Abstract
To improve treatment outcomes in real practice, useful biomarkers are desired when predicting postoperative recurrence for renal cell carcinoma (RCC). We collected data from patients who underwent definitive surgery for RCC and for benign urological tumor at our department between November 2016 and December 2019. We evaluated the differences in pre‐ and postoperative urinary metabolites with our precise quantitative method and identified predictive factors for RCC recurrence. Additionally, to clarify the significance of metabolites, we measured the intracellular metabolite concentration of three RCC cell lines. Among the 56 patients with RCC, nine had a recurrence (16.0%). When comparing 27 patients with T1a RCC and 10 with benign tumor, a significant difference was observed between pre‐ and postoperative concentrations among 10 urinary metabolites. In these 10 metabolites, multiple logistic regression analysis identified five metabolites (lactic acid, glycine, 2‐hydroxyglutarate, succinic acid, and kynurenic acid) as factors to build our recurrence prediction model. The values of area under the receiver operating characteristic curve, sensitivity, and specificity in this predictive model were 0.894%, 88.9%, and 88.0%, respectively. When stratified into low and high risk groups of recurrence based on this model, we found a significant drop of recurrence‐free survival rates among the high risk group. In in vitro studies, intracellular metabolite concentrations of metastatic tumor cell lines were much higher than those of primary tumor cell lines. By using our quantitative evaluation of urinary metabolites, we could predict postoperative recurrence with high sensitivity and specificity. Urinary metabolites could be noninvasive biomarkers to improve patient outcome.
Keywords: biomarker, intracellular metabolite, recurrence, renal cell carcinoma, urinary metabolite
Although the postoperative recurrence rate for nonmetastatic renal cell carcinoma (RCC) has been reported to be approximately 30%, there was no marker to predict recurrence. In this study, we evaluated urinary metabolites to identify new noninvasive biomarkers, and established a recurrence prediction model with high sensitivity and specificity by our accurate quantitative measurement system. Additionally, we undertook an in vitro study to investigate their intracellular concentrations in three RCC cell lines to understand their clinical significance.
Abbreviations
- 2‐HG
2‐hydroxyglutarate
- αKG
α‐ketoglutaric acid
- AUC
area under the receiver operating characteristic curve
- ccRCC
clear cell renal cell carcinoma
- DFS
disease‐free survival
- DS7P
d‐sedoheptulose 7‐phosphate
- eGFR
estimated glomerular filtration rate
- F6P
fructose 6‐phosphate
- G6P
glucose‐6‐phosphate
- IS
internal standard substance
- LC‐MS/MS
liquid chromatography–tandem mass spectrometry
- NR
not reached
- OS
overall survival
- RCC
renal cell carcinoma
- TCA
tricarboxylic acid
1. INTRODUCTION
Renal cell carcinoma accounts for approximately 3% of all adult malignancies. The American Cancer Society estimated that 73 750 men were diagnosed with RCC and 14 830 patients died of RCC in 2020. 1 , 2 Although most detected RCC are small tumors, the recurrence rate after definitive operation for nonmetastatic RCC has been reported to be approximately 30%, with most recurrence happening within 2 years after operation, due to microscopic spread of cancer prior to surgery. 3 , 4 , 5 Prognostic factors of clinically localized RCC, which include histological factors such as increasing T stage, node positive stage, greater Fuhrman grading, vascular invasion, and tumor necrosis, are already widely known. Performance status, paraneoplastic syndromes, laboratory data of calcium, albumin, hemoglobin or C‐reactive protein, and protein expression such as Ki‐67, p53, ATP‐binding cassette transporter subfamily G, or hypoxia inducible factor, have also been studied and used to predict RCC recurrence after definitive therapy. 6 , 7 Although several prognostic models and nomograms using these risk factors have been mentioned in current guidelines, standard examination of these factors in clinical practice have not been recommended for predicting localized RCC recurrence. 5
Recently, several studies have attempted to establish adjuvant systemic therapies for high‐risk RCC. 8 , 9 The purpose of adjuvant systemic therapies has been to eliminate microscopic cancer cells and increase cure rates. 9 However, there has only been one positive study in 13 randomized trials. 8 The phase III S‐TRAC trial study, which randomized 615 RCC M0 patients to sunitinib or placebo after surgery, reported improved DFS. 9 However, no improvement in DFS or OS could be observed in the other 12 trials. 8 Useful biomarkers are desired to increase accuracy in prediction of recurrence after definitive therapy for localized RCC, to improve treatment outcomes, and decrease overtreatment.
Based on available resources, metabolomics has been evaluated to identify new biomarkers in several cancers for predictive factors of recurrence. 10 , 11 , 12 , 13 A systematic review in colorectal cancer showed that several metabolites, including lactic acid, kynurenic acid, and succinic acid, were related to recurrence. 11 Loras et al 12 constructed a recurrence prediction model by measuring differences in the 128 urinary metabolome of bladder cancer patients before and after operation. Several studies have reported evaluations of urinary metabolites for biomarkers of RCC. 14 , 15 , 16 Liu et al 14 reported that nine metabolites showed the best predictive ability for RCC with an AUC value of 0.905 for the training dataset and 0.885 for the validation dataset. Falegan et al 15 showed alterations in the levels of multiple glycolytic and TCA cycle intermediate metabolites in RCC urine. However, both measurement systems applied semiquantitative analysis without the use of internal standards, and did not take the urinary matrix effect into account. We have reported the construction of a system for accurate quantitative measurement of urinary metabolites and established predictive models for diagnosis and malignant status of RCC. 17 Our quantitative measurement system includes the use of an internal standard to exclude the effect of the matrix on metabolite concentrations and the correction for dilution based on urine creatinine concentrations. Multiple logistic regression analysis revealed five metabolites (glutamic acid, lactate, DS7P, 2‐HG, and myoinositol) for the diagnostic predictive model and four metabolites (kynurenine, glutamine, F6P, and butyrylcarnitine) for the predictive model for clinical stage III/IV. Although our previous report did not show the predicting prognosis, we expect that the methods can be followed.
In the present study, we evaluated the difference between pre‐ and postoperative urinary metabolites with our quantitative measurement methods to identify predictive factors for recurrence of RCC. Additionally, we discuss the efficacy of urinary metabolites related to recurrence in real practice by considering the significance of these metabolites in RCC, based on the results of experiments using several RCC cell lines.
2. MATERIALS AND METHODS
2.1. Design and study cohort
We compiled data from 56 patients treated with radical nephrectomy or partial nephrectomy for clear cell RCC (ccRCC) at our institution between November 2016 and December 2019. None of these patients had received any neoadjuvant or adjuvant therapy, such as molecular targeted therapy or cytokine therapy. Control urine samples were also obtained from 10 patients treated with surgery for benign urological tumor. The benign urological tumor group included three angiomyolipoma patients and seven primary aldosteronism patients. Patients with a history of malignancy and those undergoing dialysis were excluded from this study.
Preoperative urine samples were collected the day before operation and postoperative samples were collected before discharge (3‐7 days after operation). The collected urine samples were immediately centrifuged at 1450 g for 10 minutes at 4°C, and the resulting supernatants were stored at −80°C until the time of analysis. By reviewing medical records, we collected clinical information including, age, sex, performance status, eGFR, body mass index, operation, and recurrence. Pathological evaluation was carried out by expert genitourinary pathologists at our institution according to the 7th edition of the American Joint Committee on Cancer staging system. After comparing the metabolites between the cT1a ccRCC group and the benign tumor group to detect the significant metabolites in ccRCC, we evaluated whether those metabolites could predict recurrence after definitive therapy in ccRCC patients. This study was approved by the institutional review board at Tohoku University Hospital. Study participants provided written informed consent.
2.2. Chemicals and reagents
Of the 33 targeted urinary metabolites, which were quantitatively measurable as previously reported, we evaluated 17 urinary metabolites that had significant reproducibility. 17 As in the previous study, we used standard substances used for the calibration curve method, and the isotope‐labeled internal standards for precise quantitative measurement. 17
2.3. Liquid chromatography‐MS/MS conditions
All LC‐MS/MS analyses were undertaken using an LC‐MS‐8050 triple‐quadrupole mass spectrometer coupled with a Nexera X2 UHPLC system (Shimadzu) and Lab Solutions software (Shimadzu). Analyses for urinary metabolites were separated into four groups (designated groups 1‐4) and analyzed with optimized methods to improve the measurement sensitivity for each metabolite.
For all four analytical groups, the column oven temperature was set at 40°C and electrospray ionization mode was selected as the ion source probe. The conditions of the ion source probe were set as follows: probe voltage, 4000 V; desolation line temperature, 100°C; block heater temperature, 150°C; interface temperature, 400°C; nebulizing gas flow, 2 L/min; drying gas, 3 L/min; and heating gas flow, 17 L/min. Column and mobile phases were selected to optimize the sensitivity for each metabolite. Those conditions, along with the preparation of the calibration standards and internal standards for each group, are reported in our previous report.
2.4. Urine sample preparation for LC‐MS/MS
At the time of analysis, 25 µL from each urine sample was combined with an equal volume of a given internal standard, along with 200 µL acetonitrile. The mixture was vortexed for 5 seconds and centrifuged at 15 000 g at 4°C for 5 minutes. 17 Aliquots (120 µL each) of the supernatant were then transferred to separate 1.5‐mL microcentrifuge tubes (cat. no. 509‐GRD‐Q; Thermo Fisher Scientific) and evaporated under reduced pressure for 1 hour.
Separate aliquots from a given sample were reconstituted with 20 µL of either water (for group 1) or a 75:25 (v:v) mixture of 20 µL acetonitrile : water (for group 2), and 20 μL of each solution was then injected into the relevant analytical system.
As sample preparation for analytical group 3, a 200‐µL aliquot of human urine was combined with 25 μL internal standard. We added 1 mL acetonitrile. The sample was mixed by vortexing for 5 seconds and then centrifuged at 15 000 g at 4°C for 5 minutes. The resulting supernatant was transferred to a new microcentrifuge tube and evaporated under reduced pressure for 1 hour. Each aliquot was reconstituted with 50 µL water. Ten microliters of the solution then was injected into the analytical system.
As sample preparation for analytical group 4, a 50‐µL aliquot of human urine was combined with 175 μL pure water in a microcentrifuge tube. We then added 50 μL IS and 225 μL acetonitrile to the mixture. The sample was mixed by vortexing for 5 second and then centrifuged at 15 000 g at 4°C for 5 minutes. After the supernatant was transferred to a new microcentrifuge tube, 1 μL was removed to be injected into the analytical system.
2.5. Urine creatinine assay
Creatinine concentrations for all urine samples were measured by an enzymatic protocol with the CRE‐CL commercial kit (Serotec). Absorbances were read on an Infinite 200 Pro microplate reader (Tecan). The measured metabolite urinary concentrations (in µmol/L) were adjusted according to the urinary creatinine concentration (in mmol/L) of their respective samples.
2.6. Cell lines and culture
Human RCC cell lines (786‐O, ACHN, and Caki‐1) were purchased from ATCC and maintained in culture medias. 786‐O cells were maintained in an incubator at 37°C and 5% CO2 in RPMI‐1640 medium (Gibco/Life Technologies) supplemented with 10% FBS. ACHN and Caki‐1 cells were maintained in DMEM (Gibco/Life Technologies) supplemented with 10% FBS. Most of the experiments using cell lines were carried out within 3‐6 months and within 10 passages after purchase from ATCC. Each cell line was seeded at 2 × 106 cells/dish for subsequent analysis and cultured at 37% with 5% CO2. After culturing for 72 hours, cells were washed with D‐PBS twice and recovered with a scraper. Cell pellets were stored at −80°C until measurement.
2.7. Cellular sample preparation for LC‐MS/MS
Mixtures of 100 µL IS and 100 µL of 50% acetonitrile were added to cell pellets. Pellets were then vortexed and disrupted by an ultrasonic vibrator. Samples were centrifuged for 5 minutes at 15 000 g and 4°C, where 100 µL supernatant was transferred to a 1.5‐mL PP tube (Lot 509‐GRD‐Q; Thermo Fisher Scientific). Supernatants were then dried with a centrifugal evaporator for 1 hour and redissolved into either 50 µL water for group 1 samples or 50 µL of a 75:25 (v:v) mixture of 20 µL acetonitrile and water for group 2 samples. For group 3, 25 µL IS, 200 µL water, and 1 mL acetonitrile were added to cell pellets and vortexed. Cell disruption was subsequently carried out with an ultrasonic vibrator. Samples were then centrifuged for 5 minutes at 15 000 g at 4°C, and supernatants were transferred to a 1.5‐mL PP tube. Samples were then dried with a centrifugal evaporator and reconstituted in 20 µL water. For group 4, 140 µL 50% acetonitrile, 10 µL IS, 175 µL water, and 175 µL acetonitrile were added to cell pellets. Samples were vortexed and cell disruption was carried out with an ultrasonic vibrator. Samples were then centrifuged for 5 minutes at 15 000 g and 4°C and supernatants were transferred to a 1.5‐mL PP tube. Approximately 50 µL supernatant was used for measurement. The obtained intracellular metabolite concentration in each cell was counted, and concentration per 10 000 cells was calculated. Cells were cultured in triplicate, and the mean metabolite concentration was calculated three times.
2.8. Statistical analysis
Clinical information and urine test results are presented as the mean and standard deviation, and were compared using Student’s t test. Clinical information including, sex, age, eGFR, body mass index, operation, and pathological diagnosis, were compared using Pearson’s χ2 test. The ratio of urine metabolites after operation to those before operation are shown as fold change values. The stepwise method was used to select variables for multivariate analysis. Multivariate analysis was undertaken using multiple logistic regression analysis. 17 Receiver operating characteristic curve analysis was used to evaluate the diagnostic performance of the resulting regression model based on its AUC, sensitivity, and specificity values. Survival curves were plotted using the Kaplan‐Meier method and statistical significance was assessed using the log‐rank test. A P value of .05 or less was considered statistically significant. Statistical analyses were undertaken using JMP Pro 15 software (SAS Institute).
3. RESULTS
3.1. Evaluation of the ratio of pre‐ to postoperative concentrations of 17 urinary metabolites between T1a ccRCC and benign tumor
Table 1 lists the patient characteristics and clinicopathologic findings of the T1a ccRCC group and the benign tumor group. No significance difference was seen in the preoperative patient characteristics between the two groups. The mean age was 62.6 years in the T1a ccRCC group and 59.4 years in the benign tumor group, respectively (P = .89). There were 21 male patients in the T1a ccRCC group (77.8%), and five male patients in the benign tumor group (P = .94). Renal dysfunction by eGFR (< 60 ml/min/1.73 m2) of the T1a ccRCC group increased from six patients to 13 patients after operation, while there was no increase in number of patients among the benign tumor group.
TABLE 1.
Characteristics and clinicopathologic findings of 27 patients with T1a clear cell renal cell carcinoma (ccRCC) and benign tumor
| Characteristic | T1a ccRCC | Benign tumor | P value |
|---|---|---|---|
| Patients, n | 27 | 10 | |
| Sex, n (%) | |||
| Male | 21 (77.8) | 5 (50.0) | |
| Female | 6 (22.2) | 5 (50.0) | NS (.10) |
| Mean age, y (range) | 62.6 (49‐83) | 59.4 (48‐90) | NS (.89) |
| eGFR preoperation, n (%) | |||
| >60 | 21 (77.8) | 7 (70.0) | NS (.94) |
| 15‐60 | 6 (22.2) | 3 (30.0) | |
| <15 | 0 (0) | 0 (0) | |
| eGFR postoperation, n (%) | |||
| >60 | 14 (51.9) | 9 (90.0) | NS (.055) |
| 15‐60 | 13 (48.1) | 1 (10.0) | |
| <15 | 0 (0) | 0 (0) | |
| BMI, n (%) | |||
| <18.5 | 0 (0) | 1 (10.0) | NS (.31) |
| 18.5‐25 | 13 (48.1) | 2 (20.0) | |
| >25 | 14 (51.9) | 7 (70.0) | |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; NS, not significant.
As described in our previous study of ccRCC tissue and urine of ccRCC patients, 17 metabolites were crucial to evaluate clinicopathologic features in the present study. Of the 17 metabolites, there were significant differences in the ratio of post‐ to preoperative concentrations of 10 urinary metabolites in a nonparametric test (Table 2). The 10 metabolites were galactose, F6P, DS7P, lactic acid, succinic acid, glutamine, glutamic acid, 2‐HG, glycine, and kynurenic acid. Although the ratios of the benign tumor group were more than one in nine urinary metabolites, those of the T1a ccRCC group were less than one in six urinary metabolites. Only the ratio of kynurenic acid was less than one in the benign tumor group, but more than one in the T1a ccRCC group.
TABLE 2.
Comparison of urinary metabolite concentrations between patients with T1a clear cell renal cell carcinoma (ccRCC) and those with benign tumor
| Variable | T1a ccRCC | Benign tumor | |||||
|---|---|---|---|---|---|---|---|
|
Mean concentration preoperation |
Mean concentration postoperation |
Mean ratio of post / pre |
Mean concentration preoperation |
Mean concentration postoperation |
Mean ratio of post / pre |
P value of mean ratio |
|
| Urinary metabolite | μmol/L/u‐Cre (mmol/L) | μmol/L/u‐Cre (mmol/L) | μmol/L/u‐Cre (mmol/L) | μmol/L/u‐Cre (mmol/L) | |||
| Galactose | 0.011 | 0.0024 | 0.39 | 0.11 | 0.23 | 5.98 | .0032 |
| F6P | 0.86 | 0.015 | 0.16 | 0.68 | 22.25 | 161.83 | .0016 |
| DS7P | 200.97 | 2.41 | 8.14 | 98.33 | 1066.1 | 79.34 | .0028 |
| Lactic acid | 191.97 | 1.48 | 0.28 | 1334.33 | 931.57 | 8.20 | .0423 |
| Propionic acid | 3619.90 | 79.99 | 14.84 | 3493.63 | 116.38 | 1.77 | .6800 |
| Succinic acid | 24.20 | 0.37 | 0.028 | 8.14 | 8.54 | 45.57 | .0028 |
| Glutamine | 124.89 | 22.28 | 1.59 | 356.61 | 449.38 | 39.1 | .0094 |
| Glutamic acid | 3.47 | 0.58 | 1.09 | 98.22 | 1065.6 | 79.16 | .0077 |
| αKG | 2.09 | 1.53 | 19.98 | 2.43 | 8.96 | 6.19 | .2800 |
| 2‐HG | 1284.83 | 4.85 | 0.071 | 172.98 | 44.85 | 2.09 | .0025 |
| Glycine | 26.51 | 0.0063 | 0.20 | 0.11 | 0.19 | 12.73 | .0036 |
| GSH | 0.45 | 1.17 | 76.02 | 0.57 | 50.7 | 206.07 | .3700 |
| Free carnitine | 7.11 | 9.39 | 3.86 | 34.5 | 46.9 | 7.88 | .6800 |
| Acylcarnitine | 13.75 | 14.27 | 2.32 | 46.31 | 70.85 | 2.93 | .4300 |
| Butylcarnitine | 4.26 | 2.61 | 0.86 | 3.06 | 2.64 | 1.06 | .3300 |
| Kynurenine | 0.062 | 0.51 | 10.03 | 0.18 | 0.20 | 2.01 | .3000 |
| Kynurenic acid | 0.22 | 0.28 | 41.87 | 0.17 | 0.006 | 0.83 | .0250 |
Abbreviations: 2‐HG, 2‐hydroxyglutarate; αKG, α‐ketoglutaric acid; DS7P, d‐sedoheptulose 7‐phosphate; F6P, fructose 6‐phosphate; GSH, glutathione.
When we classified the metabolic pathways of these 10 metabolites, we found that four of the metabolites were related to glycolysis, one was related to TCA cycle, four were related to glutaminolysis, and one was related to the tryptophan pathway (Figure 1).
FIGURE 1.
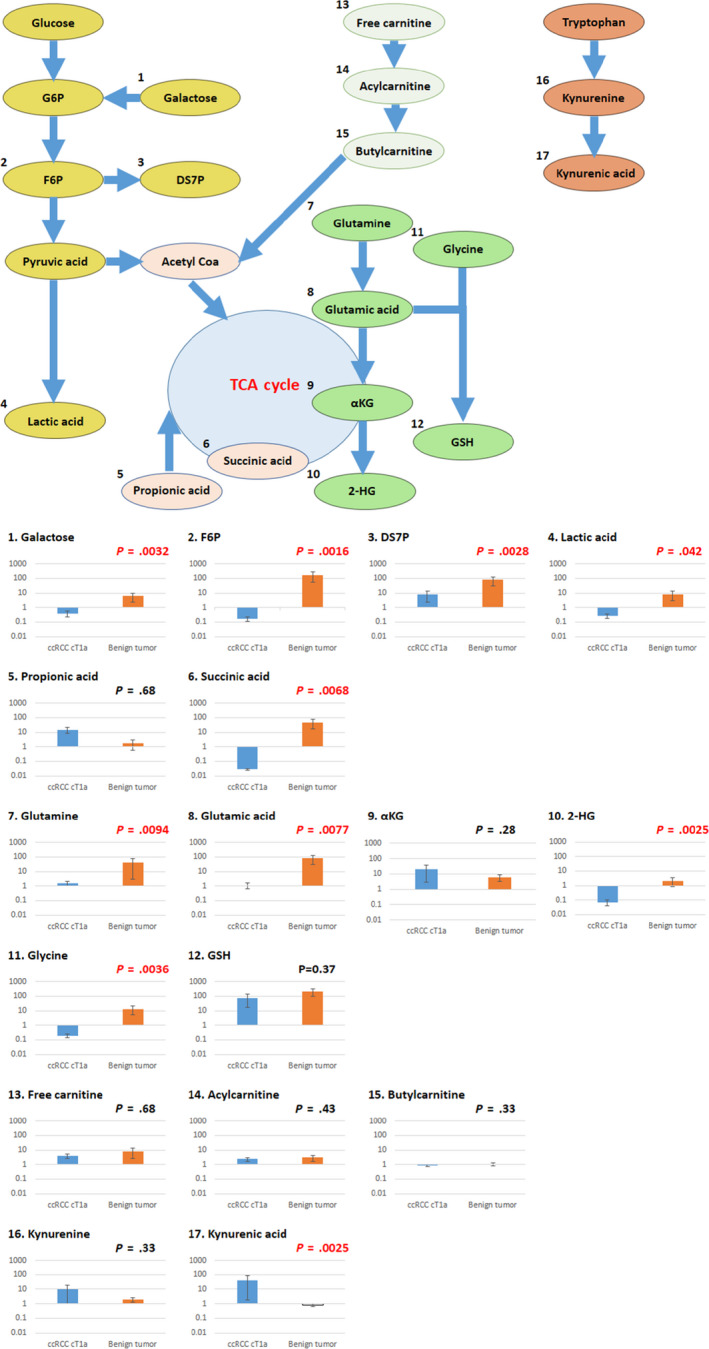
Relationships between metabolic pathways and ratios of post‐ to preoperative concentrations of 17 urinary metabolites in patients with T1a clear cell renal cell carcinoma (ccRCC) and those with benign tumor. 2‐HG, 2‐hydroxyglutarate; αKG, α‐ketoglutaric acid; DS7P, d‐sedoheptulose 7‐phosphate; F6P, fructose 6‐phosphate; G6P, glucose‐6‐phosphate; GSH, glutathione; TCA, tricarboxylic acid
3.2. Comparison of the ratio of post‐ to preoperative concentrations of 10 urinary metabolites between ccRCC with and without recurrence
Characteristics and clinicopathologic findings of ccRCC patients with and without recurrence are shown in Table 3. Of the total 56 patients, nine had recurrence with higher T stage and Fuhrman grade (P < .001) compared to patients without recurrence.
TABLE 3.
Characteristics and clinicopathologic findings of 47 clear cell renal cell carcinoma (ccRCC) patients with recurrence and nine patients without recurrence
| Characteristic | ccRCC without recurrence | ccRCC with recurrence | P value |
|---|---|---|---|
| Patients, n | 47 | 9 | |
| Sex, n (%) | |||
| Male | 38 (80.9) | 7 (77.8) | |
| Female | 9 (19.1) | 2 (22.2) | NS (.8600) |
| Mean age, y (range) | 63.0 (49‐83) | 66.7 (48‐90) | NS (.8400) |
| eGFR preoperation, n (%) | |||
| >60 | 31 (66.0) | 5 (55.6) | NS (.0880) |
| 15‐60 | 16 (34.0) | 4 (44.4) | |
| <15 | 0 (0.0) | 0 (0.0) | |
| eGFR postoperation, n (%) | |||
| >60 | 14 (29.8) | 5 (55.6) | NS (.0600) |
| 15‐60 | 33 (70.2) | 4 (44.4) | |
| <15 | 0 (0.0) | 0 (0.0) | |
| BMI, n (%) | |||
| <18.5 | 2 (4.3) | 1 (11.2) | NS (.6000) |
| 18.5‐25 | 20 (42.6) | 4 (44.4) | |
| >25 | 25 (53.2) | 4 (44.4) | |
| Operation, n (%) | |||
| Nephrectomy | 23 (50.0) | 9 (100) | .0054 |
| Nephron sparing surgery | 23 (50.0) | 0 (0.0) | |
| Pathological T stage, n (%) | |||
| T1a | 27 (67.5) | 0 (0.0) | <.0010 |
| T1b | 9 (19.1) | 0 (0.0) | |
| T2 | 2 (4.3) | 0 (0.0) | |
| T3 | 9 (19.1) | 9 (100.0) | |
| Fuhrman grade, n (%) | |||
| 1 | 13 | 0 | <.0010 |
| 2 | 32 | 4 | |
| 3 | 2 | 4 | |
| 4 | 0 | 1 | |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; NS, not significant.
In 10 urinary metabolites described in the previous results of the cT1a RCC group and control group (Table 4), we found significant differences in three urinary metabolites (glutamic acid, 2‐HG, and glycine) among patients without recurrence compared to those with recurrence. The mean ratio of glutamic acid was 2.64 in ccRCC patients without recurrence and 39.3 in patients with recurrence (P = .021). The mean ratio of 2‐HG was 0.11 in the group without recurrence and 0.37 in the group with recurrence (P = .025). The mean ratio of glycine was 0.58 in the group without recurrence and 0.048 in the group with recurrence (P = .047). Moreover, five urinary metabolites, F6P, DS7P, lactic acid, succinic acid, and kynurenic acid, had the tendency to differ between the two groups, although no significant difference was observed (Table 4). While stratifying the metabolic pathways of these eight metabolites, we found that three urinary metabolites were related to glycolysis, one was related to TCA cycle, three were related to glutaminolysis, and one was related to the tryptophan pathway (Figure 2).
TABLE 4.
Comparison of urinary metabolite concentrations between clear cell renal cell carcinoma (ccRCC) patients with and without recurrence
| Variable | ccRCC without recurrence | ccRCC with recurrence | |||||
|---|---|---|---|---|---|---|---|
| Mean concentration preoperation | Mean concentration postoperation | Mean ratio, post / pre | Mean concentration preoperation | Mean concentration postoperation | Mean ratio. post / pre | P value of mean ratio | |
| Urinary metabolite | μmol/L/u‐Cre (mmol/L) | μmol/L/u‐Cre (mmol/L) | μmol/L/u‐Cre (mmol/L) | μmol/L/u‐Cre (mmol/L) | |||
| Galactose | 0.58 | 0.0060 | 1.59 | 0.53 | 0.079 | 7.28 | .85 |
| F6P | 3.75 | 0.68 | 0.20 | 0.30 | 8.27 | 156.57 | .20 |
| DS7P | 122.24 | 15.12 | 6.11 | 3.47 | 966.77 | 19 407.3 | .35 |
| Lactic acid | 593.11 | 22.28 | 0.91 | 43.11 | 132.69 | 38.1 | .21 |
| Succinic acid | 36.7 | 0.89 | 0.036 | 15.4 | 6.1 | 6.74 | .13 |
| Glutamine | 585.97 | 101.97 | 32.7 | 173.88 | 367.42 | 3.65 | .95 |
| Glutamic acid | 115.4 | 6.44 | 2.64 | 1.76 | 11.89 | 39.3 | .021 |
| 2‐HG | 1027.58 | 4.92 | 0.11 | 372.44 | 72.34 | 0.37 | .025 |
| Glycine | 15.62 | 0.0085 | 0.58 | 0.034 | 0.048 | 3.38 | .047 |
| Kynurenic acid | 0.21 | 0.28 | 27.89 | 0.16 | 0.45 | 267.0 | .11 |
Abbreviations: 2‐HG, 2‐hydroxyglutarate; DS7P, d‐sedoheptulose 7‐phosphate; F6P, fructose 6‐phosphate.
FIGURE 2.
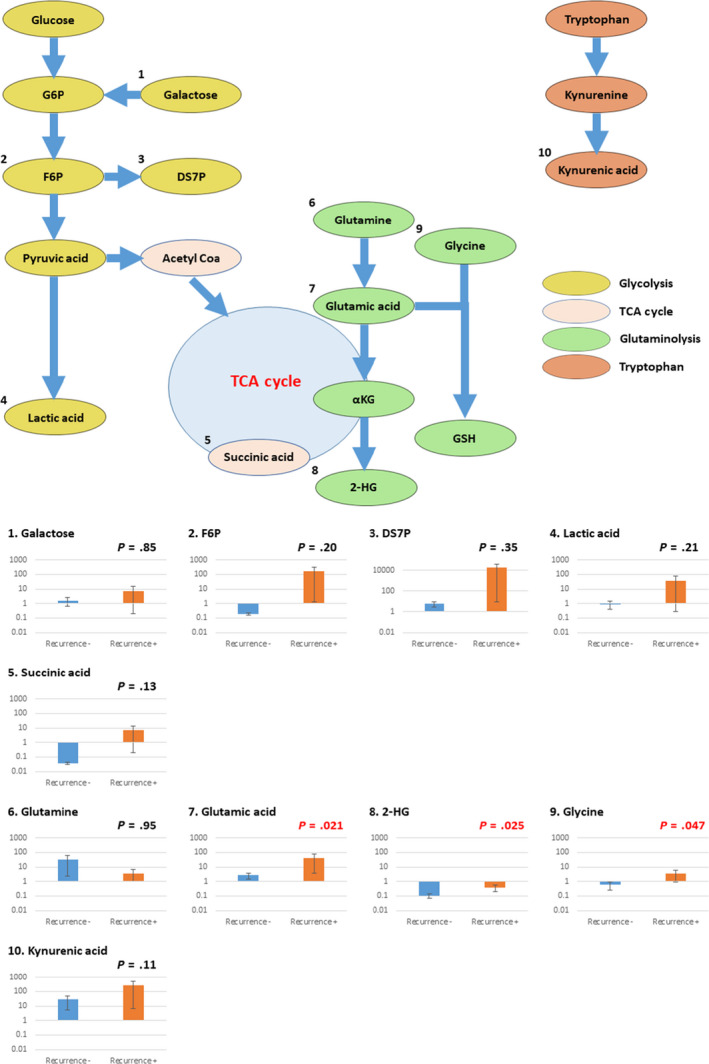
Relationships between metabolic pathways and ratios of post‐ to preoperative concentrations of 10 selected urinary metabolites in clear cell renal cell carcinoma patients with and without recurrence. 2‐HG, 2‐hydroxyglutarate; αKG, α‐ketoglutaric acid; DS7P, d‐sedoheptulose 7‐phosphate; F6P, fructose 6‐phosphate; G6P, glucose‐6‐phosphate; GSH, glutathione; TCA, tricarboxylic acid
3.3. Construction of predictive model by combing urinary metabolites for recurrence of ccRCC after operation
In this study, the median follow‐up period was 16.5 months (3‐30 months). Of the 56 ccRCC patients, nine experienced recurrence within this period (16.0%). The number of RCC patients without recurrence in each pathological stage was 36 for T1 (76.6%), two for T2 (4.3%), and nine for T3 (19.1%), while all patients with recurrence were in T3 (P < .001).
The eight urinary metabolites mentioned above (glutamic acid, 2‐HG, glycine, F6P, DS7P, lactic acid, succinic acid, and kynurenic acid) were subjected to a stepwise variable selection method to construct a predictive model for recurrence after operation. We ultimately selected five metabolites (lactic acid, glycine, 2‐HG, succinic acid, and kynurenic acid) as variables for the predictive model for recurrence. The AUC, sensitivity, and specificity values of this model were 0.894, 88.9%, and 88.0%, respectively (Figure 3A, left). When using this predictive model, among all 56 patients of this study, 47 patients were stratified to the low risk group and nine patients to the high risk group. The low risk group had two recurrences (4.3%), while there were seven recurrences among the high risk group (77.8%). The Kaplan–Meyer curve showed a significant difference in recurrence‐free survival (median OS: low risk group NR vs high risk group 20.0 months, P < .001; Figure 3A, right).
FIGURE 3.
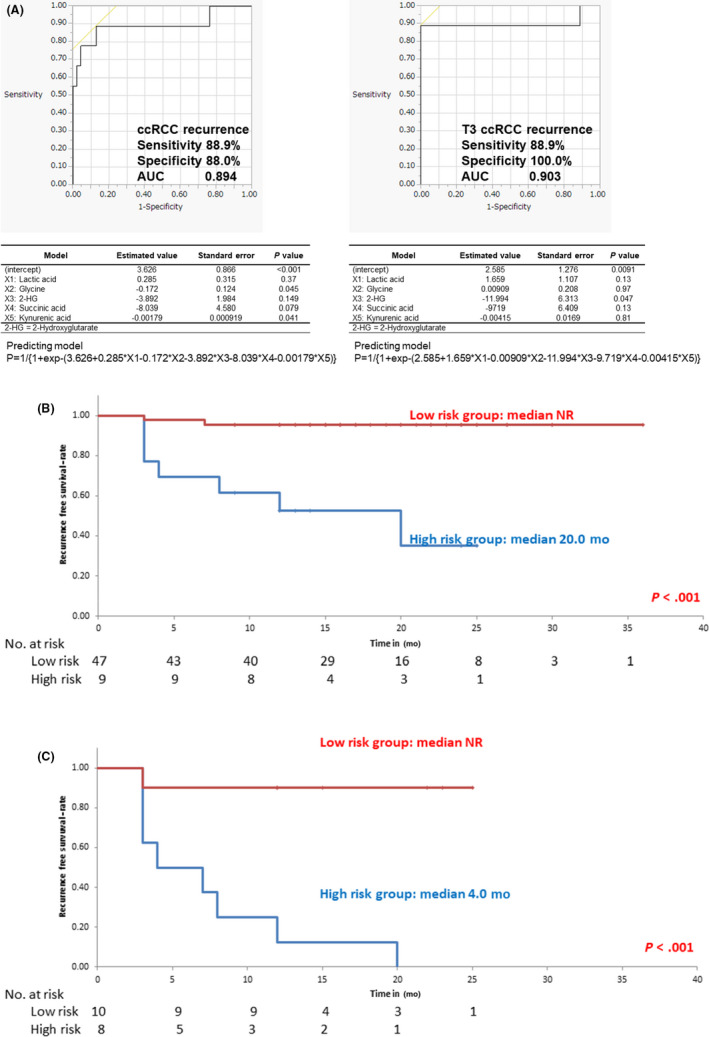
A, Recurrence predictive model for clear cell renal cell carcinoma patients (ccRCC) (left panel) and T3 ccRCC (right panel) constructed by multiple logistic analysis. The coefficients, standard error, and P values for each variable are shown in the table below the graph. The prediction formula is shown below the table. B, Survival curve using the Kaplan–Meier method, based on the ccRCC predictive model. C, Survival curve using the Kaplan–Meier method, based on the T3 ccRCC predictive model. 2‐HG, 2‐hydroxyglutarate; AUC, area under the receiver operating characteristic curve
When limiting the study to T3 patients, the recurrence rate was 50%. To determine the feasibility of this predictive model for recurrence of high stage ccRCC patients after operation, the same analyses were undertaken for T3 ccRCC patients. As a result, the five same metabolites were selected as variables for the recurrence of T3 ccRCC for the predictive model with the same analyses and same methods. The AUC, sensitivity, and specificity values of this model were 0.903, 88.9%, and 100%, respectively (Figure 3A). Consequently, these five urinary metabolites were revealed to be predictive for any stage of ccRCC. Ten patients were stratified into the low risk group and eight patients into the high risk group, using this predictive model. The low risk group had one recurrence (9.1%) while the high risk group had eight recurrences (100%). Survival analysis also showed a significant difference in recurrence‐free survival, based on the predictive model for T3 ccRCC recurrence (median OS: low risk group NR vs high risk group 4.0 months P < .001; Figure 3C).
3.4. Evaluation of five metabolites of the predictive model in intracellular metabolism based on in vitro analysis of RCC cell lines
To evaluate the five metabolites selected by a stepwise variable selection method as described above, we measured intracellular metabolite concentrations of the following three cell lines of RCC: 786‐O, which was derived from a primary tumor of ccRCC; Caki‐1, which was derived from a metastatic site of ccRCC; and ACHN, which was derived from a metastatic site of non‐ccRCC. As all cell lines were frequently used in RCC‐focused research, they could be used to understand the factors that cause urinary metabolic changes or the differences between patients with and without recurrence. Intracellular metabolite concentrations could be measured repeatedly and stably.
Intracellular metabolite concentrations of all five metabolites were significantly higher in Caki‐1 and ACHN cells, than in 786‐O cells (Figure 4). In brief, the metastatic site of RCC had higher metabolites than the primary site. Lactic acid concentrations of 786‐O, Caki‐1, and ACHN cells were 29.98 μmol/L, 118.46 μmol/L, and 150.64 μmol/L, respectively (P < .001 and P < .001, respectively). Lactic acid is the final metabolite of glycolysis and is the most well‐known metabolite of the Warburg effect. Glycine concentrations of 786‐O, Caki‐1, and ACHN cells were 0.0038 μmol/L, 0.015 μmol/L, and 0.012 μmol/L, respectively (P = .0017 and P < .001, respectively). Glycine joined glutamic acid and produced glutathione, which contributed to the reduction of reactive oxygen species. The 2‐HG concentrations of 786‐O, Caki‐1, and ACHN cells were 47.36 μmol/L, 148.13 μmol/L, and 120.46 μmol/L, respectively (P < .001 and P < .001, respectively). Succinic acid concentrations of 786‐O, Caki‐1, and ACHN were 0.037 μmol/L, 0.12 μmol/L, and 0.11 μmol/L, respectively (P = .0044 and P < .001, respectively). 2‐Hydroxyglutarate is the final metabolite of glutaminolysis, and succinic acid is a component of the TCA cycle. Both metabolites are known as oncometabolites. Kynurenic acid concentrations of 786‐O, Caki‐1, and ACHN cells were 11.10 μmol/L, 367.69 μmol/L, and 330.27 μmol/L, respectively (P < .001 and P < .001, respectively). Kynurenic acid is a final product of the tryptophan pathway and has been recently reported to be associated with poor prognosis in several types of cancer. However, no significant difference between Caki‐1 and ACHN cells were observed.
FIGURE 4.
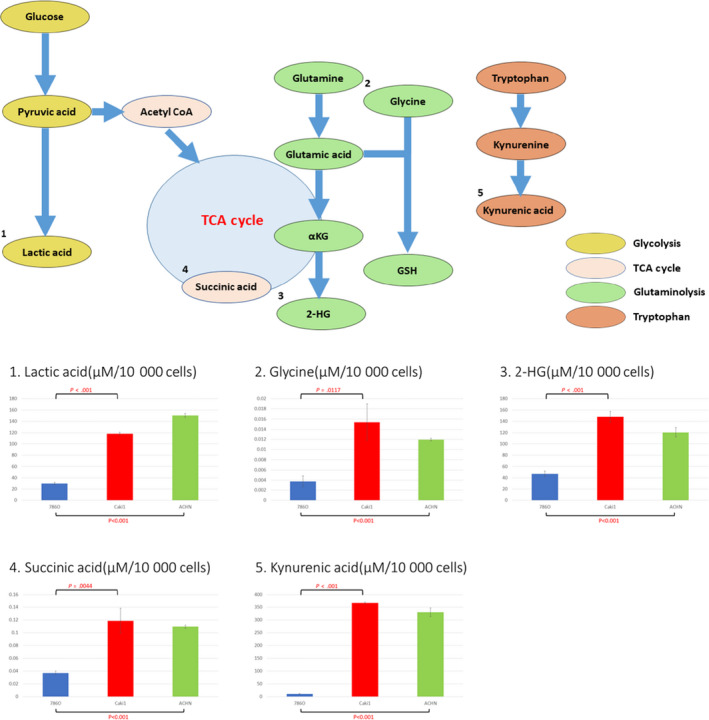
Relationships between five selected metabolites and their metabolic pathways and comparisons of their intracellular concentrations among three renal cell carcinoma cell lines, 786‐O, Caki‐1, and ACHN. 2‐HG, 2‐hydroxyglutarate; αKG, α‐ketoglutaric acid; GSH, glutathione; TCA, tricarboxylic acid
4. DISCUSSION
Data from previous studies report the recurrence rate of RCC after operation to be 40% among patients with unfavorable factors, such as high T Stage, lymphovascular invasion, or lymph metastases. 9 If recurrence could be predicted accurately by using our models, patients at a particular rate of risk of recurrence could be selected to undergo adjuvant therapy.
Microscopic spread of cancer prior to surgery has been reported to cause recurrence soon after surgery. 4 As all nine patients had recurrence within 2 years after surgery, the mechanism of recurrence seemed to be caused by this microscopic spread before surgery. When cancer metabolism differed between primary tumor and metastatic site in RCC, urinary metabolites could be affected by the microscopic spread of cancer. Our previous reports have shown associations between metabolites in ccRCC tissue and urinary metabolites in ccRCC patients. 18 We therefore believe that microscopic spread of RCC could be identified by measuring urine metabolites.
In this study, we highlighted that accurate measurement of urine metabolites before and after surgery could predict recurrence soon after surgery in ccRCC patients. We evaluated the ratio of pre‐ and postoperative concentrations of urinary metabolites. Urinary metabolites depended not only on cancer status but also on lifestyle, diet, or history of complications. 19 As comorbidities such as diabetes and dyslipidemia affected urinary metabolites, we assumed that evaluating the ratio of pre‐ and postoperative urinary metabolites was substantially more reasonable than evaluating the absolute values when establishing a model for predicting recurrence. Of 17 investigated urinary metabolites regarding ccRCC in our previous study, the ratio of pre‐ / postoperative concentrations of 10 urinary metabolites was significantly different between T1a ccRCC and control groups. By analyzing these 10 urinary metabolites, five metabolites (lactic acid, glycine, 2‐HG, succinic acid, and kynurenic acid) were identified for predictive models for recurrence after surgery. To confirm the significance of these five metabolites, we undertook an in vitro study to investigate their intracellular concentrations in three RCC cell lines to clarify their metabolic pathways, and to understand their clinical status.
Our results showed ratios of pre‐ to postoperative concentrations for all urinary metabolites except glutamine were greater in ccRCC patients with recurrence than in those without recurrence. We selected five of the nine metabolites (lactic acid, glycine, 2‐HG, succinic acid, and kynurenic acid) as significant factors for the predictive model for recurrence by a stepwise analysis. They represented intermediate and final metabolites of the metabolic pathways such as glycolysis, glutaminolysis, TCA cycle, and tryptophan. Lactic acid is a well‐known metabolite of the Warburg effect, and has been reported to be significantly elevated in urine samples of patients with RCC. 20 Glycine is an essential metabolite for nucleic acid synthesis, and the methylation sarcosine dehydrogenase gene related to a prognostic factor for recurrence‐free survival in RCC patients. 21 2‐Hydroxyglutarate is widely known as an oncometabolite common in ccRCC patients and has been reported to play a pivotal role in the fate decision of immune cells. 22 Our previous study also showed the relationship between urinary 2‐HG concentration and ccRCC diagnosis. 17 Succinic acid is also known as an oncometabolite and is significantly altered in RCC tissues. 23 Kynurenic acid is known to be one of the key metabolites concerning cancer immunity produced by tryptophan and is related to poor prognosis. 24
Metabolism of cancer patients has been reported to be distinct from that of patients without cancer. 17 We previously reported that energy metabolites related to glycolysis, carnitine, glutaminolysis, and inositol pathways increased in patients with cancer compared to those without. 17 By comparing these differences, metabolomics has been evaluated in several diseases to identify new biomarkers. 11 , 25 , 26 For example, Zhang et al 11 reported that several metabolites, including glutamic acid, succinic acid, and lactic acid, increased in patients with colorectal cancer, and six elevated urinary metabolites (L‐alanine, L‐isoleucine, L‐serine, L‐threonine, L‐proline, and L‐methionine) revealed satisfactory diagnostic values with the AUC of more than 0.75. 26 We can hypothesize that the microscopic spread of cancer caused these metabolic differences and affected the result of this study. In other words, the larger ratio of post‐ to preoperative concentrations of urinary metabolites in ccRCC patients with recurrence indicated that these metabolic changes were caused by microscopic spread of cancer.
To understand the metabolites selected for our predictive model for recurrence, we undertook an in vitro study to investigate intracellular concentrations of five metabolites in the following three cell lines of RCC: 786‐O from primary tumor, Caki‐1, and ACHN from metastatic site. So far, metabolites identified in this study have been reported in these three cell lines. 27 , 28 , 29 In 786‐O, metabolites of glycolysis and the TCA cycle were identified. 27 Damaraju et al 28 reported that nucleoside metabolites with antitumor effects, such as glycine, increased in Caki‐1. Additionally, Hirai et al 29 showed that the anticancer effect was related to an increase in the intracellular contents of glutaminolysis in ACHN. As cancer bioavailability was associated with intracellular concentrations of metabolites from these results, we can assume that evaluating intracellular metabolites would be helpful to understand the relationship between recurrence and urinary metabolites. In this study, we found that intracellular concentrations of all five metabolites in Caki‐1 and ACHN cells were significantly higher than those in 786‐O. Sullivan et al 30 reported that increased levels of metabolites, such as lactic acid, 2‐HG, and succinic acid can promote cancer initiation and progression. Jain et al 31 also reported that high expression of glycine was strongly related to rapid proliferation in cancer cells. As changes in the levels of these metabolites are associated with altered cell signaling and enzyme activity in cancer cells, higher intracellular concentrations result in an increase of malignancy. 30 Both these reports and our result suggested that metastatic sites of RCC had a higher cancer bioavailability than primary sites. As mentioned above, we could elucidate the difference in urinary metabolites between ccRCC patients with and without recurrence. The metabolic change caused by microscopic spread of cancer can be detected by using our quantitative measurement methods.
We acknowledge the following limitations of this study. First, there is a possibility of a selection bias, as our patients had heterogeneous backgrounds, including history of complications or medications. Second, patients were retrospectively enrolled in this study and had a relatively short follow‐up period. Factors such as performance status or operative procedure were not taken into account for oncologic outcomes. Finally, our result lacks a validation cohort. Despite these limitations, our predictive models could identify patients at risk of recurrence who are in need of adjuvant therapy to improve their prognosis. We tried to develop a more precise method by measuring only these five urine metabolites, to withstand actual clinical use in the future.
We have established predictive models with high sensitivity and specificity for recurrence status of ccRCC by our accurate quantitative measurement system of urinary metabolites. These models enable us to select patients in need of adjuvant therapy. Additionally, we confirmed the oncological significance of these metabolites by researching in vitro.
DISCLOSURE
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The study was approved by the ethics review boards of the Tohoku University School of Medicine (authorization number: 2019‐1‐749). This study was supported in part by Grants‐in‐Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (grant nos. 17K07210 and 20K07582).
Morozumi K, Kawasaki Y, Maekawa M, et al. Predictive model for recurrence of renal cell carcinoma by comparing pre‐ and postoperative urinary metabolite concentrations. Cancer Sci.2022;113:182–194. 10.1111/cas.15180
Funding information
Japanese Ministry of Education, Culture, Sports, Science and Technology, Grant/Award Number: 17K07210 and 20K07582
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;1:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Petejova N, Martinek A. Renal cell carcinoma: review of etiology, pathophysiology and risk factors. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:183‐194. [DOI] [PubMed] [Google Scholar]
- 3. Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75:74‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polanco PL, Herranz AF, Caño VJ, et al. Recurrence risk groups after nephrectomy for renal cell carcinoma. Actas Urol Esp. 2019;44:111‐118. [DOI] [PubMed] [Google Scholar]
- 5. Pierorazio PM, Johnson MH, Patel HD, et al. Management of renal masses and localized renal cancer: systematic review and meta‐analysis. J Urol. 2016;196:989‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol. 2018;36:1943‐1952. [DOI] [PubMed] [Google Scholar]
- 7. Wang H, Luo F, Zhu Z, et al. ABCG2 is a potential prognostic marker of overall survival in patients with clear cell renal cell carcinoma. BMC Cancer. 2017;17:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel HD, Puligandla M, Shuch BM, et al. The future of perioperative therapy in advanced renal cell carcinoma: how can we PROSPER? Future Oncol. 2019;15:1683‐1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berquist SW, Yim K, Stephen R, et al. Systemic therapy in the management of localized and locally advanced renal cell carcinoma: current state and future perspectives. Int J Urol. 2019;26:532‐542. [DOI] [PubMed] [Google Scholar]
- 10. Debik J, Euceda LR, Lundgren S, et al. Assessing treatment response and prognosis by serum and tissue metabolomics in breast cancer patients. J Proteome Res. 2019;18:3649‐3660. [DOI] [PubMed] [Google Scholar]
- 11. Zhang F, Zhang Y, Zhao W, et al. Metabolomics for biomarker discovery in the diagnosis, prognosis, survival and recurrence of colorectal cancer: a systematic review. Oncotarget. 2017;8:35460‐35472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loras A, Trassierra M, Sanjuan‐Herraez D, et al. Bladder cancer recurrence surveillance by urine metabolomics analysis. Sci Rep. 2018;8:9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takasaki S, Tanaka M, Kikuchi M, et al. Simultaneous analysis of oral anticancer drugs for renal cell carcinoma in human plasma using liquid chromatography/electrospray ionization tandem mass spectrometry. Biomed Chromatogr. 2018;32:e4184. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Zhang M, Liu X, et al. Urine metabolomics for renal cell carcinoma (RCC) prediction: tryptophan metabolism as an important pathway in RCC. Front Oncol. 2019;9:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falegan OS, Ball MW, Shaykhutdinov RA, et al. Urine and serum metabolomics analyses may distinguish between stages of renal cell carcinoma. Metabolites. 2017;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ganti S, Taylor SL, Kim K, et al. Urinary acylcarnitines are altered in human kidney cancer. Int J Cancer. 2012;130:2791‐2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sato T, Kawasaki Y, Maekawa M, et al. Accurate quantification of urinary metabolites for predictive models manifest clinicopathology of renal cell carcinoma. Cancer Sci. 2020;111:2570‐2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sato T, Kawasaki Y, Maekawa M, et al. Value of global metabolomics in association with diagnosis and clinicopathological factors of renal cell carcinoma. Int J Cancer. 2019;145:484‐493. [DOI] [PubMed] [Google Scholar]
- 19. Kim H, Rebholz CM, Wong E, Buckley JP. Urinary organophosphate ester concentrations in relation to ultra‐processed food consumption in the general US population. Environ Res. 2020;182: 109070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wettersten HI, Hakimi AA, Morin D, et al. Grade‐dependent metabolic reprogramming in kidney cancer revealed by combined proteomics and metabolomics analysis. Cancer Res. 2015;75:2541‐2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazdak M, Tezval H, Callauch J, et al. DNA methylation of sarcosine dehydrogenase (SARDH) loci as a prognosticator for renal cell carcinoma. Oncol Rep. 2019;42:2159‐2168. [DOI] [PubMed] [Google Scholar]
- 22. Du X, Hu H. The Roles of 2‐Hydroxyglutarate. Front Cell Dev Biol. 2021;26: 651317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang N, Gao R, Yang J, et al. Quantitative global proteome and lysine succinylome analyses reveal the effects of energy metabolism in renal cell carcinoma. Proteomics. 2018;18:e1800001. [DOI] [PubMed] [Google Scholar]
- 24. Li H, Bullock K, Gurjao C, et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat Commun. 2019;25:4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abooshahab R, Gholami M, Sanoie M, Azizi F, Hedayati M. Advances in metabolomics of thyroid cancer diagnosis and metabolic regulation. Endocrine. 2019;65:1‐14. [DOI] [PubMed] [Google Scholar]
- 26. Xiao S, Zhou L. Gastric cancer: metabolic and metabolomics perspectives (Review). Int J Oncol. 2017;51:5‐17. [DOI] [PubMed] [Google Scholar]
- 27. Hatakeyama H, Fujiwara T, Sato H, Terui A, Hisaka A. Investigation of metabolomic changes in sunitinib‐resistant human renal carcinoma 786‐O cells by capillary electrophoresis‐time of flight mass spectrometry. Biol Pharm Bull. 2018;41:619‐627. [DOI] [PubMed] [Google Scholar]
- 28. Damaraju VL, Mowles D, Wilson M, Kuzma M, Cass CE, Sawyer MB. Comparative in vitro evaluation of transportability and toxicity of capecitabine and its metabolites in cells derived from normal human kidney and renal cancers. Biochem Cell Biol. 2013;91:419‐427. [DOI] [PubMed] [Google Scholar]
- 29. Hirai Y, Kawabe N, Tsuda Y, Miyamoto S, Iwakawa S. Effect of 2‐methoxyestradiol, buthionine sulfoximine and hydrogen peroxide on the viability of renal carcinoma cell lines (ACHN and ACVB). Biol Pharm Bull. 2006;29:1064‐1067. [DOI] [PubMed] [Google Scholar]
- 30. Sullivan LB, Gui DY, Vander Heiden MG. Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer. 2016;16:680‐693. [DOI] [PubMed] [Google Scholar]
- 31. Jain M, Nilsson R, Sharma S, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;25:1040‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


