Abstract
Reovirus is a ubiquitous, non-pathogenic, double stranded RNA virus with anti-tumor properties. The virus’s replicative potential is regulated by phosphorylation of protein kinase receptor (PKR). In cancers with RAS pathway activation which leads to dysregulation of PKR, the virus maintains its protein translational potential and induces oncolysis. Systemic chemotherapy remains the standard of care for metastatic colorectal cancer with the addition of biologic agents in KRAS wildtype subtypes. In KRAS mutant colorectal cancers, there has been no added benefit to biologic agents. The therapeutic potential of reovirus (Reolysin®, pelareorep, Oncolytic Inc., Calgary, Canada), which induces its oncolysis with RAS activation through multimodal immune mechanisms, has been demonstrated in preclinical and clinical studies. In this review, we outline the specific immune mechanisms of reovirus induced oncolysis and provide both preclinical and clinical data on its applications in metastatic colorectal cancer patients.
Keywords: Reovirus, KRAS, colorectal cancer, immune profile, lymphocytes
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the U.S. with 149,500 estimated new cases and 52,980 estimated deaths in 2021 [1]. Approximately 50%-60% of patients develop metastatic disease [2,3]. While surgical resection is widely accepted as potential curative therapy for isolated liver and lung metastases, systemic chemotherapy remains the standard of care for patients with unresectable disease [3-7]. Treatment options for systemic therapy include FOLFOX, FOLFIRI, FOLFOXIRI, and CAPEOX, with addition of biologics depending on the mutational spectrum, which has become an integral part of directed or target therapy [8-15]. In patients with RAS wildtype metastatic CRC, EGFR inhibitors, panitumumab and cetuximab have demonstrated clinical benefit based on TAILOR, CALGB/SWOG 80405, and PRIME trials [16,17,22]. Approximately 45% of colorectal cancers are characterized, by mutations in KRAS and these patients are resistant to EGFR inhibitors [18-22]. In mutant KRAS, the addition of panitumumab to chemotherapy was associated with a reduced progression free survival (PFS) compared to chemotherapy alone (HR 1.29, P=0.02), with also a trend for decreased overall survival (OS) (HR 1.24, P=0.068) [20]. Treatment for such patients remains a challenge.
Respiratory Enteric Orphan virus (REOvirus) is a non-enveloped, widely prevalent, double-stranded RNA virus with benign pathologic implications in humans that has demonstrated anti-tumor activity through oncolysis [23-25]. The mechanism of its anti-tumor activity is multifactorial involving both the innate and adaptive immune systems [23]. The virus selectively replicates in RAS mutated cells through the regulation of protein kinase receptor (PKR). In normal cells with intact RAS pathway, PKR is phosphorylated leading to inhibition of viral protein translation, but in RAS mutant cells, the phosphorylation is inhibited, allowing viral translation and replication [26]. Due to its benign pathologic implications and its selective replication in RAS mutant cells, reovirus has been studied as a potential therapeutic agent in RAS mutated malignancies. In this review, we highlight the mechanism of oncolysis of reovirus, with an emphasis on its multimodal immune pathways and outline preclinical and clinical applications of reovirus in KRAS activated metastatic colorectal cancer.
Immune mechanism of reovirus
The immune system is composed of a complex network of antigen presenting cells and effector cells that work in conjunction to protect the host against tumor antigens. Reovirus exhibits anti-tumor properties through the activation of innate and adaptive host immune systems through dendritic cells, natural killer (NK) cells, and effector T-cells which all play an active role in tumor control (Figure 1) [25,27].
Figure 1.
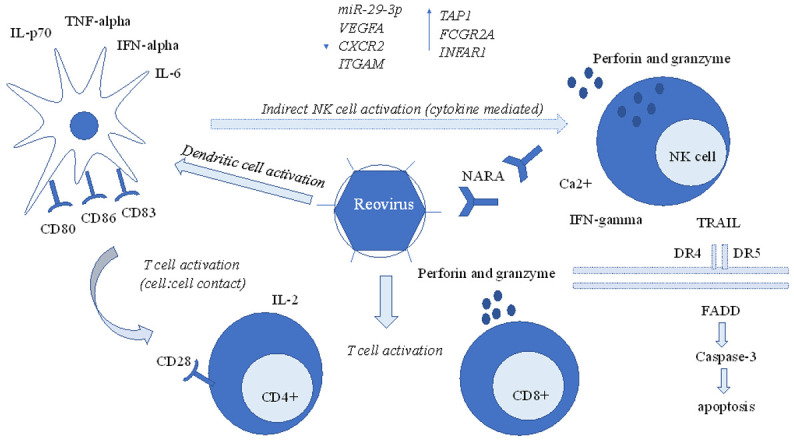
Multimodal immune characteristics of oncolysis by reovirus. Reovirus induces anti-tumor properties through multi-cellular pathways, which include activation and maturation of dendritic cells, which induce pro-inflammatory cytokines such as IL-6, IL-p70, and IFN-alpha, cytokine mediated indirect activation of NK cells through dendritic cells, that lead to perforin and granzyme mediated apoptosis, TRAIL, and direct activation of T-cells. In addition to cell-mediated apoptosis, reovirus has also demonstrated to activate the immune system at a transcriptome level, through downregulation of miR-29-3p, VEGFA, CXCR2, ITGAM, and upregulation of TAP1, FCGR2A, INFAR1.
Reovirus mediated dendritic cell activation
Antigen presenting dendritic cells have been directly and indirectly involved in reovirus mediated oncolysis. When dendritic cells from healthy individuals were incubated with reovirus, an upregulation of CD-80, CD-86, CD-83, and TAP-1, phenotypic markers of dendritic cell activation and maturation was observed, in addition to an increase in secretion of proinflammatory cytokines, TNF-alpha, IL-6, IL-p70, and INF-alpha, as well as functional markers of dendritic cell activation [25]. This dual phenotypic and functional activation of dendritic cells was also demonstrated in cell lines derived from patients with metastatic colorectal cancer, supporting the virus’s modulation of antigen presenting cells in preclinical studies [25].
Dendritic cells have also been observed to play an indirect role in activation of NK cells, a crucial part of innate and adaptive immunity, through reovirus mediated oncolysis. Rather than direct activation of NK cells by reovirus, NK cells were found to be dependent on virus infected cells for activation. When NK cells were incubated with reovirus alone, NK cell activity was not observed, but when incubated with reovirus-infected dendritic cells, an upregulation of interferon-gamma, a marker of NK cell activity, was observed [25]. Dendritic cells communicated with NK cells indirectly through production of pro-inflammatory cytokines, including IFN-alpha, TNF-alpha, IL-15, and IL-12, rather than direct cell to cell contact since even when reovirus induced NK cells were separated by a transwell, there was continued NK cell activation [25]. It is proposed that NK cells exhibited their cytotoxicity through exocytosis of perforin/granzyme since in the presence of chelating agent like egtazic acid (EGTA), the cytotoxic effect was found considerably reduced indicating a crucial role of Ca2+, an important player in perforin/granzyme mediated cytotoxicity [25].
Reovirus also exhibits its cytotoxicity through apoptotic pathways, notably through the production of proinflammatory cytokines such as tumor necrosis factor (TNF)-associated death-inducing ligand (TRAIL), which induces apoptosis through oligomerization of death receptors 4 and 5, that leads to Fas-associated death domain complex formation, which in turn activates caspase-3, leading to cell death [28]. The virus can also directly induce translocation of Smac (second mitochondria-derived activator of caspases), a mitochondrial protein encoded by the DIABLO gene, which activates Bcl-2 (B-cell lymphoma 2) and induces apoptosis [28].
Early phase clinical studies in metastatic colorectal patients have supported preclinical observations on reovirus mediated dendritic cell activation. In a phase I dose escalation trial of 30 patients with oxaliplatin refractory metastatic colorectal cancer treated with FOLFIRI/bevacizumab and reovirus, rapid maturation of dendritic cells (4.5% to 18.6% at 48 hours) was demonstrated among the 5 patients who have been studied in detail regarding their immune response [29].
Reovirus mediated T-cell cytotoxicity
In addition to dendritic cell activation, maturation, and NK cell activation, reovirus has also demonstrated anti-tumor activity through T-cell immunity. In preclinical studies, reovirus induced dendritic cells incubated with T-cells resulted in increased interferon-gamma and IL-2 production, a marker of T-cell activation, and T-cell cytotoxicity was demonstrated regardless of target cell HLA status [25]. Unlike NK cell activation, which did not require cell to cell contact and exhibited communication through proinflammatory cytokines, T-cell activation through reovirus required cell to cell contact through antigen presentation and specific receptor interaction, which suggests that NK cells and T-cells may exhibit differences in tumor microenvironment ideal for cytotoxicity. Like NK cells, cytotoxicity of T-cells was mediated by exocytosis of perforin/granzyme and activation of T-cells occurred in multiple subtypes of T-cells, including CD4+ and CD8+, rather than a subclonal T-cell population [25].
Early phase clinical trials have supported preclinical observations of reovirus mediated T-cell anti-tumor properties. In a phase I dose escalation trial of 21 heavily treated advanced stage cancer patients exposed to reovirus, an increase in CD3+CD4+ T-cells were observed in 10 (47.6%) patients and 5 patients demonstrated an increase in CD8+ perforin/granzyme+ T-cells [30]. An upregulation of T-cell related cytokine expression, including IL-5, IL-8, IL-6, IL-2, and IL-12p40, was observed in all 21 patients [30].
Reovirus and molecular implications
In addition to the cellular level, reovirus may also activate the immune system at the transcriptome level. In a trial of metastatic CRC patients, a reduction in miR-29-3p, which normally functions to express proteins that suppress IFN-gamma, was observed with an increase in IFN-gamma at 72 hours [31]. A change in several other transcription factors were also demonstrated, including an increase in TAP1, which expresses a protein essential for MHC Class I expression, FCGR2A, which encodes a protein for antibody binding, and INFAR1, which encodes a receptor essential for Type I interferon binding [31]. A reduction in VEGFA and CXCR2, which promote tumorigenic effects, and ITGAM, which express an integrin that promotes metastases were also observed, suggesting that reovirus induced anti-tumor properties involve not only the immune system at the cellular level, but also at the more intricate molecular level [31].
Reovirus and NARA
Reovirus has demonstrated to play an additional role in the adaptive immune system through stimulation of neutralizing anti-reoviral antibodies (NARA), present normally in healthy individuals [32]. In a phase I trial of intravenous reovirus, 22 of 31 heavily pretreated patients with advanced cancers demonstrated an initial NARA titer of >1/100, and post reovirus treatment led to significant increase in NARA to >1/100,000, demonstrating a neutralizing antibody response [30]. Regardless of the NARA response, oncolysis has been observed, suggesting that oncolysis is not impeded with NARA and studies have incorporated immunosuppression or chemotherapy to modulate NARA [30]. For example, gemcitabine induction has demonstrated to blunt the NARA response, which may potentially exacerbate chemotherapy or reoviral adverse effects or increase its therapeutic potential [23,33]. In combined chemotherapy trials with docetaxel and carboplatin-paclitaxel, however, NARA was not blunted and an increase in toxicity was not observed [34,35]. While a blunted oncolytic response may be observed with NARA, it has been hypothesized that reovirus may possibly minimize neutralization and be directed at metastatic colorectal cells by being carried within peripheral blood mononuclear cells with prolonged infectivity as up to 10 days from viral induction [36].
Regulation of reovirus through PKR
Oncolysis of reovirus is rather selective and its oncolytic potential is mediated and regulated by activated protein kinase R (PKR) [28]. When reovirus initially affects cells, it is recognized by multiple cellular pattern receptors, such as toll-like receptors, which activate multiple transcription factors including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and activator protein-1 (AP-1) that increase interferon-1 (Figure 2) [28,37]. This increase in interferon-1 upregulates PKR and direct binding of reovirus dsRNA to PKR, leading to dimerization and autophosphorylation of PKR, resulting in phosphorylation of eukaryotic initiation factor-2α (eIF2α), which in its active state, promotes protein synthesis [28]. The phosphorylation of eIF2α leads to a higher affinity and stable complex with eIF2B, which in turn suppresses further viral transcription, resulting in a self-limiting process [28]. In KRAS mutant cells, however, the initial phosphorylation of PKR is inhibited, leading to a disruption of the entire process, facilitating viral transcription and replication to continue unabated [26,38].
Figure 2.
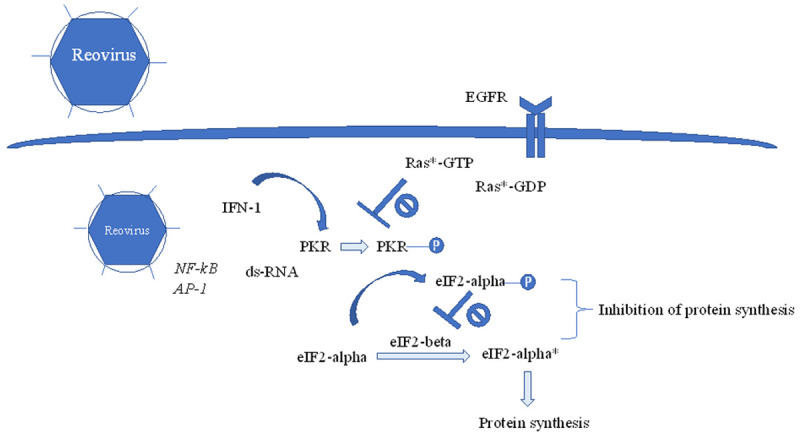
Reovirus-mediated oncolysis is negatively regulated by PKR. Upon reovirus infection, transcription factors such as NF-κB and AP-1 are activated, which leads to increase in interferon-1. Increase in interferon-1 leads to upregulation and autophosphorylation of PKR, which phosphorylates eIF2-alpha. Phosphorylated eIF2-alpha binds to eiF2-beta with high affinity, which in its free form, converts eiF2-alpha from its inactive to active state, leading to protein translation. eiF2-beta, when bound to phosphorylated eIF2-alpha, prevents recycling of active eiF2-alpha as a substrate, thereby blocking protein synthesis. In RAS-mutant cells, phosphorylation of PKR is inhibited, leading to uninhibited protein synthesis and reovirus replication.
Reovirus in clinical application
Reovirus and chemotherapy
Reovirus as monotherapy has demonstrated oncolytic potential in studies in a variety of cancers, including breast, ovarian, glioma, prostate, carcinoid, melanoma, head and neck cancer, leiomyosarcoma, and non-HIV associated Kaposi’s sarcoma, and its efficacy in combination with chemotherapy and radiotherapy has been demonstrated (Table 1) [39-43]. The first chemotherapy combination trial studied the safety and tolerability of reovirus with gemcitabine in 16 heavily pretreated cancer patients, including non-small cell lung cancer, colorectal cancer, breast cancer, cervical cancer, cholangiocarcinoma, esophageal adenocarcinoma, and poorly differentiated carcinoma [33]. Of the 10 who had an evaluable response, 1 achieved partial response, 6 stable disease, with a median duration of stable disease to be 72 days [33]. The combination of reovirus and gemcitabine was well tolerated, with most common adverse effects fever, nausea, diarrhea, and vomiting [33]. A reversible rise in liver enzymes was observed, though most also had concomitant acetaminophen use, possibly suggesting an increased possibility of gemcitabine associated liver toxicity with addition of reovirus at high titers [33].
Table 1.
Clinical trials combining chemotherapy with reovirus and immune parameters
| PMID | Cancers | Phase | Reovirus Dose | Anti-cancer agent | Results | Immune parameters |
|---|---|---|---|---|---|---|
| 32156785 | Refractory metastatic colorectal cancer | I | 1×1010 TCID50-3×1010 TCID50 | FOLFIRI-bevacizumab + reovirus | PR: 3/6 (50%) | Dendritic cell maturation: 48 hours, 4.5%-18.6% (P=000016) |
| Median PFS: 65.6 weeks | CD4-count increase: 3.5-fold (P=0.00015) | |||||
| Median OS: 25.1 months | CD8-count increase: 2.4-fold (P=0.00015) | |||||
| 18323793 | Advanced solid cancers | I | 1×108 TCID50-3×1010 TCID50 | None | N/A | CD4+: 10/21 (47.6%) |
| CD8+: 7/21 (33%) | ||||||
| NK: 6/21 (28.6%) | ||||||
| Increase in IL-5, IL-8, IL-6, IL-2, IL-12p40 | ||||||
| NARA: increase in 250-fold | ||||||
| 29653857 | Metastatic colorectal cancer, 1st line | II | 3×1010 TCID50 | FOLFOX-6-bevacizumab ± reovirus | Median PFS: 7 vs. 9 months, HR 1.59 (P=0.046) | N/A |
| Median OS: HR 1.22; (P=0.38) | ||||||
| ORR: 2.52 (P=0.03) | ||||||
| 20926400 | Advanced solid cancers | I | 3×1010 TCID50 | Docetaxel + reovirus | ORR: 14/16 | NARA: increase in 729-fold at peak |
| PR: 4/16 in breast, stomach, gastroesophageal, ocular melanoma | ||||||
| Minor response: 3/16 in mesothelioma, prostate, head and neck | ||||||
| SD: 7/16 in prostate, melanoma, esophagus, pancreas, unknown | ||||||
| 26709987 | KRAS or EGFR mutant metastatic NSCLC | II | 3×1010 TCID50 | Carboplatin, paclitaxel + reovirus | PR: 11/37 | N/A |
| SD: 20/37 | ||||||
| PD: 4/37 | ||||||
| Not evaluable: 2/37 | ||||||
| Median PFS: 4 months | ||||||
| Median OS: 13.1 months | ||||||
| 27039845 | Metastatic pancreatic adenocarcinoma | II | 3×1010 TCID50 | Carboplatin, paclitaxel ± reovirus | PFS: 4.9 vs. 5.2 (P=0.6) | Reovirus arm with increased pro-inflammatory markers, including fractalkine, IL-10, RANTES, SDF-1, VEGF-A |
| Reovirus arm with increase in CD8+ expressing CD71, CD95, CD45RO | ||||||
| 22316603 | Relapsed/metastatic Head and Neck Cancer | I | 3×1010 TCID50 | Carboplatin, paclitaxel + reovirus | CR: 1/31 (3.8%) | NARA: increase by 27-729-fold |
| PR: 6/31 (23.1%) | ||||||
| SD: 9/31 (34.6%) | ||||||
| PD: 8/31 (30.8%) | ||||||
| Duration of response: 6 months | ||||||
| Median OS: 7.1 months | ||||||
| 29748010 | NSCLC | II | 4.5×1010 TCID50 | Pemetrexed or docetaxel ± reovirus | PFS: 3.0 vs. 2.8 months (P=0.53) | N/A |
| STK11 (HR 0.29) and PIK3CA (HR 0.45) mutations with improved PFS | ||||||
| 29799479 | Advanced pancreatic adenocarcinoma, 1st line | II | 1×1010 TCID50 | Gemcitabine + reovirus | PR: 1/34 | Upregulation of PD-L1 on immunohistochemistry |
| SD: 23/34 | ||||||
| PD: 5/34 | ||||||
| Median OS: 10.2 months | ||||||
| 29027598 | Metastatic breast cancer | II | 3×1010 TCID50 | Paclitaxel ± reovirus | PFS: 3.78 months vs. 3.38 months (P=0.87) | N/A |
| OS: 17.4 months vs. 10.4 months (P=0.1) | ||||||
| 31694832 | Advanced pancreatic adenocarcinoma | Ib | 4.5×1010 TCID50 | Reovirus and pembrolizumab + 5-fluorouracil, gemcitabine, or irinotecan | Disease control in 3 of 10 patients | Creation of new T-cell clones observed |
| PR: 1/10 for 17.4 months | ||||||
| SD: 2/10 for 9 months and 4 months |
PR: partial response, SD: stable disease, PFS: progression free survival, OS: overall survival, ORR: overall response rate, NARA: neutralizing anti-reoviral antibodies, NSCLC: non-small cell lung cancer.
Reovirus has previously exhibited cytotoxic effects synergistically with irinotecan in colorectal cancer cell lines [24]. In a phase I dose escalation trial of FOLFIRI with reolysin in KRAS-mutant metastatic colorectal cancer patients with secondary endpoint of response rate, PFS, and OS, 1 patient achieved partial response, 9 patients stable disease, and 8 patients progression, with median PFS of 7.4 months in FOLFIRI-naïve patients, and not-reached in non-FOLFIRI-naïve patients [44]. Activity of reolysin in KRAS-mutant metastatic colorectal patients was also demonstrated with addition of bevacizumab to irinotecan. In a phase I dose escalation trial of FOLFIRI/bevacizumab with pelareorep in 36 oxaliplatin refractory KRAS-mutated metastatic colorectal cancer patients, 20% of patients achieved partial response and 73.3% achieved stable disease [29]. As demonstrated in preclinical studies, an increase in absolute CD-8 count (2.4-fold at 7 days) and absolute CD-4 count (3.5-fold) were observed, demonstrating the activation of T-cells as mechanism of anti-tumor properties of oncolysis in the clinical setting [29]. The addition of reovirus was safely tolerated and resulted in median progression-free survival (PFS) of 65.6 weeks and overall survival (OS) of 25.1 months at the recommended phase II dose [29]. In a prior phase II randomized trial, mixed results were observed, however, between the two cohorts of reovirus and FOLFOLX/bevacizumab versus FOLFOX/bevacizumab alone, where in KRAS-mutated patients, inferior PFS, but a trend towards superior overall response rate (ORR) was observed in the combination arm [45]. This mixed finding may suggest that reovirus may have a synergistic effect with irinotecan, but not with oxaliplatin and it is also possible that reovirus may function as an immune stimulating agent and thus may need time to demonstrate an anti-cancer effect, yielding to early cancer progression. Studies are needed to better evaluate the benefit of reovirus with combination of chemotherapy and to evaluate differential responses based on chemotherapy backbone of irinotecan vs. oxaliplatin.
Reovirus and immunotherapy
Palareorep has also been studied in combination with PD-1 (programmed cell death) checkpoint inhibitors in pre-clinical models and early phase clinical trials. PD-1 receptors are present on B, T cells, and monocytes and inhibit T-cell activation, and tumor cells have adapted to express PD-L1 receptors on their surface to escape immune surveillance [46]. Oncolytic viruses have been demonstrated to work synergistically with checkpoint inhibitors in enhancing immune-mediated tumor cell clearance [47]. In microsatellite stable (MSS) colorectal cancer cell lines, SW620 and HT29, the combined nivolumab and reovirus treated increased cell death compared to nivolumab or reovirus alone [48]. In KRAS-mutant CT26 mouse model, combined anti-PD1 and reovirus treatment significantly suppressed tumor growth and increased overall survival compared to treatment with reovirus or anti-PD-1 alone [48]. The mechanism of increased tumorigenicity in combined reovirus and anti-PD-1 treatment was suggested to be decreased nuclear expression of proliferation marker Ki67, increased expression of apoptosis markers, including cleaved-caspase 3 and TUNEL [48]. In addition to increased apoptotic markers, reovirus was observed to enhance immunogenicity through its impact on the PD-L1/PD-1 axis. CT26 mouse models treated with combined reovirus and anti-PD-1 demonstrated enhanced reduction of PD-1 compared to anti-PD-1 alone [48]. Tumor infiltrating lymphocytes, specifically CD4+ and CD8+ cells, which play a role in recognition and killing of tumor cells, and granzyme B expression was also observed to be increased in the combination treatment than either treatment alone [48]. An increase in interferon-gamma and TNF-alpha, inflammatory markers of increased cytotoxicity in CD4+, CD8+, and NK-cells, a decrease in TGF-beta, an inhibitor cytokine, and decrease in TOX, a marker of T-cell exhaustion, were observed with combined anti-PD-1 and reovirus treatment, which was not observed with monotherapy [48].
In addition to activation of the adaptive immune system, combination of anti-PD-1 and reovirus was demonstrated to synergistically activate the innate immune system through increase in pattern recognition receptors, including retinoic acid-inducible gene I (RIG-1), melanoma differentiation-associated protein 5 (MDA-5), protein kinase R (PKR), and NOD-like receptor protein 3 (NLRP3) [48].
While there is no published clinical trial combining reovirus with anti-PD1 therapy, palareorep in combination with chemo-immunotherapy has been studied in alternative gastrointestinal-related malignancies. In an open-label phase Ib study, palareorep and pembrolizumab in combination with gemcitabine, irinotecan, or 5-FU, were studied in 11 advanced pancreatic adenocarcinoma who progressed on first-line treatment, until disease progression or unacceptable toxicity [49]. Of the 10 evaluable patients, one patient achieved partial response of 17.4 months and 2 achieved stable disease of 9 months and 4 months without significant toxicity [49]. Peripheral blood samples of most patients demonstrated decreased T-cell clonality, but increased T-cell diversity, with increase in T-cell population turnover as well as statistically significant increases in CXCL10 and CXCL11, which attract CD8+ cells, without significant changes in cytokines attracting Treg [49]. It is hypothesized that the addition of checkpoint inhibitor would demonstrate a positive outcome in metastatic colorectal cancer patients based on preclinical trials. However support through clinical trials are not yet established, especially since prior trial with pancreatic adenocarcinoma comprised a substantial number of patients (6 of 11) who received gemcitabine but only 2 of 11 patients received irinotecan, which serves as a backbone in colorectal cancer [49]. Current multiple early phase clinical trials, namely NCT03206073, NCT03866525, and NCT04301011, are underway to evaluate the outcome of combined oncolytic viral therapy and checkpoint inhibitors in refractory colorectal cancer.
Conclusion
Reovirus is a multipotent immune agent with oncolytic properties that has demonstrated clinical efficacy in early phase trials in metastatic KRAS mutant colorectal cancer. Its mechanisms of anti-tumor action involve direct dendritic cell activation, by production of proinflammatory cytokines, dendritic cell mediated NK cell activation, which lead to exocytosis of perforin/granzyme and Fas-associated death domain, and activation of T-cells. Early phase clinical trials have demonstrated in prior chemotherapy refractory metastatic colorectal patients the safety, tolerability, and relatively robust response to the addition of reovirus to FOLFIRI. Further research with randomized trials is needed for to evaluate the efficacy of reovirus in treatment refractory metastatic colorectal cancer patients.
Acknowledgements
We also gratefully acknowledge Montefiore Medical Center’s oncology fellowship program.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer. 2006;6:202–207. doi: 10.3816/CCC.2006.n.036. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P European Colorectal Metastases Treatment Group. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, Cho CH, Ko HK, Lee JT, Kim NK. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009;197:728–736. doi: 10.1016/j.amjsurg.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Lee WS, Yun SH, Chun HK, Lee WY, Kim SJ, Choi SH, Heo JS, Joh JW, Choi D, Kim SH, Rhim H, Lim HK. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol. 2008;42:945–949. doi: 10.1097/MCG.0b013e318064e752. [DOI] [PubMed] [Google Scholar]
- 6.Onaitis MW, Petersen RP, Haney JC, Saltz L, Park B, Flores R, Rizk N, Bains MS, Dycoco J, D’Amico TA, Harpole DH, Kemeny N, Rusch VW, Downey R. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg. 2009;87:1684–1688. doi: 10.1016/j.athoracsur.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Wiegering A, Riegel J, Wagner J, Kunzmann V, Baur J, Walles T, Dietz U, Loeb S, Germer CT, Steger U, Klein I. The impact of pulmonary metastasectomy in patients with previously resected colorectal cancer liver metastases. PLoS One. 2017;12:e0173933. doi: 10.1371/journal.pone.0173933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maindrault-Goebel F, Louvet C, André T, Carola E, Lotz JP, Molitor JL, Garcia ML, Gilles-Amar V, Izrael V, Krulik M, de Gramont A. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer. 1999;35:1338–1342. doi: 10.1016/s0959-8049(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 9.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J. Clin. Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 10.André T, Louvet C, Maindrault-Goebel F, Couteau C, Mabro M, Lotz JP, Gilles-Amar V, Krulik M, Carola E, Izrael V, de Gramont A. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur J Cancer. 1999;35:1343–1347. doi: 10.1016/s0959-8049(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 11.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 12.Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, Athanasiadis A, Kakolyris S, Tsousis S, Kouroussis C, Vamvakas L, Kalykaki A, Samonis G, Mavroudis D, Georgoulias V. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs. FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br J Cancer. 2006;94:798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 14.Porschen R, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, Kretzschmar A, Graeven U, Grothey A, Hinke A, Schmiegel W, Schmoll HJ AIO Colorectal Study Group. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J. Clin. Oncol. 2007;25:4217–4223. doi: 10.1200/JCO.2006.09.2684. [DOI] [PubMed] [Google Scholar]
- 15.Kirstein MM, Lange A, Prenzler A, Manns MP, Kubicka S, Vogel A. Targeted therapies in metastatic colorectal cancer: a systematic review and assessment of currently available data. Oncologist. 2014;19:1156–1168. doi: 10.1634/theoncologist.2014-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, Li W, Xu N, Lin LZ, Wu Q, Li Y, Yang J, Pan H, Ouyang X, Qiu W, Wu K, Xiong J, Dai G, Liang H, Hu C, Zhang J, Tao M, Yao Q, Wang J, Chen J, Eggleton SP, Liu T. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J. Clin. Oncol. 2018;36:3031–3039. doi: 10.1200/JCO.2018.78.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Mahoney MR, O’Neil BH, Shaw JE, Polite BN, Hochster HS, Atkins JN, Goldberg RM, Mayer RJ, Schilsky RL, Bertagnolli MM, Blanke CD Cancer and Leukemia Group B (Alliance), SWOG, and ECOG. J. Clin. Oncol. 2014;32:18_suppl, LBA3–LBA3. [Google Scholar]
- 18.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 20.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 21.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 22.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Tian Y, Xu F, Sidhu R. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 23.Maitra R, Ghalib MH, Goel S. Reovirus: a targeted therapeutic--progress and potential. Mol Cancer Res. 2012;10:1514–1525. doi: 10.1158/1541-7786.MCR-12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maitra R, Seetharam R, Tesfa L, Augustine TA, Klampfer L, Coffey MC, Mariadason JM, Goel S. Oncolytic reovirus preferentially induces apoptosis in KRAS mutant colorectal cancer cells, and synergizes with irinotecan. Oncotarget. 2014;5:2807–2819. doi: 10.18632/oncotarget.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L, de Bono J, Selby P, Coffey M, Vile R, Melcher A. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 26.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gujar SA, Marcato P, Pan D, Lee PW. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. Mol Cancer Ther. 2010;9:2924–2933. doi: 10.1158/1535-7163.MCT-10-0590. [DOI] [PubMed] [Google Scholar]
- 28.Phillips MB, Stuart JD, Rodríguez Stewart RM, Berry JT, Mainou BA, Boehme KW. Current understanding of reovirus oncolysis mechanisms. Oncolytic Virother. 2018;7:53–63. doi: 10.2147/OV.S143808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel S, Ocean AJ, Parakrama RY, Ghalib MH, Chaudhary I, Shah U, Viswanathan S, Kharkwal H, Coffey M, Maitra R. Elucidation of pelareorep pharmacodynamics in a phase I trial in patients with KRAS-mutated colorectal cancer. Mol Cancer Ther. 2020;19:1148–1156. doi: 10.1158/1535-7163.MCT-19-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, Morgan R, Merrick A, Errington F, Vile RG, Melcher AA, Pandha HS, Harrington KJ. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 31.Parakrama R, Fogel E, Chandy C, Augustine T, Coffey M, Tesfa L, Goel S, Maitra R. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer. 2020;20:569. doi: 10.1186/s12885-020-07038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai JH, Williams JV, Edwards KM, Wright PF, Crowe JE Jr, Dermody TS. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J Infect Dis. 2005;191:1221–1224. doi: 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V, Twigger K, Coffey M, Mettinger K, Gill G, Evans TR, de Bono JS. A phase I study of the combination of intravenous reovirus type 3 dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2011;17:581–588. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 34.Heinemann L, Simpson GR, Boxall A, Kottke T, Relph KL, Vile R, Melcher A, Prestwich R, Harrington KJ, Morgan R, Pandha HS. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer. 2011;11:221. doi: 10.1186/1471-2407-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, Newbold K, Gore ME, Larkin J, Syrigos KN, Coffey M, Thompson B, Mettinger K, Vile RG, Pandha HS, Hall GD, Melcher AA, Chester J, Harrington KJ. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–2089. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, Beirne D, West EJ, Jennings VA, Rose A, Kyula J, Fraser S, Dave R, Anthoney DA, Merrick A, Prestwich R, Aldouri A, Donnelly O, Pandha H, Coffey M, Selby P, Vile R, Toogood G, Harrington K, Melcher AA. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra177. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maitra R, Augustine T, Dayan Y, Chandy C, Coffey M, Goel S. Toll like receptor 3 as an immunotherapeutic target for KRAS mutated colorectal cancer. Oncotarget. 2017;8:35138–35153. doi: 10.18632/oncotarget.16812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shmulevitz M, Marcato P, Lee PW. Unshackling the links between reovirus oncolysis, ras signaling, translational control and cancer. Oncogene. 2005;24:7720–7728. doi: 10.1038/sj.onc.1209041. [DOI] [PubMed] [Google Scholar]
- 39.Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D, Cairncross G, Matthews MV, Markert J, Gillespie Y, Coffey M, Thompson B, Hamilton M. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 40.Clements D, Helson E, Gujar SA, Lee PW. Reovirus in cancer therapy: an evidence-based review. Oncolytic Virother. 2014;3:69–82. doi: 10.2147/OV.S51321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thirukkumaran CM, Nodwell MJ, Hirasawa K, Shi ZQ, Diaz R, Luider J, Johnston RN, Forsyth PA, Magliocco AM, Lee P, Nishikawa S, Donnelly B, Coffey M, Trpkov K, Fonseca K, Spurrell J, Morris DG. Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. Cancer Res. 2010;70:2435–2444. doi: 10.1158/0008-5472.CAN-09-2408. [DOI] [PubMed] [Google Scholar]
- 42.Morris DG, Feng X, DiFrancesco LM, Fonseca K, Forsyth PA, Paterson AH, Coffey MC, Thompson B. REO-001: a phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest New Drugs. 2013;31:696–706. doi: 10.1007/s10637-012-9865-z. [DOI] [PubMed] [Google Scholar]
- 43.Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, Coffey M, Gill GM, Mettinger K, Mariadason JM, Mani S, Goel S. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641–649. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ocean AJ, Bekaii-Saab TS, Chaudhary I, Palmer R, Christos PJ, Mercado A, Florendo EO, Rosales VA, Ruggiero JT, Popa EC, Wilson M, Ghalib MH, Hou Y, Shah U, Rajdev L, Elrafei T, Gill GM, Coffey MC, Shah MA, Goel S. A multicenter phase I study of intravenous administration of reolysin in combination with irinotecan/fluorouracil/leucovorin (FOLFIRI) in patients (pts) with oxaliplatin-refractory/intolerant KRAS-mutant metastatic colorectal cancer (mCRC) J. Clin. Oncol. 2013;31:450. [Google Scholar]
- 45.Jonker DJ, Tang PA, Kennecke H, Welch SA, Cripps MC, Asmis T, Chalchal H, Tomiak A, Lim H, Ko YJ, Chen EX, Alcindor T, Goffin JR, Korpanty GJ, Feilotter H, Tsao MS, Theis A, Tu D, Seymour L. A randomized phase II study of FOLFOX6/bevacizumab with or without pelareorep in patients with metastatic colorectal cancer: IND. 210, a Canadian Cancer Trials Group Trial. Clin Colorectal Cancer. 2018;17:231–239. e237. doi: 10.1016/j.clcc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 47.Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L, Shim KG, Diaz RM, Pandha H, Harrington K, Coffey M, Melcher A, Vile R. Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther. 2016;24:166–174. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Augustine T, John P, Friedman T, Jiffry J, Guzik H, Mannan R, Gupta R, Delano C, Mariadason JM, Zang X, Maitra R, Goel S. Reovirus sensitizes microsatellite stable colorectal cancer to anti-PD-1 treatment via cross-talk in innate and adaptive immune systems. bioRxiv. 2021:2021.2009.2003.458915. [Google Scholar]
- 49.Mahalingam D, Wilkinson GA, Eng KH, Fields P, Raber P, Moseley JL, Cheetham K, Coffey M, Nuovo G, Kalinski P, Zhang B, Arora SP, Fountzilas C. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: a phase Ib study. Clin Cancer Res. 2020;26:71–81. doi: 10.1158/1078-0432.CCR-19-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]


