/thaw cycles | Destroy the membrane temporarily | Efficient loading | EVs aggregation, change the membrane structure and function | [125] | |
| Modification of EVs Membrane | Click chemistry | Aziridine cycloaddition reaction | Simple, maintain the integrity of the membrane | May affect the function of membrane proteins | [126-128] |
| Receptor ligand binding | Receptor ligand coupling | High specificity and efficiency | Synthetic difficulty, high cost | [129] | |
| Hydrophobic insertion | Spontaneous integration of lipophilic material into the membranes | Simple, specific targeting, no damage to membrane proteins | Efficiency is related to the hydrophobicity of the material | [130,131] | |
| Multivalent electrostatic interactions | Multiple charge interactions | High efficiency | Cytotoxicity | [132] |
EVs: extracellular vesicles; Ref.: references.
Cargo loading approaches
With their ability to maintain the stability of cargoes in vivo, EVs can exert enhanced curative effects after loading therapeutic bioactive molecules [114]. The approaches of cargo loading can be categorized into active loading and passive loading [115]. Active loading refers to the introduction of cargoes into the donor cell, followed by the release of EVs containing specific ingredients. Passive loading means to use membrane penetration methods or chemical methods to directly load cargoes into EVs.
Gene transfection is widely used for loading exogenous nucleic acids including DNA vectors, siRNAs, and miRNAs into donor cells [116]. The intracellular composition is changed through a series of biologic processes. EVs with specific cargoes are eventually isolated from the culture medium of donor cells by proper methods. This method is uncomplicated and feasible, whereas defects including low specificity and efficacy limit its application. Moreover, co-incubation is mainly applied to obtain drug-loaded EVs for disease therapy [117]. Through interacting with the lipid bilayer, drugs are absorbed by the donor cells and subsequently introduced into EVs. As an effective antiangiogenic drug, intravitreal injection of bevacizumab is a clinical therapy strategy for several retinal diseases. However, poor stability and diffusion result in the need for repeated intervention, thus increasing the treatment risk [118]. Naga et al. report that exosomes derived from RPE cells are responsible for the uptake and release of bevacizumab in the retina [119]. Therefore, exosome-mediated delivery of bevacizumab by co-incubation may improve outcome. This method is simple and maintains the integrity of EVs, but the loading efficiency is low.
Electroporation is a common approach to load exogenous cargoes into EVs. Briefly, EVs are mixed with small molecule substances, followed by high intensity electrical field stimulation to form transient transit pores on the membrane [120]. Recent studies indicate that electroporation provides a feasible scheme for the entry of mRNAs, siRNAs, miRNAs, and drugs into EVs with high efficiency [121]. However, the aggregation of EVs after electroporation hinders its application. Sonication allows the diffusion of cargos into EVs based on the increased plasma membrane permeability [122]. This method is usually used for drug loading, whereas the decreased integrity of EVs may affect their therapeutic potential. In addition, mixtures of EVs and cargoes can be fused by mechanical extrusion [123]. This violent mixing exhibits the advantage of efficient packaging, but it easily destroys the membrane structure of EVs. Saponin-mediated surface penetration is an effective strategy for protein and drug loading [124]. Pores are generated in the membrane of EVs by removing cholesterol. Nevertheless, the solution of the toxicity induced by this method requires more exploration. In a freeze/thaw method, low temperature changes the plasma membrane structure to promote cargo loading, but this method has the defect of EV aggregation [125].
Membrane modification approaches
Membrane modification of EVs has emerged as an important strategy to improve their therapeutic targeting and imaging tracing. Bioconjugation and “click chemistry” provide opportunities to form covalent binding in the membrane [126]. “Click chemistry” couples small molecules to the surface of EVs effectively and simply [127]. Wang et al. show that MSC-EVs conjugated with alendronate exert targeted treatment for osteoporosis [128]. However, such a modification approach may impair the activity of membrane proteins in EVs. Moreover, non-covalent binding strategies mainly include receptor ligand binding, hydrophobic insertion, and multivalent electrostatic interaction [121]. Based on the membrane receptors of EVs, a receptor ligand binding method exhibits the properties of specific targeting and efficient packaging. Qi et al. have developed superparamagnetic nanoparticle clusters for isolating exosomes from blood by conjugating with transferrin receptors [129]. The use of unnatural ligands for the membrane modification of EVs is challenged by difficult synthesis and high cost. For hydrophobic insertion method, hydrophobic materials integrate into EVs membrane spontaneously under ambient conditions [130]. On the basis of this method, DSPE-PEG-2000 combined with functional ligands is widely used to improve the targeting of EVs [131]. Due to the presence of negatively charged groups on the membrane, the multivalent electrostatic interaction method usually adopts cationic nanomaterials to couple with EVs [132]. Further studies need to focus on the improvement of the cationic nanomaterial-induced cytotoxic effects.
Challenges in clinical application of EVs
As a result of their value in intercellular communication, EVs are considered novel delivery vehicles to transport bioactive molecules with stability. Studies on EVs as promising diagnostic or therapeutic approaches of retinal diseases are growing, but still early in development. Due to the lack of a recognized standardized procedure, researchers adopted different methods to purify EVs according to their own actual situations. Efficient separation of high-quality EVs is still a complex problem whether using common or emerging extraction methods. Moreover, standard quality control of EVs has not been established, further limiting their application. Bioactive molecules in EVs are closely associated with the status of the donor cells, resulting in significant heterogeneity of EVs even derived from the same cell source [133]. In addition, although EVs are relatively stable at low temperature, long-term preservation is still a challenge [134].
Significant progress has been made to explore biomarkers in EVs for predicting the development of retinal diseases, whereas the content of EVs derived from specific retinal cells is inconspicuous in body fluids. The approach to accurately capture and quickly isolate specific EVs from the complex sample warrants further study. Interestingly, circulating EVs have shown an ability to package proteins and miRNAs involved in retinal disease progression. The discovery of promising biomarkers in circulating EVs is a direction of future studies.
Although studies have suggested that EVs exert therapeutic effects on several retinal diseases, the safety of this cell-free strategy needs further investigation. Traditional cell therapy has risks including low cell survival and mutation tumorigenesis [10]. Reportedly, EVs are well tolerated in several animal models such as rabbits, guinea pigs, and rats [135]. However, the biologic behavior of EVs in humans remains unclear. Immunogenicity and toxicity evaluations contribute to the clinical transformation of EV-mediated therapy. In addition, the insufficient targeting of EVs should also be considered. For the specific retinal diseases, targeting injured cells can enhance the therapeutic efficiency. Therefore, proper modification of EVs is a promising approach to strengthen their efficacy, specificity, and safety for retinal therapy.
Conclusions
EVs have exhibited potent ability in regulating biologic functions of cells due to the delivery of bioactive molecules. As discussed in this review, the emerging role of EVs as diagnostic and therapeutic approaches in retinal diseases has been revealed. However, most studies are still in the preclinical stage. The value of EVs in the field of retinal diseases remains to be comprehensively investigated. Moreover, rapid development of engineering technology provides new insight into EV modification to improve their effect. Overall, the application of EVs in early diagnosis and treatment of retinal diseases is promising. We believe that the resolution of the existing limitations will make EVs a viable strategy.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81971757), Priority Academic Program Development of Jiangsu Higher Education Institutions (Phase III) and Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application (SS2018003).
Disclosure of conflict of interest
None.
References
- 1.Ehrlich JR, Ramke J, Macleod D, Burn H, Lee CN, Zhang JH, Waldock W, Swenor BK, Gordon I, Congdon N, Burton M, Evans JR. Association between vision impairment and mortality: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e418–e430. doi: 10.1016/S2214-109X(20)30549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campochiaro PA, Akhlaq A. Sustained suppression of VEGF for treatment of retinal/choroidal vascular diseases. Prog Retin Eye Res. 2021;83:100921. doi: 10.1016/j.preteyeres.2020.100921. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata M, Yan W, Sanes JR. A cell atlas of the chick retina based on single-cell transcriptomics. Elife. 2021;10:e63907. doi: 10.7554/eLife.63907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashani AH, Asanad S, Chan JW, Singer MB, Zhang J, Sharifi M, Khansari MM, Abdolahi F, Shi Y, Biffi A, Chui H, Ringman JM. Past, present and future role of retinal imaging in neurodegenerative disease. Prog Retin Eye Res. 2021;83:100938. doi: 10.1016/j.preteyeres.2020.100938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardue MT, Allen RS. Neuroprotective strategies for retinal disease. Prog Retin Eye Res. 2018;65:50–76. doi: 10.1016/j.preteyeres.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 7.Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, Breakefield XO, Skog JK. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32:466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin L, Liu X, Shi Y, Ocansey DKW, Hu Y, Li X, Zhang C, Xu W, Qian H. Therapeutic advances of stem cell-derived extracellular vesicles in regenerative medicine. Cells. 2020;9:707. doi: 10.3390/cells9030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SY, Lin MC, Tsai JS, He PL, Luo WT, Herschman H, Li HJ. EP4 antagonist-elicited extracellular vesicles from mesenchymal stem cells rescue cognition/learning deficiencies by restoring brain cellular functions. Stem Cells Transl Med. 2019;8:707–723. doi: 10.1002/sctm.18-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12:7613–7628. doi: 10.1021/acsnano.7b07643. [DOI] [PubMed] [Google Scholar]
- 11.Ji C, Zhang J, Zhu Y, Shi H, Yin S, Sun F, Wang Q, Zhang L, Yan Y, Zhang X, Xu W, Qian H. Exosomes derived from hucMSC attenuate renal fibrosis through CK1δ/β-TRCP-mediated YAP degradation. Cell Death Dis. 2020;11:327. doi: 10.1038/s41419-020-2510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Zhang H, Gu J, Zhang J, Shi H, Qian H, Wang D, Xu W, Pan J, Santos HA. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33:e2005709. doi: 10.1002/adma.202005709. [DOI] [PubMed] [Google Scholar]
- 13.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, Chen C, Veedu RN, Wang T. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–3707. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thietart S, Rautou PE. Extracellular vesicles as biomarkers in liver diseases: a clinician’s point of view. J Hepatol. 2020;73:1507–1525. doi: 10.1016/j.jhep.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol. 2017;13:731–749. doi: 10.1038/nrneph.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 18.Vietri M, Radulovic M, Stenmark H. The many functions of ESCRTs. Nat Rev Mol Cell Biol. 2020;21:25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 19.Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, Slavík J, Machala M, Zimmermann P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 20.Varga K, Jiang ZJ, Gong LW. Phosphatidylserine is critical for vesicle fission during clathrin-mediated endocytosis. J Neurochem. 2020;152:48–60. doi: 10.1111/jnc.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, Hu X, Aubert D, Zhu S, Wu L, Yan X. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2019;9:1697028. doi: 10.1080/20013078.2019.1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu DSK, Upton FM, Rees E, Limb C, Jiao LR, Krell J, Frampton AE. Size-exclusion chromatography as a technique for the investigation of novel extracellular vesicles in cancer. Cancers (Basel) 2020;12:3156. doi: 10.3390/cancers12113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu S, Allen CL, Benjamin-Davalos S, Koroleva M, MacFarland D, Minderman H, Ernstoff MS. A rapid exosome isolation using ultrafiltration and size exclusion chromatography (REIUS) method for exosome isolation from melanoma cell lines. Methods Mol Biol. 2021;2265:289–304. doi: 10.1007/978-1-0716-1205-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9:2321–7. doi: 10.1021/nn506538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y, Wei J, Hu G, Nie G, Sun J. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano. 2017;11:6968–6976. doi: 10.1021/acsnano.7b02277. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16:489–96. doi: 10.1039/c5lc01117e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim J, Choi M, Lee H, Kim YH, Han JY, Lee ES, Cho Y. Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J Nanobiotechnology. 2019;17:1. doi: 10.1186/s12951-018-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu M, Gu J, Jiang P, Qian H, Xu W, Zhang X. Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer. 2019;18:41. doi: 10.1186/s12943-019-1001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51:1–12. doi: 10.1038/s12276-019-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durcin M, Fleury A, Taillebois E, Hilairet G, Krupova Z, Henry C, Truchet S, Trötzmüller M, Köfeler H, Mabilleau G, Hue O, Andriantsitohaina R, Martin P, Le Lay S. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 2017;6:1305677. doi: 10.1080/20013078.2017.1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835–40. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 2017;18:1153. doi: 10.3390/ijms18061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akers JC, Ramakrishnan V, Nolan JP, Duggan E, Fu CC, Hochberg FH, Chen CC, Carter BS. Comparative analysis of technologies for quantifying extracellular vesicles (EVs) in clinical cerebrospinal fluids (CSF) PLoS One. 2016;11:e0149866. doi: 10.1371/journal.pone.0149866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel R, Pal AK, Jambhrunkar S, Patel P, Thakur SS, Reátegui E, Parekh HS, Saá P, Stassinopoulos A, Broom MF. High-resolution single particle zeta potential characterisation of biological nanoparticles using tunable resistive pulse sensing. Sci Rep. 2017;7:17479. doi: 10.1038/s41598-017-14981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A. 2010;77:502–14. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoner SA, Duggan E, Condello D, Guerrero A, Turk JR, Narayanan PK, Nolan JP. High sensitivity flow cytometry of membrane vesicles. Cytometry A. 2016;89:196–206. doi: 10.1002/cyto.a.22787. [DOI] [PubMed] [Google Scholar]
- 39.Shao H, Min C, Issadore D, Liong M, Yoon TJ, Weissleder R, Lee H. Magnetic nanoparticles and microNMR for diagnostic applications. Theranostics. 2012;2:55–65. doi: 10.7150/thno.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Im H, Shao H, Weissleder R, Castro CM, Lee H. Nano-plasmonic exosome diagnostics. Expert Rev Mol Diagn. 2015;15:725–33. doi: 10.1586/14737159.2015.1041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang CY, Chen C. Toward characterizing extracellular vesicles at a single-particle level. J Biomed Sci. 2019;26:9. doi: 10.1186/s12929-019-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gézsi A, Kovács Á, Visnovitz T, Buzás EI. Systems biology approaches to investigating the roles of extracellular vesicles in human diseases. Exp Mol Med. 2019;51:1–11. doi: 10.1038/s12276-019-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldwell RB, Slapnick SM. Freeze-fracture and lanthanum studies of the retinal microvasculature in diabetic rats. Invest Ophthalmol Vis Sci. 1992;33:1610–9. [PubMed] [Google Scholar]
- 44.Elkjaer AS, Lynge SK, Grauslund J. Evidence and indications for systemic treatment in diabetic retinopathy: a systematic review. Acta Ophthalmol. 2020;98:329–336. doi: 10.1111/aos.14377. [DOI] [PubMed] [Google Scholar]
- 45.Hu J, Dziumbla S, Lin J, Bibli SI, Zukunft S, de Mos J, Awwad K, Frömel T, Jungmann A, Devraj K, Cheng Z, Wang L, Fauser S, Eberhart CG, Sodhi A, Hammock BD, Liebner S, Müller OJ, Glaubitz C, Hammes HP, Popp R, Fleming I. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature. 2017;552:248–252. doi: 10.1038/nature25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, Hsu J, Ho AC. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124:235–244. doi: 10.1016/j.ophtha.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Nittala MG, Keane PA, Zhang K, Sadda SR. Risk factors for proliferative diabetic retinopathy in a Latino American population. Retina. 2014;34:1594–9. doi: 10.1097/IAE.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabrera AP, Mankad RN, Marek L, Das R, Rangasamy S, Monickaraj F, Das A. Genotypes and phenotypes: a search for influential genes in diabetic retinopathy. Int J Mol Sci. 2020;21:2712. doi: 10.3390/ijms21082712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C, Ge HM, Liu BH, Dong R, Shan K, Chen X, Yao MD, Li XM, Yao J, Zhou RM, Zhang SJ, Jiang Q, Zhao C, Yan B. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc Natl Acad Sci U S A. 2019;116:7455–7464. doi: 10.1073/pnas.1814874116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamalden TA, Macgregor-Das AM, Kannan SM, Dunkerly-Eyring B, Khaliddin N, Xu Z, Fusco AP, Yazib SA, Chow RC, Duh EJ, Halushka MK, Steenbergen C, Das S. Exosomal microRNA-15a transfer from the pancreas augments diabetic complications by inducing oxidative stress. Antioxid Redox Signal. 2017;27:913–930. doi: 10.1089/ars.2016.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzeo A, Beltramo E, Lopatina T, Gai C, Trento M, Porta M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp Eye Res. 2018;176:69–77. doi: 10.1016/j.exer.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Tokarz A, Szuścik I, Kuśnierz-Cabala B, Kapusta M, Konkolewska M, Żurakowski A, Georgescu A, Stępień E. Extracellular vesicles participate in the transport of cytokines and angiogenic factors in diabetic patients with ocular complications. Folia Med Cracov. 2015;55:35–48. [PubMed] [Google Scholar]
- 53.Tokarz A, Konkolewska M, Kuśnierz-Cabala B, Maziarz B, Hanarz P, Żurakowski A, Szuścik I, Stępień E. Retinopathy severity correlates with RANTES concentrations and CCR 5-positive microvesicles in diabetes. Folia Med Cracov. 2019;59:95–112. [PubMed] [Google Scholar]
- 54.Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes. 2018;67:1639–1649. doi: 10.2337/db17-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao J, Zhang H, Yang F, Xiao M, Zhou L, Yu R, Shao X, Ea V, Su L, Zhang X, Li X. Proteomic analysis of plasma sEVs reveals that TNFAIP8 is a new biomarker of cell proliferation in diabetic retinopathy. J Proteome Res. 2021;20:1770–1782. doi: 10.1021/acs.jproteome.0c01048. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Cai S, Jia Y, Qi C, Sun J, Zhang H, Wang F, Cao Y, Li X. Decoding noncoding RNAs: role of microRNAs and long noncoding RNAs in ocular neovascularization. Theranostics. 2017;7:3155–3167. doi: 10.7150/thno.19646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazzeo A, Lopatina T, Gai C, Trento M, Porta M, Beltramo E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp Eye Res. 2019;184:56–63. doi: 10.1016/j.exer.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Wei J, Zhang L, Jiang G, Wang B, Jiang L. Extracellular vesicle-derived miR-26b-5p is up-regulated in the serum of patients with diabetic retinopathy. Comb Chem High Throughput Screen. 2021 doi: 10.2174/1386207324666210216092917. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–71. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu BJ, Huang X, Avula PK, Caruso E, Drysdale C, Vessey KA, Ou A, Fowler C, Liu TH, Lin Y, Horton A, Masters CL, Wiley JS, Guymer RH, Fletcher EL. Deficits in monocyte function in age related macular degeneration: a novel systemic change associated with the disease. Front Med (Lausanne) 2021;8:634177. doi: 10.3389/fmed.2021.634177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jonasson F, Arnarsson A, Eiríksdottir G, Harris TB, Launer LJ, Meuer SM, Klein BE, Klein R, Gudnason V, Cotch MF. Prevalence of age-related macular degeneration in old persons: age, gene/environment susceptibility reykjavik study. Ophthalmology. 2011;118:825–30. doi: 10.1016/j.ophtha.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grassmann F, Fritsche LG, Keilhauer CN, Heid IM, Weber BH. Modelling the genetic risk in age-related macular degeneration. PLoS One. 2012;7:e37979. doi: 10.1371/journal.pone.0037979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaarniranta K, Salminen A. Age-related macular degeneration: activation of innate immunity system via pattern recognition receptors. J Mol Med (Berl) 2009;87:117–23. doi: 10.1007/s00109-008-0418-z. [DOI] [PubMed] [Google Scholar]
- 64.Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Oxidized low-density-lipoprotein-induced injury in retinal pigment epithelium alters expression of the membrane complement regulatory factors CD46 and CD59 through exosomal and apoptotic bleb release. Adv Exp Med Biol. 2014;801:259–65. doi: 10.1007/978-1-4614-3209-8_33. [DOI] [PubMed] [Google Scholar]
- 65.Yang C, Shani S, Tahiri H, Ortiz C, Gu M, Lavoie JC, Croteau S, Hardy P. Extracellular microparticles exacerbate oxidative damage to retinal pigment epithelial cells. Exp Cell Res. 2020;390:111957. doi: 10.1016/j.yexcr.2020.111957. [DOI] [PubMed] [Google Scholar]
- 66.Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy. 2009;5:563–4. doi: 10.4161/auto.5.4.8163. [DOI] [PubMed] [Google Scholar]
- 67.Oltra M, Vidal-Gil L, Maisto R, Oltra SS, Romero FJ, Sancho-Pelluz J, Barcia JM. miR302a and 122 are deregulated in small extracellular vesicles from ARPE-19 cells cultured with H2O2. Sci Rep. 2019;9:17954. doi: 10.1038/s41598-019-54373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah N, Ishii M, Brandon C, Ablonczy Z, Cai J, Liu Y, Chou CJ, Rohrer B. Extracellular vesicle-mediated long-range communication in stressed retinal pigment epithelial cell monolayers. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2610–2622. doi: 10.1016/j.bbadis.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atienzar-Aroca S, Serrano-Heras G, Freire Valls A, Ruiz de Almodovar C, Muriach M, Barcia JM, Garcia-Verdugo JM, Romero FJ, Sancho-Pelluz J. Role of retinal pigment epithelium-derived exosomes and autophagy in new blood vessel formation. J Cell Mol Med. 2018;22:5244–5256. doi: 10.1111/jcmm.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang GY, Bang JY, Choi AJ, Yoon J, Lee WC, Choi S, Yoon S, Kim HC, Baek JH, Park HS, Lim HJ, Chung H. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J Proteome Res. 2014;13:581–95. doi: 10.1021/pr400751k. [DOI] [PubMed] [Google Scholar]
- 71.Grassmann F, Schoenberger PG, Brandl C, Schick T, Hasler D, Meister G, Fleckenstein M, Lindner M, Helbig H, Fauser S, Weber BH. A circulating microrna profile is associated with late-stage neovascular age-related macular degeneration. PLoS One. 2014;9:e107461. doi: 10.1371/journal.pone.0107461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elbay A, Ercan Ç, Akbaş F, Bulut H, Ozdemir H. Three new circulating microRNAs may be associated with wet age-related macular degeneration. Scand J Clin Lab Invest. 2019;79:388–394. doi: 10.1080/00365513.2019.1637931. [DOI] [PubMed] [Google Scholar]
- 73.Yi Z, Ouyang J, Sun W, Li S, Xiao X, Zhang Q. Comparative exome sequencing reveals novel candidate genes for retinitis pigmentosa. EBioMedicine. 2020;56:102792. doi: 10.1016/j.ebiom.2020.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newton F, Megaw R. Mechanisms of photoreceptor death in retinitis pigmentosa. Genes (Basel) 2020;11:1120. doi: 10.3390/genes11101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anasagasti A, Irigoyen C, Barandika O, López de Munain A, Ruiz-Ederra J. Current mutation discovery approaches in retinitis pigmentosa. Vision Res. 2012;75:117–29. doi: 10.1016/j.visres.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 76.Blanks JC, Spee C. Retinal degeneration in the pcd/pcd mutant mouse: accumulation of spherules in the interphotoreceptor space. Exp Eye Res. 1992;54:637–44. doi: 10.1016/0014-4835(92)90019-o. [DOI] [PubMed] [Google Scholar]
- 77.Muraoka Y, Ikeda HO, Nakano N, Hangai M, Toda Y, Okamoto-Furuta K, Kohda H, Kondo M, Terasaki H, Kakizuka A, Yoshimura N. Real-time imaging of rabbit retina with retinal degeneration by using spectral-domain optical coherence tomography. PLoS One. 2012;7:e36135. doi: 10.1371/journal.pone.0036135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li T, Snyder WK, Olsson JE, Dryja TP. Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc Natl Acad Sci U S A. 1996;93:14176–81. doi: 10.1073/pnas.93.24.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ropelewski P, Imanishi Y. RPE cells engulf microvesicles secreted by degenerating rod photoreceptors. eNeuro. 2020;7 doi: 10.1523/ENEURO.0507-19.2020. ENEURO.0507-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sahaboglu A, Vidal-Gil L, Sancho-Pelluz J. Release of retinal extracellular vesicles in a model of retinitis pigmentosa. Adv Exp Med Biol. 2019;1185:431–436. doi: 10.1007/978-3-030-27378-1_71. [DOI] [PubMed] [Google Scholar]
- 81.Vidal-Gil L, Sancho-Pelluz J, Zrenner E, Oltra M, Sahaboglu A. Poly ADP ribosylation and extracellular vesicle activity in rod photoreceptor degeneration. Sci Rep. 2019;9:3758. doi: 10.1038/s41598-019-40215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu J, Bibli SI, Wittig J, Zukunft S, Lin J, Hammes HP, Popp R, Fleming I. Soluble epoxide hydrolase promotes astrocyte survival in retinopathy of prematurity. J Clin Invest. 2019;129:5204–5218. doi: 10.1172/JCI123835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell JP, Singh P, Redd TK, Brown JM, Shah PK, Subramanian P, Rajan R, Valikodath N, Cole E, Ostmo S, Chan RVP, Venkatapathy N, Chiang MF, Kalpathy-Cramer J. Applications of artificial intelligence for retinopathy of prematurity screening. Pediatrics. 2021;147:e2020016618. doi: 10.1542/peds.2020-016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rivera JC, Holm M, Austeng D, Morken TS, Zhou TE, Beaudry-Richard A, Sierra EM, Dammann O, Chemtob S. Retinopathy of prematurity: inflammation, choroidal degeneration, and novel promising therapeutic strategies. J Neuroinflammation. 2017;14:165. doi: 10.1186/s12974-017-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barry GP, Yu Y, Ying GS, Tomlinson LA, Lajoie J, Fisher M, Binenbaum G. Retinal detachment after treatment of retinopathy of prematurity with laser versus intravitreal anti-vascular endothelial growth factor. Ophthalmology. 2021;128:1188–1196. doi: 10.1016/j.ophtha.2020.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aires ID, Santiago AR. Microglial exosomes in retinal neuroinflammation: focus in glaucoma. Neural Regen Res. 2021;16:1801–1802. doi: 10.4103/1673-5374.306084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Jiang F, Jiang Y, Wang Y, Li Z, Shi X, Zhu Y, Wang H, Zhang Z. Roles of exosomes in ocular diseases. Int J Nanomedicine. 2020;15:10519–10538. doi: 10.2147/IJN.S277190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nair KS, Srivastava C, Brown RV, Koli S, Choquet H, Kang HS, Kuo YM, Grimm SA, Sutherland C, Badea A, Johnson GA, Zhao Y, Yin J, Okamoto K, Clark G, Borrás T, Zode G, Kizhatil K, Chakrabarti S, John SWM, Jorgenson E, Jetten AM. GLIS1 regulates trabecular meshwork function and intraocular pressure and is associated with glaucoma in humans. Nat Commun. 2021;12:4877. doi: 10.1038/s41467-021-25181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mead B, Tomarev S. Extracellular vesicle therapy for retinal diseases. Prog Retin Eye Res. 2020;79:100849. doi: 10.1016/j.preteyeres.2020.100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang J, Wang Y. Altered expression of extracellular vesicles miRNAs from primary human trabecular meshwork cells induced by transforming growth factor-β2. DNA Cell Biol. 2021;40:988–997. doi: 10.1089/dna.2020.6298. [DOI] [PubMed] [Google Scholar]
- 91.Stamer WD, Hoffman EA, Luther JM, Hachey DL, Schey KL. Protein profile of exosomes from trabecular meshwork cells. J Proteomics. 2011;74:796–804. doi: 10.1016/j.jprot.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dismuke WM, Klingeborn M, Stamer WD. Mechanism of fibronectin binding to human trabecular meshwork exosomes and its modulation by dexamethasone. PLoS One. 2016;11:e0165326. doi: 10.1371/journal.pone.0165326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lerner N, Schreiber-Avissar S, Beit-Yannai E. Extracellular vesicle-mediated crosstalk between NPCE cells and TM cells result in modulation of Wnt signalling pathway and ECM remodelling. J Cell Mol Med. 2020;24:4646–4658. doi: 10.1111/jcmm.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aires ID, Ribeiro-Rodrigues T, Boia R, Catarino S, Girão H, Ambrósio AF, Santiago AR. Exosomes derived from microglia exposed to elevated pressure amplify the neuroinflammatory response in retinal cells. Glia. 2020;68:2705–2724. doi: 10.1002/glia.23880. [DOI] [PubMed] [Google Scholar]
- 95.Dismuke WM, Challa P, Navarro I, Stamer WD, Liu Y. Human aqueous humor exosomes. Exp Eye Res. 2015;132:73–7. doi: 10.1016/j.exer.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holan V, Palacka K, Hermankova B. Mesenchymal stem cell-based therapy for retinal degenerative diseases: experimental models and clinical trials. Cells. 2021;10:588. doi: 10.3390/cells10030588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaddam S, Periasamy R, Gangaraju R. Adult stem cell therapeutics in diabetic retinopathy. Int J Mol Sci. 2019;20:4876. doi: 10.3390/ijms20194876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nuzzi R, Caselgrandi P, Vercelli A. Effect of mesenchymal stem cell-derived exosomes on retinal injury: a review of current findings. Stem Cells Int. 2020;2020:8883616. doi: 10.1155/2020/8883616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deng CL, Hu CB, Ling ST, Zhao N, Bao LH, Zhou F, Xiong YC, Chen T, Sui BD, Yu XR, Hu CH. Photoreceptor protection by mesenchymal stem cell transplantation identifies exosomal MiR-21 as a therapeutic for retinal degeneration. Cell Death Differ. 2021;28:1041–1061. doi: 10.1038/s41418-020-00636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang W, Wang Y, Kong Y. Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest Ophthalmol Vis Sci. 2019;60:294–303. doi: 10.1167/iovs.18-25617. [DOI] [PubMed] [Google Scholar]
- 101.Gu C, Zhang H, Gao Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J Cell Physiol. 2021;236:5036–5051. doi: 10.1002/jcp.30213. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X, Liu J, Yu B, Ma F, Ren X, Li X. Effects of mesenchymal stem cells and their exosomes on the healing of large and refractory macular holes. Graefes Arch Clin Exp Ophthalmol. 2018;256:2041–2052. doi: 10.1007/s00417-018-4097-3. [DOI] [PubMed] [Google Scholar]
- 103.Mead B, Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. 2017;6:1273–1285. doi: 10.1002/sctm.16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mead B, Amaral J, Tomarev S. Mesenchymal stem cell-derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Invest Ophthalmol Vis Sci. 2018;59:702–714. doi: 10.1167/iovs.17-22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moisseiev E, Anderson JD, Oltjen S, Goswami M, Zawadzki RJ, Nolta JA, Park SS. Protective effect of intravitreal administration of exosomes derived from mesenchymal stem cells on retinal ischemia. Curr Eye Res. 2017;42:1358–1367. doi: 10.1080/02713683.2017.1319491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beltramo E, Lopatina T, Berrone E, Mazzeo A, Iavello A, Camussi G, Porta M. Extracellular vesicles derived from mesenchymal stem cells induce features of diabetic retinopathy in vitro. Acta Diabetol. 2014;51:1055–64. doi: 10.1007/s00592-014-0672-1. [DOI] [PubMed] [Google Scholar]
- 107.Cai C, Tahiri H, Fortin C, Ortiz C, Sintjago H, Yang C, Hardy P. Lymphocytic microparticles suppress retinal angiogenesis via targeting Müller cells in the ischemic retinopathy mouse model. Exp Cell Res. 2021;399:112470. doi: 10.1016/j.yexcr.2021.112470. [DOI] [PubMed] [Google Scholar]
- 108.Yang C, Xiong W, Qiu Q, Shao Z, Hamel D, Tahiri H, Leclair G, Lachapelle P, Chemtob S, Hardy P. Role of receptor-mediated endocytosis in the antiangiogenic effects of human T lymphoblastic cell-derived microparticles. Am J Physiol Regul Integr Comp Physiol. 2012;302:R941–9. doi: 10.1152/ajpregu.00527.2011. [DOI] [PubMed] [Google Scholar]
- 109.Qiu Q, Yang C, Xiong W, Tahiri H, Payeur M, Superstein R, Carret AS, Hamel P, Ellezam B, Martin B, Vezina M, Sapieha P, Liu G, Hardy P. SYK is a target of lymphocyte-derived microparticles in the induction of apoptosis of human retinoblastoma cells. Apoptosis. 2015;20:1613–22. doi: 10.1007/s10495-015-1177-2. [DOI] [PubMed] [Google Scholar]
- 110.Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang HG, Shao H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J Biol Chem. 2013;288:28058–67. doi: 10.1074/jbc.M113.470765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dellett M, Brown ED, Guduric-Fuchs J, O’Connor A, Stitt AW, Medina RJ, Simpson DA. MicroRNA-containing extracellular vesicles released from endothelial colony-forming cells modulate angiogenesis during ischaemic retinopathy. J Cell Mol Med. 2017;21:3405–3419. doi: 10.1111/jcmm.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lakkaraju A, Toops KA, Xu J. Should I stay or should I go? Trafficking of sub-lytic MAC in the retinal pigment epithelium. Adv Exp Med Biol. 2014;801:267–74. doi: 10.1007/978-1-4614-3209-8_34. [DOI] [PubMed] [Google Scholar]
- 113.Gu S, Liu Y, Zou J, Wang W, Wei T, Wang X, Zhu L, Zhang M, Zhu J, Xie T, Yao Y, Qiu L. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp Eye Res. 2020;201:108271. doi: 10.1016/j.exer.2020.108271. [DOI] [PubMed] [Google Scholar]
- 114.Lou P, Liu S, Xu X, Pan C, Lu Y, Liu J. Extracellular vesicle-based therapeutics for the regeneration of chronic wounds: current knowledge and future perspectives. Acta Biomater. 2021;119:42–56. doi: 10.1016/j.actbio.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 115.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, Starmann J, Tjwa M, Plate KH, Sültmann H, Altevogt P, Umansky V, Momma S. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. 2015;4:e1008371. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX, Zhang G, Feng ZH, Ye D, Huang B. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 118.Shoval A, Markus A, Zhou Z, Liu X, Cazelles R, Willner I, Mandel Y. Anti-VEGF-aptamer modified C-Dots-A hybrid nanocomposite for topical treatment of ocular vascular disorders. Small. 2019;15:e1902776. doi: 10.1002/smll.201902776. [DOI] [PubMed] [Google Scholar]
- 119.Aboul Naga SH, Dithmer M, Chitadze G, Kabelitz D, Lucius R, Roider J, Klettner A. Intracellular pathways following uptake of bevacizumab in RPE cells. Exp Eye Res. 2015;131:29–41. doi: 10.1016/j.exer.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 120.Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomedicine. 2018;13:585–599. doi: 10.2147/IJN.S154458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu P, Zhang B, Ocansey DKW, Xu W, Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. 2021;269:120467. doi: 10.1016/j.biomaterials.2020.120467. [DOI] [PubMed] [Google Scholar]
- 122.Rajendran RL, Paudel S, Gangadaran P, Oh JM, Oh EJ, Hong CM, Lee S, Chung HY, Lee J, Ahn BC. Extracellular vesicles act as nano-transporters of tyrosine kinase inhibitors to revert iodine avidity in thyroid cancer. Pharmaceutics. 2021;13:248. doi: 10.3390/pharmaceutics13020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, Fang Y, Xia Y, Cheng G, He X, Zheng SY. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018;78:798–808. doi: 10.1158/0008-5472.CAN-17-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haney MJ, Klyachko NL, Harrison EB, Zhao Y, Kabanov AV, Batrakova EV. TPP1 delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease. Adv Healthc Mater. 2019;8:e1801271. doi: 10.1002/adhm.201801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Armstrong JP, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kang YT, Hadlock T, Jolly S, Nagrath S. Extracellular vesicles on demand (EVOD) chip for screening and quantification of cancer-associated extracellular vesicles. Biosens Bioelectron. 2020;168:112535. doi: 10.1016/j.bios.2020.112535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y, Yao J, Cai L, Liu T, Wang X, Zhang Y, Zhou Z, Li T, Liu M, Lai R, Liu X. Bone-targeted extracellular vesicles from mesenchymal stem cells for osteoporosis therapy. Int J Nanomedicine. 2020;15:7967–7977. doi: 10.2147/IJN.S263756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, Qian X, Jia H, Zhao J, Sun J, Hou X, Yuan X, Kang C. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10:3323–33. doi: 10.1021/acsnano.5b06939. [DOI] [PubMed] [Google Scholar]
- 130.Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, Shiku H, Akiyoshi K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li D, Yao S, Zhou Z, Shi J, Huang Z, Wu Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr Res. 2020;493:108032. doi: 10.1016/j.carres.2020.108032. [DOI] [PubMed] [Google Scholar]
- 132.Salunkhe S, Dheeraj , Basak M, Chitkara D, Mittal A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release. 2020;326:599–614. doi: 10.1016/j.jconrel.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 133.Raposo G, Stahl PD. Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol. 2019;20:509–510. doi: 10.1038/s41580-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 134.Lőrincz Á M, Timár CI, Marosvári KA, Veres DS, Otrokocsi L, Kittel Á, Ligeti E. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J Extracell Vesicles. 2014;3:25465. doi: 10.3402/jev.v3.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, Qian H, Zhu W, Xu W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18:413–22. doi: 10.1016/j.jcyt.2015.11.018. [DOI] [PubMed] [Google Scholar]


