Abstract
The association between alcohol intake and stomach cancer risk remains controversial. We undertook a pooled analysis of data from six large‐scale Japanese cohort studies with 256 478 participants on this topic. Alcohol intake as ethanol was estimated using a validated questionnaire. The participants were followed for incidence of stomach cancer. We calculated study‐specific hazard ratios (HRs) and 95% confidence intervals (CIs) for stomach cancer according to alcohol intake using a Cox regression model. Summary HRs were estimated by pooling the study‐specific HRs using a random‐effects model. During 4 265 551 person‐years of follow‐up, 8586 stomach cancer cases were identified. In men, the multivariate‐adjusted HRs (95% CIs) of stomach cancer were 1.00 (0.87‐1.15) for occasional drinkers, and 1.00 (0.91‐1.11) for <23 g/d, 1.09 (1.01‐1.18) for 23 to <46 g/d, 1.18 (1.09‐1.29) for 46 to <69 g/d, 1.21 (1.05‐1.39) for 69 to <92 g/d, and 1.29 (1.11‐1.51) for ≥92 g/d ethanol in regular drinkers compared with nondrinkers. In women, the multivariate‐adjusted HRs were 0.93 (0.80‐1.08) for occasional drinkers, and 0.85 (0.74‐0.99) for <23 g/d, and 1.22 (0.98‐1.53) for ≥23 g/d in regular drinkers compared with nondrinkers. The HRs for proximal and distal cancer in drinkers vs nondrinkers were 1.69 (1.15‐2.47) and 1.24 (0.99‐1.55) for ≥92 g/d in men, and 1.60 (0.76‐3.37) and 1.18 (0.88‐1.57) for ≥23 g/d in women, respectively. Alcohol intake increased stomach cancer risk in men, and heavy drinkers showed a greater point estimate of risk for proximal cancer than for distal cancer.
Keywords: alcohol intake, cohort study, Japan, pooled analysis, stomach cancer
Alcohol intake increased stomach cancer risk in men, and heavy drinkers showed a greater point estimate of risk for proximal cancer than for distal cancer.

Abbreviations
- ALDH2
aldehyde dehydrogenase 2
- CI
confidence interval
- HR
hazard ratio
- IARC
International Agency for Research on Cancer
- ICD‐O‐3
International Classification of Diseases for Oncology, Third Edition
- JACC
Japan Collaborative Cohort Study
- JPHC‐I
Japan Public Health Center‐based Prospective Study I
- JPHC‐II
Japan Public Health Center‐based Prospective Study II
- LSS
Life Span Study
- MIYAGI
Miyagi Cohort Study
- OHSAKI
Ohsaki Cohort Study
1. INTRODUCTION
Stomach cancer is the fourth most common cancer and the third leading cause of cancer deaths worldwide; it remains the most frequent cancer in East Asia, despite a declining trend. 1 There is a consensus that Helicobacter pylori infection, smoking, and high salt intake are risk factors for stomach cancer. 2 , 3 , 4 , 5 Higher intake of vegetables, fruit, and green tea could be protective against stomach cancer. 6 , 7 , 8 , 9 Animal studies indicate that alcohol is harmful to the stomach, 10 partly because alcohol metabolism produces the carcinogen acetaldehyde. 11 Acetaldehyde related to alcohol intake is now considered a group 1 carcinogen by the IARC, 12 and alcohol intake is an established risk factor for several cancers, including oral cavity, head and neck, esophagus, breast, liver, and colorectal cancer. 13 A recent large meta‐analysis of mainly European and American cohort and case‐control studies showed that high alcohol intake of ≥50 g/d ethanol substantially increases the risk of stomach cancer. 14 However, meta‐analysis using publication data cannot fully examine dose–response relationships, and epidemiological evidence for the association between alcohol intake and stomach cancer risk remains controversial. 15 In addition, few cohort studies have examined whether alcohol consumption analyzed as ethanol intake is associated with stomach cancer risk in East Asian populations with a high incidence of stomach cancer. To date, six cohort studies in Japan have examined the association between alcohol intake and stomach cancer risk, and their findings are inconsistent. 16 , 17 , 18 , 19 , 20 , 21 The findings from cohort studies in Korea and China are also inconclusive. 22 , 23 , 24 Thus, it is necessary to clarify the effect of alcohol intake on stomach cancer risk in Japan and other East Asian countries, using large datasets from cohort studies.
To address this issue, we undertook a pooled analysis of data from six large‐scale Japanese cohort studies with more than 250 000 participants on the association between ethanol intake and stomach cancer risk.
2. MATERIALS AND METHODS
2.1. Study cohorts
Since 2006, the Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan has been undertaking pooled analyses using original data from major cohort studies to examine the association between lifestyle factors and major cancers in Japanese people. To maintain high quality and comparability of data, the following inclusion criteria were defined a priori for the present analysis: (a) population‐based cohort studies carried out in Japan; (b) studies initiated between the mid‐1980s and mid‐1990s; (c) studies with more than 30 000 participants; (d) studies that obtained information on alcohol intake at baseline survey using a self‐administered questionnaire; and (e) studies that collected incidence data for stomach cancer during a follow‐up period. We eventually identified six studies that met these criteria: (a) JPHC‐I 25 ; (b) JPHC‐II 25 ; (c) JACC 26 ; (d) MIYAGI 27 ; (e) OHSAKI 28 ; and (f) LSS. 29 Selected characteristics of these cohort studies are summarized in Table 1. The relevant institutional review board approved each study.
TABLE 1.
Characteristics of six Japanese cohort studies: pooled analysis of the association between alcohol intake and stomach cancer risk, 1988‐2014
| Study | Cohort | Age range at baseline survey (y) | Year of baseline survey | Cohort size | Response rate (%) for baseline questionnaire | Method of follow‐up | Present pooled analysis | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age range (mean ± SD, y) | Last follow‐up | Mean follow‐up period (y) | Size of the cohort | Number of stomach cancer cases | ||||||||||
| Men | Women | Men | Women | |||||||||||
| JPHC Study I | Japanese residents of 5 public health center areas in Japan | 40‐59 | 1990 | 61 595 | 82.0 | Cancer registry and death certificate | 40‐59 (49.5 ± 5.9) | 2013 | 21.0 | 20 239 | 21 633 | 1121 | 439 | Incidence |
| JPHC Study II | Japanese residents of 6 public health center areas in Japan | 40‐69 | 1993‐1994 | 78 825 | 80.0 | Cancer registry and death certificate | 40‐69 (53.3 ± 8.9) | 2013 | 17.3 | 28 080 | 31 909 | 1294 | 538 | Incidence |
| JACC Study | Residents from 45 areas throughout Japan | 40‐79 | 1988‐1990 | 110 585 | 83.0 | Cancer registry (selected areas: 24) and death certificate | 40‐79 (57.8 ± 10.2) | 2009 | 15.7 | 22 388 | 33 239 | 962 | 585 | Incidence |
| Miyagi Cohort Study | Residents of 14 municipalities in Miyagi Prefecture, Japan | 40‐64 | 1990 | 47 605 | 92.0 | Cancer registry and death certificate | 40‐64 (51.6 ± 7.5) | 2014 | 21.2 | 21 074 | 18 696 | 1438 | 453 | Incidence |
| Ohsaki Cohort Study | Residents of 14 municipalities in Miyagi Prefecture, Japan | 40‐79 | 1994 | 52 029 | 95.0 | Cancer registry and death certificate | 40‐79 (59.6 ± 10.4) | 2008 | 10.7 | 21 370 | 19 618 | 958 | 309 | Incidence |
| Life Span Study | Atomic bomb survivors in Hiroshima and Nagasaki, Japan a | 46‐104 | 1991 | 20 147 | 100 | Cancer registry and death certificate | 46‐104 (62.6 ± 11.6) | 2003 | 10.7 | 6800 | 11 432 | 278 | 211 | Incidence |
Abbreviations: JACC, Japan Collaborative Cohort Study; JPHC, Japan Public Health Center‐based Prospective Study.
Life Span Study originally started in 1950, and subjects who responded to the 1991 survey were analyzed for the present pooled analysis.
We excluded data from participants with a history of any cancer at baseline, those with missing information on alcohol intake, and those with exposure to atomic bomb radiation of 100 mGy or more for LSS. Data on 256 478 participants were finally included in the present pooled analysis. The JPHC‐I, MIYAGI, and JACC studies have already published results for the association between alcohol intake and stomach cancer risk. 17 , 19 , 21 In the present study, we used the updated dataset for JPHC‐I and MIYAGI with a longer follow‐up period and reanalyzed the dataset for JACC.
2.2. Assessment of alcohol intake
Information on alcohol intake in each study was collected using a self‐administered questionnaire at baseline. Although the wording of the questions varied among studies, each study calculated total alcohol intake for regular drinkers in grams per day ethanol as a continuous variable according to the alcoholic beverage type, frequency, and amount. Alcohol intake was calculated by multiplying the frequency of consumption for each type of liquor by alcohol content of the specific portion and by the portion size for one occasion. The total alcohol intake was then estimated by summing the alcohol intake over all liquor types. Each study questionnaire contained items on the intake of alcoholic beverages popular in Japan, including beer, sake, and shochu, but the style of the questions differed across studies. Thus, in the present study, we used only total alcohol intake from all beverages as the exposure. In Japan, the go is the unit most commonly used to measure the amount of alcohol intake; 1 go of sake (rice wine) is equivalent to 180 mL and contains approximately 23 g of ethanol.
According to total alcohol intake, participants were classified as follows: for men, nondrinkers (never and former drinkers), occasional drinkers (defined as those who drink less than once/week) and regular drinkers (defined as those who drink at least once/week: <23, 23 to <46, 46 to <69, 69 to <92, and ≥92 g/d ethanol); for women, nondrinkers, occasional drinkers, and regular drinkers (<23 and ≥23 g/d ethanol). Those with extremely high alcohol intake were classified into the highest categories because we did not exclude such high alcohol intake estimated with the food frequency questionnaire in each study. Sensitivity analyses were undertaken by setting never drinkers as the reference group in JPHC‐II, JACC, MIYAGI, OHSAKI, and LSS, in which former drinkers were separated from nondrinkers. We also undertook an additional sensitivity analysis in which occasional drinkers and regular drinkers were defined as those who drink less than three times/week and those who drink three times/week or more, respectively. In this analysis, LSS defined regular drinkers as those who drink at least five times/week due to the format of questionnaire.
The correlation coefficients between self‐reported alcohol intake and dietary records were 0.79 in men and 0.44 in women for JPHC‐I, 30 0.59 in men and 0.40 in women for JPHC‐II, 31 0.77 in men and 0.71 in women for MIYAGI, 32 and 0.70 in men for OHSAKI. 33 Although information on the validity of alcohol intake assessment was not available for JACC, the study used the same questionnaire on alcohol intake as that used in MIYAGI. The LSS also used a similar questionnaire for alcohol intake, although a validation study was not carried out. 34
2.3. Follow‐up and case ascertainment
Participants were followed from the baseline survey (JPHC‐I, 1990; JPHC‐II, 1993‐1994; JACC, 1988‐1990; MIYAGI, 1990; OHSAKI, 1994; LSS, 1991) until the last follow‐up date for cancer incidence in each study (JPHC‐I, 2013; JPHC‐II, 2013; JACC, 2009; MIYAGI, 2014; OHSAKI, 2008; LSS, 2003), as shown in Table 1. Vital status was confirmed through the residential registry. Information on cause of death was obtained from death certificates, and the cause of death was coded according to the International Classification of Diseases, 10th Revision. Information on cancer diagnosis was collected using population‐based cancer registries and active patient notifications from major local hospitals. In JACC, information on cancer diagnosis was collected in 24 of 45 study areas. Cases were coded using ICD‐O‐3.
The outcome in the present study was defined as stomach cancer incidence (ICD‐O‐3, topography code C16), which was diagnosed during the follow‐up period of each study. Information on cause of death from death certificates was used to complement the registry and hospital data on cancer diagnosis. If information on the date of diagnosis was not available for stomach cancer cases confirmed by death certificate, we used the date of death from stomach cancer as the date of diagnosis. The information on stomach cancer incidence by subsite allowed us to evaluate the association between alcohol intake and the risk of stomach cancer subsite; thus, we classified stomach cancer cases as proximal (upper third) (ICD‐O‐3, topography code C16.0‐C16.1) and distal (lower two‐thirds) (C16.2‐C16.6). Stomach cancer cases with no information for subsite were not included in the subsite‐specific analysis.
2.4. Statistical analyses
Person‐years of follow‐up were counted from the date of baseline survey in each study until the date of stomach cancer diagnosis, migration from the study area, death, or the end of follow‐up, whichever came first. Each study used a Cox proportional hazard regression model to estimate sex‐specific HR with 95% CI for stomach cancer incidence according to alcohol intake in the following five models. Model 1 was adjusted for age and area (JPHC‐I, JPHC‐II, JACC, and LSS only). Model 2 was adjusted for covariates in model 1 plus smoking status (for men, pack‐years: 0, <20, 20 to <40, or ≥40; for women, pack‐years: 0, <20, ≥20) and medical history of diabetes mellitus (yes, no). Model 3 was adjusted for covariates in model 2 plus total energy intake (quartiles), vegetable intake (quartiles), fruit intake (quartiles), salt intake (quartiles), and green tea consumption (cups/d: <1, 1‐2, 3‐4, and ≥5). In models 4 and 5, we excluded participants with stomach cancer diagnosis within 3 years from baseline in models 2 and 3, respectively. An indicator term for missing data was created for categorical covariates. Pack‐years in ever smokers were calculated as (daily consumption of tobacco [number of cigarettes/d]) × (duration of smoking [y])/20. Dietary intake was adjusted for total energy intake using the residual method before categorizing subjects into quartiles. 35 Trend associations were assessed by calculating the HR for 10‐g/d increase in alcohol intake and its standard error in the respective model in which nondrinkers and occasional drinkers were defined as zero for alcohol intake. In the subsite‐specific analysis, those without the outcome were considered at risk. Each study also undertook a subgroup analysis of the association between drinking and stomach cancer incidence by smoking status (nonsmokers or ever smokers); we controlled for pack‐years in the respective model for ever smokers. Interaction between drinking and smoking status for risk was also assessed with a model including a cross‐product term (ethanol intake [continuous variable] × smoking status [ever vs never]) indicating interaction.
To obtain a single pooled estimate of the HR for stomach cancer from individual studies according to alcohol intake category, we used a random‐effects model, which considers both within‐study and between‐study variation. 36 One study that had no cases for one alcohol intake category was not included in the pooled estimate for the category. The LSS was not included in models 3 or 5 because of no available data for dietary intake. Heterogeneity among studies was assessed using Q‐statistics and I 2 statistics. The correlation between drinking and smoking status was tested by a Cochran–Mantel–Haenszel test based on the pooled distribution of individual studies. To assess a single pooled interaction between drinking and smoking status for risk, we used a random‐effects model in the same manner as that used to obtain a single pooled estimate of the HR for stomach cancer from all the cohort studies. Statistical analyses were carried out using Stata statistical software version 13.1 (StataCorp). A two‐tailed P value < .05 was considered statistically significant.
3. RESULTS
This study included 256 478 participants (119 951 men and 136 527 women) and 8586 stomach cancer cases (6051 men and 2535 women) during 4 265 551 person‐years of follow‐up, as shown in Table 1. Approximately half the men habitually consumed more than 23.0 g/d of ethanol, whereas only 3% of women consumed this quantity.
Table 2 shows the association between alcohol intake and stomach cancer risk in men. Men with higher alcohol intake were at a significantly greater risk of stomach cancer. Compared with nondrinkers, the multivariate‐adjusted HRs (95% CIs) for stomach cancer were 1.00 (0.87‐1.15) for occasional drinkers, and 1.00 (0.91‐1.11) for <23 g/d ethanol, 1.09 (1.01‐1.18) for 23 to <46 g/d, 1.18 (1.09‐1.29) for 46 to <69 g/d, 1.21 (1.05‐1.39) for 69 to <92 g/d, and 1.29 (1.11‐1.51) for ≥92 g/d in regular drinkers in model 2 with adjustment for age, study area, smoking status, and medical history of diabetes mellitus. The median of alcohol intake in the category for ≥92 g/d was 115 g/d (range, 92‐4495). The HR (95% CI) for 10‐g/d increase in ethanol was 1.023 (1.011‐1.035), although the test for cross‐study heterogeneity was statistically significant (P = .019). In model 3, further adjustment for intake of total energy, vegetables, fruit, salt, and green tea did not substantially change the results. The findings did not alter after excluding participants with stomach cancer diagnosis within 3 years from baseline in both models 4 and 5. The analysis by stomach cancer subsite indicated that the point estimate of risk for heavy drinkers was greater for proximal cancer than for distal cancer. Compared with nondrinkers, the multivariate‐adjusted HRs (95% CIs) for ≥92 g/d were 1.69 (1.15‐2.47) for proximal cancer and 1.24 (0.99‐1.55) for distal cancer in model 2. These findings were almost the same when former drinkers were separated from nondrinkers as a sensitivity analysis using data from JPHC‐II, JACC, MIYAGI, OHSAKI, and LSS, as shown in Table S1. When regular drinkers were defined as those who drink at least three times/wk (at least five times/wk only for LSS), the associations were almost unchanged. Compared with nondrinkers, the multivariate‐adjusted HRs (95% CIs) for ≥92 g/d in model 2 were 1.29 (1.10‐1.51) for all stomach cancer, 1.69 (1.15‐2.49) for proximal cancer, and 1.24 (0.98‐1.55) for distal cancer. The HRs (95% CI) for 10‐g/d increase in ethanol (model 2) were 1.022 (1.010‐1.035) for all stomach cancer, 1.016 (1.002‐1.029) for proximal cancer, and 1.022 (1.009‐1.036) for distal cancer. Figure 1 shows the forest plot for HRs (95% CIs) of stomach cancer for those drinking ≥92 g/d ethanol compared with nondrinkers for men in model 2. Figure 2 shows the forest plot for HRs (95% CIs) of stomach cancer for 10‐g/day increase in ethanol for men in model 2. Almost all of the cohort in the pooled analysis showed a significant or nonsignificant positive association between alcohol intake and stomach cancer risk.
TABLE 2.
Results from a pooled analysis using a random effects model for stomach cancer incidence by alcohol intake in Japanese men, 1988‐2014
| Site of cancer | Total | Nondrinkers | Occasional drinkers (<once/wk) | Regular drinkers (≥once/wk) | P for heterogeneity for the highest category f | Alcohol intake as a continuous variable (per 10 g/d) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <23 g/d | 23 to <46 g/d | 46 to <69 g/d | 69 to <92 g/d | ≥92 g/d | HR (95% CI) | P for trend | P for heterogeneity f | |||||
| Total | ||||||||||||
| No. of subjects | 119 951 | 29 770 | 8333 | 22 039 | 24 475 | 20 883 | 9585 | 4866 | ||||
| No. of cases | 6051 | 1465 | 343 | 1001 | 1264 | 1206 | 531 | 241 | ||||
| Person‐years | 1 947 366 | 450 844 | 145 094 | 354 254 | 404 442 | 347 640 | 162 231 | 82 861 | ||||
| Incidence rate per 100 000 person‐years | 311 | 325 | 236 | 283 | 313 | 347 | 327 | 291 | ||||
| Age‐ and area‐adjusted HR (95% CI) as model 1 a | 1.00 (ref.) | 0.99 (0.86‐1.14) | 0.99 (0.89‐1.10) | 1.10 (1.02‐1.19) | 1.23 (1.12‐1.34) | 1.27 (1.09‐1.48) | 1.36 (1.17‐1.59) | .367 | 1.029 (1.015‐1.043) | <.001 | .001 | |
| Multivariate‐adjusted HR (95% CI) in model 2 b | 1.00 (ref.) | 1.00 (0.87‐1.15) | 1.00 (0.91‐1.11) | 1.09 (1.01‐1.18) | 1.18 (1.09‐1.29) | 1.21 (1.05‐1.39) | 1.29 (1.11‐1.51) | .361 | 1.023 (1.011‐1.035) | <.001 | .019 | |
| Multivariate‐adjusted HR (95% CI) in model 3 c | 1.00 (ref.) | 1.00 (0.85‐1.18) | 0.98 (0.89‐1.07) | 1.07 (0.99‐1.16) | 1.15 (1.05‐1.25) | 1.19 (1.05‐1.36) | 1.23 (1.05‐1.43) | .607 | 1.019 (1.007‐1.032) | .002 | .048 | |
| Multivariate‐adjusted HR (95% CI) in model 4 d | 1.00 (ref.) | 1.02 (0.86‐1.20) | 1.00 (0.91‐1.10) | 1.09 (1.00‐1.18) | 1.21 (1.10‐1.33) | 1.23 (1.04‐1.45) | 1.26 (1.08‐1.48) | .652 | 1.019 (1.009‐1.030) | <.001 | .095 | |
| Multivariate‐adjusted HR (95% CI) in model 5 e | 1.00 (ref.) | 1.03 (0.86‐1.23) | 0.97 (0.88‐1.07) | 1.06 (0.97‐1.16) | 1.16 (1.06‐1.27) | 1.18 (1.04‐1.33) | 1.17 (1.01‐1.37) | .571 | 1.016 (1.005‐1.027) | .005 | .123 | |
| Proximal (upper third) | ||||||||||||
| No. of subjects | 119 951 | 29 770 | 8333 | 22 039 | 24 475 | 20 883 | 9585 | 4866 | ||||
| No. of cases | 708 | 171 | 41 | 113 | 133 | 144 | 70 | 36 | ||||
| Person‐years | 1 975 692 | 457 143 | 146 827 | 358 886 | 410 636 | 353 535 | 164 704 | 83 961 | ||||
| Incidence rate per 100 000 person‐years | 36 | 37 | 28 | 31 | 32 | 41 | 43 | 43 | ||||
| Age‐ and area‐adjusted HR (95% CI) as model 1 a | 1.00 (ref.) | 1.04 (0.73‐1.47) | 0.93 (0.73‐1.19) | 1.00 (0.79‐1.26) | 1.21 (0.97‐1.52) | 1.39 (1.02‐1.88) | 1.77 (1.21‐2.58) | .985 | 1.018 (1.006‐1.030) | .004 | .494 | |
| Multivariate‐adjusted HR (95% CI) in model 2 b | 1.00 (ref.) | 1.05 (0.74‐1.49) | 0.95 (0.75‐1.22) | 0.99 (0.79‐1.25) | 1.17 (0.93‐1.48) | 1.31 (0.97‐1.77) | 1.69 (1.15‐2.47) | .987 | 1.016 (1.002‐1.029) | .021 | .700 | |
| Multivariate‐adjusted HR (95% CI) in model 3 c | 1.00 (ref.) | 1.02 (0.71‐1.47) | 0.96 (0.74‐1.25) | 1.01 (0.79‐1.30) | 1.22 (0.95‐1.56) | 1.43 (0.95‐2.17) | 1.95 (1.30‐2.94) | .770 | 1.022 (1.003‐1.042) | .020 | .267 | |
| Multivariate‐adjusted HR (95% CI) in model 4 d | 1.00 (ref.) | 1.00 (0.67‐1.46) | 0.89 (0.68‐1.16) | 1.01 (0.79‐1.30) | 1.25 (0.99‐1.59) | 1.34 (0.97‐1.85) | 1.58 (1.04‐2.40) | .830 | 1.016 (1.004‐1.028) | .010 | .624 | |
| Multivariate‐adjusted HR (95% CI) in model 5 e | 1.00 (ref.) | 0.94 (0.64‐1.39) | 0.90 (0.67‐1.20) | 1.04 (0.80‐1.35) | 1.30 (1.01‐ 1.69) | 1.41 (1.01‐1.97) | 1.79 (1.14‐2.81) | .487 | 1.024 (1.004‐1.044) | .016 | .265 | |
| Distal (lower two‐thirds) | ||||||||||||
| No. of subjects | 119 951 | 29 770 | 8333 | 22 039 | 24 475 | 20 883 | 9585 | 4866 | ||||
| No. of cases | 3573 | 803 | 221 | 573 | 767 | 737 | 324 | 148 | ||||
| Person‐years | 1 958 704 | 453 751 | 145 656 | 356 252 | 406 804 | 349 830 | 163 162 | 83 249 | ||||
| Incidence rate per 100 000 person‐years | 182 | 177 | 152 | 161 | 189 | 211 | 199 | 178 | ||||
| Age‐ and area‐adjusted HR (95% CI) as model 1 a | 1.00 (ref.) | 1.07 (0.87‐1.33) | 0.99 (0.88‐1.11) | 1.20 (1.05‐1.36) | 1.32 (1.14‐1.52) | 1.29 (1.08‐1.54) | 1.31 (1.05‐1.64) | .285 | 1.029 (1.013‐1.045) | <.001 | .008 | |
| Multivariate‐adjusted HR (95% CI) in model 2 b | 1.00 (ref.) | 1.09 (0.88‐1.34) | 1.00 (0.89‐1.11) | 1.18 (1.04‐1.34) | 1.28 (1.11‐1.47) | 1.22 (1.03‐1.46) | 1.24 (0.99‐1.55) | .284 | 1.023 (1.009‐1.037) | .001 | .034 | |
| Multivariate‐adjusted HR (95% CI) in model 3 c | 1.00 (ref.) | 1.12 (0.87‐1.46) | 0.97 (0.86‐1.09) | 1.13 (1.01‐1.28) | 1.22 (1.05‐1.41) | 1.17 (0.99‐1.38) | 1.14 (0.93‐1.39) | .496 | 1.017 (1.004‐1.030) | .013 | .135 | |
| Multivariate‐adjusted HR (95% CI) in model 4 d | 1.00 (ref.) | 1.14 (0.89‐1.47) | 1.04 (0.91‐1.18) | 1.20 (1.05‐1.37) | 1.31 (1.09‐1.57) | 1.24 (1.05‐1.48) | 1.22 (0.99‐1.49) | .632 | 1.017 (1.005‐1.030) | .006 | .148 | |
| Multivariate‐adjusted HR (95% CI) in model 5 e | 1.00 (ref.) | 1.20 (0.86‐1.64) | 0.99 (0.88‐1.12) | 1.13 (1.00‐1.27) | 1.22 (1.03‐1.44) | 1.17 (1.00‐1.36) | 1.13 (0.91‐1.40) | .508 | 1.011 (1.004‐1.018) | .002 | .452 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; ref., reference.
Adjusted for age and area (Japan Public Health Center‐based Study Prospective Study I [JPHC‐I], JPHC‐II, Japan Collaborative Cohort Study, and Life Span Study only).
Adjusted for covariates in model 1 plus smoking status (pack‐years: 0, <20, 20 to <40, and ≥40) and medical history of diabetes mellitus (yes, no).
Adjusted for covariates in model 2 plus total energy intake (quartiles), vegetable intake (quartiles), fruit intake (quartiles), salt intake (quartiles), and green tea consumption (cups/d: <1, 1‐2, 3‐4, and ≥5).
Excluding participants with stomach cancer diagnosis within 3 y from the baseline in model 2.
Excluding participants with stomach cancer diagnosis within 3 y from the baseline in model 3.
Indicating heterogeneity among the pooled cohort studies.
FIGURE 1.
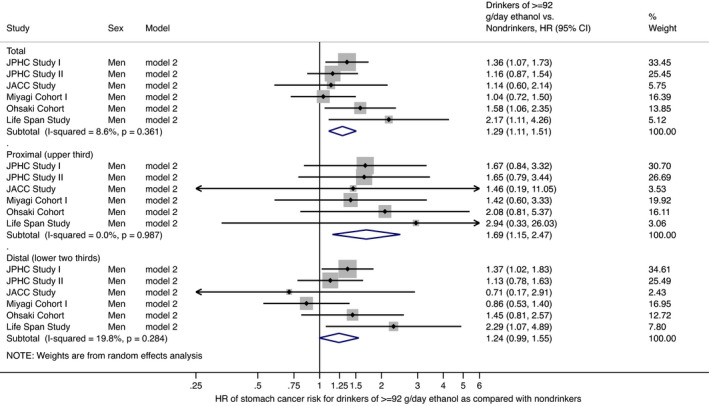
Forest plot of study‐specific and pooled hazard ratios (HRs) and 95% confidence intervals (CIs) of stomach cancer risk for Japanese men in model 2 with adjustment for age, study area, smoking status, and medical history of diabetes mellitus: comparison of drinkers (≥92 g/d ethanol) and nondrinkers. Bars show 95% CIs; arrows show that the CIs extend beyond the effect size range (−0.25 to 6.00). P values indicate heterogeneity among the pooled cohort studies. JACC, Japan Collaborative Cohort; JPHC, Japan Public Health Center‐based Prospective Study
FIGURE 2.

Forest plot of study‐specific and pooled hazard ratios (HRs) and 95% confidence intervals (CIs) of stomach cancer risk for 10‐g/d increase in ethanol in Japanese men in model 2 with adjustment for age, study area, smoking status, and medical history of diabetes mellitus. Bars show 95% CIs. P values indicate heterogeneity among the pooled cohort studies. JACC, Japan Collaborative Cohort; JPHC, Japan Public Health Center‐based Prospective Study
Table 3 shows the association between alcohol intake and stomach cancer risk in women. Consumption of ≥23 g/d ethanol was positively but nonsignificantly associated with stomach cancer risk. Compared with nondrinkers, the multivariate‐adjusted HRs (95% CIs) of stomach cancer were 0.93 (0.80‐1.08) for occasional drinkers, and 0.85 (0.74‐0.99) for <23 g/d and 1.22 (0.98‐1.53) for ≥23 g/d ethanol in regular drinkers in model 2. The median of alcohol intake in the category for ≥23 g/d was 34 g/d (range, 23‐2297). The HR (95% CI) of stomach cancer for 10‐g/d increase in ethanol was 1.031 (0.984‐1.079) with no significant cross‐study heterogeneity (P = .259). Further adjustment for dietary intake in model 3 strengthened the association; compared with nondrinkers, the multivariate‐adjusted HR (95% CI) of stomach cancer risk was 1.38 (1.10‐1.74) for ≥23 g/d ethanol. These results were essentially unchanged after excluding participants with stomach cancer diagnosis within 3 years from baseline in both models 4 and 5. As in men, the analysis stratified by stomach cancer subsite showed that the point estimate of risk associated with alcohol intake was greater for proximal cancer than for distal cancer, although the associations were not statistically significant. Compared with nondrinkers, the multivariate‐adjusted HRs (95% CIs) for ≥23 g/d ethanol were 1.60 (0.76‐3.37) for proximal cancer and 1.18 (0.88‐1.57) for distal cancer in model 2. The findings were again essentially unchanged when we separated former drinkers from nondrinkers as a sensitivity analysis using data from JPHC‐II, JACC, MIYAGI, OHSAKI, and LSS, as shown in Table S2. When regular drinkers were defined as those who drink at least three times/wk (at least five times/wk only for LSS), the associations were almost unchanged. Compared with nondrinkers, the multivariate‐adjusted HRs (95% CIs) of stomach cancer for ≥23 g/d in model 2 were 1.27 (1.01‐1.60) for all stomach cancer, 1.86 (0.88‐3.92) for proximal cancer, and 1.21 (0.88‐1.64) for distal cancer. The HRs (95% CI) for 10‐g/d increase in ethanol (model 2) were 1.031 (0.987‐1.077) for all stomach cancer, 1.031 (0.959‐1.107) for proximal cancer, and 1.044 (0.991‐1.101) for distal cancer. Figure 3 shows the forest plot for HRs (95% CIs) of stomach cancer for those drinking ≥23 g/d ethanol compared with nondrinkers for women in model 2. Figure 4 shows the forest plot for HRs (95% CIs) of stomach cancer for 10‐g/d increase in ethanol for women in model 2.
TABLE 3.
Results from a pooled analysis using a random effects model for stomach cancer incidence by alcohol intake in Japanese women, 1988‐2014
| Site of cancer | Total | Nondrinkers | Occasional drinkers (<once/wk) | Regular drinkers (≥once/wk) | P for heterogeneity for the highest category f | Alcohol intake as a continuous variable (per 10 g/d) | |||
|---|---|---|---|---|---|---|---|---|---|
| <23 g/d | ≥23 g/d | HR (95% CI) | P for trend | P for heterogeneity f | |||||
| Total | |||||||||
| No. of subjects | 136 527 | 106 537 | 11 588 | 14 068 | 4334 | ||||
| No. of cases | 2535 | 2051 | 187 | 210 | 87 | ||||
| Person‐years | 2 318 185 | 1 806 791 | 207 565 | 231 270 | 72 559 | ||||
| Incidence rate per 100 000 person‐years | 109 | 114 | 90 | 91 | 120 | ||||
| Age‐ and area‐adjusted HR (95% CI) as model 1 a | 1.00 (ref.) | 1.02 (0.87‐1.19) | 0.91 (0.75‐1.11) | 1.36 (1.10‐1.69) | .987 | 1.027 (0.995‐1.060) | .094 | .484 | |
| Multivariate‐adjusted HR (95% CI) in model 2 b | 1.00 (ref.) | 0.93 (0.80‐1.08) | 0.85 (0.74‐0.99) | 1.22 (0.98‐1.53) | .722 | 1.031 (0.984‐1.079) | .202 | .259 | |
| Multivariate‐adjusted HR (95% CI) in model 3 c | 1.00 (ref.) | 1.04 (0.88‐1.22) | 0.93 (0.75‐1.15) | 1.38 (1.10‐1.74) | .892 | 1.031 (0.992‐1.071) | .120 | .364 | |
| Multivariate‐adjusted HR (95% CI) in model 4 d | 1.00 (ref.) | 1.03 (0.88‐1.22) | 0.95 (0.79‐1.15) | 1.30 (1.02‐1.65) | .657 | 1.020 (0.983‐1.059) | .294 | .435 | |
| Multivariate‐adjusted HR (95% CI) in model 5 e | 1.00 (ref.) | 1.07 (0.91‐1.26) | 0.98 (0.79‐1.20) | 1.36 (1.05‐1.77) | .374 | 1.023 (0.985‐1.061) | .239 | .503 | |
| Proximal (upper third) | |||||||||
| No. of subjects | 136 527 | 106 537 | 11 588 | 14 068 | 4334 | ||||
| No. of cases | 172 | 139 | 12 | 13 | 8 | ||||
| Person‐years | 2 331 498 | 1 817 505 | 208 598 | 232 381 | 73 014 | ||||
| Incidence rate per 100 000 person‐years | 7 | 8 | 6 | 6 | 11 | ||||
| Age‐ and area‐adjusted HR (95% CI) as model 1 a | 1.00 (ref.) | 1.24 (0.58‐2.68) | 1.27 (0.70‐2.29) | 1.79 (0.87‐3.68) | .969 | 1.029 (0.966‐1.097) | .371 | .984 | |
| Multivariate‐adjusted HR (95% CI) in model 2 b | 1.00 (ref.) | 1.03 (0.56‐1.90) | 1.14 (0.63‐2.06) | 1.60 (0.76‐3.37) | .832 | 1.030 (0.955‐1.109) | .445 | .958 | |
| Multivariate‐adjusted HR (95% CI) in model 3 c | 1.00 (ref.) | 1.23 (0.56‐2.69) | 1.29 (0.67‐2.47) | 1.85 (0.87‐3.91) | .901 | 1.041 (0.967‐1.120) | .286 | .916 | |
| Multivariate‐adjusted HR (95% CI) in model 4 d | 1.00 (ref.) | 1.16 (0.59‐2.31) | 1.30 (0.68‐2.49) | 1.77 (0.80‐3.92) | .932 | 1.033 (0.958‐1.114) | .396 | .967 | |
| Multivariate‐adjusted HR (95% CI) in model 5 e | 1.00 (ref.) | 1.17 (0.58‐2.37) | 1.28 (0.63‐2.62) | 1.93 (0.86‐4.32) | .926 | 1.045 (0.972‐1.123) | .237 | .928 | |
| Distal (lower two‐thirds) | |||||||||
| No. of subjects | 136 527 | 106 537 | 11 588 | 14 068 | 4334 | ||||
| No. of cases | 1475 | 1176 | 123 | 125 | 51 | ||||
| Person‐years | 2 323 363 | 1 811 056 | 207 860 | 231 718 | 72 729 | ||||
| Incidence rate per 100 000 person‐years | 63 | 65 | 59 | 54 | 70 | ||||
| Age‐ and area‐adjusted HR (95% CI) as model 1 a | 1.00 (ref.) | 1.07 (0.88‐1.29) | 0.89 (0.65‐1.23) | 1.29 (0.97‐1.71) | .823 | 1.038 (0.991‐1.087) | .119 | .656 | |
| Multivariate‐adjusted HR (95% CI) in model 2 b | 1.00 (ref.) | 0.99 (0.82‐1.19) | 0.86 (0.65‐1.13) | 1.18 (0.88‐1.57) | .624 | 1.036 (0.985‐1.090) | .170 | .467 | |
| Multivariate‐adjusted HR (95% CI) in model 3 c | 1.00 (ref.) | 1.08 (0.88‐1.31) | 0.94 (0.67‐1.32) | 1.28 (0.94‐1.74) | .811 | 1.036 (0.980‐1.095) | .212 | .671 | |
| Multivariate‐adjusted HR (95% CI) in model 4 d | 1.00 (ref.) | 1.08 (0.89‐1.32) | 0.93 (0.66‐1.30) | 1.30 (0.96‐1.76) | .783 | 1.046 (0.989‐1.107) | .116 | .597 | |
| Multivariate‐adjusted HR (95% CI) in model 5 e | 1.00 (ref.) | 1.10 (0.90‐1.36) | 0.98 (0.69‐1.40) | 1.32 (0.96‐1.82) | .640 | 1.044 (0.984‐1.108) | .157 | .640 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; ref., reference.
Adjusted for age and area (Japan Public Health Center‐based Prospective Study I [JPHC I], JPHC II, Japan Collaborative Cohort Study, and Life Span Study only).
Adjusted for covariates in model 1 plus smoking status (pack‐years: 0, <20, and ≥20) and medical history of diabetes mellitus (yes, no).
Adjusted for covariates in model 2 plus total energy intake (quartiles), vegetable intake (quartiles), fruit intake (quartiles), salt intake (quartiles), and green tea consumption (cups/d: <1, 1‐2, 3‐4, and ≥5).
Excluding participants with stomach cancer diagnosis within 3 y from the baseline in model 2.
Excluding participants with stomach cancer diagnosis within 3 y from the baseline in model 3.
Indicating heterogeneity among the pooled cohort studies.
FIGURE 3.
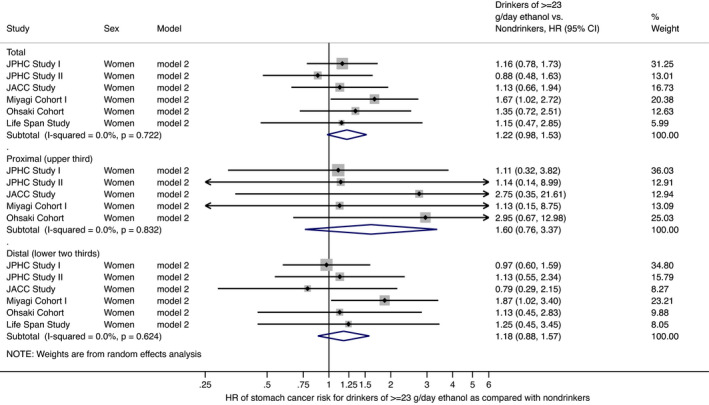
Forest plot of study‐specific and pooled hazard ratios (HRs) and 95% confidence intervals (CIs) of stomach cancer risk for Japanese women in model 2 with adjustment for age, study area, smoking status, and medical history of diabetes mellitus: comparison of drinkers (≥23 g/d ethanol) and nondrinkers. Bars show 95% CIs; arrows show that the CIs extend beyond the effect size range (−0.25 to 6.00). P values indicate heterogeneity among the pooled cohort studies. JACC, Japan Collaborative Cohort; JPHC Study, Japan Public Health Center‐based Prospective Study
FIGURE 4.

Forest plot of study‐specific and pooled hazard ratios (HRs) and 95% confidence intervals (CIs) of stomach cancer risk for 10‐g/d increase in ethanol in Japanese women in model 2 with adjustment for age, study area, smoking status, and medical history of diabetes mellitus. Bars show 95% CIs; arrows show that the CIs extend beyond the effect size range (−0.60 to 1.50). P values indicate heterogeneity among the pooled cohort studies. JACC, Japan Collaborative Cohort Study; JPHC, Japan Public Health Center‐based Prospective Study
A positive correlation between drinking and smoking status was observed in both men and women based on the pooled distribution of individual studies (P < .001, respectively). We therefore explored the association between alcohol intake and stomach cancer risk by smoking status (nonsmokers or ever smokers), as shown in Tables S3 and S4 for men and Tables S5 and S6 for women. The direction of the associations was essentially the same as for the overall results, although the number of stomach cancer cases was small for male nonsmokers and female ever smokers. We detected no significant interaction between drinking and smoking status for stomach cancer risk in any models in men (P > .10), although the interaction in women was significant in models 1‐3 (P = .003, .001 and .001, respectively) but not in models 4‐5 (P > .10).
4. DISCUSSION
In this pooled analysis of population‐based cohort studies undertaken in Japan with more than 250 000 participants and 8500 stomach cancer cases, we found that male regular drinkers had a greater risk of stomach cancer than nondrinkers. The positive associations did not substantially change after excluding participants with stomach cancer diagnosis within 3 years from baseline. These associations were especially marked in male heavy drinkers. Heavy drinkers had a greater point estimate of risk for proximal cancer than for distal cancer.
These findings on the association between alcohol intake and stomach cancer risk are consistent with the results from a recent large meta‐analysis by Tramacere et al 14 of mainly European and American cohort and case‐control studies. The risk of stomach cancer in moderate and heavy drinkers observed here was similar to the results of Tramacere et al; we found that the multivariate‐adjusted HRs (95% CIs) of stomach cancer in men were 1.18 (1.09‐1.29) for 46 to <69 g/d, 1.21 (1.05‐1.39) for 69 to <92 g/d, and 1.29 (1.11‐1.51) for ≥92 g/d, compared with nondrinkers. Tramacere et al found a relative risk of 1.20 (95% CI, 1.01‐1.44) for heavy drinkers of ≥4 drinks/d (≥50 g/d ethanol) compared with nondrinkers. Although their subgroup analysis showed no association between heavy drinking and stomach cancer risk in Asia, the present study found that drinkers of ≥50 g/d ethanol had a significantly greater risk of stomach cancer using a quantitative pooled analysis of original data from each study and common alcohol intake categories across studies. Our findings are also consistent with a report from the World Cancer Research Fund/American Institute for Cancer Research, which indicated that alcohol intake above approximately 45 g/d ethanol (approximately three drinks a day) is probably associated with stomach cancer risk. 37
Moderate Japanese drinkers could be at an increased risk for stomach cancer. Alcohol metabolism produces the carcinogen acetaldehyde, and the ALDH2 enzyme plays an important role in oxidizing harmful alcohol‐related acetaldehyde into harmless acetate. This activity depends on polymorphism in the ALDH2 gene (rs671). 38 Individuals with inactive ALDH2 alleles are exposed to higher concentrations of acetaldehyde after drinking; approximately 40% of the Japanese population has inactive ALDH2 enzyme, 39 whereas few European and American people have inactive ALDH2 enzyme. 40 Interestingly, two case‐control studies in Japan showed a substantial association and interaction between ALDH2 polymorphism (rs671), alcohol intake, and stomach cancer risk, 41 , 42 indicating that drinkers with inactive ALDH2 alleles had a higher risk of stomach cancer compared with nondrinkers carrying homozygous active alleles. Given that inactive ALDH2 alleles are specific to East Asian people, 40 alcohol intake might have a greater effect on stomach cancer risk in East Asian populations than in European and American populations. It is interesting that the subgroup analysis in the meta‐analysis by Tramacere et al 14 showed a substantial increase in stomach cancer risk for drinkers compared with nondrinkers in non‐Asian countries but not in Asia: the HRs (95% CIs) were 1.12 (1.01‐1.24) and 1.02 (0.95‐1.09), respectively. This association, however, could stem from differences in the amount of alcohol intake between the two regions, because drinkers without inactive ALDH2 alleles (ie, most drinkers in European and American populations) can consume more alcohol than drinkers with these alleles. The mediation analyses undertaken in two case‐control studies also suggest that individuals with inactive ALDH2 alleles experience two opposing effects of alcohol intake on the stomach; namely, a carcinogenic effect (ie, a direct effect mediated by increased alcohol‐related acetaldehyde after drinking due to reduced activity of ALDH2 enzymes) and a protective effect (ie, an indirect effect mediated by changing drinking behavior). 42 , 43 Therefore, the amount of alcohol intake and the direct and indirect effects of the ALDH2 polymorphism (rs671) might be associated with stomach cancer risk related to alcohol intake in a complex way. A nested case‐control study in Japan also suggested that the genes related to alcohol metabolism, including ALDH2 polymorphisms (rs671), interacted with the association between alcohol intake and stomach cancer risk. 44 In that study, individuals with inactive ALDH2 alleles who drank ≥150 g/wk ethanol had a significantly greater stomach cancer risk than those individuals without the allele who drank 0 to <150 g/wk; the multivariate‐adjusted odds ratio was 2.08 (95% CI, 1.05‐4.12) (P for interaction = .08).
We showed that heavy drinkers had a greater point estimate of risk for proximal cancer than for distal cancer, whereas Tramacere et al 14 reported that alcohol intake was (nonsignificantly) associated with noncardia stomach cancer rather than with cardia stomach cancer. In that study, drinkers of ≥50 g/d ethanol had a summary relative risk of 1.17 (95% CI, 0.78‐1.75) for gastric noncardia cancer and 0.99 (0.67‐1.47) for gastric cardia cancer, compared with nondrinkers. Although it is unclear why these findings differed from our own, a partial explanation might be the difference in study regions. For example, noncardia stomach cancer cases caused by H. pylori infection and individuals with inactive ALDH2 enzymes are much more common in East Asian populations, including the Japanese population, than in European and American populations. 40 , 45 As the meta‐analysis by Tramacere et al 14 mainly featured Western populations, their findings may reflect a Western‐specific association of alcohol intake with stomach cancer risk by subsite; however, a recent large cohort study with more than 490 000 participants in the United States found no association between higher alcohol intake and gastric noncardia cancer. 15 The observed difference in the association of alcohol intake with stomach cancer risk between subsites could be mediated by different risk factors. Additional studies are needed to elucidate the relevant factors and mechanisms.
We provided evidence for a positive association between alcohol intake and stomach cancer risk among Japanese people using a pooled analysis of data from six large‐scale cohort studies. A strength of this study was that all the studies analyzed had a prospective design, a large population with a large number of stomach cancer cases, a long follow‐up period, used a validated questionnaire to assess alcohol intake, and adjusted for multiple confounders. Our pooled analysis using common alcohol intake categories between studies enabled us to properly examine the dose‐response relationship between alcohol intake and stomach cancer. Pooled analysis using datasets from individual studies yields more precise estimates of the association between exposure and outcome than meta‐analysis using data from publications. 46 Our findings could apply not only to the Japanese population but also to other East Asian populations because they share many factors, such as a high incidence of stomach cancer, high prevalence of H. pylori infection, and genetic background. 1 , 40 , 45 However, the following limitations should be considered. First, we did not consider the effects of the prevalence of H. pylori infection on the association between alcohol intake and stomach cancer, although this is a known strong risk factor for stomach cancer. 2 Several cross‐sectional studies showed that alcohol intake was not associated with H. pylori infection. 47 , 48 In addition, the IARC also states that confounding by H. pylori infection is not a major concern. 49 If alcohol intake is related to H. pylori infection, however, it could confound the association between alcohol intake and stomach cancer. Interestingly, a large‐scale pooled analysis of case‐control studies showed the significant interaction between alcohol intake and H. pylori infection for stomach cancer risk (ie, the synergistic positive effect of alcohol intake and H. pylori infection on stomach cancer risk). 50 Therefore, further studies that take into account H. pylori infection are needed. Second, we did not consider the effects of the relationship between participants’ genetic background (eg, ALDH2 polymorphisms) and alcohol metabolism on the association between alcohol intake and stomach cancer. As previous case‐control and nested case‐control studies suggest an important role of ALDH2 polymorphisms for the association between alcohol intake and stomach cancer risk, 41 , 42 , 43 , 44 cohort studies that examine this role could further elucidate the effect of alcohol intake on stomach cancer risk. Third, we did not examine the association between heavy drinking and stomach cancer risk in women because of the small number of female heavy drinkers. Fourth, although we controlled for the confounding effect of smoking through statistical adjustment or subgroup analysis by smoking status, it is difficult to completely rule out a possible residual confounding effect of smoking. We detected a significant interaction between drinking and smoking status for stomach cancer risk in women; however, the number of stomach cancer cases was limited in female ever smokers. In addition, as the number of heavy drinkers was limited in male nonsmokers, further examinations are required to confirm the association. Fifth, our evaluation of alcohol intake using a self‐administered questionnaire at baseline could have led to misclassification in each study. If present, however, this would have been nondifferential and resulted in underestimation of the associations. Differences in information bias for drinking would not occur between participants with stomach cancer and those without, because this information was recorded before the stomach cancer diagnosis. Finally, we were unable to consider changes in drinking habits and potential confounders (eg, smoking) during the follow‐up period because the information was obtained only at baseline. As people tend to reduce alcohol intake with age due to various reasons, we might overestimate their alcohol intake during the follow‐up; the overestimated exposure could lead to underestimation of the associations.
In conclusion, we provide evidence for a positive association between alcohol intake and stomach cancer risk in men using a pooled analysis of population‐based cohort studies. Better understanding of this relationship could help physicians and policymakers to develop intervention strategies to reduce stomach cancer risk caused by alcohol intake.
DISCLOSURE
The authors have declared no conflicts of interest.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
ACKNOWLEDGMENTS
This study was supported by the National Cancer Center Research and Development Fund (30‐A‐15, 27‐A‐4, 24‐A‐3) and the Health and Labour Sciences Research Grants for the Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare, Japan (H21‐3jigan‐ippan‐003, H18‐3jigan‐ippan‐001, H16‐3jigan‐010). The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan, is a public interest incorporated foundation funded by the Japanese Ministry of Health, Labour and Welfare and the US Department of Energy. This publication was supported by RERF Research Protocol A2‐15. The views of the authors do not necessarily reflect those of the two governments.
The members of the research group comprise the following: Manami Inoue (principal investigator), Sarah K. Abe, Motoki Iwasaki, Michihiro Muto, Eiko Saito, Norie Sawada, Taichi Shimazu, Shiori Tanaka, Shoichiro Tsugane, Taiki Yamaji, Hadrien Charvat (until 2017), Tetsuya Otani (until 2006), Shizuka Sasazuki (until 2017) (National Cancer Center, Tokyo); Akiko Tamakoshi (until 2018) (Hokkaido University, Sapporo); Yumi Sugawara, Ichiro Tsuji, Yoshikazu Nishino (until 2006), Yoshitaka Tsubono (until 2003) (Tohoku University, Sendai); Tetsuya Mizoue (National Center for Global Health and Medicine, Tokyo); Shuhei Nomura (The University of Tokyo, Tokyo); Hidekazu Suzuki (Keio University, Tokyo); Hidemi Ito, Keitaro Matsuo, Isao Oze (Aichi Cancer Center, Nagoya); Kenji Wakai (until 2017) (Nagoya University, Nagoya); Yingsong Lin (Aichi Medical University, Aichi); Chisato Nagata, Keiko Wada (Gifu University, Gifu); Tetsuhisa Kitamura, Yuri Kitamura (until 2019) (Osaka University, Osaka); Tomio Nakayama (until 2017) (Osaka International Cancer Institute, Osaka); Mariko Naito (Hiroshima University, Hiroshima); Kotaro Ozasa, Mai Utada, Atsuko Sadakane (until 2019) (Radiation Effects Research Foundation, Hiroshima); Keitaro Tanaka (Saga University, Saga).
Tamura T, Wakai K, Lin Y, et al; for the Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan . Alcohol intake and stomach cancer risk in Japan: A pooled analysis of six cohort studies. Cancer Sci.2022;113:261–276. doi: 10.1111/cas.15172
Funding information
National Cancer Center Research and Development Fund, Grant/Award Number: 30‐A‐15, 27‐A‐4, 24‐A‐3; Health and Labour Sciences Research Grants for the Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare, Japan, Grant/Award Number: H21‐3jigan‐ippan‐003, H18‐3jigan‐ippan‐001, H16‐3jigan‐010.
[Correction added on 10 December 2021, after first online publication: the first name of the 20th author has been corrected from ‘Mamami’ to ‘Manami’.]
Contributor Information
Takashi Tamura, Email: ttamura@med.nagoya-u.ac.jp.
for the Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan:
Sarah K. Abe, Motoki Iwasaki, Michihiro Muto, Eiko Saito, Shiori Tanaka, Taiki Yamaji, Hadrien Charvat, Tetsuya Otani, Shizuka Sasazuki, Yoshikazu Nishino, Yoshitaka Tsubono, Shuhei Nomura, Hidekazu Suzuki, Isao Oze, Keiko Wada, Yuri Kitamura, Tomio Nakayama, and Atsuko Sadakane
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457‐466. [DOI] [PubMed] [Google Scholar]
- 3. Nishino Y, Inoue M, Tsuji I, et al. Tobacco smoking and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2006;36:800‐807. [DOI] [PubMed] [Google Scholar]
- 4. Kim MK, Sasaki S, Sasazuki S, Tsugane S; Japan Public Health Center‐based Prospective Study Group . Prospective study of three major dietary patterns and risk of gastric cancer in Japan. Int J Cancer. 2004;110:435‐442. [DOI] [PubMed] [Google Scholar]
- 5. Tsugane S, Sasazuki S, Kobayashi M, Sasaki S. Salt and salted food intake and subsequent risk of gastric cancer among middle‐aged Japanese men and women. Br J Cancer. 2004;90:128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang XQ, Yan H, Terry PD, et al. Interaction between dietary factors and Helicobacter pylori infection in noncardia gastric cancer: a population‐based case‐control study in China. J Am Coll Nutr. 2012;31:375‐384. [DOI] [PubMed] [Google Scholar]
- 7. Shimazu T, Wakai K, Tamakoshi A, et al. Association of vegetable and fruit intake with gastric cancer risk among Japanese: a pooled analysis of four cohort studies. Ann Oncol. 2014;25:1228‐1233. [DOI] [PubMed] [Google Scholar]
- 8. Sasazuki S, Tamakoshi A, Matsuo K, et al. Green tea consumption and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:335‐346. [DOI] [PubMed] [Google Scholar]
- 9. Inoue M, Sasazuki S, Wakai K, et al. Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut. 2009;58:1323‐1332. [DOI] [PubMed] [Google Scholar]
- 10. Soffritti M, Belpoggi F, Cevolani D, et al. Results of long‐term experimental studies on the carcinogenicity of methyl alcohol and ethyl alcohol in rats. Ann NY Acad Sci. 2002;982:46‐69. [DOI] [PubMed] [Google Scholar]
- 11. Baan R, Straif K, Grosse Y, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292‐293. [DOI] [PubMed] [Google Scholar]
- 12. IARC, Personal Habits and Indoor Combustions. International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 100(E). IARC, . [PMC free article] [PubMed] [Google Scholar]
- 13. LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and cancer: a statement of the American Society of Clinical Oncology. J Clin Oncol. 2018;36:83‐93. [DOI] [PubMed] [Google Scholar]
- 14. Tramacere I, Negri E, Pelucchi C, et al. A meta‐analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23:28‐36. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Freedman ND, Loftfield E, Hua X, Abnet CC. Alcohol consumption and risk of gastric cardia adenocarcinoma and gastric noncardia adenocarcinoma: a 16‐year prospective analysis from the NIH‐AARP diet and health cohort. Int J Cancer. 2018;143:2749‐2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato I, Tominaga S, Matsumoto K. A prospective study of stomach cancer among a rural Japanese population: a 6‐year survey. Jpn J Cancer Res. 1992;83:568‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasazuki S, Sasaki S, Tsugane S; Japan Public Health Center Study Group . Cigarette smoking, alcohol consumption and subsequent gastric cancer risk by subsite and histologic type. Int J Cancer. 2002;101:560‐566. [DOI] [PubMed] [Google Scholar]
- 18. Kono S, Ikeda M, Tokudome S, Nishizumi M, Kuratsune M. Cigarette smoking, alcohol and cancer mortality: a cohort study of male Japanese physicians. Jpn J Cancer Res. 1987;78:1323‐1328. [PubMed] [Google Scholar]
- 19. Nakaya N, Tsubono Y, Kuriyama S, et al. Alcohol consumption and the risk of cancer in Japanese men: the Miyagi cohort study. Eur J Cancer Prev. 2005;14:169‐174. [DOI] [PubMed] [Google Scholar]
- 20. Tamura T, Wada K, Tsuji M, et al. Association of alcohol consumption with the risk of stomach cancer in a Japanese population: a prospective cohort study. Eur J Cancer Prev. 2018;27:27‐32. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Eshak ES, Shirai K, et al. Alcohol consumption and risk of gastric cancer: The Japan Collaborative Cohort study. J Epidemiol. 2021;31:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sung NY, Choi KS, Park EC, et al. Smoking, alcohol and gastric cancer risk in Korean men: the National Health Insurance Corporation Study. Br J Cancer. 2007;97:700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran GD, Sun X‐D, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456‐463. [DOI] [PubMed] [Google Scholar]
- 24. Moy KA, Fan Y, Wang R, Gao YT, Yu MC, Yuan JM. Alcohol and tobacco use in relation to gastric cancer: a prospective study of men in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2010;19:2287‐2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44:777‐782. [DOI] [PubMed] [Google Scholar]
- 26. Tamakoshi A, Yoshimura T, Inaba Y, et al. Profile of the JACC study. J Epidemiol. 2005;15:S4‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsuji I, Nishino Y, Tsubono Y, et al. Follow‐up and mortality profiles in the Miyagi Cohort Study. J Epidemiol. 2004;14:S2‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsuji I, Nishino Y, Ohkubo T, et al. A prospective cohort study on National Health Insurance beneficiaries in Ohsaki, Miyagi Prefecture, Japan: study design, profiles of the subjects and medical cost during the first year. J Epidemiol. 1998;8:258‐263. [DOI] [PubMed] [Google Scholar]
- 29. Ozasa K, Grant EJ, Kodama K. Japanese legacy cohorts: the life span study atomic bomb survivor cohort and survivors' offspring. J Epidemiol. 2018;28:162‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsubono Y, Kobayashi M, Sasaki S, Tsugane S, JPHC . Validity and reproducibility of a self‐administered food frequency questionnaire used in the baseline survey of the JPHC Study Cohort I. J Epidemiol. 2003;13:S125‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Otani T, Iwasaki M, Yamamoto S, et al. Alcohol consumption, smoking, and subsequent risk of colorectal cancer in middle‐aged and elderly Japanese men and women: Japan Public Health Center‐ based prospective study. Cancer Epidemiol Biomarkers Prev. 2003;12:1492‐1500. [PubMed] [Google Scholar]
- 32. Ogawa K, Tsubono Y, Nishino Y, et al. Validation of a food‐ frequency questionnaire for cohort studies in rural Japan. Public Health Nutr. 2003;6:147‐157. https://pubmed.ncbi.nlm.nih.gov/12675957/ (DOI: 10.1079/PHN2002411 [DOI] [PubMed] [Google Scholar]
- 33. Nakaya N, Kikuchi N, Shimazu T, et al. Alcohol consumption and suicide mortality among Japanese men: the Ohsaki Study. Alcohol. 2007;41:503‐510. [DOI] [PubMed] [Google Scholar]
- 34. Sauvaget C, Allen N, Hayashi M, Spencer E, Nagano J. Validation of a food frequency questionnaire in the Hiroshima/Nagasaki Life Span Study. J Epidemiol. 2002;12:394‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S‐1228S. [DOI] [PubMed] [Google Scholar]
- 36. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 37. World Cancer Research Fund/American Institute for Cancer Research . Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and stomach cancer. dietandcancerreport.org
- 38. Lai C‐L, Yao C‐T, Chau G‐Y, et al. Dominance of the inactive Asian variant over activity and protein contents of mitochondrial aldehyde dehydrogenase 2 in human liver. Alcohol Clin Exp Res. 2014;38:44‐50. [DOI] [PubMed] [Google Scholar]
- 39. Wakai K, Hamajima N, Okada R, et al. Profile of participants and genotype distributions of 108 polymorphisms in a cross‐sectional study of associations of genotypes with lifestyle and clinical factors: a project in the Japan Multi‐Institutional Collaborative Cohort (J‐MICC) Study. J Epidemiol. 2011;21:223‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. International HapMap Consortium . The International HapMap Project. Nature. 2003;426:789‐796. [DOI] [PubMed] [Google Scholar]
- 41. Matsuo K, Oze I, Hosono S, et al. The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis. 2013;34:1510‐1515. [DOI] [PubMed] [Google Scholar]
- 42. Ishioka K, Masaoka H, Ito H, et al. Association between ALDH2 and ADH1B polymorphisms, alcohol drinking and gastric cancer: a replication and mediation analysis. Gastric Cancer. 2018;21:936‐945. [DOI] [PubMed] [Google Scholar]
- 43. Koyanagi YN, Suzuki E, Imoto I, et al. Across‐site differences in the mechanism of alcohol‐induced digestive tract carcinogenesis: an evaluation by mediation analysis. Cancer Res. 2020;80:1601‐1610. [DOI] [PubMed] [Google Scholar]
- 44. Hidaka A, Sasazuki S, Matsuo K, et al. Genetic polymorphisms of ADH1B, ADH1C and ALDH2, alcohol consumption, and the risk of gastric cancer: the Japan Public Health Center‐based prospective study. Carcinogenesis. 2015;36:223‐231. [DOI] [PubMed] [Google Scholar]
- 45. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ferro A, Morais S, Rota M, et al. Alcohol intake and gastric cancer: meta‐analyses of published data versus individual participant data pooled analyses (StoP Project). Cancer Epidemiol. 2018;54:125‐132. [DOI] [PubMed] [Google Scholar]
- 47. Tsugane S, Kabuto M, Imai H, et al. Helicobacter pylori, dietary factors, and atrophic gastritis in five Japanese populations with different gastric cancer mortality. Cancer Causes Control. 1993;4:297‐305. [DOI] [PubMed] [Google Scholar]
- 48. Tamura T, Morita E, Kawai S, et al. No association between Helicobacter pylori infection and diabetes mellitus among a general Japanese population: a cross‐sectional study. Springerplus. 2015;4:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. International Agency for Research on Cancer . Personal habits and indoor combustions. Monograph, Consumption of Alcoholic Beverages. 2012;100E:373‐499.https://publications.iarc.fr/Book‐And‐Report‐Series/Iarc‐Monographs‐On‐The‐Identification‐Of‐Carcinogenic‐Hazards‐To‐Humans/Personal‐Habits‐And‐Indoor‐Combustions‐2012 [Google Scholar]
- 50. Collatuzzo G, Pelucchi C, Negri E, et al. Exploring the interactions between Helicobacter pylori (Hp) infection and other risk factors of gastric cancer: a pooled analysis in the Stomach cancer Pooling (StoP) Project. Int J Cancer. 2021;149:1228‐1238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6


