Abstract
Objective: The aim of this study was to elucidate the role of miR-200c-3p in cochlear hair cells injured by oxidative stress (OS) and the underlying mechanisms. Methods: The OS injury model of HEI-OC1 cells was induced by 100 μmol/L tert-butyl hydroperoxide (t-BHP). The expression of miR-200c-3p in HEI-OC1 was detected by RT-PCR, the levels of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), Catalase (CAT), and malondialdehyde (MDA) were determined with ELISA, and the expression levels of Taok1 and apoptosis-related proteins were measured by Western Blot. Flow cytometry was used to detect cell apoptosis. Results: Real-time polymerase chain reaction (RT-qPCR) analysis identified down-regulated miR-200c-3p and up-regulated Taok1 in HEI-OC1 cells damaged by OS, as well as an inverse association between miR-200c-3p and Taok1. Cell tests confirmed that miR-200c-3p overexpression could effectively inhibit the OS response and apoptosis of HEI-OC1 cells. Bioinformatics prediction and dual luciferase reporter assay revealed that Taok1 was a direct target of miR-200c-3p. Taok1 overexpression could reverse the protective action of miR-200c-3p overexpression on the OS injury of HEI-OC1 cells. Conclusions: Given the capacity of miR-200c-3p to suppress the OS and apoptosis of HEI-OC1 cells via targeting Taok1, it can be a novel and potential therapeutic target for cochlear hair cell injury.
Keywords: miR-200c-3p, cochlear hair cells, taok1, oxidative stress, apoptosis
Introduction
Deafness is one of the most common inner ear sensory impairments. According to the statistics from the World Health Organization (WHO) in 2019, nearly one-third of the elderly over 65 years old and one in every 500 newborns globallysuffer from hearing loss to varying degrees [1,2]. Besides, hearing loss will cause serious communication barriers and severely affect the quality of life of patients [3].
Hair cells are an important component of auditory receptors. Studies have shown that the damage of cochlear hair cells caused by inner ear ischemia, hypoxia, noise and ototoxic drugs is closely associated with the generation of oxygen free radicals [4,5]. miRNAs are non-coding microRNAs with a length of about 18-25 nucleotides, which can participate in a series of essential biological processes such as cell development, proliferation, differentiation and apoptosis via modulating gene expression by means of specific translation inhibition or mRNA cleavage [6]. It is found that miRNAs are abundantly expressed in inner ear hair cells and are significantly associated with auditory function [7]. Previous literature has unveiled the abnormal expression of some miRNAs in cochlear hair cells damaged by oxidative stress (OS). For example, miR-133a was found to be down-regulated in cochlear hair cells damaged by tert-butyl hydroperoxide (t-BHP) [8]. Some scholars [9] have analyzed miRNA expression profiles in cochlea tissue after acoustic loss. miR-200c was found significantly down-regulated in cochlea tissue and Taok1 was an potential target of miR-200c as predicted by bioinformatics analysis. However, little is known regarding the mechanism of miRNAs, including miR-200c-3p, in OS-induced cochlear hair cell loss. This study aimed at determining the involvement of the two genes in oxidative damage of cochlear hair cells as well as the regulatory function of miR-200c on Taok1.
Materials and methods
Cell culture and transfection
Purchased from Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, HEI-OC1 cells (as a model system for cochlear hair cell research in vitro, it can express a variety of hair cell markers) were cultured in dulbecco’s modified eagle medium (DMEM, high sugar; Hyclone, USA) containing 10% fetal bovine serum (Gibco, USA) in an incubator (37°C, 10% CO2). Medium renewal was performed on alternate days, and the cells were subcultured once 48-72 h. The cells were counted by a blood counting chamber before inoculation. After passage, cells in a good growth state were selected and digested into suspension. Thereafter, they were counted and inoculated into 6-well plates (2×105/well). The cells were induced to suffer OS injury by 100 μmol/L tert-butyl hydroperoxide (t-BHP, Sigma Company, USA), and a control group (cells without the intervention of OS injury) was set up. Subsequently, miR-200c-3p mimic (5’-UAAUACUGCCCGGGUAAUGAUGGA-3’) and its negative control (miR-NC, 5’-UUGUACUACACAAAAGUACUG-3’), Taok1 small interfering RNA (siRNA) (si-Taok1) and its negative control (si-NC), as well as Taok1 overexpression plasmid established using pcDNA3.1 vector (pcDNA3.1-Taok1) and its negative control (pcDNA3.1-NC) (GenePharma, Shanghai, China) were transfected into cells using LipofectamineTM 2000 kits (Invitrogen, Carlsbad, USA), strictly following the instructions.
RT-PCR
Cells intervened by t-BHP for 12 h were subjected to total RNA isolation with the Trizol reagent (Invitrogen, USA), and subsequent purity and concentration determination using an ultraviolet spectrophotometer. Then, 5 μg of the total RNA was collected for reverse transcription of cDNA according to kit instructions. The PCR reaction system was as follows: Bestar ®Sybr Green qPCR Master Mix: 10 μL, forward primer (10 μM): 0.5 μL, reverse primer (10 μM): 1 μL, and dddH2O to a final volume of 10 μL. Amplification conditions: PCR reaction conditions (40 cycles): 94°C, 30 s; 94°C, 5 s; 60°C, 30 s. In this study, U6 was used as the internal reference for miR-200C-3p, and GAPDH was used as the internal reference for Taok1. The data were analyzed using 2-ΔΔCT. Primer sequences are presented in Table 1.
Table 1.
Primer sequences
| Gene | Upstream primer 5’-3’ | Downstream primer 5’-3’ |
|---|---|---|
| miR-200c-3p | TCGTCTTACCCAGCAGTG | CGGCAGTATTAGAGACTCC |
| U6 | CTCGCTTCGGCAGCACATA | AACGATTCACGAATTTGCGT |
| Taok1 | AAG AGC ATC AGC TCC ACA GT | GCC GAT GTT CGT CCA TTT CT |
| GAPDH | CCTCGTCTCATAGACAAGATGGT | GGGTAGAGTCATACTGGAACATG |
Detection of intracellular OS indexes
The cells were collected after digestion and rinsed with PBS once. They were then lysed using an ultrasonic crushing apparatus, and centrifuged at low speed at 3000 r/min for 15 min. The resulting lysed supernatant was gathered for the determination of oxidative damage-related molecules glutathione peroxidase (GSH-px), superoxide dismutase (SOD), catalase (CAT) and malondialdehyde (MDA) using ELISA kits all purchased from Jiancheng Bioengineering Institute, Nanjing, China.
Western blot (WB)
Radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China) was used for total protein extraction. The protein concentration, measured by bicinchoninic acid (BCA) method (Beyotime, Shanghai, China), was made to 4 μg/μL, which was subsequently processed for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 12%), polyvinylidene fluoride (PVDF) membrane transfer, dyeing in Ponceau S Solution, and soaking in PBST (5 min). After closing in 5% Nonfat-Dried Milk for 2 h, it was immersed in primary antibodies Bax (1:500), Bcl-2 (1:500), Taok1 (1:1,000) and β-Actin (1:1,000) (Abcam, ab32503, ab32124, ab197891, ab6276), for overnight blocking at 4°C. Following the removal of primary antibodies, the membrane was cultured in the secondary antibody horseradish peroxidase (HRPO)-labeled goat anti-rabbit (1:1,000) (Abcam, ab6728) for 1 h (37°C), and then washed with PBS three times (5 min/time). The membrane was blotted with filter paper and finally developed by enhanced chemiluminescent (ECL) reagent (Pierce Biotechnology, Rockford, USA).
Apoptosis detection
The collected cells were adjusted to 4×105/mL and immersed in 500 μL Binding Buffer for resuspension referring to the instructions of Annexin V-FITC apoptosis kit (MultiSciences, 70-AP101-100). Then they were mixed with Annexin V (5 μL) and PI (10 μL) staining solution successively in dark. At the same time, blank cell samples, Annexin V single-stained and PI single-stained samples were used to adjust the optical path compensation. After staining (15 min), the apoptotic rate of cells was detected by flow cytometry (FCM).
Dual luciferase reporter (DLR)
The bioinformatics database Targetscan identified Taok1 as a direct target of miR-200c-3p. After amplifying the oligonucleotide containing Taok1 target sequence, it was introduced into pmirGLO plasmid (WT) (Promega, Madison WI, USA). Following the construction of pmirGLO-Taok1-3’UTR wild type (WT) and Mutant (Mut), they were transferred to the luciferase reporter gene downstream to sequence and identify the constructed plasmids. The luciferase reporter plasmid was co-transfected into 293T cells (Huzhen Biotech, Shanghai, China, HZ-H321) with either miR-200c-33p mimic or miR-NC by Lipofectamine 2000. After 24 h of culture, the cells were collected for luciferase activity detection with a luciferase detection kit (Promega, Madison, Wisconsin, USA), and the results were statistically analyzed.
Statistical processing
In this study, the statistical analysis and image rendering employed SPSS 20.0 and GraphPad Prism 6 respectively. Measurement data, recorded in the form of SD ± means, were analyzed by t-test; The methods for inter- and multi-group comparisons were independent samples t-test (denoted by t) and one-way ANOVA plus LSD-t post-hoc test, respectively, with the difference considered significant when P<0.05.
Results
Effect of t-BHP intervention on HEI-OC1 cells
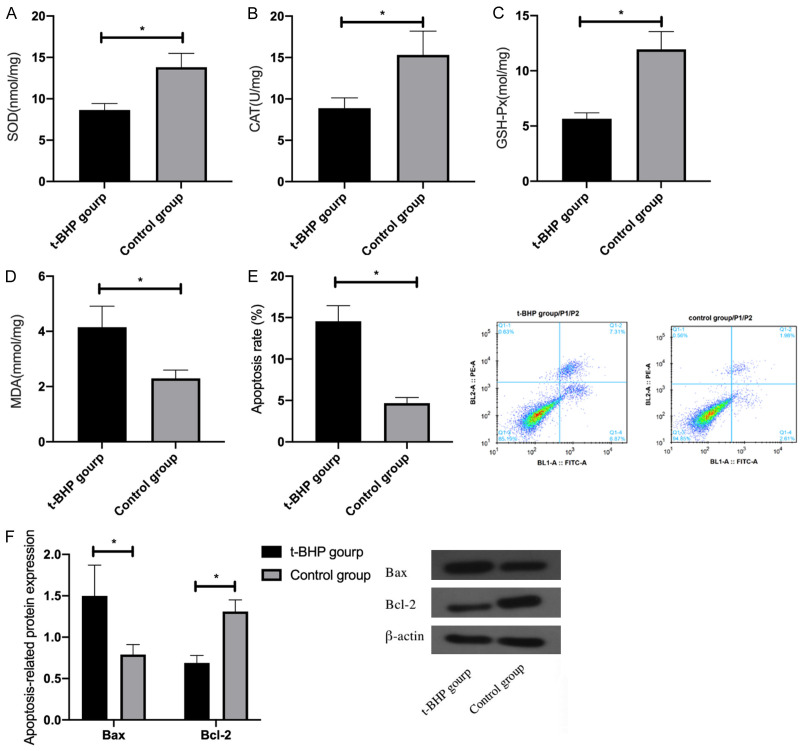
Compared with Control group, the levels of antioxidant stress factors SOD, CAT and GSH-Px in t-BHP treated HEI-OC1 reduced remarkably, while the expression of MDA, an OS factor, increased significantly. Meanwhile, statistically increased apoptosis rate and pro-apoptotic protein Bax as well as remarkably declined anti-apoptotic protein Bcl-2 were observed in HEI-OC1 cells (P<0.05). See Figure 1.
Figure 1.
Effect of t-BHP treatment on HEI-OC1 cells. A: Expression of SOD in HEI-OC1 cells treated with t-BHP; B: Expression of CAT in HEI-OC1 cells treated with t-BHP; C: Expression of GSH-Px in HEI-OC1 cells treated with t-BHP; D: Expression of MDA in HEI-OC1 cells treated with t-BHP; E: Apoptosis rate of HEI-OC1 cells treated with T-BHP; F: Expression of apoptosis-related proteins Bax and Bcl-2 in HEI-OC1 cells treated with T-BHP. *indicates P<0.05 (independent samples t-test).
miR-200c-3p and Taok1 in t-BHP-treated HEI-OC1 cells
After t-BHP treatment, the expression of miR-200c-3p and Taok1 mRAN in HEI-OC1 cells was detected by qRT-PCR, and the expression of Taok1 protein was detected by WB. The results identified down-regulated miR-200c-3p and up-regulated Taok1 mRNA and protein in t-BHP treated HEI-OC1 cells, versus Control group (P<0.05). See Figure 2.
Figure 2.
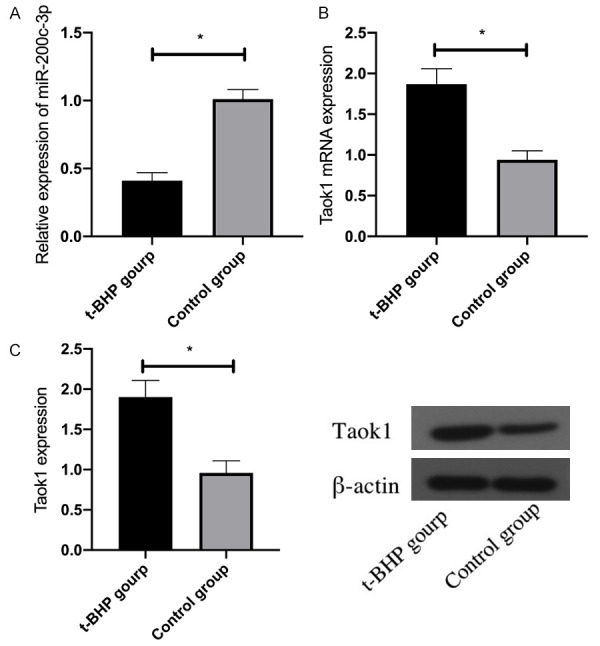
Expression of miR-200c-3p and Taok1 in HEI-OC1 cells after t-BHP treatment. A: Expression of miR-200c-3p in HEI-OC1 cells after t-BHP treatment; B: Expression of Taok1 mRNA in HEI-OC1 cells after t-BHP treatment; C: Expression of Taok1 protein in HEI-OC1 cells after t-BHP treatment. *indicates P<0.05 (independent samples t-test).
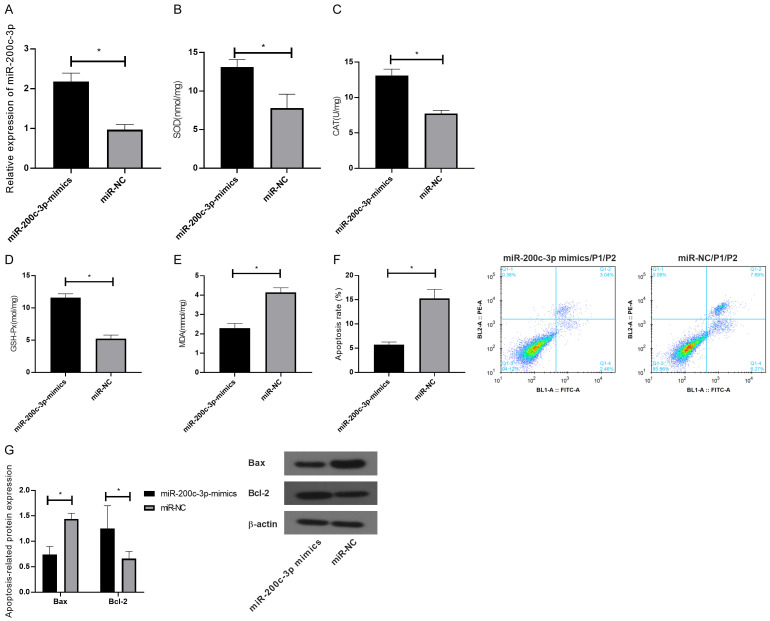
Effect of miR-200c-3p overexpression on HEI-OC1 cells
miR-200c-3p-mimic and its negative control were transfected into HEI-OC1 cells injured by OS. The results showed that the expression of miR-200c-3p in HEI-OC1 cells was significantly up-regulated compared with miR-NC group (P<0.05). SOD, CAT and GSH-Px in transfected HEI-OC1 cells were obviously up-regulated while MDA was notably down-regulated, as indicated by ELISA (P<0.05). FCM revealed that the apoptosis of HEI-OC1 cells decreased obviously after transfection (P<0.05). Meanwhile, WB identified reduced Bax protein and elevated Bcl-2 protein. See Figure 3.
Figure 3.
Effect of overexpression of miR-200c-3p on HEI-OC1 cells. A: Expression of miR-200c-3p in HEI-OC1 cells after transfection; B: Effect of overexpression of miR-200c-3p on the expression of SOD in HEI-OC1 cells; C: Effect of overexpression of miR-200c-3p on the expression of CAT in HEI-OC1 cells; D: Effect of overexpression of miR-200c-3p on the expression of GSH-Px in HEI-OC1 cells; E: Effect of overexpression of miR-200c-3p on the expression of MDA in HEI-OC1 cells; F: Effect of overexpression of miR-200c-3p on the apoptosis rate of HEI-OC1 cells; G: Effect of overexpression of miR-200c-3p on the expression of Bax and Bcl-2 in HEI-OC1 cells. *indicates P<0.05 (independent samples t-test).
Taok1 is a target gene of miR-200c-3p
DLR assay showed that miR-200c-3p overexpression decreased the luciferase activity of Taok1 3’UTR Wt (P<0.05), but did not affect Taok1 3’UTR Mut. Furthermore, WB showed down-regulated Taok1 protein in HEI-OC1 cells transfected with miR-200c-3p mimic (P<0.05). Figure 4.
Figure 4.
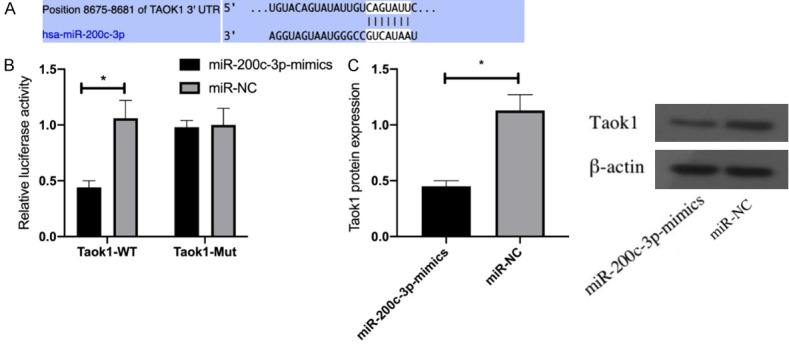
Dual luciferase reporter assay. A: Targeted binding sites; B: Effect of miR-200c-3p on the luciferase activity of Taok1; C: Effect of miR-200c-3p on Taok1 protein expression. *indicates P<0.05 (independent samples t-test).
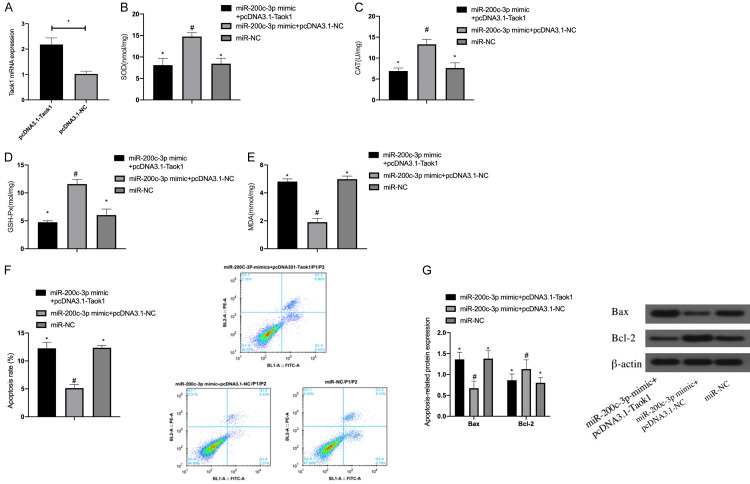
miR-200c-3p targets Taok1 to protect HEI-OC1 cells injured by OS
After co-transfecting miR-200c-3p mimic and pcDNA3.1-Taok1 into HEI-OC1 cells, it was found that the expression of Taok1 mRNA in HEI-OC1 cells was significantly increased after pcDNA3.1-Taok1 transfection. In addition, pcDNA3.1-Taok1 reversed the inhibitory effect of miR-200c-3p mimic on OS and apoptosis of HEI-OC1 (P<0.05), indicating that miR-200c-3p exerted a protective effect on HEI-OC1 cells damaged by OS via targeting Taok1 Figure 5.
Figure 5.
Effect of co-transfection of miR-200c-3p mimic and pcDNA3.1-Taok1 on HEI-OC1 cells. A: Transfection efficiency of pcDNA3.1-Taok1 in HEI-OC1 cells; B: Effect of co-transfection of miR-200c-3p mimic and pcDNA3.1-Taok1 on the expression of SOD in HEI-OC1 cells; C: Effect of co-transfection of miR-200c-3p mimic and pcDNA3.1-Taok1 on the expression of CAT in HEI-OC1 cells; D: Effect of co-transfection of miR-200c-3p mimic and pcDNA3.1-Taok1 on the expression of GSH-Px in HEI-OC1 cells; E: Effect of co-transfection of miR-200c-3p mimic and pcDNA3.1-Taok1 on the expression of MDA in HEI-OC1 cells; F: Effect of co-transfection of miR-200c-3p mimic and pcDNA3.1-Taok1 on the apoptosis rate of HEI-OC1 cells; G: Effect of co-transfection of miR-200c-3p mimic and pcDNA3.1-Taok1 on the expression of apoptosis related proteins Bax and Bcl-2 in HEI-OC1 cells. **indicates P<0.05; *compared with #, P<0.05 (independent samples t test was used for Figure A, one-way ANOVA was used for the rest, and LSD-T test was used for post-hoc pairwise comparison).
Discussion
Generally, all mammals, including humans, cannot regenerate cochlear hearing cells after the inner ear matures. When the inner ear encounts irreversible damage to cochlear hair cells and cannot be repaired, people will experience varying degrees of hearing loss [10,11]. In recent years, miRNAs have been shown to be abundantly expressed in inner ear hair cells and are strongly correlated with auditory function [12]. It was previously reported that, using 100 μmol/L t-BHP to simulate the OS injury of HEI-OC1 cells induced by noise in vitro, miR-200c can protect HEI-OC1 cells from OS injury by targeting Taok1 [13].
miR-200c belongs to the miR-200 family and is located on chromosome 12p13 [14]. Evidence has shown that miR-200c is down-regulated in OS-induced glaucoma [15]. OS is also responsible for cochlear hair cell injury. However, no research has explored the role and related mechanisms of miR-200c in OS-induced cochlear hair cell injury. After using t-BHP to induce OS damage in HEI-OC1 cells, we observed significantly down-regulated antioxidant stress factors SOD, CAT and GSH-Px in HEI-OC1 cells, notably up-regulated OS factor MDA, and statistically reduced apoptosis rate of HEI-OC1 cells, which indicated that t-BHP successfully induced the OS injury of HEI-OC1 cells. Besides, miR-200c in cells was found to be down-regulated, indicating that abnormally expressed miR-200c may be related to OS injury of HEI-OC1 cells.
The results of bioinformatics analysis demonstrated that Taok1 was a potential target of miR-200c-3p, which was subsequently confirmed by double luciferase reporter gene assay. In cancer tissues, Taok1 was able to stimulate the c-Jun N-terminal kinase/mitogen-activated protein (MAP) kinase axis [16]. In human neuroblastoma cells, Taok1 transfection induced apoptosis [17]. While in noise-impaired cochlea, the MAP kinase pathway is related to cochlea apoptosis, and inhibition of this pathway can reduce apoptosis [18,19]. All these observations indicate that Taok1 is involved in regulation of cochlear responses to acoustic injury, possibly by modulating apoptosis. However, the involvement of Taok1 in HEI-OC1 cells is rarely reported. A previous study proved that OS may increase Taok1 expression, and in our study, Taok1 in HEI-OC1 cells was up-regulated after t-BHP treatment.
In subsequent studies, we overexpressed miR-200c-3p in t-BHP-intervened HEI-OC1 cells, and found that miR-200c-3p overexpression significantly inhibited OS injury and apoptosis of HEI-OC1 cells. Meanwhile, it was observed that Bax and Bcl-2, which were pro-apoptotic and anti-apoptotic proteins respectively, showed consistent expression profiling with the changing trend of apoptosis rate. Bax is an essential factor for OS-induced apoptosis, while Bcl-2 shows a negative response to OS [21].
Some miRNAs are thought to regulate OS or apoptosis of HEI-OC1 cells. For example, a study found that miRNA-207 can enhance radiation-induced cochlear hair cell apoptosis by targeting Akt3. Another study [22] found that by regulating Bcl-2, miR-204-5p can influence lipopolysaccharids-induced cochlea cell apoptosis. miR-200c and Taok1 are both involved in regulating apoptosis [23,24]. The present study found an inverse association between Taok1 and miR-200c-3p. In addition, rescue experiments showed that up-regulation of Taok1 expression could offset the inhibitory action of miR-200c-3p overexpression on OS and apoptosis of HEI-OC1 cells. It demonstrates that miR-200c-3p reduces OS response and apoptosis of HEI-OC1 cells by targeting Taok1. Furthermore, it indicates that miR-200c-3p functions as an anti-apoptotic gene and Taok1 acts as a pro-apoptotic factor in t-BHP intervened HEI-OC1 cells.
In summary, miR-200c-3p overexpression inhibits the OS response and apoptosis of t-BHP-treated HEI-OC1 cells, possibly through targeted regulation of Taok1. Therefore, miR-200c-3p may be a potential therapeutic target for cochlear hair cell injury. However, this study also has some limitations. For example, the reverse efficiency of pcDNA3.1-Taok1 on miR-200c-3p mimic in this study is extremely high, which we believed might be due to a certain contingency. Although the reversal effect of PCDNA3.1-TaOK1 on miR-200C-3p mimic is undeniable, further analysis of its specific transfection efficiency is still needed to avoid errors.
Acknowledgements
The Startup Fund for Scientific Research, Fujian Medical University [grant number 2017XQ1141]; the Fujian Provincial Clinical Priority Specialty Construction Project (Fujian Medical Health (2015)) [grant number 593]; the National Natural Science Foundation of China [grant number 81730029]; the National Key Research and Development Program of China [grant number 2016YFC1000700].
Disclosure of conflict of interest
None.
References
- 1.Oh KS, Walls D, Joo SY, Kim JA, Yoo JE, Koh YI, Kim DH, Rim JH, Choi HJ, Kim HY, Yu S, Smith RJ, Choi JY, Gee HY, Jung J. COCH-related autosomal dominant nonsyndromic hearing loss: a phenotype-genotype study. Hum Genet. 2021;9:16. doi: 10.1007/s00439-021-02368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mei H, Zhao L, Li W, Zheng Z, Tang D, Lu X, He Y. Inhibition of ferroptosis protects House Ear Institute-Organ of Corti 1 cells and cochlear hair cells from cisplatin-induced ototoxicity. J Cell Mol Med. 2020;24:12065–12081. doi: 10.1111/jcmm.15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goutman JD, Elgoyhen AB, Gomez-Casati ME. Cochlear hair cells: the sound-sensing machines. FEBS Lett. 2015;589:3354–3361. doi: 10.1016/j.febslet.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Z, Li S, Liu L, Yang Q, Zhang H, Gao C. [Corrigendum] NADPH oxidase 3-associated oxidative stress and caspase 3-dependent apoptosis in the cochleae of D-galactose-induced aged rats. Mol Med Rep. 2016;13:1056. doi: 10.3892/mmr.2015.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SH, Choi CH. Noise-induced neural degeneration and therapeutic effect of antioxidant drugs. J Audiol Otol. 2015;19:111–119. doi: 10.7874/jao.2015.19.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bak MJ, Truong VL, Ko SY, Nguyen XN, Ingkasupart P, Jun M, Shin JY, Jeong WS. Antioxidant and hepatoprotective effects of procyanidins from wild grape (vitis amurensis) seeds in ethanol-induced cells and rats. Int J Mol Sci. 2016;17:758. doi: 10.3390/ijms17050758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YM, Li XD, Guo X, Liu B, Lin AH, Rao SQ. Association between polymorphisms in SOD1 and noise-induced hearing loss in Chinese workers. Acta Otolaryngol. 2010;130:477–486. doi: 10.3109/00016480903253587. [DOI] [PubMed] [Google Scholar]
- 8.Cheng X, Wang Z, Yang J, Ma M, Lu T, Xu G, Liu X. Acidic fibroblast growth factor delivered intranasally induces neurogenesis and angiogenesis in rats after ischemic stroke. Neurol Res. 2011;33:675–680. doi: 10.1179/1743132810Y.0000000004. [DOI] [PubMed] [Google Scholar]
- 9.Patel M, Cai Q, Ding D, Salvi R, Hu Z, Hu BH. The miR-183/Taok1 target pair is implicated in cochlear responses to acoustic trauma. PLoS One. 2013;8:e58471. doi: 10.1371/journal.pone.0058471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La JH, Gebhart GF. Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2011;301:G165–174. doi: 10.1152/ajpgi.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulivi P, Foschi G, Mengozzi M, Scarpi E, Silvestrini R, Amadori D, Zoli W. Peripheral blood miR-328 expression as a potential biomarker for the early diagnosis of NSCLC. Int J Mol Sci. 2013;14:10332–10342. doi: 10.3390/ijms140510332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Q, Fang Y, Lu F, Pan J, Wang L, Gong W, Fei F, Cui J, Zhong J, Hu R, Liang M, Fang L, Wang H, Yu M, Zhang ZF. Analysis of differential expression profile of miRNA in peripheral blood of patients with lung cancer. J Clin Lab Anal. 2019;33:e23003. doi: 10.1002/jcla.23003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Liu Y, Han N, Chen X, Yu W, Zhang W, Zou F. Profiles of oxidative stress-related microRNA and mRNA expression in auditory cells. Brain Res. 2010;1346:14–25. doi: 10.1016/j.brainres.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 14.Feng X, Wang Z, Fillmore R, Xi Y. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344:166–173. doi: 10.1016/j.canlet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romano GL, Platania CB, Forte S, Salomone S, Drago F, Bucolo C. MicroRNA target prediction in glaucoma. Prog Brain Res. 2015;220:217–240. doi: 10.1016/bs.pbr.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Wojtala RL, Tavares IA, Morton PE, Valderrama F, Thomas NS, Morris JD. Prostate-derived sterile 20-like kinases (PSKs/TAOKs) are activated in mitosis and contribute to mitotic cell rounding and spindle positioning. J Biol Chem. 2011;286:30161–30170. doi: 10.1074/jbc.M111.228320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu MF, Wang SG. Human TAO kinase 1 induces apoptosis in SH-SY5Y cells. Cell Biol Int. 2008;32:151–156. doi: 10.1016/j.cellbi.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan PX, Du SS, Ren C, Yao QW, Zheng R, Li R, Yuan YW. MicroRNA-207 enhances radiation-induced apoptosis by directly targeting Akt3 in cochlea hair cells. Cell Death Dis. 2014;5:e1433. doi: 10.1038/cddis.2014.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Yang X, Ge X, Zhang F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed Pharmacother. 2019;109:726–733. doi: 10.1016/j.biopha.2018.10.161. [DOI] [PubMed] [Google Scholar]
- 22.Xie L, Zhou Q, Chen X, Du X, Liu Z, Fei B, Hou J, Dai Y, She W. Elucidation of the Hdac2/Sp1/miR-204-5p/Bcl-2 axis as a modulator of cochlear apoptosis via in vivo/in vitro models of acute hearing loss. Mol Ther Nucleic Acids. 2021;23:1093–1109. doi: 10.1016/j.omtn.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan C, Xu M, Rong R, Mei Y, Cai W, Li L, Xue Y, Zhu B, Sun K, Han L. miR-200c regulates endothelin-1 induced PASMCs abnormal proliferation and apoptosis. IUBMB Life. 2017;69:877–886. doi: 10.1002/iub.1686. [DOI] [PubMed] [Google Scholar]
- 24.Dulovic-Mahlow M, Trinh J, Kandaswamy KK, Braathen GJ, Di Donato N, Rahikkala E, Beblo S, Werber M, Krajka V, Busk OL, Baumann H, Al-Sannaa NA, Hinrichs F, Affan R, Navot N, Al Balwi MA, Oprea G, Holla OL, Weiss MER, Jamra RA, Kahlert AK, Kishore S, Tveten K, Vos M, Rolfs A, Lohmann K. De Novo variants in TAOK1 cause neurodevelopmental disorders. Am J Hum Genet. 2019;105:213–220. doi: 10.1016/j.ajhg.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]