Abstract
Fusion of RET with different partner genes has been detected in papillary thyroid, lung, colorectal, pancreatic, and breast cancer. Approval of selpercatinib for treatment of lung and thyroid cancer with RET gene mutations or fusions calls for studies to explore RET fusion partners and their eligibility for RET‐based targeted therapy. In this study, RET fusion patterns in a large group of Chinese cancer patients covering several cancer types were identified using next‑generation sequencing. A total of 44 fusion patterns were identified in the study cohort with KIF5B, CCDC6, and ERC1 being the most common RET fusion partners. Notably, 17 novel fusions were first reported in this study. Prevalence of functional RET fusions was 1.05% in lung cancer, 6.03% in thyroid cancer, 0.39% in colorectal cancer, and less than 0.1% in gastric cancer and hepatocellular carcinoma. Analysis showed a preference for fusion partners in different tumor types, with KIF5B being the common type in lung cancer, CCDC6 in thyroid cancer, and NCOA4 in colorectal cancer. Co‐occurrence of EGFR mutations and RET fusions with rare partner genes (rather than KIF5B) in lung cancer patients was correlated with epidermal growth factor receptor‐tyrosine kinase inhibitor resistance and could predict response to targeted therapies. Findings from this study provide a guide to clinicians in determining tumors with specific fusion patterns as candidates for RET targeted therapies.
Keywords: EGFR‐TKI resistance, lung cancer, multicancer, NGS, RET fusion
A total of 12 888 lung cancer patients, 2848 colorectal cancer patients, 1785 hepatocellular carcinoma patients, 1169 gastric cancer patients, and 232 papillary thyroid cancer patients from China were retrospectively analyzed for RET fusion using next‐generation sequencing. A total of 164 functional RET fusions and 58 nonfunctional fusions were identified; 17 of the 164 functional RET fusions were novel. Identification of these genomic fusion patterns will facilitate rationalization of clinical treatment strategies.
![]()
Abbreviations
- cfDNA
cell‐free DNA
- CRC
colorectal cancer
- FFPE
formalin‐fixed paraffin‐embedded
- GC
gastric cancer
- HCC
hepatocellular carcinoma
- IHC
immunohistochemistry
- LC
lung cancer
- NGS
next‐generation sequencing
- NSCLC
non‐small‐cell lung cancer
- PTC
papillary thyroid cancer
- RET
rearranged during transfection
- TKI
tyrosine kinase inhibitor
1. INTRODUCTION
RET was initially discovered as a rearranged oncogene in a 3T3 fibroblast cell line transfected with a human lymphoma DNA. 1 The RET gene encodes a receptor‐tyrosine kinase, which plays an important role in cell proliferation, migration, and differentiation. 2 , 3 , 4 , 5 RET fusion induces oncogenic activation and occurs in approximately 5%‐10% of sporadic PTC types and in 1%‐2% of lung cancer cases with low frequency in other solid cancer types (breast cancer, <0.21%. colorectal cancer, <0.26%. esophageal cancer, <0.17%. ovarian cancer, <0.17%. prostate cancer, <0.08% and stomach cancer, <0.81%). 6 , 7 , 8 , 13 , 14 , 15 , 16 In tumors with activating RET fusions, a 5′‐terminal partner gene coding sequence is fused to 3′‐terminal RET kinase domain coding sequence including the kinase domain (NM_020975: exons 12‐18). 17 , 18
The most common breakpoints in RET occur in intron 11, followed by intron 10. 19 Multiple N‐terminal partner genes of RET fusion have been identified. In PTCs, the most common RET fusions include CCDC6‐RET (RET/PTC1) and NCOA4‐RET (RET/PTC3), which are detected in approximately 90% of RET fusion‐positive cases. 8 , 20 The most common RET fusions in NSCLC are KIF5B‐RET, NCOA4‐RET, and CCDC6‐RET. 21 , 22 Multiple rare RET fusions have been discovered and reported in different cancers. 23 , 24 , 25 , 26 , 27 , 28 , 31
Selpercatinib (LOXO‐292) was approved by the US FDA for treatment of NSCLC, thyroid cancer, and medullary thyroid cancer with RET mutations or fusions. 32 In addition, it has shown effectiveness in other solid cancer types including brain cancer and pancreatic cancer. 33 , 34 , 35 Various molecular testing methods have been developed for detection of RET fusions, including NGS, RT‐PCR, FISH, and IHC. Immunohistochemistry is limited for general application due to its low sensitivity and specificity. 13 , 36 , 37 Reverse transcription‐PCR can only detect RET fusions with known fusion partners. 29 , 38 , 39 Although FISH is highly sensitive, it requires special technical expertise and is not effective for identification of fusion partners. 38 , 40 The NGS platform provides a more feasible way for comprehensive and accurate diagnostic testing of RET fusion for cancer patients who could benefit from RET inhibitors. In addition, it can be used to identify other genetic alterations.
In this study, 12 888 LC patients, 2848 CRC patients, 1785 HCC patients, 1169 GC patients, and 232 PTC patients from China were retrospectively analyzed for RET fusion using NGS. A total of 164 functional RET fusions and 58 nonfunctional fusions were identified. Notably, 17 of the 164 functional RET fusions were novel. Identification of these genomic fusion patterns will facilitate rationalization of clinical treatment strategies.
2. MATERIALS AND METHODS
2.1. Patients and samples
Tumor samples (tissues or plasma fractions) obtained from patients between January 2017 and December 2019 were used for NGS RET fusion detection (Genetron Health).
2.2. DNA sequencing
DNA samples from LC, CRC, HCC, and GC patients were analyzed using targeted deep sequencing using NGS technology. Genomic DNA was extracted from FFPE samples using QIAamp DNA FFPE tissue kit (Qiagen) following the manufacturer’s instructions. Plasma cfDNA was extracted using MagMAX Cell Free DNA Isolation Kit (Thermo Fisher Scientific). DNA samples were quantified with the Qubit 2.0 Fluorometer using Qubit dsDNA HS Assay kit (Life Technologies) following the manufacturer’s instructions. Genomic DNA from each FFPE sample was sheared into 150‐200‐bp fragments using the M220 Focused‐ultrasonicator (Covaris). Fragmented genomic DNA and cfDNA libraries were constructed with the KAPA HTP Library Preparation Kit (KAPA Biosystems) following the manufacturer’s protocol. Concentration of DNA in the library was determined using the Qubit dsDNA HS Assay kit. DNA libraries were analyzed using Onco PanScan (Genetron Health), which is an 825‐gene panel including major tumor‐related genes. Quality control was undertaken on raw sequencing data to remove adapters and low‐quality regions using Trimmomatic (version 0.36). Local alignments of reads to the hg19 genome (GRch37) were carried out using the Burrows‐Wheeler Aligner tool (version 0.7.10). 41 Somatic single‐nucleotide variants were retrieved using muTect (https://software.broadinstitute.org/cancer/cga/mutect), 42 somatic insertions and deletions were retrieved using Strelka (https://github.com/Illumina/strelka), 43 and structural variations were determined using GeneFuse version 0.6.1 (https://github.com/OpenGene/GeneFuse). 44 A total of 1000 genomes and variants with population frequency over 0.1% were excluded based on guidelines by the Exome Aggregation Consortium. The other variants were annotated with Oncotator and Vep.
2.3. Papillary thyroid cancer sample sequencing
DNA and RNA extracted from PTC samples were analyzed with the FSZ‐Thyroid‐V1 NGS Panel using one‐step multiplex PCR targeted amplicons as described previously. 45 DNA and total RNA were isolated from FNA samples using AllPrep DNA/RNA Mini Kit (Qiagen) according to the manufacturer’s instructions. DNA and RNA concentrations were determined using a Qubit 3.0 Fluorometer (Thermo Fisher Scientific). Ten nanograms of RNA was reverse transcribed into cDNA using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific). Libraries were prepared from 10 ng DNA and 10 ng cDNA and normalized for template preparation, on the Ion Chef System (Thermo Fisher Scientific). The libraries were sequenced on the Ion Proton (Thermo Fisher Scientific) platform following the manufacturer’s protocol. Data analysis and interpretation were carried out using Torrent Suite (version 5.2.2; Thermo Fisher Scientific).
2.4. RNA sequencing
A 395‐gene RNA panel was analyzed to identify gene fusions at the transcript level. Total RNA was isolated using the AllPrep DNA/RNA Mini Kit (Qiagen), then reverse transcribed to cDNA using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific). The libraries were constructed with the KAPA HTP Library Preparation Kit (KAPA Biosystems). DNA libraries were captured with an Agilent SureSelect V5 system (Agilent) and the captured samples were subjected to Illumina HiSeq X‐Ten for paired end sequencing. Sequencing reads were mapped to a human reference genome (hg19) using Hisat2‐2.0.5. 46 Gene fusions were identified using FusionMap. 47
2.5. Statistical analyses
Categorical variables were compared using Pearson’s analysis and χ2 test. Analyses and data presentation were undertaken using GraphPad Prism (8.0.1) and R (version 4.1.1).
3. RESULTS
3.1. Patient characteristics
Functional RET fusions occur when the RET gene is located in the 3′‐terminal with final transcripts containing RET kinase domain (exons 12‐18). 40 , 45 , 48 This fusion can generate a constitutively active chimeric protein with an N‐terminal kinase domain characteristic of RET protein. A total of 222 RET fusions in 185 patients were identified using this criterion, including 164 functional fusions and 58 nonfunctional fusions (Tables S1 and S2, Figure 1A). Most of the functional fusions identified in this study have been reported previously; however, 17 functional fusions were identified for the first time. Analysis of all samples (12 888 LC patients, 2848 CRC patients, 1785 HCC patients, 1169 GC patients, and 232 PTC patients) showed that 162 (0.86%) patients harbored functional RET fusions with 1.05% (135/12888) in the LC group, 0.39% (11/2848) in the CRC group, 0.06% (1/1785) in the HCC group, 0.09% (1/1169) in the GC group, and 6.03% (14/232) of PTC patients (Figure 1B). Analysis of the 135 RET fusion‐positive LC patients showed that 55 (40.74%) of them were men and 80 (59.26%) were women. The fusions occurred more frequently in younger patients (P < .001), women (P < .001), and patients with adenocarcinoma (P < .001). Analysis of patients in the CRC and PTC cohorts showed no preference pattern in terms of gender or age in the RET fusion‐positive cohort. However, there was significant difference (P = .002) in RET fusion‐positive rates between colon and rectum cancers (Table 1).
FIGURE 1.
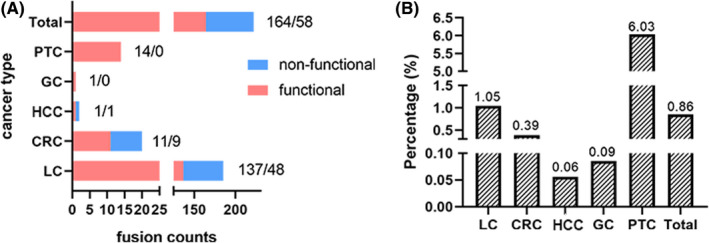
RET fusions identified in different cancers. A, Counts of functional and nonfunctional RET fusions identified in different cancer types. B, Proportion of functional RET fusions identified in different cancer types. CRC, colorectal cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; LC, lung cancer; PTC, papillary thyroid cancer
TABLE 1.
Relationships between RET fusion and clinicopathologic features in cancer patients
| Feature | Total | RET fusion | P value | ||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Lung cancer | Age, y | ||||
| Mean | 62.9 | 55.6 | 62.9 | <.001 | |
| Median | 63 | 57 | 64 | ||
| Range | 17‐101 | 23‐92 | 17‐101 | ||
| Sex | |||||
| Male | 7211 | 55 | 7156 | <.001 | |
| Female | 5677 | 80 | 5597 | ||
| Histotype | |||||
| ADC | 7991 | 102 | 7889 | <.001 | |
| Non‐ADC | 1169 | 1 | 1168 | ||
| Unknown | 3728 | 32 | 3696 | ||
| Colorectal cancer | Age, y | ||||
| Mean | 59.1 | 65.8 | 59.1 | .062 | |
| Median | 61 | 62 | 61 | ||
| Range | 18‐94 | 52‐83 | 18‐94 | ||
| Sex | |||||
| Male | 1744 | 9 | 1735 | .274 | |
| Female | 1104 | 2 | 1102 | ||
| Tumor location | |||||
| Colon | 1449 | 11 | 1438 | .002 | |
| Rectum | 1223 | 0 | 1223 | ||
| Unknown | 176 | 0 | 176 | ||
| Thyroid cancer | Age, y | ||||
| Mean | 44.0 | 38.7 | 44.4 | .092 | |
| Median | 45 | 37 | 45 | ||
| Range | 13‐75 | 25‐57 | 13‐75 | ||
| Sex | |||||
| Male | 63 | 2 | 61 | .420 | |
| Female | 169 | 12 | 157 | ||
| Histotype | |||||
| PTC | 232 | 14 | 218 | ||
Abbreviations: ADC, adenocarcinoma; PTC, papillary thyroid cancer.
3.2. Identification of RET fusion partners in patients with different cancer types
Analysis of the functional RET fusions in LC showed that the most common partner genes were KIF5B, with 85 KIF5B‐RET fusion events identified (62.04%). The second and third most frequent fusion partners were CCDC6 and ERC1 (21.17%, 29/137 and 2.19%, 3/137, respectively) (Figure 2A). Several rare and novel RET fusion partners were identified in this study, including DNER, DPP6, FGD5, GADL1, GLI3, GPRC6A, IL1RAPL2, KIAA1598, KIF13A, MALRD1, SPECC1, TLN1, and ZNF33B (Table 2). In addition, multiple RET fusions were identified in one individual patient (such as KIF5B‐RET and GLI3‐RET identified in one patient, KIF5B‐RET and MALRD1‐RET in another patient).
FIGURE 2.
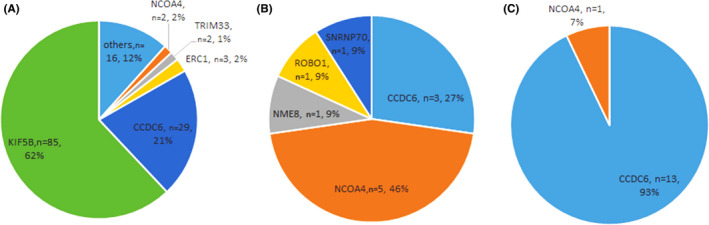
Distribution of fusion partners identified in cancer patients with RET fusions. A, In 135 lung cancer patients with RET fusions, 137 fusion events were identified with two patients carrying two different RET fusions. B, C, Fusion events identified in 11 colorectal cancer patients (B) and 14 papillary thyroid cancer patients (C). Each patient carried only one functional RET fusion. Different colors and sizes indicate the frequency of each RET fusion partner in all RET fusion events identified
TABLE 2.
Patterns of functional RET fusions in cancer patients
| Fusion (no.) | Pos1: Pos2 | Counts | Cancer type | Fusion (no.) | Pos1: Pos2 | Counts | Cancer type |
|---|---|---|---|---|---|---|---|
| KIF5B‐RET (85) | E15: E12 | 63 | LC | GLI3‐RET a | E2: E11 | 1 | LC |
| E15: E11 | 8 | LC | GPRC6A‐RET a | E1: E12 | 1 | LC | |
| E23: E12 | 4 | LC | IL1RAPL2‐RET a | E2: E12 | 1 | LC | |
| E24: E11 | 3 | LC | KIAA1598‐RET a | E2: E12 | 1 | LC | |
| E24: E9 | 2 | LC | KIF13A‐RET | E18: E12 | 1 | LC | |
| E24: E10 | 1 | LC | MALRD1‐RET a | E32: E8 | 1 | LC | |
| E16: E12 | 1 | LC | PRKAR1A‐RET | E2: E10 | 1 | LC | |
| E17: E11 | 1 | LC | SPECC1‐RET a | E4: E12 | 1 | LC | |
| E19: E12 | 1 | LC | TLN1‐RET a | E54: E12 | 1 | LC | |
| E22: E12 | 1 | LC | TRIM24‐RET | E9: E12 | 1 | LC | |
| CCDC6‐RET (29) | E1: E12 | 28 | LC | ZNF33B‐RET a | E4: E11 | 1 | LC |
| E2: E12 | 1 | LC | CCDC6‐RET (3) | E2:E12 | 1 | CRC | |
| ERC1‐RET (3) | E3: E12 | 1 | LC | E8:E12 | 1 | CRC | |
| E5: E12 | 1 | LC | E1:E10 | 1 | CRC | ||
| E7: E12 | 1 | LC | NCOA4‐RET (5) | E11:E12 | 4 | CRC | |
| NCOA4‐RET (2) | E10: E12 | 1 | LC | E9:E12 | 1 | CRC | |
| E8: E12 | 1 | LC | NME8‐RET a | E14:E9 | 1 | CRC | |
| TRIM33‐RET (2) | E16: E10 | 1 | LC | ROBO1‐RET a | E3:E12 | 1 | CRC |
| E10: E12 | 1 | LC | SNRNP70‐RET a | E2:E12 | 1 | CRC | |
| DNER‐RET a | E1: E12 | 1 | LC | NCOA4‐RET | E8:E12 | 1 | PTC |
| DPP6‐RET a | E2: E12 | 1 | LC | CCDC6‐RET | E1:E12 | 13 | PTC |
| EML4‐RET | E17: E12 | 1 | LC | GABRG3‐RET a | E5:E9 | 1 | HCC |
| FGD5‐RET a | E1: E12 | 1 | LC | OPALIN‐RET a | E6:E11 | 1 | GC |
| GADL1‐RET a | E14: E9 | 1 | LC |
Abbreviations: CRC, colorectal cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; LC, lung cancer; PTC, papillary thyroid cancer.
Novel fusions first reported in this study.
In addition to lung cancer and papillary thyroid carcinoma, RET fusions have been found in 0.6%‐0.7% of patients with other types of cancer, including breast, colon, esophageal, ovarian, prostate, and stomach cancers. 6 , 7 , 8 , 13 , 14 , 15 , 16 In this study, 2848 CRC patients, 1785 HCC patients, 1169 GC patients, and 232 PTC patients were retrospectively analyzed. The findings showed that 27 patients had functional RET fusions (11 with CRC; 1 with HCC, 1 with GC, and 14 with PTC) (Table 2). Common fusion partner genes in these groups were NCOA4 and CCDC6, whereas no KIF5B‐RET fusion was identified in this group of samples (Figure 2B,C). Notably, the common partner genes were different in different cancers, implying that the hotspots of chromosome breakpoints in the partner genes are different, which might be associated with difference in sensitivity to RET inhibitors.
3.3. Genomic breakpoints in RET of patients with different cancer types
Fusion‐mediated RET activation is induced by different mechanisms, including increased kinase expression due to replacement of the 5′‐upstream RET promoter with that of fusion partners, 7 , 49 and dimerization/oligomerization of the RET kinase domain mediated by a C‐terminal domain present in the fusion partners that leads to ligand‐independent kinase activation. 40 , 45 , 48 , 50 Breakpoints in RET and its fusion partners mainly occur in the intronic regions, therefore, the ORF is retained after mRNA splicing. A RET in intron 11, the most common breakpoint in LC patients, allowed exon 12 to be retained in the fusion product. In addition, breakpoints in introns 7, 8, 9, and 10 of RET were observed in this study (Figure 3). Notably, breakpoints in intron 11 were the most common types in these malignancies, and breakpoints in intron 8, 9, and 10 were also observed (Figure 3). The functional RET fusion might result in oncogenic activation due to the remaining intact RET kinase domain (Figure 4). Various upstream 5′ gene partners contribute different domains, typically coiled‐coil domains, to RET fusion proteins and mediate ligand‐independent dimerization of the chimeric oncoproteins. They thereby mediate autophosphorylation of the RET kinase domain, activating downstream signaling pathways that drive tumor cell proliferation (Figure 4). Of the proteins encoded by the partner genes in this study, 13 (encoded by EML4, CCDC6, ERC1, KIF13A, KIF5B, NCOA4, TRIM24, TRIM33, FGD5, KIAA1598, SNRNP70, SPECC1 and TLN1) have coiled‐coil domains that can provide a dimerization motif and seven (encoded by PRKAR1A, GABRG 3, GPRC6A, ROBO1, GLI3, ZNF33B and DPP6) can form a dimerization through other mechanisms. 51 , 52 , 53 , 54 , 55 , 56 However, there are still six partners (encoded by DNER, GADL1, IL1RAPL2, MALRD1, NME8 and OPALIN) that lack the known motifs to form dimerization or oligomerization and need more exploration.
FIGURE 3.
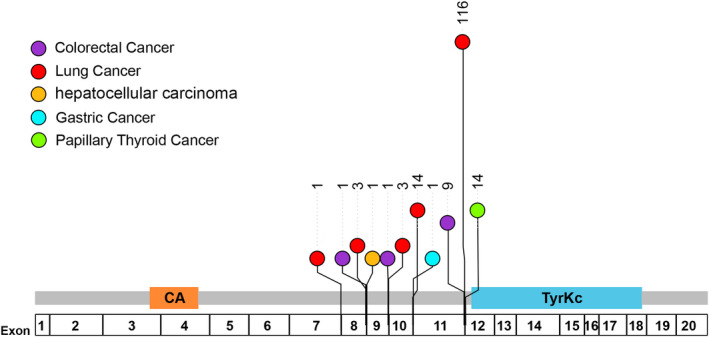
Breakpoint positions in RET. Different colors represent different cancer types: purple, colorectal cancer; red, lung cancer; orange, hepatocellular carcinoma; cyan, gastric cancer; green, papillary thyroid cancer. Numbers beyond circles represent the counts of functional RET fusions detected in different cancer type
FIGURE 4.
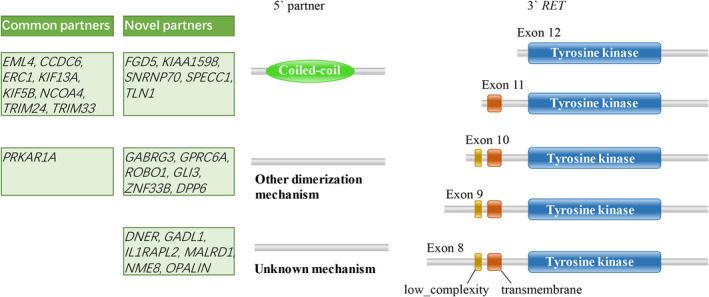
Ligand‐independent activation of the RET fusion protein. RET fusions maintain the tyrosine kinase domain of the 3′ RET gene. A variety of fusion partners contribute different domains, such as coiled‑coil, to RET fusion proteins. These motifs mediate ligand‑independent dimerization of the RET fusion protein. Identification and annotation of genetically mobile domains and analysis of domain architectures (http://smart.embl‐heidelberg.de/)
3.4. mRNA features of cases with novel RET fusion
Of the 17 novel fusions first reported in this study, five cases were sent for RNA sequencing to verify the breakpoint locations and fusion partners at the transcript level (Table 3). We observed that fusion partners and breakpoints at the transcript level matched those predicted by DNA sequencing in four of the five cases, including SNRNP70‐RET in CRC and GABRG3‐RET in HCC. In addition, two LC samples harbored both common and novel RET fusions (KIF5B‐RET and GLI3‐RET, and KIF5B‐RET and MALRD1‐RET), which can be detected by DNA and RNA sequencing. However, OPALIN‐RET in the GC sample was not detected at the transcript level. The inconsistency between DNA and RNA for fusion detection has been reported recently. 57 , 58 , 59 However, the mechanism of this inconsistency needs more investigation.
TABLE 3.
Novel fusion partners of RET identified by DNA and RNA next‐generation sequencing
| Patient | Gender | Age, y | Cancer type | DNA_fusion | RNA_fusion |
|---|---|---|---|---|---|
| W002899T | Male | 41 | HCC | GABRG3‐RET_E5:E9 | GABRG3‐RET_E5:E9 |
| W027998T | Male | 62 | CRC | SNRNP70‐RET_E2:E12 | SNRNP70‐RET_E2:E12 |
| W001013T | Female | 46 | GC | OPALIN‐RET_E6:E11 | Negative |
| W016284T | Female | 61 | LC | KIF5B‐RET_E15:E12 | KIF5B‐RET_E15:E12 |
| GLI3‐RET_E2:E11 | GLI3‐RET_E2:E11 | ||||
| W044019T | Female | 33 | LC | KIF5B‐RET_E24:E9 | KIF5B‐RET_E24:E9 |
| MALRD1‐RET_E32:E8 | MALRD1‐RET_E32:E8 |
Abbreviations: CRC, colorectal cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; LC, lung cancer.
At the same time, we analyzed the average per‐base coverage for RET exons 2‐19 in RNA sequencing, which can represent the relative quantity of mRNA transcript for each exon (Figures 5 and S1). Due to the different preferences for each exon when constructing the library, we selected six samples without RET fusions as negative controls to observe the distribution of coverage depth. Generally, negative samples had high coverage depth on exons 3, 11, 12 13, and 18, while coverage on exons 4‐7 and exons 14‐16 were poor (Figures 5 and S1A–F). Two samples with common RET fusions (KIF5B‐RET_E15:E12 and CCDC6‐RET_E1:E12) were chosen as positive controls. The two samples showed low coverage on the exons before the RET breakpoint, and there was a sharp rise of the coverage on the exons after the breakpoint (Figures 5 and S1G,H). The OPALIN‐RET fusion (E6:E11) detected in case W001013T by DNA sequencing was negative in the RNA test, and the distribution of RNA sequencing coverage for each exon was consistent with the negative sample (Figures 5 and S1C,I). For the GABRG3‐RET fusion (E5:E9) in HCC (case W002899T), although the fusion was detected by both DNA and RNA sequencing, there was no transcription enhancement on exons 9‐19 (Figures 5 and S1J). A novel RET fusion, SNRNP70‐RET (E2:E12), was confirmed by DNA and RNA sequencing in case W027998T, and the covered depth rose from exon 12 (Figures 5 and S1L). Interestingly, in the other two LC cases (W016284T and W044019T) harboring two different fusions in RET, the mRNA level went up from the exon fused with KIF5B (exon 12 and exon 9, respectively) rather than the novel partners (exon 11 and exon 8, respectively). This results shows that not all the novel fusions at the DNA level can be detected at the transcript level, and the mRNA levels of these fusion genes might not necessarily increase. The carcinogenic mechanisms of RET fused with novel and common partners could be different, which deserves more research and discussion in the future.
FIGURE 5.
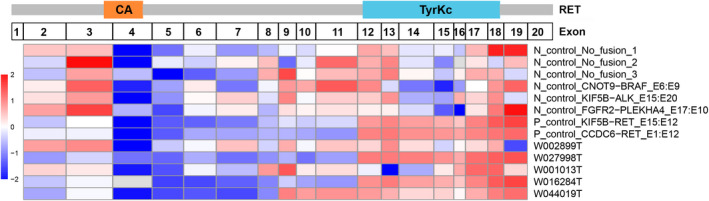
Heatmap of the average per‐base coverage for RET exons 2‐19 (Z‐score). Data for each sample was log2‐transformed and then Z‐score standardized using scale function
3.5. Coexistence of RET fusion with other actionable variations in LC patients
Previous studies reported that driver mutations are commonly mutually exclusive. 60 , 61 However, a coexistence of RET fusions with other driver variations was identified in the panel sequencing of lung cancer in this study. In 6.67% (9/135) of LC samples, RET fusions coincided with other driver mutations, such as EGFR L858R, EGFR exon 19 deletion, EGFR T790M, KRAS G12D, and/or EML4‐ALK fusion (Tables S3 and S4, Figure 6).
FIGURE 6.
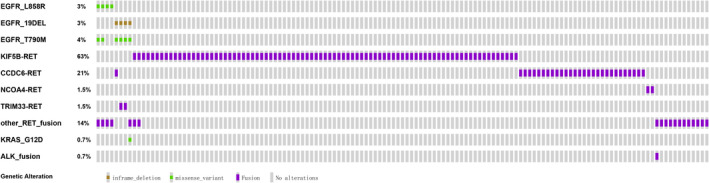
Coexistence status of actionable genes with RET fusions in 135 lung cancer patients. Driver mutations EGFR/L858R, EGFR/19DeL, and ALK fusion in nine individuals with RET fusions were exclusive to each other. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor resistance mutation, EGFR T790M, was present in six of eight EGFR‐driven patient tumors. The oncoprint of RET fusion and other driver mutations was identified using next‐generation sequencing. Different colors represent different categories of mutations
Notably, seven of the eight patients who harbored EGFR driver mutations in RET fusion‐positive tumors had undergone EGFR‐TKI treatment and had developed drug resistance, and six patients developed resistance to first‐generation EGFR‐TKIs with acquired resistance mutation EGFR T790M (Table 4). Furthermore, RET fusions were identified in six patients who had undergone treatment with osimertinib, including one patient (W054297T) who had never received first‐generation EGFR‐TKIs. Occurrence of RET fusion could contribute to resistance to third‐generation EGFR‐TKIs, as previously reported. 60 , 61 In addition, RET fusions from these eight lung cancer patients with co‐occurring EGFR driver mutations partnered with rare genes rather than the most frequent (KIF5B) in LC. The mechanism behind the “selective” RET fusions in contributing to acquired resistance should be explored further. In LC patients with no other well‐known driver mutations, the frequency of having a rare fusion partner of RET was 16.78% (15/126), lower compared with that in patients with other driver mutations (88.89%, 8/9) (P < .001, Pearson’s χ2 test) (Table S3). This finding validates the function of KIF5B‐RET fusion protein as a driver mutation in LC (Figure 6). In addition, it implies that different fusion partners might have different functions during oncogenesis.
TABLE 4.
RET fusion and EGFR comutation in lung cancer patients
| Sample ID | Age, y/gender | Sample type | RET fusion/AF | EGFR mutation/AF | EGFR‐TKI history |
|---|---|---|---|---|---|
| W025319T | 36/F | Tissue | GPRC6A‐RET/0.021 | p.Leu858Arg/0.247 | EGFR‐TKI naïve |
| LAL1965T | 61/F | Tissue | TLN1‐RET/0.071 | p.Leu858Arg/0.598 | Gefitinib, osimertinib |
| p.Thr790Met/0.228 | |||||
| LBD9835T | 63/M | Tissue | TRIM33‐RET/0.064 | p.Glu746_Thr751delinsAla/0.468 | Gefitinib, erlotinib, osimertinib |
| p.Thr790Met/0.199 | |||||
| LBE1673NX | 42/F | Plasma | TRIM33‐RET/0.012 | p.Leu747_Thr751del/0.254 | Erlotinib |
| p.Thr790Met/0.008 | |||||
| W054297T | 38/F | Plasma | KIAA1598‐RET/0.003 | p.Leu858Arg/0.548 | Osimertinib |
| W033932T | 70/F | Plasma | SPECC1‐RET/0.009 | p.Leu858Arg/0.082 | Gefitinib, osimertinib |
| p.Thr790Met/0.003 | |||||
| W045845T | 42/M | Plasma | TRIM24‐RET/0.052 | p.Glu746_Ala750del/0.169 | Gefitinib, osimertinib |
| p.Thr790Met/0.048 | |||||
| W005941N | 26/F | Plasma | CCDC6‐RET/0.018 | p.Glu746_Ala750del/0.087 | Gefitinib, osimertinib |
| p.Thr790Met/0.027 |
Abbreviations: AF, allele frequency; F, female; M, male; TKI, tyrosine kinase inhibitor.
4. DISCUSSION
Cancer‐associated RET fusions are recognized as RET if they occur at the 3′‐terminal, thus retaining the complete kinase domain, and can be targeted with recently approved RET inhibitors. RET fusion is frequent in PTC, CRC, and LC and can be present in several other cancer types. An accurate detection of RET fusion partners and breakpoints is critical for clinical management.
Various molecular testing methods have been developed to detect RET fusions, including NGS, RT‐PCR, FISH, and IHC. Immunohistochemistry is limited for general application due to its low sensitivity and specificity. 13 , 36 , 37 Reverse transcription‐PCR can only detect RET fusions with known fusion partners. 29 , 38 , 39 Although FISH is highly sensitive, it requires special technical expertise and it is not effective for identification of fusion partners, which could be critical for determining oncogenicity of fusion products. 38 , 40 The kinase domain of RET spans from exon 12 to 18. Breakpoints in RET and its fusion partners mainly occur in the intronic regions and can retain the ORF after mRNA splicing. In this study, breakpoints of 3′‐terminal RET fusion in intron 11 were the most common types, and breakpoints in RET introns 7, 8, 9, 10, 12, and 16 were also observed. Breakpoints in the kinase domain of RET destroy the activity of the protease, resulting in a nonfunctional fusion product. Therefore, it is necessary to identify the breakpoints of RET fusion and other gene fusions. In addition, FISH assay could result in false negative results when fusion partners are in close proximity with RET (for instance, ZNF33B is ~0.5 Mb away from RET), or false positive results when breakpoints are located in the kinase domain (shown as nonfunctional fusions CCDC60‐RET E2:E17, SLX4IP‐RET E2:E13, and UPP2‐RET, E3:E17 in this study). However, it is necessary to clarify the partner genes fused with RET, as different fusion partners could activate RET through different mechanisms, which means that the sensitivity to inhibitors will also be different. Additionally, RET fusion with rare partners could be the cause of resistance to EGFR‐TKI. Next‐generation sequencing can identify alterations of multiple genes simultaneously with precise identification of fusion partners and breakpoints; therefore, it has become the most widely used procedure in clinical testing. 33
Co‐occurrence of RET fusion with other oncogenic driver mutations (eg, EGFR, BRAF, ALK, and ROS1) indicates a sensitization or resistance to existing targeted therapies. 27 , 62 , 63 , 64 Biological functions of these comutations have not been fully explored for effective guidance in clinical therapies. Discrimination between different RET fusion types and targeted drug sensitivity is crucial for clinical applications. The function of fusion products with different partners has not been explored. A preference for KIF5B (62.04%) in LC, NCOA4 (45.45%) in CRC, and CCDC6 (93.33%) in PTC and different fusion partners between acquired TKI resistance and driver mutations in LC reflects different biological functions of fusion products (Figures 2 and 5).
In summary, a series of novel and previously reported RET fusions were identified by screening large‐scale NGS data from a large sample of Chinese patients with different cancer types. In addition to LC and PTC, the analysis showed that patients with other cancers also occasionally carry RET fusions. RET‐activating fusions, which can be targeted using RET inhibitors, were identified by filtering out those with 5′‐terminal RET fusions and fusions whose breakpoints occurred in the kinase domain. The findings of this study have potential clinical application as these RET fusions can be used to guide cancer diagnosis and/or stratify patients for targeted therapies across different cancer types. Further clinical studies should be undertaken to explore the sensitivity of different fusions in response to RET inhibitors.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
Figure S1
Table S1‐S4
ACKNOWLEDGMENTS
This paper was supported by grants from the Wenzhou Municipal Science and Technology Bureau of China (No. Y20180175). The study was approved by clinical research ethics board of Wenzhou Medical University (No. 2016–197).
Shi M, Wang W, Zhang J, et al. Identification of RET fusions in a Chinese multicancer retrospective analysis by next‐generation sequencing. Cancer Sci.2022;113:308–318. 10.1111/cas.15181
Minke Shi, Weiran Wang, and Jinku Zhang contributed equally to this work.
Funding information
The paper was supported by grants from the Wenzhou Municipal Science and Technology Bureau of China (No. Y20180175)
Contributor Information
Dezhi Cheng, Email: dezhicheng@sina.com.
Tonghui Ma, Email: tonghuima@yeah.net.
REFERENCES
- 1. Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581‐588. [DOI] [PubMed] [Google Scholar]
- 2. Alberti L, Carniti C, Miranda C, Roccato E, Pierotti MA. RET and NTRK1 proto‐oncogenes in human diseases. J Cell Physiol. 2003;195:168‐186. [DOI] [PubMed] [Google Scholar]
- 3. McCarty MF. Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integr Cancer Ther. 2004;3:349‐380. [DOI] [PubMed] [Google Scholar]
- 4. Gainor JF, Shaw AT. Novel targets in non‐small cell lung cancer: ROS1 and RET fusions. Oncologist. 2013;18:865‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qian YY, Chai S, Liang Z, et al. KIF5B‐RET fusion kinase promotes cell growth by multilevel activation of STAT3 in lung cancer. Mol Cancer. 2014;13:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET‐driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15:151‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14:173‐186. [DOI] [PubMed] [Google Scholar]
- 8. Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto‐oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12:192‐202. [DOI] [PubMed] [Google Scholar]
- 9. Schram AM, Chang MT, Jonsson P, Drilon A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol. 2017;14:735‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Consortium ITP‐CAoWG . Pan‐cancer analysis of whole genomes. Nature. 2020;578:82‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bounacer A, Wicker R, Schlumberger M, Sarasin A, Suarez HG. Oncogenic rearrangements of the ret proto‐oncogene in thyroid tumors induced after exposure to ionizing radiation. Biochimie. 1997;79:619‐623. [DOI] [PubMed] [Google Scholar]
- 12. Hamatani K, Eguchi H, Ito R, et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008;68:7176‐7182. [DOI] [PubMed] [Google Scholar]
- 13. Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohno T, Tabata J, Nakaoku T. REToma: a cancer subtype with a shared driver oncogene. Carcinogenesis. 2020;41:123‐129. [DOI] [PubMed] [Google Scholar]
- 15. Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET aberrations in diverse cancers: next‐generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23:1988‐1997. [DOI] [PubMed] [Google Scholar]
- 16. Rich TA, Reckamp KL, Chae YK, et al. Analysis of cell‐free DNA from 32,989 advanced cancers reveals novel co‐occurring activating RET alterations and oncogenic signaling pathway aberrations. Clin Cancer Res. 2019;25:5832‐5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santoro M, Moccia M, Federico G, Carlomagno F. RET gene fusions in malignancies of the thyroid and other tissues. Genes. 2020;11(4):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim P, Jia P, Zhao Z. Kinase impact assessment in the landscape of fusion genes that retain kinase domains: a pan‐cancer study. Brief Bioinform. 2018;19:450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang K, Chen H, Wang Y, et al. Clinical characteristics and molecular patterns of RET‐rearranged lung cancer in Chinese patients. Oncol Res. 2019;27:575‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yakushina VD, Lerner LV, Lavrov AV. Gene fusions in thyroid cancer. Thyroid. 2018;28:158‐167. [DOI] [PubMed] [Google Scholar]
- 21. Li AY, McCusker MG, Russo A, et al. RET fusions in solid tumors. Cancer Treat Rev. 2019;81:101911. [DOI] [PubMed] [Google Scholar]
- 22. Saito M, Shimada Y, Shiraishi K, et al. Development of lung adenocarcinomas with exclusive dependence on oncogene fusions. Cancer Res. 2015;75:2264‐2271. [DOI] [PubMed] [Google Scholar]
- 23. Hamatani K, Eguchi H, Koyama K, Mukai M, Nakachi K, Kusunoki Y. A novel RET rearrangement (ACBD5/RET) by pericentric inversion, inv(10)(p12.1;q11.2), in papillary thyroid cancer from an atomic bomb survivor exposed to high‐dose radiation. Oncol Rep. 2014;32:1809‐1814. [DOI] [PubMed] [Google Scholar]
- 24. Iyama K, Matsuse M, Mitsutake N, et al. Identification of three novel fusion oncogenes, SQSTM1/NTRK3, AFAP1L2/RET, and PPFIBP2/RET, in thyroid cancers of young patients in Fukushima. Thyroid. 2017;27:811‐818. [DOI] [PubMed] [Google Scholar]
- 25. Cancer Genome Atlas Research N . Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Staubitz JI, Musholt TJ, Schad A, et al. ANKRD26‐RET – a novel gene fusion involving RET in papillary thyroid carcinoma. Cancer Genet. 2019;238:10‐17. [DOI] [PubMed] [Google Scholar]
- 27. Xu H, Shen J, Xiang J, et al. Characterization of acquired receptor tyrosine‐kinase fusions as mechanisms of resistance to EGFR tyrosine‐kinase inhibitors. Cancer Manag Res. 2019;11:6343‐6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lira ME, Choi Y‐L, Lim SM, et al. A single‐tube multiplexed assay for detecting ALK, ROS1, and RET fusions in lung cancer. J Mol Diagn. 2014;16:229‐243. [DOI] [PubMed] [Google Scholar]
- 29. Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET‐rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35:1403‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paratala BS, Chung JH, Williams CB, et al. RET rearrangements are actionable alterations in breast cancer. Nat Commun. 2018;9:4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann Oncol. 2018;29:1394‐1401. [DOI] [PubMed] [Google Scholar]
- 32. Selpercatinib MA. First approval. Drugs. 2020;80:1119‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stinchcombe TE. Current management of RET rearranged non‐small cell lung cancer. Ther Adv Med Oncol. 2020;12:1758835920928634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET‐altered cancers. Ann Oncol. 2018;29:1869‐1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU‐667 for RET‐driven cancers. Cancer Discov. 2018;8:836‐849. [DOI] [PubMed] [Google Scholar]
- 36. Ferrara R, Auger N, Auclin E, Besse B. Clinical and translational implications of RET rearrangements in non‐small cell lung cancer. J Thorac Oncol. 2018;13:27‐45. [DOI] [PubMed] [Google Scholar]
- 37. Go H, Jung YJ, Kang HW, et al. Diagnostic method for the detection of KIF5B‐RET transformation in lung adenocarcinoma. Lung Cancer. 2013;82:44‐50. [DOI] [PubMed] [Google Scholar]
- 38. Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non‐small‐cell lung cancer. J Clin Oncol. 2012;30:4352‐4359. [DOI] [PubMed] [Google Scholar]
- 39. Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern‐specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84:121‐126. [DOI] [PubMed] [Google Scholar]
- 40. Tsuta K, Kohno T, Yoshida A, et al. RET‐rearranged non‐small‐cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer. 2014;110:1571‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li H, Durbin R. Fast and accurate short read alignment with Burrows‐Wheeler transform. Bioinformatics. 2009;25:1754‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK. Strelka: accurate somatic small‐variant calling from sequenced tumor‐normal sample pairs. Bioinformatics. 2012;28:1811‐1817. [DOI] [PubMed] [Google Scholar]
- 44. Chen S, Liu M, Huang T, Liao W, Xu M, Gu J. GeneFuse: detection and visualization of target gene fusions from DNA sequencing data. Int J Biol Sci. 2018;14:843‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cancer Genome Atlas Research N . Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ge H, Liu K, Juan T, Fang F, Newman M, Hoeck W. FusionMap: detecting fusion genes from next‐generation sequencing data at base‐pair resolution. Bioinformatics. 2011;27:1922‐1928. [DOI] [PubMed] [Google Scholar]
- 48. Pierotti MA, Santoro M, Jenkins RB, et al. Characterization of an inversion on the long arm of chromosome 10 juxtaposing D10S170 and RET and creating the oncogenic sequence RET/PTC. Proc Natl Acad Sci USA. 1992;89:1616‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Attie‐Bitach T, Abitbol M, Gerard M, et al. Expression of the RET proto‐oncogene in human embryos. Am J Med Genet. 1998;80:481‐486. [DOI] [PubMed] [Google Scholar]
- 50. Ju YS, Lee W‐C, Shin J‐Y, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole‐genome and transcriptome sequencing. Genome Res. 2012;22:436‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Norskov‐Lauritsen L, Jorgensen S, Brauner‐Osborne H. N‐glycosylation and disulfide bonding affects GPRC6A receptor expression, function, and dimerization. FEBS Lett. 2015;589:588‐597. [DOI] [PubMed] [Google Scholar]
- 52. Aleksandrova N, Gutsche I, Kandiah E, et al. Robo1 forms a compact dimer‐of‐dimers assembly. Structure. 2018;26(2):320‐328 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krishna SS, Majumdar I, Grishin NV. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. 2003;31:532‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bezerra GA, Dobrovetsky E, Seitova A, Fedosyuk S, Dhe‐Paganon S, Gruber K. Structure of human dipeptidyl peptidase 10 (DPPY): a modulator of neuronal Kv4 channels. Sci Rep. 2015;5:8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET‐driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15:150. [DOI] [PubMed] [Google Scholar]
- 56. Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking‐mediated plasticity of inhibitory synapses. Neuron. 2011;70:385‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li W, Guo L, Liu Y, et al. Potential unreliability of uncommon ALK, ROS1, and RET genomic breakpoints in predicting the efficacy of targeted therapy in NSCLC. J Thorac Oncol. 2021;16:404‐418. [DOI] [PubMed] [Google Scholar]
- 58. Cui M, Han Y, Li P, et al. Molecular and clinicopathological characteristics of ROS1‐rearranged non‐small‐cell lung cancers identified by next‐generation sequencing. Mol Oncol. 2020;14:2787‐2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davies KD, Le AT, Sheren J, et al. Comparison of molecular testing modalities for detection of ROS1 rearrangements in a cohort of positive patient samples. J Thorac Oncol. 2018;13:1474‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shaw AT, Yeap BY, Mino‐Kenudson M, et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol. 2009;27:4247‐4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horn L, Pao W. EML4‐ALK: honing in on a new target in non‐small‐cell lung cancer. J Clin Oncol. 2009;27:4232‐4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Klempner SJ, Bazhenova LA, Braiteh FS, et al. Emergence of RET rearrangement co‐existing with activated EGFR mutation in EGFR‐mutated NSCLC patients who had progressed on first‐ or second‐generation EGFR TKI. Lung Cancer. 2015;89:357‐359. [DOI] [PubMed] [Google Scholar]
- 63. Offin M, Somwar R, Rekhtman N, et al. Acquired ALK and RET gene fusions as mechanisms of resistance to osimertinib in EGFR‐mutant lung cancers. JCO Precis. Oncol. 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR‐mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU‐667 for acquired RET fusion. Cancer Discov. 2018;8:1529‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1‐S4


