Abstract
Objectives: This study discussed and analyzed the predictive value of the prognostic nutritional index (PNI) to postoperative prognosis and nursing intervention measures for colorectal cancer. Methods: 196 patients with colorectal cancer who underwent radical resection in gastrointestinal surgery were retrospectively analyzed. The patients’ data and follow-up results were collected and classified into two groups based on the PNI, i.e., the high PNI group (≥45.61, 107 cases) and the low PNI group (<45.61, 89 cases) by reregarding PNI 45.61 as the threshold value. The differences in clinical materials and prognosis between the two groups were compared, and the multivariate analysis of 5-year survival after radical resection of colorectal cancer was conducted by Cox proportional hazard model. Results: The incidence of postoperative complications and severe complications in low PNI group was critically higher than those in high PNI group (P<0.05). Besides, the postoperative disease-free survival and overall survival of the high PNI patients were obviously superior to those of the counterpart (P<0.05). In addition, the results of univariate and multivariate analysis showed that age, TNM staging and PNI were independent risk factors that affected the postoperative survival of patients with colorectal cancer (P<0.05). Conclusion: The preoperative PNI is an independent risk factor that affects the survival of colorectal cancer patients after radical resection. PNI assessment of patients with colorectal cancer helps predict the clinical prognosis of patients. At the same time, the corresponding nursing countermeasures were provided according to the PNI score to improve patients’ nutritional status and immune function, which may eventually improve the clinical prognosis.
Keywords: Prognostic nutritional index, colorectal cancer, postoperative prognosis, predictive value, nursing strategy
Introduction
Colorectal cancer (CRC) is a common malignant tumor of the digestive tract. If it is not effectively treated and controlled, as the patient’s condition further develops, it will become colorectal cancer with liver or other organs metastasis, which will seriously threaten the life of the patient [1,2]. The key to clinical treatment is to clarify the patient’s disease progression in time and give targeted therapeutic and nursing schedules [3]. Therefore, the accurate postoperative prognosis for colorectal cancer patients before surgery is of great significance for guiding the clinical treatment and nursing work [4]. Studies have shown [5] that patients’ preoperative nutrition, immune and inflammatory status are related to the surgical risk and the progression of malignant tumors. The prognostic nutritional index (PNI), in particular, is a simple but effective index that reflects the preoperative nutritional status and immune function of patients [5,6]. In order to further improve the postoperative prognosis of patients with colorectal cancer, this study explored and analyzed the predictive value of PNI to postoperative prognosis and nursing intervention strategies for colorectal cancer.
Materials and methods
Research subjects
196 patients with colorectal cancer who underwent radical resection in gastrointestinal surgery from March 2013 to May 2015 were enrolled as subjects for retrospectively analysis. This study was approved by the ethics committee of our hospital.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients underwent radical resection of colorectal cancer; (2) The patients were diagnosed as colorectal tumor by histopathology; (3) No radiotherapy or chemotherapy was performed before operation; (4) No distant metastasis; (5) No other infectious diseases; (6) Patients had complete blood test result for the first admission; (7) Patients received at least 2 rounds of chemotherapy after surgery; and (8) The patients and their family member were informed and consented.
Exclusion criteria: (1) The tumor cannot be resected or had distant metastasis; (2) Patients with diseases of blood or immune system; (3) Preoperative radiotherapy and chemotherapy were performed; (4) Patients with intestinal obstruction, perforation, bleeding or other emergency operations; (5) Patients with incomplete clinical data; or (6) Patients who did not receive chemotherapy or radiotherapy after surgery or the treatment duration was less than 2 rounds.
Data collection
Clinicopathological data such as gender, age, body mass, size of tumor, TNM staging, histological typing, and embryonic antigen (CEA)/CA19-9 were collected and recorded.
Postoperative complications
The postoperative complications involved in this study referred to various complications related to surgery or treatment that occurred within 30 days after surgery. The complications were classified by using Clavien-Dindo classification method [7]: Grade I: Slightly deviated from the normal postoperative rehabilitation process, but no treatment is required; Grade II: Complications that require drug intervention; Grade III: Complications that require surgical, endoscopic, or radiotherapy intervention; Grade IV: Complications that require intensive care and the complications threaten the lives of patients; Grade V: Death. Grade II indicates complications and Grade III or above indicates severe complications.
Calculation of PNI
The neutrophil count, lymphocyte count, monocyte count and serum albumin concentration obtained from the first blood sampling before admission were taken as the criterion. NLR = neutrophil count ÷ lymphocyte count; LMR = lymphocyte count ÷ mononuclear cell count; and PNI = serum albumin concentration (g/L) + 5 × lymphocyte count (×109/L).
Subjects grouping
According to previous research results [8], patients were classified into high PNI group (≥45.61, 107 cases) and low PNI group (<45.61, 89 cases) by regarding PNI 45.61 as the threshold value.
Postoperative follow-up
Patients were followed up after surgery, and the follow-up deadline was October 1, 2020. The Overall survival (OS) and disease-free survival (DFS) of the subjects were recorded. OS is defined as: the time from the postoperative day to the death of the patient (for any reason); DFS is defined as the time between the beginning of the patient’s postoperative day and the recurrence of the disease or (for any reason) death.
Nursing countermeasures plan
In order to further improve the clinical prognosis of colorectal cancer patients after surgery, our hospital summarized and analyzed the previous nursing experience, and put forward targeted nursing countermeasures as follows: (1) A nutrition intervention team was established, which was composed of clinicians with the title of attending or above, specialist nurse with over 5 years’ working experience, and nutritionist who had acquired the qualification and worked in the field over 5 years. Guided by nutritionist, the study of PNI concept, the assessment of patients’ nutritional status and the corresponding nutritional intervention methods were carried out by the team. The training was conducted twice a week for two consecutive weeks, and a unified assessment after the training wascarried out. (2) A nutritional intervention plan was constructed. When PNI≥50, patient needed to be instructed to consume foods with high protein and vitamins, and to avoid cold, spicy or pungent foods. When 45≤PNI<50, personalized recipes were customized according to patient’s dietary habits and nutritional needs. Patients should take strictly according to the recipes with clear type and quantity of diets consumed. When 40≤PNI<45, professional on-site nutritional preparations for cancer patients will be added on the basis of the above-mentioned personalized diet plan. The preparation was taken orally 500 ml per day, 200-250 ml each time. When PNI<40, the patient would be given a personalized diet plan, oral enteral nutrition preparations and combined intervention of parenteral nutrition support. At the same time, nutrients such as water-soluble vitamins, compound amino acid injection and fat milk injection would be injected intravenously. When necessary, enteral nutrition catheter shall be inserted through the nasal cavity for enteral nutrition. (3) The nutrition intervention program was implemented. The nutritional status of patient was evaluated by PNI on the first day after admission, and the nutritional intervention program was established based on the evaluation results. The evaluation was performed every 3 days postoperatively, and the program shall be adjusted promptly according to the evaluation results until the patient is discharged. After discharge, patients would be followed up by WeChat or telephone for diet guidance. Thereafter, a PNI evaluation would be conducted 1 day before each chemotherapy, and an individualized nutritional intervention program would be developed.
Statistical analysis
Data was settled and analyzed via statistical software SPSS 22.0. Measurement data were compared by t test, enumeration data were compared by X2 test, Kaplan-Meier survival curve was drawn for survival analysis, and group comparison was by Log-rank test. Multivariate analysis of survival at 5 years after radical resection of colorectal cancer was performed by Cox proportional hazards model, and the test level was = 0.05. The figures were plotted by Graphpad Prism 8.0.
Results
Clinical materials
The differences in gender, BMI, histological typing, tumor site, operation time and intraoperative blood loss in two sets of subjects were statistically insignificant (P>0.05). The age of subjects in low PNI group was critically higher than that of the high PNI group (P<0.05), and the proportion of patients with TNM stage III, CEA≥5 μg/ml, CA-199≥37 U/ml in low PNI group was obviously higher than those of high PNI counterpart (P<0.05) (Table 1).
Table 1.
Comparison of clinical data between two groups of subjects
| Clinical materials | High PNI group (n = 107) | Low PNI group (n = 89) | t/X2 | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 66 | 52 | 0.215 | 0.643 |
| Female | 41 | 37 | ||
| Age (years old, x̅±s) | 62.64±7.33 | 65.38±9.27 | 2.310 | 0.022 |
| BMI (kg/m2) | ||||
| <18.5 | 19 | 18 | 0.193 | 0.660 |
| ≥18.5 | 88 | 71 | ||
| Histological classification | ||||
| Adenocarcinoma | 85 | 70 | 0.018 | 0.893 |
| Others | 22 | 19 | ||
| Site of tumor | ||||
| Colon | 61 | 50 | 0.014 | 0.907 |
| Rectum | 46 | 39 | ||
| Size of tumor (cm) | ||||
| <5 | 67 | 48 | 1.511 | 0.219 |
| ≥5 | 40 | 41 | ||
| TNM staging | ||||
| I~II | 86 | 57 | 6.567 | 0.010 |
| II | 21 | 32 | ||
| CEA (μg/ml) | ||||
| ≥5 | 35 | 42 | 4.271 | 0.039 |
| <5 | 72 | 47 | ||
| CA-199 (U/ml) | ||||
| ≥37 | 26 | 35 | 5.118 | 0.024 |
| <37 | 81 | 54 | ||
| Operation time (h) | ||||
| ≤4 | 56 | 47 | 0.004 | 0.947 |
| >4 | 51 | 42 | ||
| Intraoperative blood loss (ml) | ||||
| ≤400 | 54 | 49 | 0.410 | 0.522 |
| >400 | 53 | 40 |
Comparison of postoperative complications
In the high PNI group, the incidence of postoperative complications was 24.30%, and the incidence of severe complications was 4.67%, while those in the low PNI group were 41.57%, and 20.22%, respectively. The incidence of postoperative complications and severe complications in low PNI group was critically higher than those in high PNI group (P<0.05) (Table 2).
Table 2.
Comparison of postoperative complications incidence between the two groups [n (%)]
| Group | Number of cases | Complications | Severe complications |
|---|---|---|---|
| High PNI group | 107 | 26 (24.30) | 5 (4.67) |
| Low PNI group | 89 | 37 (41.57) | 18 (20.22) |
| X2 | - | 6.647 | 11.345 |
| P | - | 0.010 | 0.001 |
Comparison of postoperative DFS
The median disease-free survival time of high PNI group and low PNI group was 62 months and 58 months, respectively. The postoperative disease-free survival of patients with high PNI was obviously superior to that of the low group (P<0.05) (Figure 1).
Figure 1.
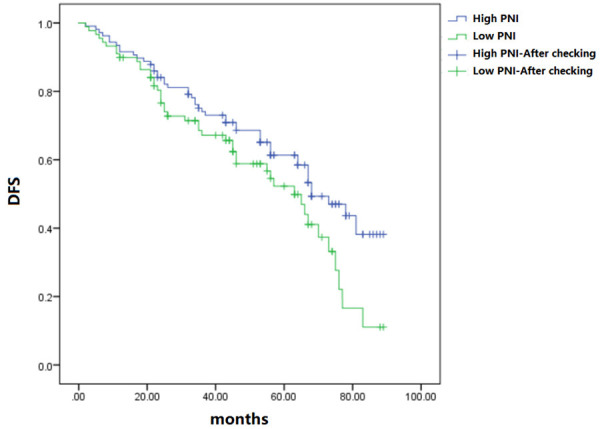
Comparison of postoperative DFS.
Comparison of postoperative OS
The postoperative overall survival of patients with high PNI was obviously superior to that of the low PNI group (P<0.05) (Figure 2).
Figure 2.
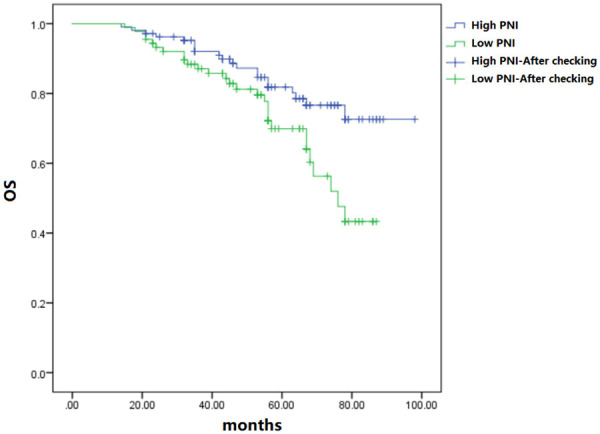
Comparison of postoperative OS.
Analysis of risk factors that affected survival and prognosis of patients
The factors that had an impact on the prognosis of patients were included in Cox proportional hazard model for univariate and multivariate analysis. The results of univariate and multivariate analysis showed that age, TNM staging and PNI were independent risk factors that affected postoperative survival of patients with colorectal cancer (P<0.05) (Table 3).
Table 3.
Analysis of univariate and multivariate factors that affected the survival and prognosis of patients
| Factor | Single factor analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Gender (female) | 1.089 | 0.527~1.983 | 0.738 | - | - | - |
| Age (≥60 years old) | 2.173 | 1.462~3.876 | 0.001 | 1.897 | 1.287~3.426 | 0.010 |
| BMI (≥18.5 kg/m2) | 0.722 | 0.334~1.273 | 0.364 | - | - | - |
| Histological type (adenocarcinoma) | 1.082 | 0.573~1.462 | 0.783 | - | - | - |
| Tumor site (colon) | 1.673 | 0.978~2.374 | 0.088 | - | - | - |
| Tumor size (≥5 cm) | 1.583 | 0.734~1.658 | 0.102 | - | - | - |
| TNM staging (III) | 1.895 | 0.762~1.732 | 0.032 | 1.705 | 1.387~4.207 | 0.039 |
| CEA (≥5 μg/ml) | 1.574 | 0.755~1.827 | 0.073 | - | - | - |
| CA-199 (≥37 U/ml) | 1.337 | 0.812~1.695 | 0.082 | - | - | - |
| Operation time (>4 h) | 1.042 | 0.663~2.174 | 0.092 | - | - | - |
| Intraoperative blood loss (>400 ml) | 1.306 | 0.387~1.930 | 0.117 | - | - | - |
| PNI (<45.61) | 3.074 | 1.937~5.672 | 0.000 | 1.937 | 1.271~4.263 | 0.019 |
Discussion
Colorectal cancer is one of the most common gastrointestinal malignancies clinically. Colorectal cancer morbidity has been increasing year by year in recent years, and becomes the third cause of tumor-related death [9,10]. Due to the gastrointestinal symptoms, which affect the appetite and eating, most of the CRC patients have varying degrees of malnutrition risk [11,12]. Studies have revealed that immune function and nutritional status of human body are crucial indicators for evaluating the patients’ prognosis after surgery [13,14]. Preoperative PNI, which is related to the serum albumin level and peripheral blood lymphocyte count, can reflect the preoperative nutritional status and immune status of patients [15,16]. Literature reports demonstrated that patients with high lymphocyte counts had a higher 5-year survival rate than those with low lymphocyte counts; and the low serum albumin levels partially reflect the poor nutritional status of the host and insufficient defense system capabilities, which may be related to the poor prognosis of patients [17,18].
We have shown that the incidence of postoperative complications and severe complications in low PNI group was critically higher than those in high PNI group; and the postoperative disease-free survival and overall survival of the high PNI patients were obviously superior to those of the control group. The results, which were similar to the relevant studies by other reserachers [19,20], indicate that PNI is tightly correlated with the early postoperative complications and long-term prognosis of colorectal cancer patients. The overall survival and disease-free survival of patients with low PNI decreased remarkably, possibly due to the poor nutritional status and immune function of patients. Moreover, several studies have shown [21,22] that PNI is an independent predictor of postoperative prognosis in colorectal cancer patients, which is consistent with the results of this study. The state of low PNI is related to the degree of tumor invasion, lymph node metastasis and other invasive clinicopathological features in colorectal cancer patients, which may be an important cause of the poor prognosis.
However, due to the limited quantity of objects included, deviation may exist in the results of the study. At the same time, we did not conduct a prospective study on the countermeasures of nursing intervention in this study, suggesting that further expansion of the sample size is needed in subsequent studies to acquire more reliable research data.
In summary, the preoperative PNI is an independent risk factor that affects the survival of colorectal cancer sufferers after radical resection. The conduction of PNI assessment in colorectal cancer patients is beneficial to predict their clinical prognosis. At the same time, the corresponding nursing countermeasures were provided according to the PNI score, and the improvement of nutritional status and immune function of patients may be crucial measures that promoting the clinical prognosis of patients.
Disclosure of conflict of interest
None.
References
- 1.Aguirre-Portolés C, Fernández LP, Ramírez DE, Molina A. Precision nutrition for targeting lipid metabolism in colorectal cancer. Nutrients. 2017;9:1076. doi: 10.3390/nu9101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerman D, Laszlo M, Provisor A, Yu A. Nutrition management for the head and neck cancer patient. Cancer Treat Res. 2018;174:187–208. doi: 10.1007/978-3-319-65421-8_11. [DOI] [PubMed] [Google Scholar]
- 3.Hui D, Dev R, Bruera E. The last days of life: symptom burden and impact on nutrition and hydration in cancer patients. Curr Opin Support Palliat Care. 2015;9:346–54. doi: 10.1097/SPC.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molfino A, Amabile MI, Muscaritoli M. Nutrition support for treating cancer-associated weight loss: an update. Curr Opin Support Palliat Care. 2018;12:434–438. doi: 10.1097/SPC.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 5.Gaynor EP, Sullivan PB. Nutritional status and nutritional management in children with cancer. Arch Dis Child. 2015;100:1169–1172. doi: 10.1136/archdischild-2014-306941. [DOI] [PubMed] [Google Scholar]
- 6.Laviano A, Di Lazzaro L, Koverech A. Nutrition support and clinical outcome in advanced cancer patients. Proc Nutr Soc. 2018;77:388–393. doi: 10.1017/S0029665118000459. [DOI] [PubMed] [Google Scholar]
- 7.Wang SM, Taylor PR, Fan JH, Pfeiffer RM, Gail MH, Liang H, Murphy GA, Dawsey SM, Qiao YL, Abnet CC. Effects of nutrition intervention on total and cancer mortality: 25-year post-trial follow-up of the 5.25-year Linxian nutrition intervention trial. J Natl Cancer Inst. 2018;110:1229–1238. doi: 10.1093/jnci/djy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, Pless M, Ballmer PE. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr. 2018;37:1202–1209. doi: 10.1016/j.clnu.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Song M, Chan AT. The potential role of exercise and nutrition in harnessing the immune system to improve colorectal cancer survival. Gastroenterology. 2018;155:596–600. doi: 10.1053/j.gastro.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace TC, Bultman S, D’Adamo C, Daniel CR, Debelius J, Ho E, Eliassen H, Lemanne D, Mukherjee P, Seyfried TN, Tian Q, Vahdat LT. Personalized nutrition in disrupting cancer - proceedings from the 2017 American college of nutrition annual meeting. J Am Coll Nutr. 2019;38:1–14. doi: 10.1080/07315724.2018.1500499. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Chen W, Li H, Zhao B Chinese Oncology Nutrition Survey Group. Nutrition support in hospitalized cancer patients with malnutrition in China. Asia Pac J Clin Nutr. 2018;27:1216–1224. doi: 10.6133/apjcn.201811_27(6).0007. [DOI] [PubMed] [Google Scholar]
- 12.Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. 2018;153:1081–1089. doi: 10.1001/jamasurg.2018.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangadharan A, Choi SE, Hassan A, Ayoub NM, Durante G, Balwani S, Kim YH, Pecora A, Goy A, Suh KS. Protein calorie malnutrition, nutritional intervention and personalized cancer care. Oncotarget. 2017;8:24009–24030. doi: 10.18632/oncotarget.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–86. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bail J, Meneses K, Demark-Wahnefried W. Nutritional status and diet in cancer prevention. Semin Oncol Nurs. 2016;32:206–214. doi: 10.1016/j.soncn.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Petzel MQB, Hoffman L. Nutrition implications for long-term survivors of pancreatic cancer surgery. Nutr Clin Pract. 2017;32:588–598. doi: 10.1177/0884533617722929. [DOI] [PubMed] [Google Scholar]
- 17.Joffe L, Ladas EJ. Nutrition during childhood cancer treatment: current understanding and a path for future research. Lancet Child Adolesc Health. 2020;4:465–475. doi: 10.1016/S2352-4642(19)30407-9. [DOI] [PubMed] [Google Scholar]
- 18.De Cicco P, Catani MV, Gasperi V, Sibilano M, Quaglietta M, Savini I. Nutrition and breast cancer: a literature review on prevention, treatment and recurrence. Nutrients. 2019;11:1514. doi: 10.3390/nu11071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan T, Mastnak DM, Palamar N, Kozjek NR. Nutritional therapy for patients with esophageal cancer. Nutr Cancer. 2018;70:23–29. doi: 10.1080/01635581.2017.1374417. [DOI] [PubMed] [Google Scholar]
- 20.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 21.Greenlee H, Santiago-Torres M, McMillen KK, Ueland K, Haase AM. Helping patients eat better during and beyond cancer treatment: continued nutrition management throughout care to address diet, malnutrition, and obesity in cancer. Cancer J. 2019;25:320–328. doi: 10.1097/PPO.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 22.Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol. 2019;17:275–289. doi: 10.1016/j.cgh.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


